Abstract
Many bacterial pathogens assemble surface fibers termed pili or fimbriae that facilitate attachment to host cells and colonization of host tissues. The chaperone/usher (CU) pathway is a conserved secretion system that is responsible for the assembly of virulence-associated pili by many different Gram-negative bacteria. Pilus biogenesis by the CU pathway requires a dedicated periplasmic chaperone and an integral outer membrane (OM) assembly and secretion platform termed the usher. Nitazoxanide (NTZ), an antiparasitic drug, was previously shown to inhibit the function of aggregative adherence fimbriae and type 1 pili assembled by the CU pathway in enteroaggregative Escherichia coli, an important causative agent of diarrhea. We show here that NTZ also inhibits the function of type 1 and P pili from uropathogenic E. coli (UPEC). UPEC is the primary causative agent of urinary tract infections, and type 1 and P pili mediate colonization of the bladder and kidneys, respectively. By analysis of the different stages of the CU pilus biogenesis pathway, we show that treatment of bacteria with NTZ causes a reduction in the number of usher molecules in the OM, resulting in a loss of pilus assembly on the bacterial surface. In addition, we determine that NTZ specifically prevents proper folding of the usher β-barrel domain in the OM. Our findings demonstrate that NTZ is a pilicide with a novel mechanism of action and activity against diverse CU pathways. This suggests that further development of the NTZ scaffold may lead to new antivirulence agents that target the usher to prevent pilus assembly.
INTRODUCTION
Adhesive surface structures termed pili or fimbriae are key virulence factors for many bacterial pathogens (1–3). Pili are hair-like fibers composed of multiple different subunit proteins, one or more of which function as adhesins that confer binding to a variety of surfaces. Pilus-mediated adhesion is critical for early stages of infection, allowing invading bacteria to establish a foothold within the host. Following bacterial attachment, pili may also function to modulate host cell signaling pathways, promote or inhibit invasion inside host cells, and facilitate bacterial-bacterial interactions leading to the formation of community structures such as biofilms. Pili thus function to initiate and sustain infection and, therefore, represent attractive therapeutic targets (4, 5).
The chaperone/usher (CU) pathway is a conserved secretion system dedicated to the biogenesis of pili in Gram-negative bacteria (1, 6–8), including pathogens such as Klebsiella pneumoniae, Pseudomonas aeruginosa, Proteus mirabilis, Yersinia pestis, Acinetobacter baumannii, and intestinal and extraintestinal pathogenic Escherichia coli (9–16). Pilus biogenesis by the CU pathway requires two specialized assembly components: a periplasmic chaperone and an integral outer membrane (OM) assembly and secretion platform termed the usher. The chaperone allows proper folding of pilus subunits in the periplasm, maintains subunits in an assembly competent state, and prevents premature subunit-subunit interactions (17, 18). The usher catalyzes the exchange of chaperone-subunit for subunit-subunit interactions, promotes ordered polymerization of the pilus fiber, and provides the channel for secretion of the pilus fiber to the cell surface (19–22).
The type 1 and P pili expressed by uropathogenic Escherichia coli (UPEC) are prototypical pili assembled by the CU pathway. UPEC is the primary causative agent of urinary tract infections (UTIs) and is responsible for ∼85% of all uncomplicated and catheter-associated forms of the disease (23). Type 1 and P pili are key UPEC virulence factors, mediating adhesion to and colonization of the bladder and kidney, respectively (1, 2, 10, 11). Type 1 and P pili have composite architectures that consist of a helical rod segment that extends from the bacterial surface and a distal tip fiber that contains the adhesin (24–26). The type 1 pilus adhesin FimH binds to a variety of surfaces and host tissues in a mannose-sensitive manner (27). UPEC uses type 1 pili to bind to mannosylated proteins present in the bladder, which leads to bacterial colonization, bladder epithelial cell invasion, and the development of cystitis (10). The P pilus adhesin PapG binds to di-galactose moieties present in the globoseries of glycolipids found in kidney epithelial cells (28). The expression of P pili by UPEC is strongly associated with the ability of the bacteria to colonize the kidney and cause pyelonephritis (11, 29). The glycolipid receptor is also part of the P blood group antigen, thus allowing P-pilus-mediated agglutination of human erythrocytes (30).
UTIs are one of the most commonly acquired infections of the human body, afflicting >50% of women and accounting for 40% of all hospital-acquired infections (31, 32). UTIs lead to over 7 million office visits per year at a cost of more than $2 billion annually in the United States alone (32, 33). Although standard antibiotic treatment is often successful in clearing UTIs, high rates of recurrence are associated with the disease. In addition, with the increasing prevalence of antibiotic resistance among UPEC and other pathogenic bacteria, there is an urgent need for the development of new and alternative therapeutics (34). Pili assembled by the CU pathway represent a promising target for clinical intervention, and a number of approaches have been taken to develop antipilus therapeutics, including vaccines against pilus proteins, competitive inhibitors of pilus-mediated adhesion, and small molecules that disrupt pilus biogenesis (35–42). An example of the latter is a class of small molecules termed pilicides, which interfere with the CU pathway and block assembly of P and type 1 pili by UPEC (39, 40).
Nitazoxanide (NTZ) is a synthetic nitrothiazolyl-salicylamide therapeutic that is used to treat intestinal parasitic diseases such as giardiasis and cryptosporidiosis (43, 44). NTZ also exhibits broad-spectrum activity in vitro against strictly anaerobic bacteria (45), including Clostridium difficile, and against members of the Epsilonproteobacteria, including Helicobacter pylori and Campylobacter jejuni, by inhibition of pyruvate ferredoxin oxidoreductase in these organisms (46, 47). In contrast, bacteria such as the Enterobacteriaceae lack this drug target and are generally insensitive to the drug (48, 49). Although NTZ does not exhibit bactericidal activity against E. coli, previous work demonstrated that NTZ inhibits biofilm formation by enteroaggregative E. coli (EAEC), a leading cause of acute and persistent diarrhea (50). NTZ inhibited the assembly of aggregative adherence fimbriae (AAF), which are assembled by the CU pathway and are responsible for biofilm formation and attachment of EAEC to intestinal epithelial cells. The loss of AAF assembly in response to NTZ was not due to repression of pilus gene expression or to interference with disulfide bond formation. In addition to inhibition of AAF-mediated biofilm formation, treatment of EAEC with NTZ also inhibited AAF-mediated and type 1 pilus-mediated hemagglutination (HA) activity of the bacteria (50). Consistent with a loss of pilus-mediated colonization, subsequent modeling studies in mice demonstrated that treatment of EAEC-infected animals with NTZ shortened the duration of infection by causing washout of the bacteria (51).
In this study, we demonstrate that NTZ inhibits biogenesis of the UPEC type 1 and P pili, suggesting that NTZ is broadly active against CU pathways. Furthermore, we have determined that pilus inhibition by NTZ is due to a specific interference with proper maturation of the usher protein in the bacterial OM. These findings point to a novel mechanism of action for NTZ that is distinct from current pilicides.
MATERIALS AND METHODS
Nitazoxanide preparation.
NTZ powder (Waterstone Technology) was dissolved in dimethyl sulfoxide (DMSO) to give a stock concentration of 10 mg/ml. A final concentration range of 0 to 20 μg/ml NTZ was used, as previous studies indicated that NTZ is inhibitory to growth at higher concentrations (50).
Strains, plasmids, and growth conditions.
All E. coli strains and plasmids used in this study are listed in Table 1. Host strain BW25113ΔompT was obtained from the Keio collection (52, 53) and was cured of its kanamycin resistance cassette by introduction of Flp recombinase on the temperature-sensitive plasmid pCP20 (54). Strain BW25113ΔdegP was generated by P1 transduction (55) using donor strain KS474 (56). Unless otherwise stated, E. coli overnight cultures were diluted 1:100 into fresh LB medium containing appropriate antibiotics (ampicillin, 100 μg/ml; kanamycin, 50 μg/ml; tetracycline, 15 μg/ml) and 0 to 20 μg/ml NTZ as indicated. For 0 μg/ml NTZ, a final concentration of 0.2% DMSO was added as a vehicle-only control, and a 0.2% DMSO final concentration was maintained for all NTZ concentrations. Cultures were grown at 37°C with aeration. When the cultures reached an optical density at 600 nm (OD600) of 0.3, the expression of plasmid-borne genes was induced for 1 h by addition of the appropriate inducing agent (50 or 100 μM isopropyl-β-d-thiogalactopyranoside [IPTG], 0.1% arabinose).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)b | Reference or source |
|---|---|---|
| Strainsa | ||
| AAEC185 | MM294 λ- F- supE44 hsdRI7 mcrA mcrB endAl thi-l ΔfimB-fimH ΔrecA | 58 |
| BW25113 | LacIq rrnB ΔlacZ hsdR514 ΔaraBAD ΔrhaBAD | 62 |
| BW25113ΔompT | BW25113 ΔompT | This study |
| BW25113ΔdegP | BW25113 degP::kan | This study |
| Plasmids | ||
| pCP20 | Carries FLP recombinase | 54 |
| pACYC184 | Cloning vector, Ptrc, Clmr, Tetr | 91 |
| pMMB66 | Cloning vector, Ptac, Ampr | 92 |
| pMMB91 | Cloning vector, Ptac, Kanr | 93 |
| pMON6235Δcat | Cloning vector, Para, Ampr | 71 |
| pFJ29 | pACYC184 derivative carrying the whole pap operon | 59 |
| pETS4 | pMMB66 derivative carrying His-tagged FimD | 68 |
| pETS9 | pMMB66 derivative carrying the whole fim operon | 60 |
| pETS11 | pMMB66 derivative carrying a ΔfimD fim operon | 60 |
| pPAP58 | pMMB91 derivative carrying PapDJKEFG | 63 |
| pFJ20 | pMON6235Δcat derivative carrying PapC | 59 |
| pTN1Δ2-11 | pMON6235Δcat derivative carrying PapCΔ2-11 | T. Ng and D. G. Thanassi, unpublished |
| pMJ3 | pMON6235Δcat derivative carrying His-tagged PapC | 66 |
| pDG2ΔN2ΔC640 | pMON6235Δcat derivative carrying His-tagged PapC129–640, deleted for the N- and C-terminal periplasmic domains | 20 |
| pNH281 | pMON6235Δcat derivative carrying His-tagged PapCΔ252–333, R251G, deleted for the plug domain | 67 |
All strains are E. coli K-12.
