Abstract
Increasing rates of fluoroquinolone resistance (FQ-R) have limited empirical treatment options for Gram-negative infections, particularly in patients with severe beta-lactam allergy. This case-control study aims to develop a clinical risk score to predict the probability of FQ-R in Gram-negative bloodstream isolates. Adult patients with Gram-negative bloodstream infections (BSI) hospitalized at Palmetto Health System in Columbia, South Carolina, from 2010 to 2013 were identified. Multivariate logistic regression was used to identify independent risk factors for FQ-R. Point allocation in the fluoroquinolone resistance score (FQRS) was based on regression coefficients. Model discrimination was assessed by the area under receiver operating characteristic curve (AUC). Among 824 patients with Gram-negative BSI, 143 (17%) had BSI due to fluoroquinolone-nonsusceptible Gram-negative bacilli. Independent risk factors for FQ-R and point allocation in FQRS included male sex (adjusted odds ratio [aOR], 1.97; 95% confidence intervals [CI], 1.36 to 2.98; 1 point), diabetes mellitus (aOR, 1.54; 95% CI, 1.03 to 2.28; 1 point), residence at a skilled nursing facility (aOR, 2.28; 95% CI, 1.42 to 3.63; 2 points), outpatient procedure within 30 days (aOR, 3.68; 95% CI, 1.96 to 6.78; 3 points), prior fluoroquinolone use within 90 days (aOR, 7.87; 95% CI, 4.53 to 13.74; 5 points), or prior fluoroquinolone use within 91 to 180 days of BSI (aOR, 2.77; 95% CI, 1.17 to 6.16; 3 points). The AUC for both final logistic regression and FQRS models was 0.73. Patients with an FQRS of 0, 3, 5, or 8 had predicted probabilities of FQ-R of 6%, 22%, 39%, or 69%, respectively. The estimation of patient-specific risk of antimicrobial resistance using FQRS may improve empirical antimicrobial therapy and fluoroquinolone utilization in Gram-negative BSI.
INTRODUCTION
Bloodstream infection (BSI) is the seventh leading cause of death in the United States (1, 2). Inappropriate empirical antimicrobial therapy has been associated with increased mortality in patients with BSI (3–6). Increasing antimicrobial resistance rates, particularly among Gram-negative bloodstream isolates, have limited empirical antimicrobial treatment options. It has been reported that fluoroquinolone resistance (FQ-R) rates among Gram-negative bloodstream isolates exceeded 20% in multiple geographical settings in North America and Europe (7–9). This increase in FQ-R has severely limited safe and effective empirical antimicrobial treatment options in patients with severe beta-lactam allergy (8). Patients with BSI due to fluoroquinolone-nonsusceptible (FQ-NS) Gram-negative bacilli were more likely than those with fluoroquinolone-susceptible (FQ-S) isolates to receive inappropriate empirical antimicrobial therapy, leading to higher mortality and increased hospital length of stay (10, 11).
In addition, this increase in FQ-R rates among Gram-negative bloodstream and urinary isolates in population-based settings (7, 12) has made empirical fluoroquinolone therapy for acute pyelonephritis obsolete given the national treatment guideline recommendations which favor agents with >90% overall susceptibility rates (13).
The stratification of patients based on the risk of FQ-R using clinical variables would provide valuable information in the empirical setting. The identification of patients with high risk of FQ-R at the time of initial presentation with suspected Gram-negative BSI may optimize the selection of empirical antimicrobial therapy and subsequently improve patient outcomes. Such information also might expand treatment options in patients with low risk of FQ-R, particularly in those with severe beta-lactam allergy, and provide oral options with high bioavailability for patients who may not otherwise require hospitalization.
In this case-control study, independent risk factors for FQ-R in patients with Gram-negative BSI were identified. A clinical score that estimates the probability of FQ-R at the time of initial presentation with Gram-negative BSI was developed based on predictors for FQ-R, including prior fluoroquinolone use and other clinical variables.
(The preliminary results of this study were presented in part at the IDWeek Annual Meeting on 9 October 2015 in San Diego, CA [14].)
MATERIALS AND METHODS
Definitions.
Gram-negative BSI was defined as the growth of any aerobic Gram-negative bacillus in a blood culture. The primary source of BSI was defined according to the Centers for Disease Control and Prevention (CDC) criteria (15). The sites of infection acquisition were classified as community-acquired, health care-associated, and hospital-acquired as previously defined (16). Outpatient procedures included both invasive (bladder, colon, and prostate biopsy specimens, etc.) and noninvasive procedures (colonoscopy, cystoscopy, duodenoscopy, etc.). FQ-NS isolates were defined as Gram-negative bacilli that were resistant in vitro (MIC, ≥4) or intermediate to ciprofloxacin (MIC = 2) according to the Clinical and Laboratory Standards Institute (CLSI) guidelines.
Setting.
The study was conducted at Palmetto Health Richland and Baptist Hospitals in Columbia, SC. The two hospitals combined have nearly 1,100 licensed beds, provide medical, surgical, and subspecialty care mostly for Richland County residents, and receive regional referrals. The Institutional Review Board at Palmetto Health approved the study.
Case ascertainment.
