Abstract
We have morphologically characterized Candida tropicalis isolates resistant to amphotericin B (AmB). These isolates present an enlarged cell wall compared to isolates of regular susceptibility. This correlated with higher levels of β-1,3-glucan in the cell wall but not with detectable changes in chitin content. In line with this, AmB-resistant strains showed reduced susceptibility to Congo red. Moreover, mitogen-activated protein kinases (MAPKs) involved in cell integrity were already activated during regular growth in these strains. Finally, we investigated the response elicited by human blood cells and found that AmB-resistant strains induced a stronger proinflammatory response than susceptible strains. In agreement, AmB-resistant strains also induced stronger melanization of Galleria mellonella larvae, indicating that the effect of alterations of the cell wall on the immune response is conserved in different types of hosts. Our results suggest that resistance to AmB is associated with pleiotropic mechanisms that might have important consequences, not only for the efficacy of the treatment but also for the immune response elicited by the host.
INTRODUCTION
Amphotericin B (AmB) is a widely used antifungal drug that presents strong killing activity against fungi after binding to ergosterol. Classically, it has been described that AmB induced cell death by forming pores at the level of the cell membrane (1, 2). However, recent findings suggest that AmB elicits its fungicidal effect through multiple mechanisms. In this sense, AmB induces ergosterol sequestration, which causes alterations in the cell membrane that result in killing of the cells (3, 4). In addition, AmB induces a significant accumulation of reactive oxygen species (ROS), which has been correlated with cell damage, apoptosis induction, and death (5–9).
Resistance to AmB is a complex process, and multiple mechanisms have been described to be responsible for it. Lack of ergosterol at the cell membrane has been frequently correlated with reduced susceptibility to AmB (10–14). However, there are also some cases in which resistance to AmB can occur in cells with normal ergosterol content (15, 16). Aspergillus terreus, which is a mold that presents reduced susceptibility to AmB, contains similar ergosterol content to Aspergillus fumigatus, which is fully susceptible to this antifungal. In this case, increase in catalase and reduction of ROS production have been associated with increased resistance to AmB (17). Moreover, mechanisms that protect against the production of ROS also confer reduced susceptibility to AmB (6, 9). These results indicate that AmB exerts multiple responses in the cells and that resistance to this antifungal can be acquired by different mechanisms.
The correlation between the cell wall and resistance to AmB has been poorly described. A relationship between resistance to AmB and changes in the cell wall has been observed in different fungi (18–20), although it is not known to what extent changes in the cell wall contribute to amphotericin B resistance.
We have previously characterized Candida tropicalis strains resistant to AmB that have increased catalase activity, changes in mitochondrial potential, growth delay, and low accumulation of ROS and are deficient in ergosterol synthesis (9, 21). In the present work, we extended these findings and describe that AmB-resistant strains also present an enlarged cell wall. Interestingly, these changes correlate with increased detection of wall β-1,3-glucans in AmB-resistant strains of yeast and with increased immune activation compared to susceptible isolates. Our results highlight a new aspect of the resistance to AmB and also suggest that the appearance of resistant strains during infection might interfere with the regular immune response elicited by the host.
MATERIALS AND METHODS
Strains and growth conditions.
The following C. tropicalis strains resistant to AmB were used in this work: ATCC 200956 and CL-6835 (from the yeast collection of the Mycology Reference Laboratory of the Spanish National Centre for Microbiology). The MIC values reported for these strains to AmB and fluconazole were 2 and >64 mg/liter, respectively (following the EUCAST antifungal susceptibility testing method [9, 21]). In addition, as susceptible isolates, the strains ATCC 750, CL-7099, CL-7119, and CL-7869 were used (MIC values to AmB and fluconazole of 0.25 and 0.5 mg/liter, respectively). Also a strain resistant to azoles but not to AmB was included (TP-13650) (21) (MIC values to AmB and fluconazole of 0.25 and >64 mg/liter, respectively). The strains were grown in liquid Sabouraud medium (Oxoid, Ltd., Basingstoke, Hampshire, England) or yeast extract-peptone-dextrose (YEPD) medium at 30°C with moderate shaking (150 rpm).
TEM.
For ultrastructural analysis by transmission electron microscopy (TEM), wild-type and AmB-resistant C. tropicalis strains (3 × 108 cells in the exponential growth phase) were chemically fixed in 0.1 M Na2HPO4 (pH 7.4), 2% glutaraldehyde, and 4% p-formaldehyde for 2 h at 4°C. Cells were washed three times in Na2HPO4 buffer and postfixed with 1% potassium permanganate for 1 h at 4°C, 0.15% tannic acid for 1 min at room temperature, and 2% uranyl acetate for 1 h at room temperature. Dehydration was carried out in increasing concentrations of ethanol (50, 75, 90, 95, and 100%) for 10 min at 4°C. Infiltration was done at room temperature using increasing concentrations of epoxy resin (25, 50, 75, and 100%), and polymerization was performed at 60°C for 48 h. Ultrathin sections (50 to 70 nm) were obtained with an RMC MT6000_XL ultramicrotome and stained following standard procedures with saturated uranyl acetate and 2% lead citrate. Images were recorded on a 1k Gatan charge-coupled device (CCD) camera with a Tecnai 12 FEI microscope operated at 120 kV.