Ampr, ampicillin resistance; Kanr, kanamycin resistance; Clmr, chloramphenicol resistance; Tetr, tetracycline resistance; Para, arabinose-inducible promoter; Ptac or Ptrc, IPTG-inducible promoter.
Hemagglutination assay.
Hemagglutination (HA) assays were conducted as previously described (57) by serial dilution in microtiter plates. E. coli strain AAEC185 (58), transformed with pFJ29 (59) or pETS9 (60), was used to test P and type 1 pilus functionality, respectively. Plasmids pACYC184 and pETS11 served as negative controls for pFJ29 and pETS9, respectively. Following growth in 0 to 20 μg/ml NTZ and induction with 50 μM IPTG, bacteria were harvested and washed with phosphate-buffered saline (PBS) before being resuspended and normalized to an OD540 of 1.0. Following serial dilution of the bacteria in microtiter plates, either human or guinea pig (Colorado Serum Company) erythrocytes were added, and agglutination titers were determined visually as the highest dilution of bacteria able to maintain agglutination. For each assay, three independent experiments were performed, and each experiment contained three replicates.
Pilus isolation from the bacterial surface.
Using a previously established pilus isolation protocol (61), P pili were purified from host strain AAEC185/pFJ29 by heat extraction and by MgCl2 precipitation. Strain AAEC185/pACYC184 served as a vector-only control. Following growth in 0 to 20 μg/ml NTZ and induction with 50 μM IPTG, bacteria were harvested and washed with 5 mM Tris-HCl (pH 8.0) and 75 mM NaCl. Cultures were then normalized to an OD600 of 1.5 in the same buffer to ensure equal bacterial numbers. Pili were dissociated from the bacterial surface by heating equal sample volumes at 65°C for 30 min and were precipitated from supernatant fractions by addition of MgCl2. Pelleted protein precipitate was resuspended in 1 mM Tris-HCl (pH 8.0) with 2× SDS sample buffer containing 4 M urea and was analyzed by SDS-PAGE. PapA was visualized by Coomassie blue staining, and bands were quantified by gel densitometry using a LI-COR Odyssey CLx imager. The pilus tip adhesin PapG was detected by immunoblotting with a rabbit polyclonal antibody raised against purified PapD-PapG complex (anti-PapD-PapG; used at 1:50,000 dilution) and was visualized with secondary LI-COR IRDye 800CW goat anti-rabbit infrared conjugated antibody (1:15,000) (LI-COR Biosciences) using the LI-COR Odyssey CLx imager.
Periplasm isolation.
Periplasmic fractions were isolated from E. coli strain BW25113 (62), which was transformed with plasmid pPAP58 (63) or pMMB91, as a vector control. Following growth in 0 to 20 μg/ml NTZ and induction with 100 μM IPTG, bacteria were harvested, washed, and normalized to an OD600 of 2.0 using 20 mM Tris-HCl (pH 8.0). Equal sample volumes were processed to isolate the periplasm fractions as described previously (64). The periplasm fractions were analyzed by SDS-PAGE, and PapD-PapG was detected by immunoblotting as described for the pilus isolation. Alternatively, the PapE pilus tip subunit was detected by immunoblotting with a rabbit polyclonal antibody raised against purified P pilus tip fibers consisting of PapG, PapF, PapE, and PapK (anti-P pilus tips; used at 1:1,000 dilution), followed by an anti-rabbit alkaline phosphatase-conjugated secondary antibody. The blot was developed by treatment with BCIP (5-bromo-4-chloro-3-indolylphosphate)-nitroblue tetrazolium (NBT) substrate (KPL).
Chaperone-subunit complex copurification with the usher.
A previously described copurification method (57, 65) was used to isolate P pilus usher-chaperone-subunit complexes from the OM. Host strain BW25113ΔompT was transformed with plasmids pMJ3 (66) and pPAP58. Following growth in 0 to 20 μg/ml NTZ and induction with 0.1% l-arabinose and 100 μM IPTG, bacteria were harvested, washed, and normalized to an OD600 of 2.0 in 20 mM Tris-HCl (pH 8.0) containing 1× complete protease inhibitor cocktail (Roche). OM fractions were isolated and solubilized with n-dodecyl-β-d-maltoside (DDM) (Anatrace) as described previously (57). The His6-tagged PapC usher, together with stably bound pilus assembly intermediates, was purified by nickel affinity chromatography using an AKTA chromatography system (GE Life Sciences). Fractions eluted from the column using imidazole were analyzed for the presence of PapC by SDS-PAGE and Coomassie blue staining. Corresponding peak fractions were then pooled and treated with a final concentration of 9% trichloroacetic acid (TCA) for 1 h on ice to precipitate chaperone-subunit-usher complexes. Samples were centrifuged (5 min at 16,100 × g and 4°C), supernatants were carefully removed, and protein pellets were washed 3 times with 1 ml cold acetone. Protein pellets were left to air dry to remove access acetone and were then resuspended in 1× SDS sample buffer for SDS-PAGE. P pilus tip proteins were detected by immunoblotting with anti-P pilus tips antibody as described for the periplasm isolation.
OM isolation and analysis of the usher.
OM fractions were isolated from E. coli strains BW25113 or BW25113ΔdegP. Strain BW25113 was transformed with PapC plasmids pMJ3, pDG2ΔN2ΔC640 (20), pNH281 (67), or the FimD plasmid pETS4 (68). Plasmids pMON6235Δcat and pMMB66 served as vector controls for the PapC and FimD plasmids, respectively. Strain BW25113ΔdegP was transformed with pMJ3. Bacteria were grown in the presence of 0 to 20 μg/ml NTZ and were induced with 0.1% l-arabinose for the PapC plasmids or 100 μM IPTG for FimD. Bacteria were harvested, washed, resuspended, and normalized to an OD600 of 1.5 in 20 mM Tris-HCl (pH 8.0) plus 1× complete protease inhibitor cocktail, and OM fractions were isolated as previously described (64). For all strains except BW25113/pETS4, OM pellets were resuspended in 20 mM Tris-HCl and 0.3 M NaCl, followed by the addition of SDS sample buffer. For BW25113/pETS4, to remove any inclusion bodies, OM pellets were resuspended in 20 mM Tris-HCl, 0.3 M NaCl, and 1% DDM and were rocked overnight at 4°C. The next day, samples were centrifuged (30 min at 16,100 × g and 4°C), and supernatant fractions were collected, to which SDS sample buffer was added. The OM samples were incubated at 25°C or were heated to 95°C for 10 min. Samples were analyzed by SDS-PAGE and immunoblotted with a mouse monoclonal anti-His6 (1:1,000) antibody (Covance) followed by a secondary IRDye 680 goat anti-mouse conjugated infrared antibody (1:40,000) (LI-COR Biosciences). Blots were visualized, and band densitometry was performed using a LI-COR Odyssey CLx imager.
Overlay assay.
Overlay assays were performed as described previously (65). OM fractions were obtained as described above from strain BW25113 transformed with PapC plasmids pFJ20 (69) or pTN1Δ2-11 (T. Ng and D. G. Thanassi, unpublished data) or with pMON6235Δcat as the vector control. The bacteria were grown without the addition of DMSO or NTZ. OM fractions were subjected to SDS-PAGE and were either stained with Coomassie blue or transferred to a polyvinylidene difluoride (PVDF) membrane (Osmonics Inc.). Separately, periplasm fractions were extracted from BW25113/pPAP58 as described above. The bacteria were grown without the addition of DMSO or NTZ. NTZ (0 to 20 μg/ml) was then added to the isolated periplasm fractions, and these samples were incubated with the PVDF membranes containing the immobilized OM for 1 h with shaking. The PVDF membranes were washed with Tris-buffered saline, 0.05% Tween 20, and 0.02% sodium-azide (TBST) and were probed with an anti-PapD (1:10,000) primary antibody. Secondary anti-rabbit alkaline phosphatase antibody followed by incubation with BCIP-NBT substrate was used to reveal chaperone-subunit complexes bound to the usher.
Disk diffusion assay.
An overnight culture of E. coli strain BW25113 was spread on LB agar plates containing 0 to 20 μg/ml NTZ. Filter disks (6 mm diameter) impregnated with novobiocin or vancomycin were placed on the agar plates and were incubated overnight at 37°C. Zones of inhibition (diameter) surrounding the disks were recorded the next day. The experiment was performed in duplicate.
Statistical analysis.
Densitometry analysis of protein bands was performed using a LI-COR Odyssey CLx imager on samples from three independent experiments. Statistical significance was calculated by one-way analysis of variance and by Bonferroni's multiple-comparison posttest using Prism 6 (GraphPad Software). For comparison of folded versus unfolded PapC usher levels, a two-way analysis of variance was used. P values of <0.05 were considered significant.
RESULTS
NTZ has inhibitory activity against type 1 and P pili.
Previous work by Shamir et al. (50) demonstrated that treatment of EAEC with NTZ ablated the assembly of AAF on the bacterial surface and inhibited HA activity mediated by AAF as well as by type 1 pili. AAF and type 1 pili are assembled by the CU pathway. To extend this observation to other strains of E. coli and to other types of pili assembled by the CU pathway, we examined the effect of NTZ on the type 1 and P pili expressed by UPEC.