All patients with BSI due to aerobic Gram-negative bacilli from 1 January 2010 to 31 December 2013 were identified through microbiology laboratory databases at Palmetto Health. Overall, 824 unique adults with first episodes of Gram-negative BSI were included in the study. Children <18 years old (n = 98) were excluded due to infrequent use of fluoroquinolones in this age group. Other exclusions included recurrent BSI (n = 34), to avoid potential inclusion of same patient as case and control, and polymicrobial BSI (n = 121), to exclude growth of microorganisms with discrepant fluoroquinolone susceptibilities in the same blood culture. In addition, bloodstream isolates without ciprofloxacin in vitro susceptibility testing (n = 8) were excluded. Demographics, microbiology, and clinical data, including potential risk factors for antimicrobial resistance, were collected from electronic medical records. The receipt of prior fluoroquinolones in the 6 months preceding BSI was ascertained from medication administration records from current and prior hospitalizations, electronic prescriptions in medical records from prior visits to affiliated hospitals or ambulatory clinics, and clinical notes from current or prior visits, including emergency department, hospital admission, and consultation notes.
Statistical analysis.
Logistic regression was used to identify risk factors for FQ-R among Gram-negative bloodstream isolates in this case-control study. For the simplification of statistical analysis, Gram-negative bacilli that were in vitro resistant or intermediate to ciprofloxacin were analyzed as FQ-NS. Demographics and clinical variables were collected in patients with BSI due to FQ-NS (cases) and FQ-S Gram-negative bacilli (controls). Fluoroquinolone use within 180 days prior to BSI was analyzed as a categorical variable: none, within 90 days, and within 91 to 180 days from BSI. Variables that were associated with FQ-R in the univariate logistic regression model with a P value of <0.10 were included in multivariate logistic regression models to identify independent risk factors for FQ-R. Variables were retained in the final model if they were independently associated with FQ-R with a P value of <0.05 in multivariate logistic regression models using the likelihood ratio test method and individually retained in ≥70% of 200 bootstrap samples (17). Each bootstrap sample was derived by applying the same model selection criteria. The frequency of selected variables was computed as a percentage across all bootstrap samples.
The fluoroquinolone resistance score (FQRS) was derived from the final model. Points were assigned for each independent risk factor of FQ-R and weighted approximately by the corresponding regression coefficients in the final model. The area under receiver operating characteristic curve (AUC) was used to quantify the discriminative ability of the FQRS, with a value of 0.5 denoting random predictions and a value of 1.0 indicating perfect predictions. To visually assess calibration, deciles of predicted risk of FQ-R from the FQRS model were plotted by the actual fraction of patients who had BSI due to FQ-NS bacteria. Predicted probabilities obtained directly from the scoring model were plotted by FQRS values to visualize the estimated risk of FQ-R.
After FQRS was developed, its potential to improve the adequacy of empirical therapy and to avoid unnecessary broad-spectrum antimicrobial therapy was evaluated in this cohort. First, the ability of the FQRS to improve the adequacy of empirical therapy was evaluated by estimating the proportion of patients with inadequate empirical fluoroquinolone therapy who had a high predicted risk of FQ-R at baseline and, thus, potentially could have been switched to alternative adequate empirical therapy. Second, a subgroup analysis of noncritically ill (Pitt bacteremia score of <4 [18]) women with a urinary source of BSI was conducted to identify potential opportunities to spare broad-spectrum antimicrobial therapy in the setting of low predicted probability of FQ-R.
Finally, the performance of FQRS, which was developed using the CLSI ciprofloxacin susceptibility breakpoint (MIC of ≤1), was evaluated using the European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoint (MIC of ≤0.5) and United States Committee on Antimicrobial Susceptibility Testing (USCAST) breakpoints (MIC of ≤0.25 for Enterobacteriaceae and ≤0.5 for nonfermenting Gram-negative bacilli). Sensitivity, specificity, and negative predictive value (NPV) were calculated from the respective receiver operating characteristic curve for the best cutoff points.
JMP Pro (version 12.0; SAS Institute, Inc., Cary, NC) was used for statistical analysis. The level of significance for statistical testing was defined as P < 0.05 (2-sided) unless otherwise specified.
RESULTS
During the 4-year study period, 824 patients with Gram-negative BSI were included. Overall, the median age was 66 years and 382 (46%) were men. The site of acquisition was mostly community acquired (343; 42%) or health care associated (313; 38%). Hospital-acquired BSI accounted for 168 (20%) cases. Over one-half of patients had a urinary source of BSI (442; 54%). The remaining patients had BSI secondary to intra-abdominal (106; 13%), central venous catheter (59; 7%), respiratory tract (48; 6%), other (48; 6%), and undetermined sources of infection (121; 15%). Escherichia coli was the most common bloodstream isolate (439; 53%), followed by Klebsiella pneumoniae (146; 18%), Proteus mirabilis (58; 7%), Pseudomonas aeruginosa (50; 6%), and Enterobacter cloacae (30; 4%).
Among this cohort, 143 (17%) had BSI due to FQ-NS Gram-negative bacilli (131 were in vitro resistant to ciprofloxacin and 12 were intermediate). The baseline demographics and clinical characteristics of patients with BSI due to FQ-NS and FQ-S bloodstream isolates are shown in Table 1.
TABLE 1.