The cell wall thickness was determined measuring its width in at least 20 cells from each strain using Adobe Photoshop software (San Jose, CA). Then the volumes of the yeast cell and the cell wall were calculated by applying the formula for determining the volume of ellipsoidal structures, V = (π/6) × b2a, where b corresponds to the length of the organelle and a to its width (22).
Detection of β-1,3-glucan at the cell wall by immunofluorescence.
The cell wall content of β-1,3-glucans was estimated by an indirect immunofluorescence assay with monoclonal antibodies against this polysaccharide (23, 24). Detection was performed on heat-treated cells, since this process has been reported to increase the exposure of β-glucans on the surface (25–28). Briefly, a cell suspension of 108 yeast cells/ml was incubated in boiling water for 30 min and fixed with 4% p-formaldehyde at room temperature for 40 min and washed with phosphate-buffered saline (PBS). The cells were then suspended in PBS containing 1% bovine serum albumin (BSA [Sigma-Aldrich]) for 30 min and incubated with 5 μg/ml of mouse IgG monoclonal antibody against β-1,3-glucans (Biosupplies Australia Pty., Ltd., Bundoora, Victoria, Australia) for 1 h at 37°C. The cells were then washed with PBS plus BSA three times and incubated with 10 μg/ml of goat anti mouse IgG conjugated to Alexa 488 (Invitrogen) for 1 h at 37°C in darkness. Finally, samples were washed with PBS and were visualized under a fluorescence microscope Leica DMI 3000B. Pictures were taken with a DFC300FX digital camera using the LAS AF software (Leica Microsystems). The fluorescence intensity was quantified using the ImageJ software (http://rsb.info.nih.gov/ij) in at least 20 cells for each strain.
Estimation of chitin content in the cell wall.
The yeast cells were collected after overnight growth in Sabouraud, and the cultures were filtered to discard clumps and washed with PBS. The inocula were prepared at 107 yeast cells/ml. Yeast cells were fixed with p-formaldehyde (4%) for 40 min and stained with calcofluor white (CFW) at a concentration of 10 mg/liter (Sigma-Aldrich, St. Louis, MO) at 37°C for 30 min and washed twice with PBS before being examined. The chitin content was estimated by flow cytometry using a FACSAria cytometer (Becton Dickinson) with an excitation maximum of 405 nm and emission maximum of 440 nm. Chitin content was also estimated using a Cary Eclipse fluorescence spectrophotometer (Varian) at an excitation maximum of 400 nm and emission maximum of 490 nm. In all cases, unstained cells were included as controls.
Susceptibility to Congo red.
The susceptibility of the strains to the cell-wall-disturbing agent Congo red was evaluated by observing the growth on solid medium. Congo red (Sigma) binds to the cell wall and alters its structure, which results in inhibition of yeast replication (29). Spots of 5 μl containing 105, 104, 103, or 102 yeast cells from each strain were cultivated on Sabouraud medium supplemented with different concentrations of Congo red (200, 150, 100, or 50 μg/ml) and incubated at 30 or 37°C. Pictures of the plates were taken every 24 h with a Nikon digital camera using a macro lens.
Protein extracts and immunoblot assays.
For Western blot assays, exponentially growing cultures in YEPD medium at 37°C with moderate shaking were treated with 5 mg/liter tunicamycin (Sigma) for 60 min or 1 mg/liter AmB for 60 and 120 min. The procedures employed for cell collection, cell lysis, protein extraction, fractionation by SDS-polyacrylamide gel electrophoresis (PAGE), and transfer to nitrocellulose membranes have been described previously (30, 31). Anti-phospho-p44/p42 mitogen-activated protein kinase (MAPK) (Thr202 Tyr204) antibody (New England BioLabs) was used to detect dually phosphorylated Mkc1 and Cek1 MAPKs (indicated as “Phospho-Mkc1” and “Phospho-Cek1” in the figures); phospho-p38 MAPK (Thr180 Tyr182) 28B10 monoclonal antibody (Cell Signaling Technology, Inc.) was used to detect the phosphorylated Hog1 (indicated as “Phospho-Hog1” in the figures). Saccharomyces cerevisiae Hog1 (ScHog1) polyclonal antibody (Santa Cruz Biotechnology) was used to detect C. tropicalis Hog1 protein homologues. Western blots were developed according to the manufacturer's instructions using the Hybond ECL (enhanced chemiluminescence) kit (Amersham Pharmacia Biotech). To equalize the amount of protein loaded in each lane, similar amounts of protein (determined by the absorbance of the sample at 280 nm) were loaded on SDS-PAGE gels and stained with Coomassie blue.