To examine pilus assembly and function separate from any effects that NTZ might have on pilus gene expression, we recombinantly expressed the type 1 (fim) or P (pap) operons from UPEC strain J96 (70) under the control of an inducible promoter in the E. coli K-12 background. We first assessed the assembly of functional type 1 pili on the bacterial surface by measuring agglutination of guinea pig erythrocytes using an HA assay. Bacteria (AAEC185/pETS9) were grown in the presence of DMSO (as a vehicle-only control) or increasing concentrations of NTZ and were induced for expression of type 1 pili. As shown in Table 2, growth in the presence of 10 or 20 μg/ml (30 or 60 μM) NTZ caused a dose-dependent decrease in HA activity. This confirms the previous observation of Shamir et al. (50) that NTZ inhibits type 1 pilus function and demonstrates that NTZ is effective against type 1 pili expressed by different strains of E. coli.
TABLE 2.
Effect of NTZ on pilus-mediated hemagglutination
| Pili expresseda | NTZ (μg/ml)b | HA titerc |
|---|---|---|
| Type 1 pili | ||
| − | 0 | 0 |
| + | 0 | 128 |
| + | 10 | 32 |
| + | 20 | 16 |
| P pili | ||
| − | 0 | 0 |
| + | 0 | 256 |
| + | 5 | 256 |
| + | 10 | 64 |
| + | 15 | 0 |
| + | 20 | 0 |
+, expressed; −, not expressed.
Strain AAEC185 was grown in the presence of the indicated concentrations of NTZ and induced for expression of type 1 pili (pETS9) or P pili (pFJ29).
Hemagglutination (HA) titer is the maximum fold dilution of bacteria able to agglutinate guinea pig (type 1 pili) or human (P pili) red blood cells.
We next determined the effect of NTZ on P pili. Bacteria (AAEC185/pFJ29) grown in the presence of 0 to 20 μg/ml NTZ were induced for expression of P pili and were then used to agglutinate human erythrocytes. As observed for type 1 pili, growth in the presence of NTZ caused a dose-dependent decrease in HA titer, with concentrations greater than 10 μg/ml inhibiting P pilus function completely (Table 2). To determine if the decrease in HA activity correlated with a loss of pilus assembly on the bacterial surface, we isolated P pili from bacteria grown in the absence or presence of NTZ. Analysis of the purified pili by gel electrophoresis revealed that yields of the major pilus rod subunit PapA (∼1,000 copies per pilus) and of the tip-located adhesin PapG (1 copy per pilus) decreased in a dose-dependent manner in response to NTZ treatment of the bacteria (Fig. 1A). Quantitation of the PapA band by densitometry showed that growth in the presence of 10 μg/ml NTZ caused an ∼50% decrease in pilus levels compared to those of the vehicle-only control, while 20 μg/ml NTZ caused an ∼80% decrease in pilus assembly (Fig. 1B). Together with the previous findings of Shamir et al. (50), these results indicate that NTZ is active against genetically distinct CU pilus systems and that NTZ functions by interfering with pilus assembly on the bacterial surface.
FIG 1.
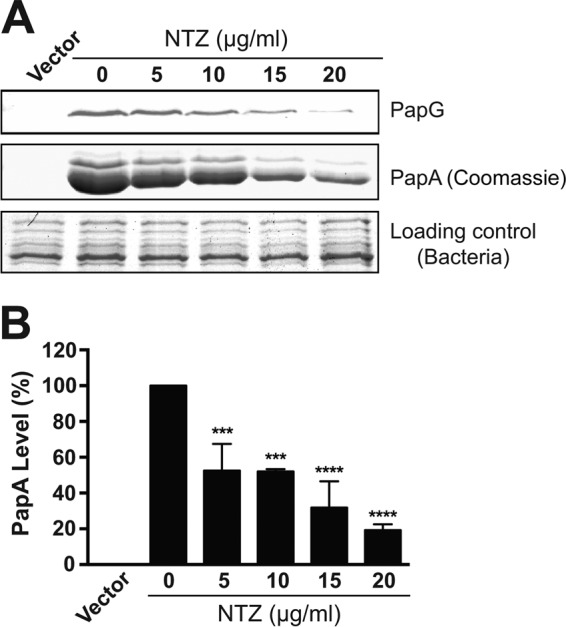
Effect of NTZ on P pilus assembly on the bacterial surface. (A) Strain AAEC185/pJF29 was grown in the presence of increasing concentrations of NTZ and was induced for expression of P pili. Pili isolated from the bacterial surface were separated by SDS-PAGE and were blotted with anti-PapD-PapG antibody to visualize the PapG adhesin (top panel) or stained with Coomassie blue to visualize the major pilus subunit PapA (middle panel). The bottom panel shows the Coomassie-stained whole bacteria used for pilus isolation as a loading control. E. coli containing vector only (pACYC184) served as a negative control for pilus isolation. (B) Quantitation of PapA levels in the isolated pili. PapA levels were measured by densitometry of the middle section of panel A, and the percentages of PapA levels were calculated relative to 0 μg/ml NTZ. Bars represent means ± standard errors of the means (SEM) from three independent experiments. ***, P < 0.001; ****, P < 0.0001 for comparison with 0 μg/ml NTZ.
NTZ inhibits pilus biogenesis by decreasing levels of the usher protein in the OM.
Pilus biogenesis by the CU pathway is a well-characterized, multistep assembly process (8). Upon entry into the periplasm, nascent pilus subunits must interact with the periplasmic chaperone (PapD for P pili) for proper folding (17, 18). If complex formation between pilus subunits and the chaperone is disrupted, the subunits will misfold and will be degraded by periplasmic proteases (69, 71). To determine if NTZ decreases pilus biogenesis by interfering with the formation of chaperone-subunit complexes, we analyzed periplasm fractions isolated from bacteria (BW25113/pPAP58) that were grown in the absence or presence of NTZ and that were induced for expression of PapD and P pilus tip subunits. Immunoblotting with anti-PapD-PapG or anti-P pilus tip antibodies showed no change in the levels of the PapD chaperone, PapG adhesin, or PapE major pilus tip subunit in response to the addition of 10 or 20 μg/ml NTZ (Fig. 2). Therefore, NTZ does not interfere with chaperone-subunit interactions. Note that PapD contains an essential disulfide bond, which is catalyzed by the oxidoreductases DsbA and DsbB (69). Failure to form this disulfide bond leads to the degradation of PapD by periplasmic proteases. Therefore, our results confirm that NTZ does not interfere with disulfide bond formation in the periplasm as previously suggested (50). Additionally, since levels of the P pilus proteins in the periplasm were unchanged by drug treatment, these results confirm that NTZ does not alter transcription or translation of the pilus genes in our recombinant system.
FIG 2.
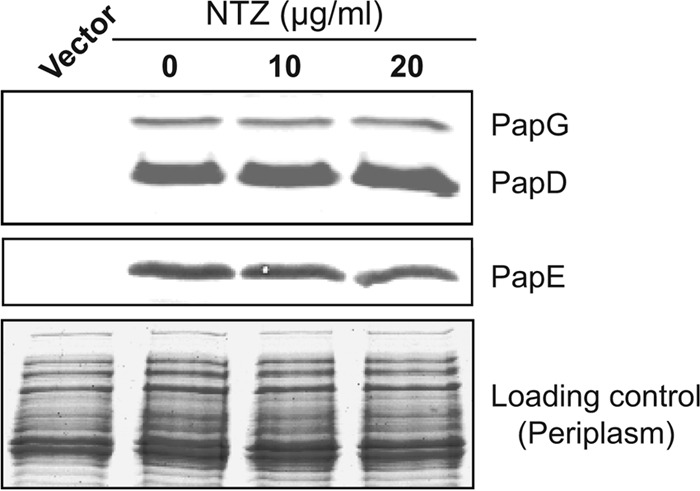
Effect of NTZ on chaperone-subunit interactions in the periplasm. Strain BW25113/pPAP58 was grown in the presence of increasing concentrations of NTZ and was induced for expression of the PapD chaperone and P pilus tip subunits (PapG, E, F, and K). Periplasm fractions isolated from the bacteria were separated by SDS-PAGE and were blotted with anti-PapD-PapG antibody (top panel) to visualize the chaperone and adhesin or with anti-P pilus tip antibody to visualize the PapE major tip subunit (middle panel). The bottom panel shows the periplasm fractions stained with Coomassie blue as a loading control.
The second step of P pilus biogenesis requires that periplasmic chaperone-subunit complexes bind to the usher protein PapC, which functions as the pilus assembly and secretion platform in the OM. The N-terminal periplasmic domain of PapC is the initial binding site for chaperone-subunit complexes (64). To determine if NTZ interferes with this step of pilus biogenesis, we used an in vitro overlay assay, which measures binding of chaperone-subunit complexes to the usher N-terminal domain (64). OM fractions from E. coli expressing the PapC usher were isolated and transferred to a PVDF membrane. Separately, periplasm fractions were isolated from E. coli that was expressing PapD and the P pilus tip subunits. NTZ (0, 10, or 20 μg/ml) was added to the isolated periplasm fractions, and the supplemented fractions were then incubated with the PVDF membrane to allow binding of chaperone-subunit complexes to the usher. Binding was detected by immunoblotting with an anti-PapD antibody. Equivalent binding of chaperone-subunit complexes to the usher was detected under all conditions (data not shown). These results indicate that NTZ does not interfere with the initial binding of chaperone-subunit complexes to the usher N-terminal domain.
We next used a copurification assay to examine the effect of NTZ on the formation of stable usher-pilus assembly intermediates in vivo (57). Bacteria were grown in the absence or presence of NTZ and were induced for expression of P pilus tip subunits and His-tagged PapC usher. OM fractions were isolated, and the His-tagged usher was purified by affinity chromatography. Coomassie staining was used to detect the purified PapC usher, and immunoblotting was performed to detect copurifying pilus complexes. If NTZ interfered with the formation of pilus assembly intermediates at the usher, we would expect levels of the purified usher protein to remain constant but yields of copurifying pilus subunits to decrease in response to increasing concentrations of NTZ. Instead, we observed a parallel decrease in the usher and in copurifying subunits (Fig. 3). The corresponding decrease in the usher and in the bound subunits suggests that NTZ does not interfere with the formation of usher-chaperone-subunit interactions in bacteria. Instead, we hypothesized that NTZ may act by reducing levels of the usher protein in the OM.