Demographics and clinical characteristics of patients with BSI due to FQ-NS and FQ-S organisms
| Variable | Value fora: |
|
|---|---|---|
| FQ-NS (n = 143) | FQ-S (n = 681) | |
| Age in yr, median (IQR) | 68 (58–77) | 65 (53–78) |
| Male sex | 84 (59) | 298 (44) |
| Ethnicity | ||
| White | 71 (50) | 327 (48) |
| African-American | 69 (48) | 329 (48) |
| Other | 3 (2) | 25 (4) |
| Diabetes mellitus | 61 (43) | 236 (35) |
| End-stage renal disease | 16 (11) | 61 (9) |
| Liver cirrhosis | 5 (4) | 25 (4) |
| Cancer | 27 (19) | 124 (18) |
| Immunocompromised host | 14 (10) | 92 (14) |
| Indwelling urinary catheter | 27 (19) | 75 (11) |
| Recent outpatient procedureb | 20 (14) | 36 (5) |
| Residence at skilled nursing facility | 36 (25) | 95 (14) |
| Recent hospitalizationc | 57 (40) | 205 (30) |
| Recent chemotherapyb | 8 (6) | 64 (9) |
| Central venous catheter | 31 (22) | 149 (22) |
| Hospital-acquired infection | 24 (17) | 144 (21) |
| Urinary source of infection | 78 (55) | 364 (54) |
| Prior fluoroquinolone used | ||
| None | 103 (72) | 625 (92) |
| Within 90 days | 30 (21) | 36 (5) |
| Within 91–180 days | 10 (7) | 20 (3) |
Data are shown as number (percent) unless otherwise specified. FQ-NS, fluoroquinolone nonsusceptible; FQ-S, fluoroquinolone susceptible; IQR, interquartile range.
Within 30 days of bloodstream infection.
Within 90 days of bloodstream infection.
Within 180 days of bloodstream infection.
In the univariate logistic regression model, male sex, diabetes mellitus, indwelling urinary catheter, recent outpatient procedure, residence at a skilled nursing facility, recent hospitalization, and prior fluoroquinolone use were associated with FQ-R. Notably, the nosocomial acquisition of BSI was not associated with FQ-R (Table 2).
TABLE 2.
Univariate logistic regression results for risk factors of FQ-R in Gram-negative bloodstream isolates
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Age (per decade) | 1.10 | 0.99–1.23 | 0.08 |
| Male sex | 1.83 | 1.27–2.65 | 0.001 |
| White ethnicity | 1.07 | 0.74–1.53 | 0.72 |
| Diabetes mellitus | 1.40 | 0.97- 2.02 | 0.07 |
| End-stage renal disease | 1.28 | 0.69- 2.24 | 0.41 |
| Liver cirrhosis | 0.95 | 0.32–2.33 | 0.92 |
| Cancer | 1.05 | 0.65–1.64 | 0.85 |
| Immunocompromised host | 0.69 | 0.37–1.22 | 0.23 |
| Indwelling urinary catheter | 1.88 | 1.14–3.02 | 0.01 |
| Recent outpatient procedurea | 2.91 | 1.61–5.15 | <0.001 |
| Residence at skilled nursing facility | 2.08 | 1.33–3.19 | 0.001 |
| Recent hospitalizationb | 1.54 | 1.06–2.23 | 0.02 |
| Recent chemotherapya | 0.57 | 0.25–1.15 | 0.15 |
| Central venous catheter | 0.99 | 0.63–1.51 | 0.96 |
| Hospital-acquired infection | 0.75 | 0.46–1.19 | 0.24 |
| Urinary source of infection | 1.05 | 0.73–1.50 | 0.81 |
| Prior fluoroquinolone usec | |||
| None | 1 | Reference value | Reference value |
| Within 90 days | 5.90 | 3.50–9.95 | <0.001 |
| Within 91–180 days | 3.13 | 1.37–6.74 | 0.008 |
Within 30 days of bloodstream infection.
Within 90 days of bloodstream infection.
Within 180 days of bloodstream infection.
After adjustments for potential confounders in multivariate logistic regression models, age, indwelling urinary catheter (odds ratio [OR], 1.50; 95% confidence interval [CI], 0.87 to 2.53; P = 0.14) and recent hospitalization (OR, 0.94; 95% CI, 0.60 to 1.45; P = 0.78) were not independently associated with FQ-R. Male sex, diabetes mellitus, recent outpatient procedure, residence at a skilled nursing facility, and prior use of fluoroquinolones were independently associated with FQ-R and were retained in the final model after bootstrap resampling (Table 3). The AUC for the final logistic regression model was 0.73.
TABLE 3.
Final logistic regression results for independent risk factors of FQ-R among Gram-negative bloodstream isolates and FQRS point allocation
| Variable | aORa | 95% CI | P value | Regression coefficient | Point allocation |
|---|---|---|---|---|---|
| Male sex | 1.97 | 1.36–2.98 | <0.001 | 0.31 | 1 |
| Diabetes mellitus | 1.54 | 1.03–2.28 | 0.03 | 0.21 | 1 |
| Residence at skilled nursing facility | 2.28 | 1.42–3.63 | <0.001 | 0.41 | 2 |
| Recent outpatient procedureb | 3.68 | 1.96–6.78 | <0.001 | 0.65 | 3 |
| Prior fluoroquinolone usec | |||||
| None | 1 | Reference value | Reference value | Reference value | 0 |
| Within 90 days | 7.87 | 4.53–13.74 | <0.001 | 0.98 | 5 |
| Within 91–180 days | 2.77 | 1.17–6.16 | 0.02 | 0.55 | 3 |
aOR, adjusted odds ratio.