Cytokine stimulation in human PBMCs.
Samples of blood from 15 healthy volunteers were analyzed anonymously. Buffy coats from blood were obtained from the Sanquin Biobank Nijmegen, after written consent was given by the healthy volunteers. Peripheral blood mononuclear cells (PBMCs) were isolated by density centrifugation of PBS-diluted blood (dilution 1:1) over a Ficoll-Opaque Plus gradient (GE Healthcare). PBMCs were washed three times with sterile cold PBS. For stimulation experiments, a total of 5 × 105 cells/well were plated in round-bottom 96-well plates (Greiner Bio-One) suspended in RPMI 1640 culture medium supplemented with 1% gentamicin, 1% pyruvate, and 1% l-glutamine. For PBMC stimulation, yeast cells were grown in Sabouraud medium as described above and fixed with 4% p-formaldehyde for 40 min at room temperature. Thereafter, the yeast cells were washed in sterile PBS twice and suspended in the same buffer. Stimulation experiment was performed with two different PBMC/yeast ratios (1:2 and 1:20). The cells were incubated at 37°C with 5% CO2, and cytokine concentrations were determined in the supernatants after 1 and 7 days. All of the cytokine concentrations were measured by enzyme-linked immunosorbent assay (ELISA) using commercial kits. To determine interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), IL-17, interleukin-1 receptor a (IL-1Ra), and IL-2, we used an ELISA kit from R&D Systems (Abingdon, United Kingdom), and for IL-10, IL-6, and gamma interferon (IFN-γ), we used an ELISA kit from Sanquin (Amsterdam, The Netherlands).
Effect of yeast cells on Galleria mellonella response.
The differential immune responses of G. mellonella to infection by strains with different AmB susceptibility profiles were evaluated by measuring larval melanization according to the methodology described in reference 32. Groups of 10 larvae were infected with three different inoculum concentrations (106, 2 × 106, or 4 × 106 yeast cells/larva [see the video at https://www.youtube.com/watch?v=2XEu_5aF1qk]). In addition, a control group inoculated with PBS-ampicillin was included. After 1 h of incubation at 37°C, the hemolymph was obtained by an apical incision in each larva with a sterile scalpel. Hemolymph was diluted 10 times in cold PBS and immediately centrifuged at 12,000 rpm to remove cellular debris. Finally, the optical density at 405 nm (OD405) was determined on an MRX-Dynex Technologies spectrophotometer (Worthing, United Kingdom) to evaluate the melanin content in the hemolymph.
Statistical analyses.
The nonparametric Kruskal-Wallis test and Dunn's multiple comparison test were used to evaluate the differences between AmB-sensitive and -resistant strains regarding three parameters: cell wall thickness, cell volume, and mean fluorescent intensity (MFI) of β-glucan content. Results obtained in the human PBMC stimulation were analyzed by one-way analysis of variance (ANOVA) and the Bonferroni test. All statistical analyses were carried out with the GraphPad Prism 5, and P values of <0.05 were considered significant.
RESULTS
Structure of the cell wall study by TEM.
In a previous study, we characterized the resistance mechanisms of different C. tropicalis strains to AmB (9, 21). In particular, these strains were resistant to AmB and azoles due to mutations in ERG genes and presented lack of ergosterol and increased tolerance to ROS. During these studies, we observed that resistance was associated with pleiotropic changes, some of them involving the cell wall. Since this structure is important for the stability of the cell and for the recognition by the immune system, we decided to characterize this phenotype in detail.