FIG 3.
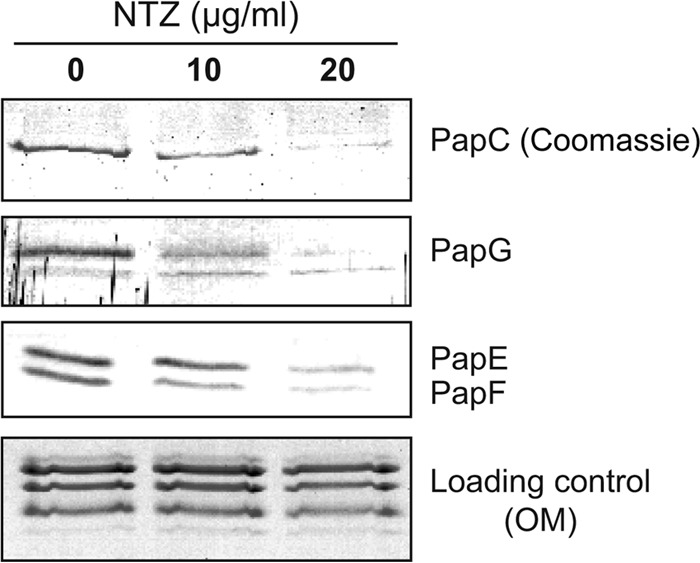
Effect of NTZ on formation of usher-chaperone-subunit complexes in bacteria. Strain BW25113ΔompT was grown in the presence of increasing concentrations of NTZ and was induced for expression of the His-tagged PapC usher (pMJ3), along with the PapD chaperone and the P pilus tip subunits (pPAP58). PapC-His, together with any stably bound pilus assembly complexes, was purified from solubilized OM fractions by nickel affinity chromatography and was separated by SDS-PAGE. The purified PapC was visualized by Coomassie blue staining (top panel). Copurifying pilus tip subunits were visualized by blotting with anti-P pilus tip antibody to detect the PapG, PapE, and PapF tip subunits (middle panels). The bottom panel shows the solubilized OM fractions stained with Coomassie blue as a loading control.
To directly test the effect of NTZ on usher levels, bacteria (BW25113/pMJ3) were grown in the absence or presence of NTZ and were induced for expression of His-tagged PapC alone. Analysis of OM fractions that were isolated from these bacteria revealed that PapC levels decreased in a dose-dependent manner in response to NTZ (Fig. 4A). Densitometry analysis of PapC, as detected by immunoblotting with anti-His tag antibody, revealed that 10 μg/ml NTZ caused an ∼40% decrease in OM usher levels compared to those of the vehicle-only control, while 20 μg/ml NTZ caused an ∼80% decrease in usher levels (Fig. 4B). This closely matches the decrease observed for pilus assembly on the bacterial surface in response to NTZ (Fig. 1B). Notably, analysis of the OM fraction by Coomassie staining revealed little to no effect of NTZ on levels of other OM proteins, including the major porins (OmpA, OmpC, and OmpF) (Fig. 4A). Moreover, immunoblotting for the OM lipopolysaccharide (LPS) transporter LptD also revealed no changes in response to NTZ (Fig. 4A). Additionally, bacteria grown in the presence NTZ did not exhibit increased sensitivity to novobiocin or to vancomycin, as measured by a disk diffusion assay, demonstrating no alteration in membrane integrity in response to the drug (data not show). To extend these results to the type 1 pilus system, OM fractions were isolated from bacteria (BW25113/pETS4) that were grown in the absence or presence of NTZ and that were induced for expression of the His-tagged FimD usher. Analysis of the OM fractions revealed that FimD levels decreased in a dose-dependent manner in response to NTZ (Fig. 5), as found for the P pilus usher PapC. Thus, NTZ treatment leads to a decrease in usher protein levels in the OM, and this effect appears to be specific for usher proteins.
FIG 4.
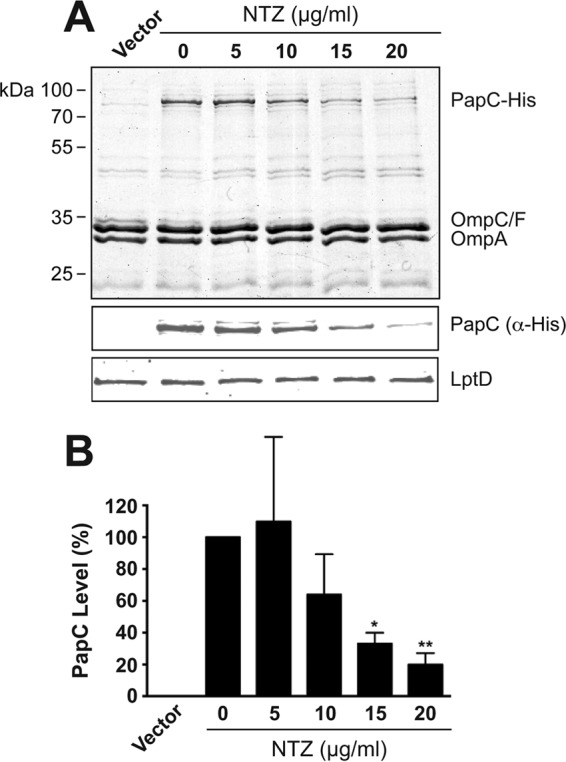
Effect of NTZ on levels of the PapC usher in the OM. (A) Strain BW25113/pMJ3 was grown in the presence of increasing concentrations of NTZ and was induced for expression of the His-tagged PapC usher. OM fractions isolated from the bacteria were subjected to SDS-PAGE and Coomassie staining to observe PapC and the major OM protein constituents (top panel). Samples were also probed with anti-His antibody to visualize PapC (middle panel) or anti-LptD antibody to visualize the LPS transporter LptD (bottom panel). E. coli containing vector only (pMON6235Δcat) served as a negative control for PapC expression. (B) Quantitation of PapC levels in the OM. PapC levels were measured by densitometry of the anti-His blot in panel A, and the percentages of PapC levels were calculated relative to 0 μg/ml NTZ. Bars represent means ± SEM from three independent experiments. *, P < 0.05; **, P < 0.01 for comparison with 0 μg/ml NTZ.
FIG 5.
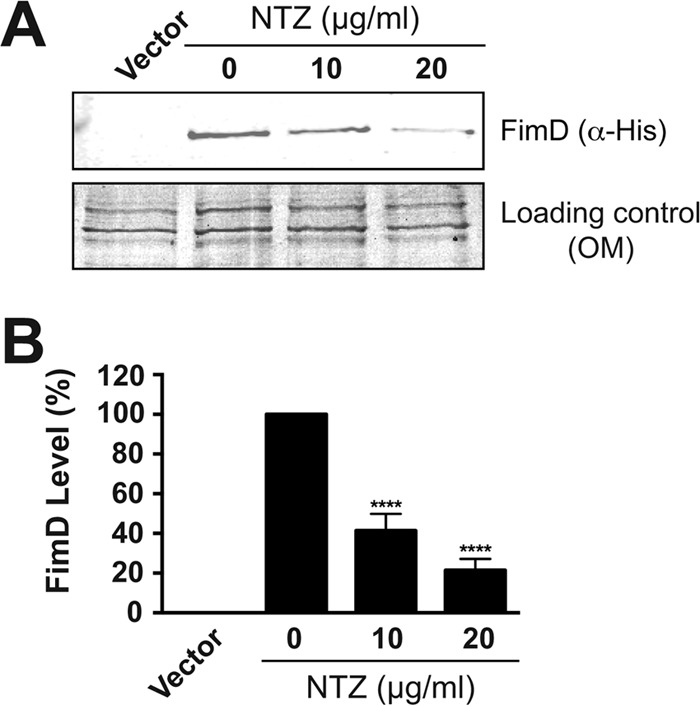
Effect of NTZ on levels of the FimD usher in the OM. (A) Strain BW25113/pETS4 was grown in the presence of increasing concentrations of NTZ and was induced for expression of the His-tagged FimD usher. OM fractions isolated from the bacteria were subjected to SDS-PAGE and were blotted with anti-His antibody to visualize FimD (top panel). The bottom panel shows the OM fractions stained with Coomassie blue as a loading control. E. coli containing vector only (pMMB66) served as a negative control for FimD expression. (B) Quantitation of FimD levels in the OM. FimD levels were measured by densitometry of the anti-His blot in panel A, and the percentages of FimD levels were calculated relative to 0 μg/ml NTZ. Bars represent means ± SEM from three independent experiments. ****, P < 0.0001 for comparison with 0 μg/ml NTZ.
NTZ prevents proper folding of the usher β-barrel domain in the OM.
Ushers are integral OM proteins comprising five distinct domains: a transmembrane β-barrel channel that resides in the OM, a plug domain that functions as a channel gate, a periplasmic N-terminal domain, and two periplasmic C-terminal domains (20, 21, 72). To localize the effect of NTZ to a specific region of the usher, we examined different domain deletion mutants of PapC. A PapC mutant lacking the N- and C-terminal periplasmic domains (PapCΔNΔC) displayed the same sensitivity to NTZ as that found for the full-length PapC usher (Fig. 6A and B). OM levels of a PapC mutant lacking the plug domain (PapCΔplug) also decreased in response to NTZ (Fig. 6A). Note that deletion of the plug domain destabilizes the usher, resulting in low levels of PapCΔplug in the OM even in the absence of drug. However, addition of NTZ caused a further decrease in the usher below levels detectable by immunoblotting (Fig. 6A). Taken together, the sensitivity of the PapCΔNΔC and the PapCΔplug mutants to NTZ suggests that the effect of NTZ is specific to the usher β-barrel domain.
FIG 6.