Within 30 days of bloodstream infection.
Within 180 days of bloodstream infection.
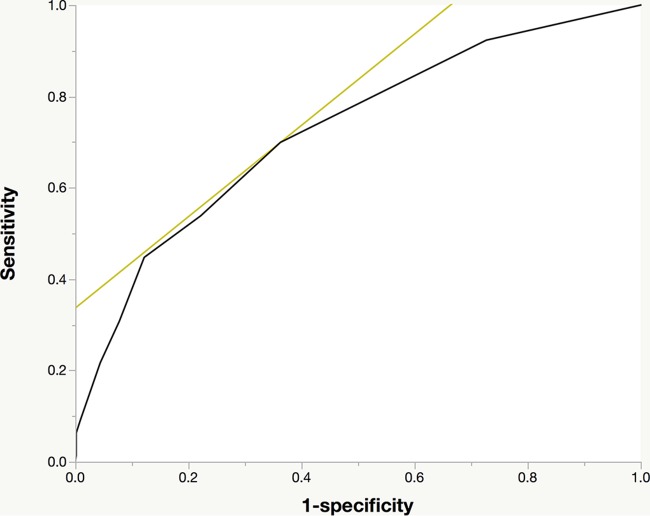
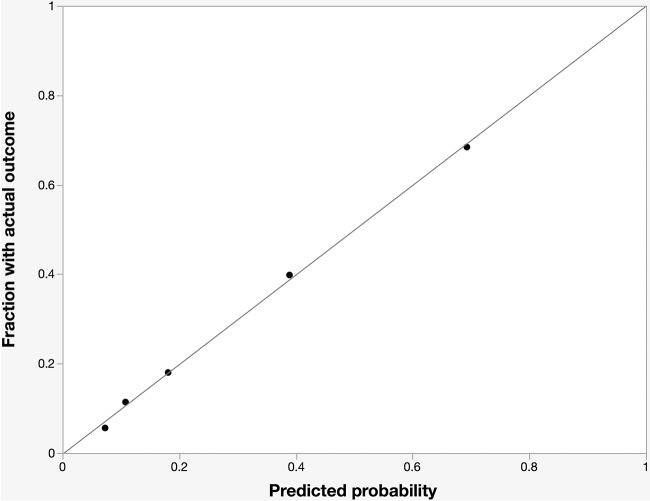
The FQRS was developed by assigning points for each variable in the final model, weighted approximately by the corresponding regression coefficients (Table 3). FQRS is the cumulative number of points for each individual from their risk factors, and it ranges in value from 0 to 12. The AUC for the FQRS model was 0.73 (Fig. 1). Model calibration looked satisfactory, as the observed outcomes appeared fairly close to the predictions (Fig. 2).
FIG 1.
Receiver operating characteristic plot of the fluoroquinolone resistance score. The black line indicates the receiver operating characteristic curve. The light-colored tangent line highlights the point in the curve that represents the best performance of the model. The area under the curve is 0.73.
FIG 2.
Calibration plot of fluoroquinolone resistance score. The observed frequency of fluoroquinolone resistance plotted by deciles of predicted probability from the fluoroquinolone resistance score (black dots) is shown. Perfect calibration is represented by the gray y = x line.
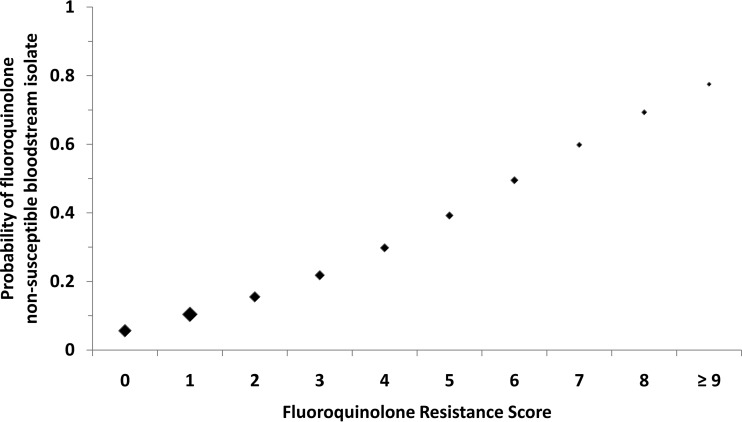
The FQRS provides clinicians with a simple tool with which to predict the risk of FQ-R in patients with Gram-negative BSI (Fig. 3). A higher score corresponds to an increased risk of FQ-R. For example, patients with an FQRS of 0 have a relatively low predicted probability of FQ-R of nearly 6%. The estimated risk of FQ-R increases to 22% with an FQRS of 3 and approaches 40% in patients with an FQRS of 5.
FIG 3.
Predicted probability of fluoroquinolone-nonsusceptible bloodstream isolates by fluoroquinolone resistance score. The size of the marker for point estimates is weighted approximately by the relative number of subjects with the corresponding score.
The performance characteristics of FQRS as a binary classification test for selected cutoff points are shown in Table 4. Using a low cutoff score of ≥1 to indicate high risk of resistance provides high sensitivity and NPV. A higher cutoff point of ≥3 improves specificity at the expense of sensitivity and NPV. A cutoff point of ≥2 provides the best performance of FQRS in the statistical model.
TABLE 4.