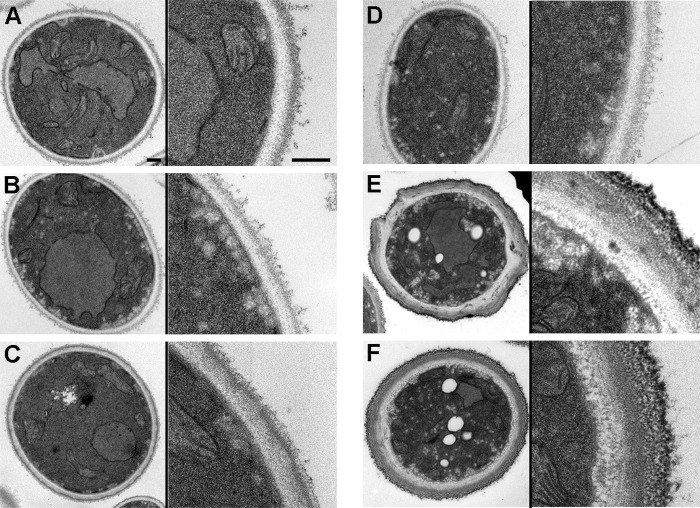
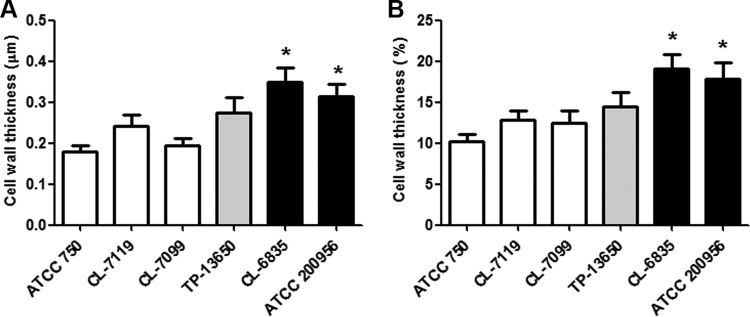
As a first approach, we performed TEM to observe the structure of the cell wall. We found that in strains resistant to AmB, there were a high proportion of cells with a thickened cell wall. As shown in Fig. 1, the cell wall of AmB-susceptible strains was composed of two main layers that had different electron densities. In contrast, in the two AmB-resistant isolates, a significant proportion of cells presented an abnormally enlarged cell wall. Since these strains showed resistance to AmB and azoles, we decided to investigate whether these changes were present also in azole-resistant (but not AmB-resistant) strains. For this purpose, we used strain TP-13650. This strain presented a normal cell wall thickness (Fig. 1), indicating that changes in cell wall composition correlated with reduced susceptibility to AmB but not to azoles. We measured the thickness of the cell wall and confirmed that in AmB-resistant strains the width of the cell wall was statistically significantly thicker (P < 0.05) (Fig. 2A). We also calculated the volume of the cell wall and found that the percentage of the volume of the wall compared to the total volume of the cell was significantly (P < 0.05) higher in the yeast cells from AmB-resistant strains (Fig. 2B).
FIG 1.
Electron microscopy of ultrathin sections from C. tropicalis strains with different antifungal susceptibility profiles. Shown are representative fields of yeast cell wall from strains ATCC 750 (A), CL-7099 (B), CL-7119 (C), TP13650 (D), CL-6835 (E), and ATCC 200956 (F). In each panel, a picture of the whole cell (left part) and a magnification of the cell wall (right part) are shown. The scale bars correspond to 0.2 μm.
FIG 2.
Measurement of the cell wall thickness of AmB-resistant C. tropicalis strains. The width (A) and percentage of the volume of the cell wall relative to the total volume of the cell (B) were calculated from the TEM images. The mean value and the standard deviation are plotted as bar graphs. Asterisks indicate statistically significant difference (P < 0.05) between each strain and the ATCC 750 strain. Gray bars correspond to the strain resistant to azoles and black bars to the strains resistant to AmB.
Estimation of the contents of β-1,3-glucans and chitin.
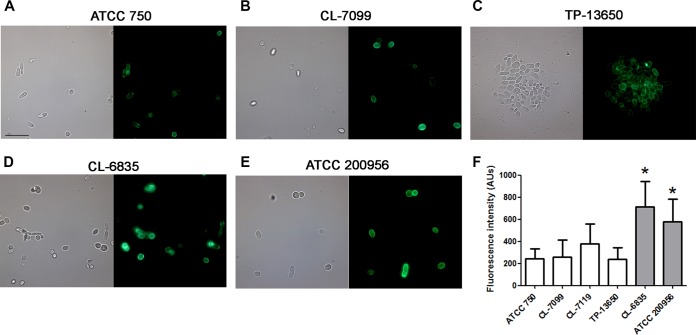
We studied by fluorescence microscopy if the amount of β-1,3-glucan, which is one of the main structural components of the cell wall, was altered in cells from AmB-resistant strains. In regular cells, β-1,3-glucans are distributed through the cell wall but are mainly exposed at the bud scars. For this reason, antibodies against this polysaccharide show low binding at the surface of regular untreated cells. However, several circumstances, such as heat treatment of the cells or during infection, expose this polysaccharide and allow stronger binding of the antibodies (33). As shown in Fig. 3, binding of the antibody produced a stronger fluorescent signal in the two AmB-resistant strains, suggesting that the total content of this polysaccharide is higher in these strains. We quantified the intensity of the fluorescent signal, and as shown in Fig. 3F, estimated that AmB-resistant strains presented an increase of 2- to 3-fold in the amount of β-1,3-glucans. We also tried to quantify the content of chitin by staining with calcofluor white and detection of the fluorescence signal by flow cytometry. However, in contrast to what was observed with the β-1,3-glucan, we found no relationship between the content of chitin and resistance to AmB (data not shown).
FIG 3.