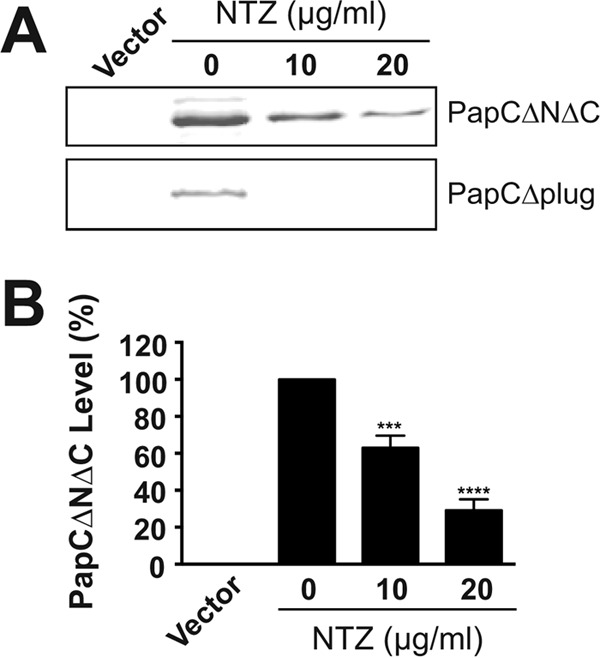
Analysis of domain deletion mutants of the PapC usher. (A) Strain BW25113 transformed with plasmid pDG2ΔN2ΔC640 or pNH281 was grown in the presence of increasing NTZ concentrations and was induced for expression of PapC lacking the N- and C-terminal periplasmic domains (ΔNΔC) or the plug domain (Δplug), respectively. OM fractions isolated from the bacteria were subjected to SDS-PAGE and were blotted with anti-His antibody to visualize PapCΔNΔC (upper panel) or PapCΔplug (lower panel). E. coli containing vector only (pMON6235Δcat) served as a negative control for PapC expression. (B) Quantitation of PapCΔNΔC levels in the OM. PapC levels were measured by densitometry of the upper section of panel A, and the percentages of PapC levels were calculated relative to 0 μg/ml NTZ. Bars represent means ± SEM from three independent experiments. ***, P < 0.001; ****, P < 0.0001 for comparison with 0 μg/ml NTZ.
To test if NTZ interferes with proper folding of the usher β-barrel domain, we took advantage of the characteristic resistance of OM β-barrel proteins, including the usher, to denaturation by SDS (64, 73). This resistance results in heat-modifiable mobility on SDS-PAGE, in which a fraction of the usher remains folded in the absence of heating and migrates with faster mobility compared to a fully denatured protein. The OM isolated from bacteria (BW25113/pMJ3) that were grown in the absence or presence of NTZ was incubated at 25°C in SDS sample buffer prior to analysis by SDS-PAGE. Levels of the folded and the unfolded PapC species decreased similarly in response to increasing concentrations of NTZ (Fig. 7A). One interpretation for this result is that NTZ causes misfolding of the usher and that the improperly folded usher is then subject to degradation. DegP, which functions as a chaperone and as a protease in the periplasm, is known to target misfolded OM proteins such as the usher for degradation (74–76). We therefore reasoned that analysis of PapC levels in a strain lacking DegP would result in the retention of the misfolded usher and would allow discrimination between the effects of NTZ on usher folding and usher degradation. Indeed, repetition of the experiment in a ΔdegP mutant background revealed that, whereas levels of the folded usher species decreased almost completely in response to NTZ, levels of the unfolded species decreased only slightly (Fig. 7B). Taken together, these results demonstrate that treatment of bacteria with NTZ interferes with proper folding of the usher in the OM, with DegP then largely responsible for degrading the misfolded protein.
FIG 7.
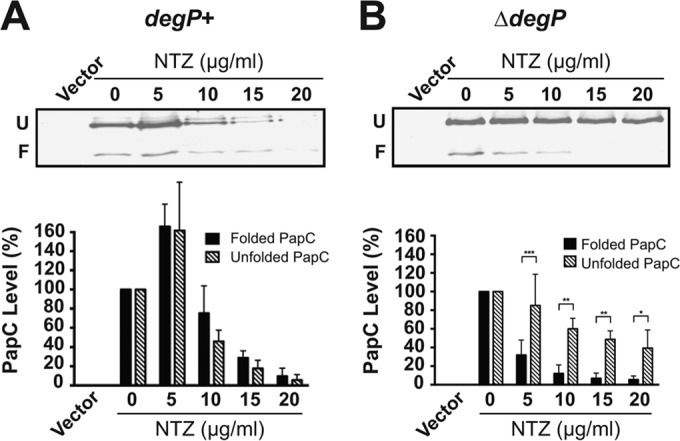
Analysis of usher folding in the OM. Strains BW25113 (A) or BW25113ΔdegP (B) transformed with pMJ3 were grown in the presence of increasing concentrations of NTZ and were induced for expression of the His-tagged PapC usher. OM fractions isolated from the bacteria were incubated in SDS sample buffer at 25°C, subjected to SDS-PAGE, and probed with anti-His antibody to visualize PapC. Positions of the folded (F) and unfolded (U) PapC species are indicated on the left of each gel image. E. coli containing vector only (pMON6235Δcat) served as a negative control for PapC expression. Quantitation of the folded and unfolded PapC bands is presented below each gel image. The PapC levels were measured by densitometry of the anti-His blots, and the percentages of PapC levels were calculated relative to the respective folded or unfolded species present at 0 μg/ml NTZ. Bars represent means ± SEM from three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 for comparison of folded to unfolded PapC at each NTZ concentration.
DISCUSSION
The CU pathway is a conserved and widespread secretion system that is responsible for biogenesis of virulence-associated surface structures by Gram-negative bacteria. Adhesive pili or fimbriae assembled by the CU pathway mediate binding to host cells and colonization of host tissues and, thus, perform key roles in the ability of bacterial pathogens to establish and maintain infection. In this study, we demonstrate that the small-molecule drug NTZ inhibits type 1 and P pili expressed by UPEC by interfering with pilus assembly on the bacterial surface. Through analysis of different steps of the CU pathway, we show that NTZ interferes with pilus biogenesis by preventing proper folding of the usher protein in the bacterial OM. Together with previous results showing that NTZ inhibits the assembly and function of AAF and of type 1 pili expressed by EAEC (50), our findings confirm that NTZ is a pilicide with activity against different CU pathways. Furthermore, compared to previously characterized pilicides that target chaperone function, we demonstrate that NTZ has a novel mechanism of action and is inhibitory in the low micromolar concentration range typical for most antibiotics.
We found that growth of bacteria in the presence of NTZ inhibited type 1 and P pilus-mediated agglutination of red blood cells in a dose-dependent manner, with inhibition observed beginning at ∼10 μg/ml (30 μM) NTZ. This is below the growth-inhibitory concentrations of NTZ (50) and within the range achievable in human plasma (77). Our further analysis of P pilus assembly revealed a dose-dependent decrease in the levels of pili on the bacterial surface in response to NTZ, corresponding with the decrease in HA activity. This agrees with the previous analysis of AAF assembly on the surface of EAEC (50) and demonstrates that the anti-pilus activity of NTZ is due to disruption of pilus biogenesis on the bacterial surface. A difference between the current and previous studies is that we expressed our CU pilus genes recombinantly under an artificial promoter, rather than from the native chromosomal loci. Although Shamir et al. (50) did not detect changes in aaf transcript levels in EAEC in response to NTZ, our experimental setup allowed us to examine pilus assembly separate from any potential regulatory effects on pilus gene expression. In agreement with a lack of NTZ effect on transcription and translation of the pilus genes, we detected no changes in periplasmic levels of P pilus proteins in response to NTZ (Fig. 2).
We took advantage of the detailed understanding of the CU secretion pathway to gain insight into the mechanism by which NTZ inhibits pilus assembly. Using the P pilus system, we found no evidence that NTZ interferes with the formation of chaperone-subunit complexes or with the stability of pilus proteins in the periplasm. NTZ did not affect PapD chaperone function in stabilizing pilus subunits (69), which is dependent on disulfide bonding catalyzed by DsbA (78). Therefore, our studies and those of Shamir et al. (50) formally rule out DsbA as a target of NTZ action. Periplasmic chaperone-subunit complexes must dock at the OM usher for exchange of chaperone-subunit for subunit-subunit interactions, leading to polymerization of the pilus fiber and secretion through the usher to the bacterial surface. Using overlay and copurification assays, we found no evidence that NTZ disrupts the formation of usher-chaperone-subunit interactions. However, the copurification assay did reveal a dose-dependent decrease in the levels of the PapC usher and the bound pilus subunits in response to NTZ (Fig. 3), suggesting that the drug might be acting to reduce levels of available usher protein in the OM. This would explain the loss in pilus assembly on the bacterial surface in response to NTZ, as the usher is the rate-limiting component for pilus biogenesis (19, 21, 79). Indeed, analysis of bacteria expressing the PapC or FimD ushers in the absence of any other pilus proteins showed that usher protein levels in the OM decreased in a dose-dependent manner in response to NTZ. This decrease in usher protein levels closely paralleled the decreases in HA activity and the loss of pilus fibers on the bacterial surface in response to NTZ, which is consistent with the amount of functional ushers dictating the number of pili assembled. We expect NTZ to have a similar mechanism of action against CU pili expressed in their native context by clinical E. coli strains. Indeed, studies of UPEC isolates indicate that NTZ inhibits pilus-mediated agglutination and decreases usher protein levels as was found in our recombinant E. coli system (P. Chahales and D. G. Thanassi, unpublished data).