Performance of FQRS as a binary classification testa
| FQRS | Sensitivity (%) | Specificity (%) | NPV (%) |
|---|---|---|---|
| ≥1 | 92, 91, 90 | 27, 28, 28 | 94, 92, 90 |
| ≥2b | 70, 69, 68 | 64, 65, 67 | 91, 89, 87 |
| ≥3 | 56, 53, 51 | 78, 79, 79 | 89, 88, 85 |
Data show results for each variable using Clinical and Laboratory Standards Institute (CLSI), European Committee on Antimicrobial Susceptibility Testing (EUCAST), and United States Committee on Antimicrobial Susceptibility Testing (USCAST) breakpoints, respectively.
Using a cutoff score of ≥2 to indicate high predicted risk of fluoroquinolone resistance provides the best performance in statistical models.
The potential impact of FQRS on the adequacy of empirical fluoroquinolone therapy for Gram-negative BSI was examined. In the current cohort, 108 patients were treated empirically with fluoroquinolones, of which 19 (18%) had BSI due to FQ-NS Gram-negative bacilli. The application of FQRS (using a score of ≥2 to indicate high risk of resistance) would have eliminated nearly two-thirds (13/19) of inadequate empirical fluoroquinolone therapy, which would have reduced the overall proportion of inadequate empirical fluoroquinolones to 6% (6/108).
In order to assess the implications of FQRS on the utilization of broad-spectrum antimicrobial agents, FQRS was evaluated in a subgroup of 238 noncritically ill women with a urinary source of BSI in the current cohort. Among these women, 171 had low predicted risk of fluoroquinolone resistance (FQRS of <2), of whom 161 (94%) had BSI due to FQ-S Gram-negative bacilli. Empirical fluoroquinolones were used in 44/171 (26%) women with a FQRS of <2, whereas 34 (20%) were empirically treated with antipseudomonal beta-lactams, 8 (5%) with carbapenems, 41 (24%) with combination therapy of beta-lactams plus fluoroquinolones or aminoglycosides, and the remaining 44 (26%) with non-antipseudomonal beta-lactams. The application of FQRS would have spared approximately one-half (83/171) of patients in this subgroup from broad-spectrum antipseudomonal beta-lactams, carbapenems, and combination therapy.
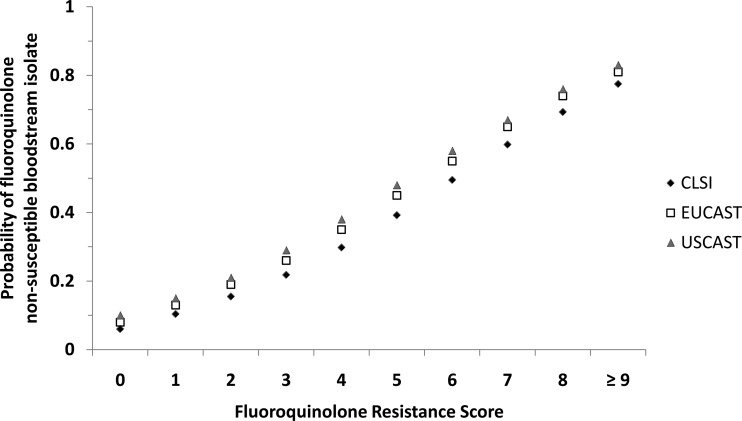
Finally, FQRS performance was evaluated using EUCAST and USCAST ciprofloxacin susceptibility breakpoints to improve generalizability and account for the potential reappraisal of CLSI breakpoints in the future. FQRS had good discrimination in predicting FQ-R using both EUCAST and USCAST susceptibility breakpoints (AUC of 0.72 and 0.71, respectively). An FQRS of ≥2 represented the best cutoff with fairly comparable sensitivity, specificity, and NPV (Table 4). There was an upshift in predicted FQ-R using EUCAST and USCAST compared to the level predicted by CLSI criteria throughout the curve (Fig. 4). However, the impact appeared less substantial at the lower end of the curve (FQRS of <2), with an increase in FQ-R of 2 to 3% using EUCAST and 3 to 4% using USCAST compared to CLIS breakpoints. There was a substantial increase in resistance at the high end of the curve (FQRS of ≥2) of 4 to 5% and 6 to 8% using EUCAST and USCAST breakpoints, respectively.
FIG 4.
Comparison of predicted probabilities of ciprofloxacin-nonsusceptible bloodstream isolates by fluoroquinolone resistance score using different MIC susceptibility breakpoint criteria. CLSI, Clinical Laboratory and Standards Institute (MIC of ≤1); EUCAST, European Committee on Antimicrobial Susceptibility Testing (MIC of ≤0.5); USCAST, United States Committee on Antimicrobial Susceptibility Testing (MIC of ≤0.25 for Enterobacteriaceae and ≤0.5 for nonfermenting Gram-negative bacilli).
DISCUSSION
The study demonstrated that the risk of FQ-R can be estimated with good discrimination based on prior use of fluoroquinolones and other readily available demographics and clinical variables at the time of initial presentation with Gram-negative BSI.