Detection of β-1,3-glucans at the cell wall. Immunofluorescent detection of β-1,3-glucans in the cell wall of C. tropicalis strains ATCC 750 (A), CL-7099 (B), TP-13650 (C), CL-6835 (D), and ATCC 200956 (E) after heat treatment. The right panel corresponds to the same samples in a bright field. (F) Quantification of the fluorescent signal obtained in the cells from panel A. Images were processed with ImageJ, and the quantification of the fluorescent signal was obtained as arbitrary units in each case. Asterisks indicate statistically significant difference (P < 0.05) between each strain and the ATCC 750 strain.
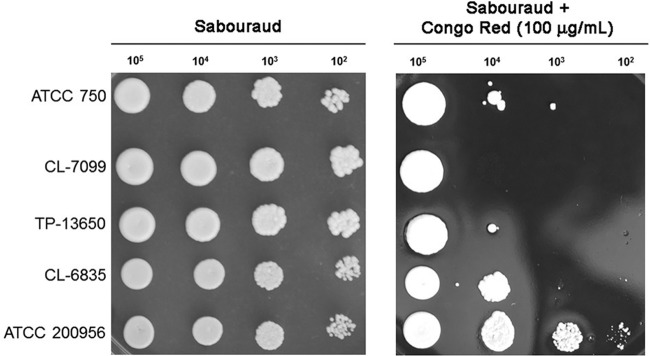
Susceptibility to Congo red.
Changes in the cell wall can result in differences in the susceptibility to agents that bind and alter the structure of the cell wall, such as Congo red. For this reason, we investigated if AmB-resistant strains had different susceptibilities to this dye. AmB-resistant strains showed slower growth in regular solid medium compared to AmB-susceptible strains, which is in agreement with previous findings of our group (9). All strains, independently of their susceptibility profile, were sensitive to the highest concentrations of Congo red assayed (150 and 200 μg/ml) at 30 and 37°C. Nevertheless, the two strains resistant to AmB (CL-6835 and ATCC 200956) showed enhanced growth in Congo red at 50 and 100 μg/ml at both temperatures (30 and 37°C), this phenotype being more evident at 100 μg/ml at 37°C (Fig. 4A) (data not shown), and in particular, for strain ATCC 200956. These results suggest that changes at the cell wall of AmB-resistant strains promote increased or reduced resistance to agents that alter its structure.
FIG 4.
Growth of C. tropicalis strain in Congo red. Candida tropicalis strains with different susceptibility profiles were incubated overnight in Sabouraud liquid medium, and after being washed with PBS, different amounts of cells were placed on Sabouraud medium plates or on the same plates containing Congo red (100 μg/ml). The plates were incubated at 37°C, and pictures were taken after 48 h.
Cytokine stimulation in human PBMCs.
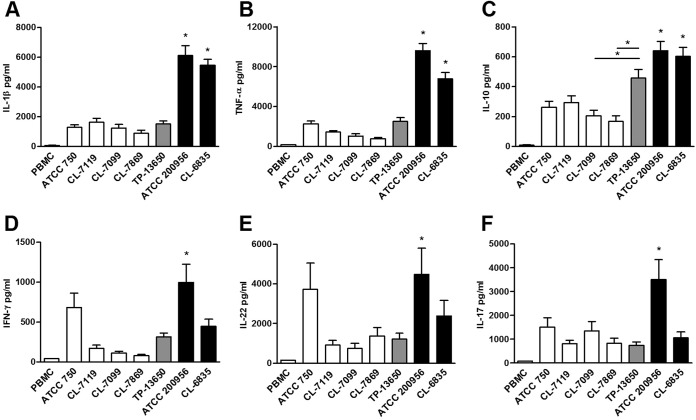
Since the cell wall contains the most important structures recognized by the immune cells and because the relative abundances of the various cell wall polysaccharides differ in strains resistant to AmB, we investigated if AmB-resistant isolates induce a different response in human PBMCs. For this experiment, we isolated PBMCs from healthy blood donors and exposed them to different C. tropicalis strains (previously fixed with p-formaldehyde) with different susceptibility profiles to antifungals at a 1:20 PBMC/yeast cell ratio. After 1 or 7 days of incubation, we measured the levels of production of different cytokines. We found that AmB-resistant strains induced higher production of cytokines by PBMCs than AmB-susceptible isolates (Fig. 5), this phenotype being more noticeable for strain ATCC 200956. The only exceptions were the anti-inflammatory cytokine IL-4 and the proinflammatory cytokine IL-6. In these two cases, we did not find differences in the amount of cytokines produced by PBMCs exposed to different yeast strains (data not shown). The cytokine profile induced by the azole-resistant strain TP-13650 was comparable to that of the susceptible strains, except for the anti-inflammatory cytokine IL-10 (Fig. 5C).
FIG 5.