Using domain deletion mutants of PapC, we were further able to localize the effect of NTZ to the usher's transmembrane β-barrel channel domain. The majority of integral bacterial OM proteins adopt a β-barrel fold (80). The usher has a more complicated architecture compared to that of major OM proteins, such as the porins, but other OM proteins, such as the LPS transporter LptD, have similarly complicated architectures (81, 82). Therefore, a surprising and interesting finding from our studies is the apparent specificity of NTZ for the usher, as we did not observe changes in the general OM protein profile or in levels of LptD in response to NTZ (Fig. 4A). This specificity demonstrates that the pilicide activity of NTZ is not due to broad physiological or pleiotropic effects on the bacterial envelope. NTZ does exhibit toxicity to E. coli at concentrations higher than those used in our experiments (50). At these higher drug concentrations, NTZ may cause a more general accumulation of misfolded OM β-barrel proteins. An important clue for how NTZ affects the usher was obtained by using heat-modifiable mobility to monitor the folding of the usher in the OM. Comparison of the folded and unfolded usher species in wild-type (WT) and ΔdegP E. coli strain backgrounds revealed that growth in the presence of NTZ causes a loss of properly folded ushers in the OM. The misfolded ushers are then subject to degradation by DegP, which explains the decrease in usher protein levels observed in the OM in response to NTZ.
How can treatment of bacteria with NTZ lead to misfolding of the usher? We envision several possibilities. First, the drug may directly bind to a conserved region of usher molecules, either preventing proper folding or destabilizing already-folded ushers. In studies to date, we have not observed a direct effect of NTZ on purified usher proteins or on isolated OM fractions (Chahales and Thanassi, unpublished data). Second, the drug may interfere with a periplasmic chaperone, such as SurA, Skp, or DegP, which are required for proper passage of nascent β-barrel proteins to the OM (83). Maturation of the PapC and FimD ushers is known to involve SurA (75, 84, 85). However, this might be unlikely since these chaperones are involved in the delivery of porins and other proteins to the OM, whereas we observe a specific effect on the usher. Third, the drug may interfere with proper insertion of nascent usher molecules into the OM. As for the majority of integral OM proteins, insertion of the usher occurs via the β-barrel assembly machinery (Bam) complex (75, 86). Studies have shown that, compared to other OM proteins such as the porins and LptD, FimD has a distinct requirement for BamB (75, 87), which suggests a unique role for this component of the Bam complex in usher assembly. Therefore, it is possible that NTZ may affect Bam complex function in a manner that is specific to usher proteins.
Rates of antibiotic resistance among pathogenic bacteria have risen to alarming levels (34). The development of antivirulence therapeutics such as pilicides represents an alternative to traditional antibiotics that may limit the development of drug resistance and avoid the detrimental side effects of conventional antibiotics (88). The antipilus activity of NTZ is distinct from that of previously characterized pilicides. Previous pilicides were shown to target the periplasmic chaperone and to disrupt pilus biogenesis by interfering with binding of chaperone-subunit complexes to the usher or by inhibiting chaperone-subunit interactions (39, 89). Small molecules have also been identified that disrupt polymerization of the pilus fiber, and effects of pilicides on pilus expression have also been observed (42, 90). In contrast, we show here that NTZ disrupts pilus biogenesis by decreasing the number of usher molecules in the OM, which is a novel mechanism of action. The usher functions as an essential pilus assembly and secretion platform, making it an excellent target for therapeutic development. Moreover, our results together with those of Shamir et al. (50) demonstrate that NTZ has activity against diverse CU pili, suggesting a target common to all CU systems. Further investigation is required to determine the range of CU pathways affected by NTZ and whether the drug interferes with a common requirement for usher folding in the OM, such as the Bam system. NTZ holds promise as a novel pilicide with broad therapeutic potential against Gram-negative pathogens.
ACKNOWLEDGMENTS
We thank A. W. Karzai and the Karzai laboratory (Stony Brook University) for access to and assistance with the Keio E. coli mutant collection. We thank T. J. Silhavy (Princeton University) for the anti-LptD antibody and for helpful discussions.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Thanassi DG, Bliska JB, Christie PJ. 2012. Surface organelles assembled by secretion systems of Gram-negative bacteria: diversity in structure and function. FEMS Microbiol Rev 36:1046–1082. doi: 10.1111/j.1574-6976.2012.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kline KA, Falker S, Dahlberg S, Normark S, Henriques-Normark B. 2009. Bacterial adhesins in host-microbe interactions. Cell Host Microbe 5:580–592. doi: 10.1016/j.chom.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Sauer FG, Mulvey MA, Schilling JD, Martinez JJ, Hultgren SJ. 2000. Bacterial pili: molecular mechanisms of pathogenesis. Curr Opin Microbiol 3:65–72. doi: 10.1016/S1369-5274(99)00053-3. [DOI] [PubMed] [Google Scholar]
- 4.Cusumano CK, Hultgren SJ. 2009. Bacterial adhesion—a source of alternate antibiotic targets. IDrugs 12:699–705. [PubMed] [Google Scholar]
- 5.Steadman D, Lo A, Waksman G, Remaut H. 2014. Bacterial surface appendages as targets for novel antibacterial therapeutics. Future Microbiol 9:887–900. doi: 10.2217/fmb.14.46. [DOI] [PubMed] [Google Scholar]
- 6.Nuccio SP, Baumler AJ. 2007. Evolution of the chaperone/usher assembly pathway: fimbrial classification goes Greek. Microbiol Mol Biol Rev 71:551–575. doi: 10.1128/MMBR.00014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zav'yalov V, Zavialov A, Zav'yalova G, Korpela T. 2010. Adhesive organelles of Gram-negative pathogens assembled with the classical chaperone/usher machinery: structure and function from a clinical standpoint. FEMS Microbiol Rev 34:317–378. doi: 10.1111/j.1574-6976.2009.00201.x. [DOI] [PubMed] [Google Scholar]
- 8.Geibel S, Waksman G. 2014. The molecular dissection of the chaperone-usher pathway. Biochim Biophys Acta 1843:1559–1567. doi: 10.1016/j.bbamcr.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Hatkoff M, Runco LM, Pujol C, Jayatilaka I, Furie MB, Bliska JB, Thanassi DG. 2012. Roles of chaperone/usher pathways of Yersinia pestis in a murine model of plague and adhesion to host cells. Infect Immun 80:3490–3500. doi: 10.1128/IAI.00434-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, Hultgren SJ. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 11.Roberts JA, Marklund B-I, Ilver D, Haslam D, Kaack MB, Baskin G, Louis M, Mollby R, Winberg J, Normark S. 1994. The Gal(alpha1-4)Gal-specific tip adhesin of Escherichia coli P-fimbriae is needed for pyelonephritis to occur in the normal urinary tract. Proc Natl Acad Sci U S A 91:11889–11893. doi: 10.1073/pnas.91.25.11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boisen N, Struve C, Scheutz F, Krogfelt KA, Nataro JP. 2008. New adhesin of enteroaggregative Escherichia coli related to the Afa/Dr/AAF family. Infect Immun 76:3281–3292. doi: 10.1128/IAI.01646-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jansen AM, Lockatell V, Johnson DE, Mobley HL. 2004. Mannose-resistant Proteus-like fimbriae are produced by most Proteus mirabilis strains infecting the urinary tract, dictate the in vivo localization of bacteria, and contribute to biofilm formation. Infect Immun 72:7294–7305. doi: 10.1128/IAI.72.12.7294-7305.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomaras AP, Dorsey CW, Edelmann RE, Actis LA. 2003. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: involvement of a novel chaperone-usher pili assembly system. Microbiology 149:3473–3484. doi: 10.1099/mic.0.26541-0. [DOI] [PubMed] [Google Scholar]
- 15.Vallet I, Olson JW, Lory S, Lazdunski A, Filloux A. 2001. The chaperone/usher pathways of Pseudomonas aeruginosa: identification of fimbrial gene clusters (cup) and their involvement in biofilm formation. Proc Natl Acad Sci U S A 98:6911–6916. doi: 10.1073/pnas.111551898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy CN, Mortensen MS, Krogfelt KA, Clegg S. 2013. Role of Klebsiella pneumoniae type 1 and type 3 fimbriae in colonizing silicone tubes implanted into the bladders of mice as a model of catheter-associated urinary tract infections. Infect Immun 81:3009–3017. doi: 10.1128/IAI.00348-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choudhury D, Thompson A, Stojanoff V, Langermann S, Pinkner J, Hultgren SJ, Knight SD. 1999. X-ray structure of the FimC-FimH chaperone-adhesin complex from uropathogenic Escherichia coli. Science 285:1061–1066. doi: 10.1126/science.285.5430.1061. [DOI] [PubMed] [Google Scholar]
- 18.Sauer FG, Fütterer K, Pinkner JS, Dodson KW, Hultgren SJ, Waksman G. 1999. Structural basis of chaperone function and pilus biogenesis. Science 285:1058–1061. doi: 10.1126/science.285.5430.1058. [DOI] [PubMed] [Google Scholar]
- 19.Nishiyama M, Ishikawa T, Rechsteiner H, Glockshuber R. 2008. Reconstitution of pilus assembly reveals a bacterial outer membrane catalyst. Science 320:376–379. doi: 10.1126/science.1154994. [DOI] [PubMed] [Google Scholar]