Prior fluoroquinolone use was the strongest risk factor for FQ-R in this study, with more recent use (within 3 months) representing higher risk of resistance than relatively more distant use (within 3 to 6 months). The association between prior fluoroquinolone use and FQ-R is consistent with the results of previous studies (8, 9). It is conceivable that exposure to fluoroquinolones increases the risk of colonization with FQ-NS Gram-negative bacilli, particularly colonic flora, and subsequently infections with these bacteria. The current study demonstrates that the effect of fluoroquinolone exposure on resistance lasts for at least 6 months. The inverse association between the timing of prior fluoroquinolone use and risk of FQ-R deserves further examination. It is possible that FQ-NS Gram-negative bacilli no longer represent the dominant colonic flora in some patients who do not receive further fluoroquinolone therapy in the subsequent 3 months. However, it remains possible that this is due to recall bias, as patients may not remember antibiotics used over 3 months ago.
Residence at skilled nursing facilities has been described as a risk factor for antimicrobial resistance and acquisition of E. coli serotype ST131, a predominantly FQ-NS strain (19). The increased risk of antimicrobial resistance in elderly residents of skilled nursing facilities is not surprising, since fluoroquinolone use, particularly for the treatment of suspected urinary tract infections, is commonplace in these facilities. The increased risk of FQ-R in residents of skilled nursing facilities likely is due to close proximity to other patients who have recently received fluoroquinolones (19). The implementation of antimicrobial stewardship programs and better infection control measures in these facilities may improve fluoroquinolone utilization and antimicrobial resistance rates.
Outpatient procedures have been associated with the risk of acquisition of bacteria that harbor antimicrobial resistance in some settings. Infections due to FQ-NS Gram-negative bacilli have been described recently following invasive urologic procedures such as transrectal prostate biopsies (20). Outbreaks of infections due to multidrug-resistant bacteria, including carbapenem-resistant Enterobacteriaceae (CRE), have been reported recently following noninvasive gastrointestinal procedures such as duodenoscopies (21). The current study results suggest that CRE infections following some outpatient procedures likely represent the tip of the iceberg, and other resistance mechanisms, such as FQ-R, may be more common after such procedures. These data provide opportunities to review infection control practices in the settings of invasive and noninvasive urologic and gastrointestinal procedures.
The identification of male sex and diabetes mellitus as risk factors for FQ-R deserves further investigation. Speculative causes include differences in colonic microbiota, dietary habits, and recall bias rates across genders. In addition, FQ-R in men and diabetics may be a reflection of undocumented or more distant (>6 months) use of fluoroquinolones. Future studies comparing the effect of antibiotics on microbiota and the duration of this effect across genders and in patients with diabetes mellitus are warranted. However, male sex and diabetes were minor risk factors compared to the other three predictors of FQ-R in this study. Diabetes mellitus was also the least retained variable in bootstrapping and would have been eliminated if more strict retention criteria were applied.
It is also interesting that neither current nor recent hospitalization was independently associated with FQ-R in patients with Gram-negative BSI. Although the difference was not statistically significant, hospital-acquired Gram-negative bloodstream isolates were less likely than community-onset isolates to be FQ-NS (14% versus 18%). This is consistent with the results of a recent study from a nearby geographical location in North Carolina (8). This may be due to local antimicrobial stewardship program initiatives to minimize unnecessary fluoroquinolone use in hospitals and the lack of such programs in the community (22). Recent hospitalization was likely a marker for prior fluoroquinolone use, as it was associated with FQ-R in the univariate, but not multivariate, model. However, this finding may not be generalizable to hospitals in other geographical locations, particularly those with higher rates of FQ-R. The external validation of the FQRS in those settings is necessary.
The current study adds to existing knowledge about risk factors for FQ-R. It confirms the increased risk of FQ-R in skilled nursing facility residents and patients undergoing outpatient procedures (19, 20). It also emphasizes the importance of enquiring about prior fluoroquinolone use not only over the past 1 to 3 months (8, 9) but also up to 6 months prior to index infection, as this appears to be the most important predictor of FQ-R. Efforts to improve provider awareness of prior antibiotic exposure should be explored going forward. The most notable addition to previous studies, however, is the development of a clinical risk score that takes into account the strength of association between each risk factor and FQ-R, since not all risk factors have the same impact.
The FQRS provides clinicians with a useful tool to improve the selection of empirical antimicrobial regimens in patients with Gram-negative BSI. The nonstratified use of empirical fluoroquinolones in patients with Gram-negative BSI has been discouraged due to increasing rates of FQ-R (23). In the current cohort, the inadequacy of fluoroquinolone empirical therapy based on providers' individual selections was very close to the overall rate of FQ-R in Gram-negative bloodstream isolates (18% and 17%, respectively). A systematic approach with the stratification of patients based on the predicted risk of antimicrobial resistance using FQRS would have reduced the inadequacy of empirical fluoroquinolone therapy from 18% to 6%.
In addition, this increase in FQ-R rates has limited empirical treatment options for serious Gram-negative infections, particularly in patients with severe beta-lactam allergy (8). A recent study demonstrated that overall resistance rates of Gram-negative bacilli have exceeded 20% for both aztreonam and ciprofloxacin in these patients (8). However, using FQRS allows the estimation of the probability of FQ-R in each individual patient based on specific risk factors rather than using an overall resistance rate. For example, the probability of FQ-R is approximately 6% and 10% in patients with FQRS of 0 and 1, respectively. In such patients with relatively low predicted risk of FQ-R, fluoroquinolones may be the best remaining empirical antimicrobial agents for monotherapy, providing the highest susceptibility rates (compared to aztreonam) and safest side effect profiles (compared to aminoglycosides). Although an FQRS of ≥2 provides the best performance in the statistical model, a more conservative approach using an FQRS of ≥1 to indicate high risk of predicted FQ-R may be more appropriate in critically ill patients with Gram-negative BSI.