Cytokines produced by PBMCs after exposure to C. tropicalis. PBMCs from healthy human donors were exposed to different C. tropicalis strains, and the concentrations of IL-1β (A), TNF-α (B), IL-10 (C), IFN-γ (D), IL-22 (E), and IL-17 (F) in the supernatants were determined by ELISA after 24 h (IL-1β, TNF-α, and IL-10) or 7 days (IFN-γ, IL-22, and IL-17). Significant statistical differences (P < 0.05) between AmB-resistant and AmB-susceptible strains are shown by asterisks.
Quantification of melanin production in Galleria mellonella as an indicator of the immune response following infection with C. tropicalis strains with different susceptibility profiles.
Melanin production in insects is a sign of early response to an infectious agent or a foreign body (34). Therefore, we examined whether C. tropicalis strains with different antifungal susceptibility profiles induced this response when inoculated into G. mellonella larvae. Candida tropicalis stimulated melanin production, which was detected in larval hemolymph (35), but we found differences in the kinetics of melanin production. AmB-resistant strains produced a faster accumulation of melanin in larvae than AmB-susceptible strains. The total amount of melanin found in the hemolymph was also higher when larvae were infected with AmB-resistant isolates (Fig. 6).
FIG 6.
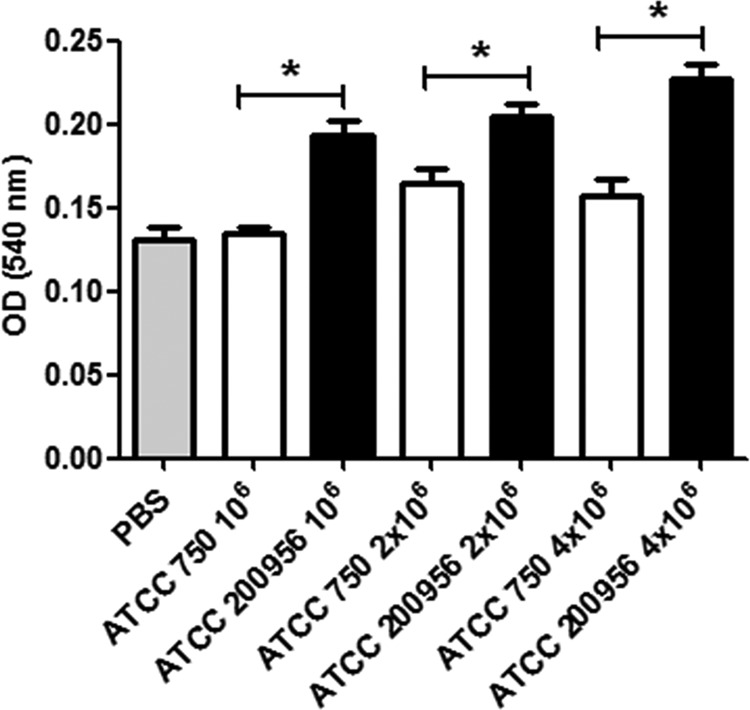
Quantification of melanin in the hemolymph of G. mellonella larvae infected with C. tropicalis. Galleria mellonella larvae were infected with 106, 2 × 106, or 4 × 106 C. tropicalis cells from strain ATCC 750 or ATCC 200956. The larvae were incubated at 37°C, and after 1 h, the hemolymph was isolated and melanin was estimated as described in Materials and Methods. The results correspond to the average ± standard deviation of the OD values obtained from each larva in each group. The asterisks indicate significant differences (P < 0.05).
Western blots to detect kinase activation.
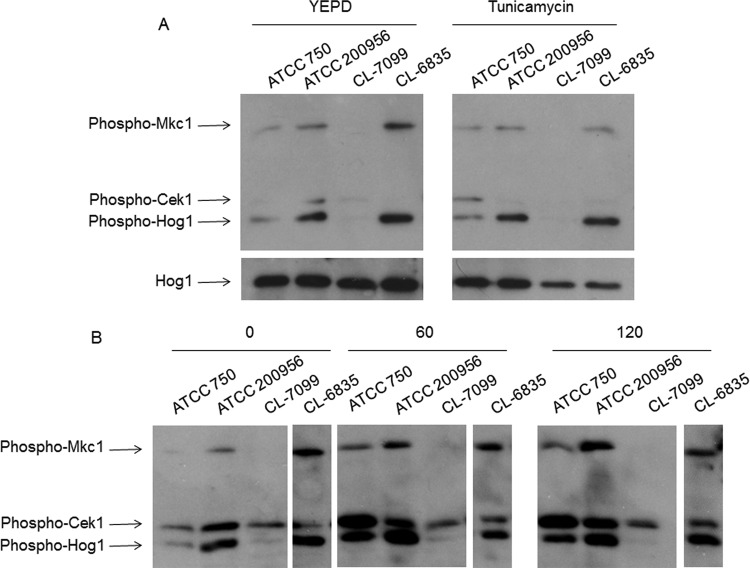
The Cek1 and Mkc1 MAPKs are activated following cell wall damage in C. albicans (31, 36–39). In order to determine if the presumed changes in cell wall correlated with an altered MAPK phosphorylation pattern, we analyzed their behavior under different cell-wall-related stress conditions in these strains. Log-phase cells at 37°C were treated for 1 h with tunicamycin (an inhibitor of N-linked glycosylation). AmB-resistant strain ATCC 200956 showed both Mkc1 and Hog1 MAPKs activated under basal conditions—that is, even before the drug was added (Fig. 7A). However, the activation of Cek1 observed in the ATCC 750 strain was not detected in the rest of the strains analyzed and it is remarkable that no phosphorylation was detected in any of the MAPKs in the AmB-sensitive CL-7099 strain. We then examined the pattern upon AmB treatment (1 mg/liter). Again, under non-stress conditions AmB-resistant strains showed significant Mkc1, Hog1, and Cek1 MAPK phosphorylation compared to the susceptible strains. AmB induced phosphorylation of the three MAPKs in all the strains tested except CL-7099 (AmB sensitive).