- 20.Remaut H, Tang C, Henderson NS, Pinkner JS, Wang T, Hultgren SJ, Thanassi DG, Waksman G, Li H. 2008. Fiber formation across the bacterial outer membrane by the chaperone/usher pathway. Cell 133:640–652. doi: 10.1016/j.cell.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phan G, Remaut H, Wang T, Allan WJ, Pirker KF, Lebedev A, Henderson NS, Geibel S, Volkan E, Yan J, Kunze MBA, Pinkner JS, Ford B, Kay CWM, Li H, Hultgren S, Thanassi DG, Waksman G. 2011. Crystal structure of the FimD usher bound to its cognate FimC-FimH substrate. Nature 474:49–53. doi: 10.1038/nature10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Werneburg GT, Henderson NS, Portnoy EB, Sarowar S, Hultgren SJ, Li H, Thanassi DG. 2015. The pilus usher controls protein interactions via domain masking and is functional as an oligomer. Nat Struct Mol Biol 22:540–546. doi: 10.1038/nsmb.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silverman JA, Schreiber HL IV, Hooton TM, Hultgren SJ. 2013. From physiology to pharmacy: developments in the pathogenesis and treatment of recurrent urinary tract infections. Curr Urol Rep 14:448–456. doi: 10.1007/s11934-013-0354-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones CH, Pinkner JS, Roth R, Heuser J, Nicholes AV, Abraham SN, Hultgren SJ. 1995. FimH adhesin of type-1 pili is assembled into a fibrillar tip structure in the Enterobacteriaceae. Proc Natl Acad Sci U S A 92:2081–2085. doi: 10.1073/pnas.92.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuehn MJ, Heuser J, Normark S, Hultgren SJ. 1992. P pili in uropathogenic E. coli are composite fibres with distinct fibrillar adhesive tips. Nature 356:252–255. doi: 10.1038/356252a0. [DOI] [PubMed] [Google Scholar]
- 26.Hahn E, Wild P, Hermanns U, Sebbel P, Glockshuber R, Haner M, Taschner N, Burkhard P, Aebi U, Muller SA. 2002. Exploring the 3D molecular architecture of Escherichia coli type 1 pili. J Mol Biol 323:845–857. doi: 10.1016/S0022-2836(02)01005-7. [DOI] [PubMed] [Google Scholar]
- 27.Abraham SN, Sun D, Dale JB, Beachey EH. 1988. Conservation of the d-mannose-adhesion protein among type 1 fimbriated members of the family Enterobacteriaceae. Nature 336:682–684. doi: 10.1038/336682a0. [DOI] [PubMed] [Google Scholar]
- 28.Bock K, Breimer ME, Brignole A, Hansson GC, Karlsson K-A, Larson G, Leffler H, Samuelsson BE, Strömberg N, Svanborg-Edén C, Thurin J. 1985. Specificity of binding of a strain of uropathogenic Escherichia coli to Gala(1-4)Gal-containing glycosphingolipids. J Biol Chem 260:8545–8551. [PubMed] [Google Scholar]
- 29.Dodson KW, Pinkner JS, Rose T, Magnusson G, Hultgren SJ, Waksman G. 2001. Structural basis of the interaction of the pyelonephritic E. coli adhesin to its human kidney receptor. Cell 105:733–743. doi: 10.1016/S0092-8674(01)00388-9. [DOI] [PubMed] [Google Scholar]
- 30.Kallenius G, Mollby R, Svenson SB, Windberg J, Lundblud A, Svenson S, Cedergen B. 1980. The Pk antigen as receptor for the haemagglutinin of pyelonephritogenic Escherichia coli. FEMS Microbiol Lett 7:297–302. doi: 10.1111/j.1574-6941.1980.tb01608.x. [DOI] [Google Scholar]
- 31.Dielubanza EJ, Schaeffer AJ. 2011. Urinary tract infections in women. Med Clin North Am 95:27–41. doi: 10.1016/j.mcna.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 32.Foxman B. 2010. The epidemiology of urinary tract infection. Nat Rev Urol 7:653–660. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 33.Foxman B, Barlow R, D'Arcy H, Gillespie B, Sobel JD. 2000. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol 10:509–515. doi: 10.1016/S1047-2797(00)00072-7. [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 35.Roberts JA, Kaack MB, Baskin G, Chapman MR, Hunstad DA, Pinkner JS, Hultgren SJ. 2004. Antibody responses and protection from pyelonephritis following vaccination with purified Escherichia coli PapDG protein. J Urol 171:1682–1685. doi: 10.1097/01.ju.0000116123.05160.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langermann S, Mollby R, Burlein JE, Palaszynski SR, Auguste CG, DeFusco A, Strouse R, Schenerman MA, Hultgren SJ, Pinkner JS, Winberg J, Guldevall L, Soderhall M, Ishikawa K, Normark S, Koenig S. 2000. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J Infect Dis 181:774–778. doi: 10.1086/315258. [DOI] [PubMed] [Google Scholar]
- 37.Han Z, Pinkner JS, Ford B, Obermann R, Nolan W, Wildman SA, Hobbs D, Ellenberger T, Cusumano CK, Hultgren SJ, Janetka JW. 2010. Structure-based drug design and optimization of mannoside bacterial FimH antagonists. J Med Chem 53:4779–4792. doi: 10.1021/jm100438s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwardt O, Rabbani S, Hartmann M, Abgottspon D, Wittwer M, Kleeb S, Zalewski A, Smiesko M, Cutting B, Ernst B. 2011. Design, synthesis and biological evaluation of mannosyl triazoles as FimH antagonists. Bioorg Med Chem 19:6454–6473. doi: 10.1016/j.bmc.2011.08.057. [DOI] [PubMed] [Google Scholar]
- 39.Pinkner JS, Remaut H, Buelens F, Miller E, Aberg V, Pemberton N, Hedenstrom M, Larsson A, Seed P, Waksman G, Hultgren SJ, Almqvist F. 2006. Rationally designed small compounds inhibit pilus biogenesis in uropathogenic bacteria. Proc Natl Acad Sci U S A 103:17897–17902. doi: 10.1073/pnas.0606795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cegelski L, Pinkner JS, Hammer ND, Cusumano CK, Hung CS, Chorell E, Aberg V, Walker JN, Seed PC, Almqvist F, Chapman MR, Hultgren SJ. 2009. Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nat Chem Biol 5:913–919. doi: 10.1038/nchembio.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Totsika M, Kostakioti M, Hannan TJ, Upton M, Beatson SA, Janetka JW, Hultgren SJ, Schembri MA. 2013. A FimH inhibitor prevents acute bladder infection and treats chronic cystitis caused by multidrug-resistant uropathogenic Escherichia coli ST131. J Infect Dis 208:921–928. doi: 10.1093/infdis/jit245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lo AW, Van de Water K, Gane PJ, Chan AW, Steadman D, Stevens K, Selwood DL, Waksman G, Remaut H. 2014. Suppression of type 1 pilus assembly in uropathogenic Escherichia coli by chemical inhibition of subunit polymerization. J Antimicrob Chemother 69:1017–1026. doi: 10.1093/jac/dkt467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romero Cabello R, Guerrero LR, Munoz Garcia MR, Geyne Cruz A. 1997. Nitazoxanide for the treatment of intestinal protozoan and helminthic infections in Mexico. Trans R Soc Trop Med Hyg 91:701–703. doi: 10.1016/S0035-9203(97)90531-9. [DOI] [PubMed] [Google Scholar]
- 44.Gilles HM, Hoffman PS. 2002. Treatment of intestinal parasitic infections: a review of nitazoxanide. Trends Parasitol 18:95–97. doi: 10.1016/S1471-4922(01)02205-X. [DOI] [PubMed] [Google Scholar]
- 45.Pankuch GA, Appelbaum PC. 2006. Activities of tizoxanide and nitazoxanide compared to those of five other thiazolides and three other agents against anaerobic species. Antimicrob Agents Chemother 50:1112–1117. doi: 10.1128/AAC.50.3.1112-1117.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoffman PS, Sisson G, Croxen MA, Welch K, Harman WD, Cremades N, Morash MG. 2007. Antiparasitic drug nitazoxanide inhibits the pyruvate oxidoreductases of Helicobacter pylori, selected anaerobic bacteria and parasites, and Campylobacter jejuni. Antimicrob Agents Chemother 51:868–876. doi: 10.1128/AAC.01159-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Warren CA, van Opstal E, Ballard TE, Kennedy A, Wang X, Riggins M, Olekhnovich I, Warthan M, Kolling GL, Guerrant RL, Macdonald TL, Hoffman PS. 2012. Amixicile, a novel inhibitor of pyruvate: ferredoxin oxidoreductase, shows efficacy against Clostridium difficile in a mouse infection model. Antimicrob Agents Chemother 56:4103–4111. doi: 10.1128/AAC.00360-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dubreuil L, Houcke I, Mouton Y, Rossignol JF. 1996. In vitro evaluation of activities of nitazoxanide and tizoxanide against anaerobes and aerobic organisms. Antimicrob Agents Chemother 40:2266–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sisson G, Goodwin A, Raudonikiene A, Hughes NJ, Mukhopadhyay AK, Berg DE, Hoffman PS. 2002. Enzymes associated with reductive activation and action of nitazoxanide, nitrofurans, and metronidazole in Helicobacter pylori. Antimicrob Agents Chemother 46:2116–2123. doi: 10.1128/AAC.46.7.2116-2123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shamir ER, Warthan M, Brown SP, Nataro JP, Guerrant RL, Hoffman PS. 2010. Nitazoxanide inhibits biofilm production and hemagglutination by enteroaggregative Escherichia coli strains by blocking assembly of AafA fimbriae. Antimicrob Agents Chemother 54:1526–1533. doi: 10.1128/AAC.01279-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bolick DT, Roche JK, Hontecillas R, Bassaganya-Riera J, Nataro JP, Guerrant RL. 2013. Enteroaggregative Escherichia coli strain in a novel weaned mouse model: exacerbation by malnutrition, biofilm as a virulence factor and treatment by nitazoxanide. J Med Microbiol 62:896–905. doi: 10.1099/jmm.0.046300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baneyx F, Georgiou G. 1990. In vivo degradation of secreted fusion proteins by the Escherichia coli outer membrane protease OmpT. J Bacteriol 172:491–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cherepanov PP, Wackernagel W. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9–14. doi: 10.1016/0378-1119(95)00193-A. [DOI] [PubMed] [Google Scholar]
- 55.Thomason LC, Costantino N, Court DL. 2007. E. coli genome manipulation by P1 transduction. Curr Protoc Mol Biol 79:1.17.1–1.17.8. doi: 10.1002/0471142727.mb0117s79. [DOI] [PubMed] [Google Scholar]