Other clinical applications for the FQRS include women with acute pyelonephritis who may not otherwise require hospitalization. The practice guidelines recommend the administration of 1 dose of intravenous ceftriaxone prior to discharge from the emergency room on oral fluoroquinolones (13). The current study results argue that ceftriaxone is necessary in patients with low FQRS. For example, in patients with an FQRS of <2, the estimated risk of antimicrobial resistance to fluoroquinolones and third-generation cephalosporins may be comparable (7). The utilization of FQRS may simplify antimicrobial management in these patients and spare unnecessary or additional broad-spectrum antibiotic exposure and associated risk of toxicities, as demonstrated in the current study. However, FQRS should not replace in vitro antimicrobial susceptibility testing. Empirical antimicrobial therapy should be adjusted based on organism identification and in vitro antimicrobial susceptibility testing results whenever available.
It is intuitive to utilize FQRS in the treatment of patients with intra-abdominal infections where the yield of blood cultures is relatively low (24). However, since FQRS was derived from a cohort of patients with confirmed Gram-negative BSI, its use should not be extrapolated to culture-negative infections without validation.
Current CLSI susceptibility breakpoints for fluoroquinolones were used in the development of the FQRS, which are fairly similar to U.S. Food and Drug Administration (FDA) breakpoints, except that the CLSI does not report moxifloxacin susceptibility breakpoints for Enterobacteriaceae. The FQRS performed well in predicting FQ-R using EUCAST and USCAST susceptibility breakpoints. The upshift in predicted FQ-R using EUCAST and USCAST compared to CLSI breakpoints was minimal when fluoroquinolone use seemed appropriate at low FQRS. The substantial increase in resistance at high FQRS is unlikely to influence clinical decisions, since fluoroquinolones are not recommended due to the high predicted probability of resistance using any susceptibility breakpoint criteria. This is reassuring for providers who use EUCAST or USCAST criteria or who anticipate the reappraisal of current CLSI or FDA susceptibility breakpoints.
The study had some limitations. First, in a retrospective cohort design, it remains possible that some patients received fluoroquinolones in other unaffiliated hospitals or clinics without documentation in electronic medical records. Second, the results were derived from two hospitals within the same health care system and geographical location. Data from multiple geographical settings may provide external validity and generalizability of the model.
In summary, the prediction of antimicrobial resistance opens a new horizon in the selection of empirical antimicrobial therapy for Gram-negative infections. It allows health care providers to initiate therapy based on patient-specific risk of antimicrobial resistance rather than average antimicrobial resistance rates from large multinational, nationwide, regional, or local surveillance, population-based, or institutional data. The application of FQRS may improve empirical antimicrobial therapy, particularly in patients with severe or undocumented beta-lactam allergy. It also may simplify antimicrobial management in patients without indications for hospital admission, such as some cases of acute pyelonephritis.
ACKNOWLEDGMENTS
We thank the Antimicrobial Stewardship and Support Team at Palmetto Health in Columbia, SC, for their help in facilitating the conducting of this study.
S.D. and M.N.A.-H. have full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
S.D., A.S., J.K., H.A., and M.N.A.-H. have no conflicts of interest to report. P.B.B. is on the research advisory board for Actavis Pharmaceuticals (now Allergan) and has received research funding from Actavis Pharmaceuticals (now Allergan). J.A.J. is an advisory board member for Cempra Pharmaceuticals.
REFERENCES
- 1.Goto M, Al-Hasan MN. 2013. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 19:501–509. doi: 10.1111/1469-0691.12195. [DOI] [PubMed] [Google Scholar]
- 2.Uslan DZ, Crane SJ, Steckelberg JM, Cockerill FR III, St Sauver JL, Wilson WR, Baddour LM. 2007. Age- and sex-associated trends in bloodstream infection: a population-based study in Olmsted County, Minnesota. Arch Intern Med 167:834–839. doi: 10.1001/archinte.167.8.834. [DOI] [PubMed] [Google Scholar]
- 3.Bryan CS, Reynolds KL, Brenner ER. 1983. Analysis of 1,186 episodes of Gram-negative bacteremia in non-university hospital: the effects of antimicrobial therapy. Rev Infect Dis 5:629–638. doi: 10.1093/clinids/5.4.629. [DOI] [PubMed] [Google Scholar]