FIG 7.
Activation of MAPKs in C. tropicalis strains by Western blotting. The activation of the Mkc1, Hog1, and Cek1 MAPKs was detected by Western blotting in C. tropicalis strains with different susceptibility profiles. (A) Cells were grown in YEPD (left panel) and then exposed to tunicamycin (TM; 5 μg/ml [right panel]). Western blotting was performed to detect the activated forms of Mkc1, Cek1, and Hog1 with specific MAbs. The detection of nonphosphorylated form of Hog1 was used as a loading control. (B) The cells were grown as described above, but they were treated with AmB (1 μg/ml) for 60 or 120 min. Detection of activated forms of the MAPs was performed as in panel A.
DISCUSSION
The action mechanism of AmB has been extensively studied. This molecule binds to ergosterol and with lower affinity to cholesterol and therefore has deleterious effects on many different types of organisms. However, the mechanisms of action seem to be different in different cells. For example, in mammalian cells, AmB toxicity does not seem to rely only on increasing the permeability of the plasma membrane, since the antifungal is localized mainly in internal vesicles (40, 41). This internal binding would mediate toxicity by triggering endosomes and lysosomes to release their content into the cytoplasm, which leads to cell damage. In contrast, in yeast cells AmB localizes mainly at the plasma membrane (42), where it has various effects, such as pore formation and ergosterol sequestration (reviewed in reference 43). However, AmB has also intracellular effects, mainly due to an increase in the production of ROS (5–9). It is therefore reasonable to believe that this effect in yeast is mediated through signal transduction pathways that result in an accumulation of ROS. For these reasons, resistance to AmB has been related to multiple mechanisms, such as absence of ergosterol in the cell membrane, activation of antioxidant mechanisms, and decrease in mitochondrial activity (6, 9–14, 17). Interestingly, petite mutants from Candida glabrata show resistance to fluconazole but are more susceptible to AmB (44), and this phenomenon has been related to a higher content of free ergosterol in these mutants (45). In general, these data indicate that tolerance to AmB is a complex phenomenon that might require the presence of several resistant mechanisms concomitantly. In this work, we provide further evidence that adds complexity to the adaptation mechanisms to AmB, which involve changes at the cell wall level.
The cell wall is an important structure for the growth of fungi because it confers osmotic stability and protects against stress. In addition, it is responsible for maintaining the shape of the cells. From an immunological point of view, many of the cell wall components such as β-glucans, chitin, and mannans are recognized by the immune system (33, 46, 47). In particular, β-glucans are recognized by Dectin-1 and constitute one of the main pathogen-associated molecular patterns (28, 48–50). Alterations of the cell wall composition lead to the activation of integrity pathways that result in activation of compensation mechanisms (51–53). The pathways involved in cell wall rearrangements in C. tropicalis are not known, but we describe in this work that AmB-resistant strains present a basal level of activation of Mkc1, Hog1, and Cek1 MAPKs, supporting that these strains have alterations in the normal composition of the cell wall. Changes at the cell wall have been related to alterations in the susceptibility to AmB. Therefore, we hypothesize that the association between the susceptibility to AmB and changes in the cell wall could provide a link between antifungal resistance and immune response.
The mechanisms by which alterations at the cell wall influence the susceptibility to AmB remain unknown, and several hypotheses are possible. Removal of β-1,3-glucans results in decreased resistance to AmB in C. albicans (54). It has also been suggested that components of the fungal cell wall such as β-glucans may physically interact with antifungals and inhibit penetration to the site of action (18). These findings are consistent with our data. Other reports have also suggested a correlation between AmB resistance and cell wall changes (55). However, at the moment, it cannot be discarded that the cell wall changes described in this article do not directly participate in AmB resistance, and they could be the result of a regulatory mechanism to compensate for an abnormal fluidity of the membrane due to the lack of ergosterol. Previous work from our group has shown that ergosterol is absent in the plasma membrane of the AmB-resistant strains used in this work and that this defect is compensated for by accumulation of other sterols (21). Since β-1,3-glucan synthase is located at the cell membrane, changes in the functionality of this structure could alter the activity of this enzyme. Until now, the lack of standardization of molecular biology tools for C. tropicalis has been a limitation to fully understand the relationship between the cell wall and AmB resistance.