- 56.Strauch KL, Johnson K, Beckwith J. 1989. Characterization of degP, a gene required for proteolysis in the cell envelope and essential for growth of Escherichia coli at high temperature. J Bacteriol 171:2689–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henderson NS, Ng TW, Talukder I, Thanassi DG. 2011. Function of the usher N-terminus in catalysing pilus assembly. Mol Microbiol 79:954–967. doi: 10.1111/j.1365-2958.2010.07505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blomfield IC, McClain MS, Eisenstein BI. 1991. Type 1 fimbriae mutants of Escherichia coli K-12: characterization of recognized afimbriate strains and construction of new fim deletion mutants. Mol Microbiol 5:1439–1445. doi: 10.1111/j.1365-2958.1991.tb00790.x. [DOI] [PubMed] [Google Scholar]
- 59.Jacob-Dubuisson F, Striker R, Hultgren SJ. 1994. Chaperone-assisted self-assembly of pili independent of cellular energy. J Biol Chem 269:12447–12455. [PubMed] [Google Scholar]
- 60.Saulino ET, Bullitt E, Hultgren SJ. 2000. Snapshots of usher-mediated protein secretion and ordered pilus assembly. Proc Natl Acad Sci U S A 97:9240–9245. doi: 10.1073/pnas.160070497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thanassi DG, Stathopoulos C, Dodson KW, Geiger D, Hultgren SJ. 2002. Bacterial outer membrane ushers contain distinct targeting and assembly domains for pilus biogenesis. J Bacteriol 184:6260–6269. doi: 10.1128/JB.184.22.6260-6269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hultgren SJ, Lindberg F, Magnusson G, Kihlberg J, Tennent JM, Normark S. 1989. The PapG adhesin of uropathogenic Escherichia coli contains separate regions for receptor binding and for the incorporation into the pilus. Proc Natl Acad Sci U S A 86:4357–4361. doi: 10.1073/pnas.86.12.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ng TW, Akman L, Osisami M, Thanassi DG. 2004. The usher N terminus is the initial targeting site for chaperone-subunit complexes and participates in subsequent pilus biogenesis events. J Bacteriol 186:5321–5331. doi: 10.1128/JB.186.16.5321-5331.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.So SS, Thanassi DG. 2006. Analysis of the requirements for pilus biogenesis at the outer membrane usher and the function of the usher C-terminus. Mol Microbiol 60:364–375. doi: 10.1111/j.1365-2958.2006.05111.x. [DOI] [PubMed] [Google Scholar]
- 66.Thanassi DG, Saulino ET, Lombardo MJ, Roth R, Heuser J, Hultgren SJ. 1998. The PapC usher forms an oligomeric channel: implications for pilus biogenesis across the outer membrane. Proc Natl Acad Sci U S A 95:3146–3151. doi: 10.1073/pnas.95.6.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mapingire OS, Henderson NS, Duret G, Thanassi DG, Delcour AH. 2009. Modulating effects of the plug, helix and N- and C-terminal domains on channel properties of the PapC usher. J Biol Chem 284:36324–36333. doi: 10.1074/jbc.M109.055798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saulino ET, Thanassi DG, Pinkner JS, Hultgren SJ. 1998. Ramifications of kinetic partitioning on usher-mediated pilus biogenesis. EMBO J 17:2177–2185. doi: 10.1093/emboj/17.8.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jacob-Dubuisson F, Pinkner J, Xu Z, Striker R, Padmanhaban A, Hultgren SJ. 1994. PapD chaperone function in pilus biogenesis depends on oxidant and chaperone-like activities of DsbA. Proc Natl Acad Sci U S A 91:11552–11556. doi: 10.1073/pnas.91.24.11552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marklund BI, Tennent JM, Garcia E, Hamers A, Baga M, Lindberg F, Gaastra W, Normark S. 1992. Horizontal gene transfer of the Escherichia coli pap and prs pili operons as a mechanism for the development of tissue-specific adhesive properties. Mol Microbiol 6:2225–2242. doi: 10.1111/j.1365-2958.1992.tb01399.x. [DOI] [PubMed] [Google Scholar]
- 71.Jones CH, Danese PN, Pinkner JS, Silhavy TJ, Hultgren SJ. 1997. The chaperone-assisted membrane release and folding pathway is sensed by two signal transduction systems. EMBO J 16:6394–6406. doi: 10.1093/emboj/16.21.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nishiyama M, Horst R, Eidam O, Herrmann T, Ignatov O, Vetsch M, Bettendorff P, Jelesarov I, Grutter MG, Wuthrich K, Glockshuber R, Capitani G. 2005. Structural basis of chaperone-subunit complex recognition by the type 1 pilus assembly platform FimD. EMBO J 24:2075–2086. doi: 10.1038/sj.emboj.7600693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sugawara E, Steiert M, Rouhani S, Nikaido H. 1996. Secondary structure of the outer membrane proteins OmpA of Escherichia coli and OprF of Pseudomonas aeruginosa. J Bacteriol 178:6067–6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krojer T, Sawa J, Schafer E, Saibil HR, Ehrmann M, Clausen T. 2008. Structural basis for the regulated protease and chaperone function of DegP. Nature 453:885–890. doi: 10.1038/nature07004. [DOI] [PubMed] [Google Scholar]
- 75.Palomino C, Marin E, Fernandez LA. 2011. The fimbrial usher FimD follows the SurA-BamB pathway for its assembly in the outer membrane of Escherichia coli. J Bacteriol 193:5222–5230. doi: 10.1128/JB.05585-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ge X, Wang R, Ma J, Liu Y, Ezemaduka AN, Chen PR, Fu X, Chang Z. 2014. DegP primarily functions as a protease for the biogenesis of β-barrel outer membrane proteins in the Gram-negative bacterium Escherichia coli. FEBS J 281:1226–1240. doi: 10.1111/febs.12701. [DOI] [PubMed] [Google Scholar]
- 77.Stockis A, De Bruyn S, Gengler C, Rosillon D. 2002. Nitazoxanide pharmacokinetics and tolerability in man during 7 days dosing with 0.5 g and 1 g b.i.d. Int J Clin Pharmacol Ther 40:221–227. doi: 10.5414/CPP40221. [DOI] [PubMed] [Google Scholar]
- 78.Bardwell JC, McGovern K, Beckwith J. 1991. Identification of a protein required for disulfide bond formation in vivo. Cell 67:581–589. doi: 10.1016/0092-8674(91)90532-4. [DOI] [PubMed] [Google Scholar]
- 79.Norgren M, Baga M, Tennent JM, Normark S. 1987. Nucleotide sequence, regulation and functional analysis of the papC gene required for cell surface localization of Pap pili of uropathogenic Escherichia coli. Mol Microbiol 1:169–178. doi: 10.1111/j.1365-2958.1987.tb00509.x. [DOI] [PubMed] [Google Scholar]
- 80.Fairman JW, Noinaj N, Buchanan SK. 2011. The structural biology of β-barrel membrane proteins: a summary of recent reports. Curr Opin Struct Biol 21:523–531. doi: 10.1016/j.sbi.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dong H, Xiang Q, Gu Y, Wang Z, Paterson NG, Stansfeld PJ, He C, Zhang Y, Wang W, Dong C. 2014. Structural basis for outer membrane lipopolysaccharide insertion. Nature 511:52–56. doi: 10.1038/nature13464. [DOI] [PubMed] [Google Scholar]
- 82.Qiao S, Luo Q, Zhao Y, Zhang XC, Huang Y. 2014. Structural basis for lipopolysaccharide insertion in the bacterial outer membrane. Nature 511:108–111. doi: 10.1038/nature13484. [DOI] [PubMed] [Google Scholar]
- 83.Mogensen JE, Otzen DE. 2005. Interactions between folding factors and bacterial outer membrane proteins. Mol Microbiol 57:326–346. doi: 10.1111/j.1365-2958.2005.04674.x. [DOI] [PubMed] [Google Scholar]
- 84.Justice SS, Hunstad DA, Harper JR, Duguay AR, Pinkner JS, Bann J, Frieden C, Silhavy TJ, Hultgren SJ. 2005. Periplasmic peptidyl prolyl cis-trans isomerases are not essential for viability, but SurA is required for pilus biogenesis in Escherichia coli. J Bacteriol 187:7680–7686. doi: 10.1128/JB.187.22.7680-7686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Watts KM, Hunstad DA. 2008. Components of SurA required for outer membrane biogenesis in uropathogenic Escherichia coli. PLoS One 3:e3359. doi: 10.1371/journal.pone.0003359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ricci DP, Silhavy TJ. 2012. The Bam machine: a molecular cooper. Biochim Biophys Acta 1818:1067–1084. doi: 10.1016/j.bbamem.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ruiz N, Falcone B, Kahne D, Silhavy TJ. 2005. Chemical conditionality: a genetic strategy to probe organelle assembly. Cell 121:307–317. doi: 10.1016/j.cell.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 88.Allen RC, Popat R, Diggle SP, Brown SP. 2014. Targeting virulence: can we make evolution-proof drugs? Nat Rev Microbiol 12:300–308. doi: 10.1038/nrmicro3232. [DOI] [PubMed] [Google Scholar]
- 89.Svensson A, Larsson A, Emtenas H, Hedenstrom M, Fex T, Hultgren SJ, Pinkner JS, Almqvist F, Kihlberg J. 2001. Design and evaluation of pilicides: potential novel antibacterial agents directed against uropathogenic Escherichia coli. Chembiochem 2:915–918. doi:. [DOI] [PubMed] [Google Scholar]
- 90.Greene SE, Pinkner JS, Chorell E, Dodson KW, Shaffer CL, Conover MS, Livny J, Hadjifrangiskou M, Almqvist F, Hultgren SJ. 2014. Pilicide ec240 disrupts virulence circuits in uropathogenic Escherichia coli. mBio 5:e02038-14. doi: 10.1128/mBio.02038-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rose RE. 1988. The nucleotide sequence of pACYC184. Nucleic Acids Res 16:355. doi: 10.1093/nar/16.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morales VM, Backman A, Bagdasarian M. 1991. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene 97:39–47. doi: 10.1016/0378-1119(91)90007-X. [DOI] [PubMed] [Google Scholar]
- 93.Dodson KW, Jacob-Dubuisson F, Striker RT, Hultgren SJ. 1993. Outer-membrane PapC molecular usher discriminately recognizes periplasmic chaperone-pilus subunit complexes. Proc Natl Acad Sci U S A 90:3670–3674. doi: 10.1073/pnas.90.8.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]