- 4.Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. 2010. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother 54:4851–4863. doi: 10.1128/AAC.00627-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Retamar P, Portillo MM, López-Prieto MD, Rodríguez-López F, de Cueto M, García MV, Gómez MJ, Del Arco A, Muñoz A, Sánchez-Porto A, Torres-Tortosa M, Martín-Aspas A, Arroyo A, García-Figueras C, Acosta F, Corzo JE, León-Ruiz L, Escobar-Lara T, Rodríguez-Baño J, SAEI/Bacteremia Group SAMPAC . 2012. Impact of inadequate empirical therapy on the mortality of patients with bloodstream infections: a propensity score-based analysis. Antimicrob Agents Chemother 56:472–478. doi: 10.1128/AAC.00462-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cain SE, Kohn J, Bookstaver PB, Albrecht H, Al-Hasan MN. 2015. Stratification of the impact of inappropriate empirical antimicrobial therapy for Gram-negative bloodstream infections by predicted prognosis. Antimicrob Agents Chemother 59:245–250. doi: 10.1128/AAC.03935-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peirano G, Pitout JD. 2014. Fluoroquinolone-resistant Escherichia coli sequence type 131 isolates causing bloodstream infections in a Canadian region with a centralized laboratory system: rapid emergence of the H30-Rx sublineage. Antimicrob Agents Chemother 58:2699–2703. doi: 10.1128/AAC.00119-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koliscak LP, Johnson JW, Beardsley JR, Miller DP, Williamson JC, Luther VP, Ohl CA. 2013. Optimizing empiric antibiotic therapy in patients with severe β-lactam allergy. Antimicrob Agents Chemother 57:5918–5923. doi: 10.1128/AAC.01202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortega M, Marco F, Soriano A, Almela M, Martínez JA, Muñoz A, Mensa. 2009. Analysis of 4758 Escherichia coli bacteraemia episodes: predictive factors for isolation of an antibiotic-resistant strain and their impact on the outcome. J Antimicrob Chemother 63:568–574. doi: 10.1093/jac/dkn514. [DOI] [PubMed] [Google Scholar]
- 10.Lautenbach E, Metlay JP, Bilker WB, Edelstein PH, Fishman NO. 2005. Association between fluoroquinolone resistance and mortality in Escherichia coli and Klebsiella pneumoniae infections: the role of inadequate empirical antimicrobial therapy. Clin Infect Dis 41:923–929. doi: 10.1086/432940. [DOI] [PubMed] [Google Scholar]
- 11.Brigmon M, Bookstaver BP, Kohn J, Albrecht H, Al-Hasan MN. 2015. Impact of fluoroquinolone resistance on community-onset gram-negative bloodstream infections. Clin Microbiol Infect 21:843–849. doi: 10.1016/j.cmi.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee R, Johnston B, Lohse C, Chattopadhyay S, Tchesnokova V, Sokurenko EV, Johnson JR. 2013. The clonal distribution and diversity of extraintestinal Escherichia coli isolates vary according to patient characteristics. Antimicrob Agents Chemother 57:5912–5917. doi: 10.1128/AAC.01065-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 14.Dan S, Shah A, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. 2015. Abstr IDWeek 2015 Ann Meet, abstr 50548. [Google Scholar]
- 15.Horan TC, Andrus M, Dudeck MA. 2008. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W, Clark C, MacFarquhar J, Walton AL, Reller LB, Sexton DJ. 2002. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 17.Al-Hasan MN, Lahr BD, Eckel-Passow JE, Baddour LM. 2013. Predictive scoring model of mortality in Gram-negative bloodstream infection. Clin Microbiol Infect 19:948–954. doi: 10.1111/1469-0691.12085. [DOI] [PubMed] [Google Scholar]
- 18.Paterson DL, Ko WC, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, Mulazimoglu L, Trenholme G, Klugman KP, Bonomo RA, Rice LB, Wagener MM, McCormack JG, Yu VL. 2004. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann Intern Med 140:26–32. doi: 10.7326/0003-4819-140-1-200401060-00008. [DOI] [PubMed] [Google Scholar]
- 19.Banerjee R, Johnston B, Lohse C, Porter SB, Clabots C, Johnson JR. 2013. Escherichia coli sequence type 131 is a dominant, antimicrobial-resistant clonal group associated with healthcare and elderly hosts. Infect Control Hosp Epidemiol 34:361–369. doi: 10.1086/669865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williamson DA, Roberts SA, Paterson DL, Sidjabat H, Silvey A, Masters J, Rice M, Freeman JT. 2012. Escherichia coli bloodstream infection after transrectal ultrasound-guided prostate biopsy: implications of fluoroquinolone-resistant sequence type 131 as a major causative pathogen. Clin Infect Dis 54:1406–1412. doi: 10.1093/cid/cis194. [DOI] [PubMed] [Google Scholar]
- 21.Epstein L, Hunter JC, Arwady MA, Tsai V, Stein L, Gribogiannis M, Frias M, Guh AY, Laufer AS, Black S, Pacilli M, Moulton-Meissner H, Rasheed JK, Avillan JJ, Kitchel B, Limbago BM, MacCannell D, Lonsway D, Noble-Wang J, Conway J, Conover C, Vernon M, Kallen AJ. 2014. New Delhi metallo-β-lactamase-producing carbapenem-resistant Escherichia coli associated with exposure to duodenoscopes. JAMA 312:1447–1455. doi: 10.1001/jama.2014.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fishman N. 2006. Antimicrobial stewardship. Am J Med 119:S53–S61. doi: 10.1016/j.amjmed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Al-Hasan MN, Lahr BD, Eckel-Passow JE, Baddour LM. 2009. Antimicrobial resistance trends of Escherichia coli bloodstream isolates: a population-based study, 1998-2007. J Antimicrob Chemother 64:169–174. doi: 10.1093/jac/dkp162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ, O'Neill PJ, Chow AW, Dellinger EP, Eachempati SR, Gorbach S, Hilfiker M, May AK, Nathens AB, Sawyer RG, Bartlett JG. 2010. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis 50:133–164. doi: 10.1086/649554. [DOI] [PubMed] [Google Scholar]