The C. tropicalis strains described in this work also showed changes in mitochondrial membrane potential and defects in respiration (9). Consistent with our results, an association between mitochondrial alterations (specifically at the level of cytochrome c), with functional and/or structural cell wall changes in the yeast Kluyveromyces lactis has been described (56).
AmB-resistant strains elicited different immune responses compared to AmB-susceptible isolates (Fig. 5 and 6). Since cell wall elements are strong immunogens, we hypothesize that the differential response elicited by leukocytes is due to the differences at the cell wall. At the moment, the fungal components responsible for differential response elicited by PBMCs and G. mellonella are unknown, but our results suggest a correlation between the amount of β-glucans present in the yeasts and the host immune response. β-Glucans are well known to possess significant and wide biological and physiological activities, including antitumor, antioxidant, anticoagulant, antifungal, and immunomodulatory activities (57–60). These polysaccharides induce protective responses during infection (61, 62). Most of the biological studies on the effects of glucans have focused on β-1,3-glucans (28, 63–67). In agreement with these data, the increase of β-1,3-glucans in AmB-resistant strains correlates with the enhanced response of PBMCs exposed to these isolates. Interestingly, treatment with cell-wall-perturbing agents also results in exposure of β-glucans and differential induction of immune responses (24, 68, 69). Candida albicans also increases the exposure of β-glucans during infection (69), so it is reasonable to argue that cells with a higher content of this polysaccharide will elicit stronger responses (70). We have used an alternative infection model, the wax moth Galleria mellonella, to confirm our results. In agreement with the in vitro data, AmB-resistant strains also increased the production of melanin by G. mellonella, which is one of the main immune responses of this lepidopteran (32, 71–73). Cryptococcus neoformans, which does not contain β-glucans, does not induce melanization of G. mellonella (72). Globally, our results are in agreement with the fact that β-1,3-glucans prime the insect innate immune system (74, 75).
Our work has important clinical implications because it provides a link between antimicrobial resistance and interaction with the host. Resistance to antifungals is a major factor that determines the effectiveness of the antifungal treatments, but this work also suggests that the immune response elicited is also different, with unpredictable consequences. An exaggerated proinflammatory response also has deleterious effects in the host (see the review in reference 76), so an uncontrolled priming of the immune system by AmB-resistant strains could contribute to the lack of response of the patients to the treatment. Antifungal resistance has been also frequently associated with reduced fitness and virulence (35, 77–79). For this reason, the full phenotypic characterization of AmB-resistant isolates is needed to understand the clinical outcome of the infection and to improve the treatment of the patients.
ACKNOWLEDGMENTS
We thank Teresa Peláez (Gregorio Marañon Hospital, Madrid) and Emilia Mellado (Instituto de Salud Carlos III, Madrid) for the kind gift of strain TP-13650 and Miguel Calero (Functional Unit for Research on Chronic Diseases, Instituto de Salud Carlos III) for his help with the experiments on chitin determination using the fluorescence spectrophotometer.
A. C. Mesa-Arango has been supported by fellowships from Fundación Carolina and Instituto de Salud Carlos III. C. Rueda has a Sara Borrell contract from the Fondo de Investigaciones Sanitarias (reference no. CD11/00110).
Oscar Zaragoza would like to dedicate this article to Steven Wright from the Library Special Collections of the University College of London for his gesture of extreme generosity and his contributions to the diffusion of the history of science.
REFERENCES
- 1.Finkelstein A, Holz R. 1973. Aqueous pores created in thin lipid membranes by the polyene antibiotics nystatin and amphotericin B. Membranes 2:377–408. [PubMed] [Google Scholar]
- 2.Brajtburg J, Powderly WG, Kobayashi GS, Medoff G. 1990. Amphotericin B: current understanding of mechanisms of action. Antimicrob Agents Chemother 34:183–188. doi: 10.1128/AAC.34.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray KC, Palacios DS, Dailey I, Endo MM, Uno BE, Wilcock BC, Burke MD. 2012. Amphotericin primarily kills yeast by simply binding ergosterol. Proc Natl Acad Sci U S A 109:2234–2239. doi: 10.1073/pnas.1117280109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palacios DS, Anderson TM, Burke MD. 2007. A post-PKS oxidation of the amphotericin B skeleton predicted to be critical for channel formation is not required for potent antifungal activity. J Am Chem Soc 129:13804–13805. doi: 10.1021/ja075739o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips AJ, Sudbery I, Ramsdale M. 2003. Apoptosis induced by environmental stresses and amphotericin B in Candida albicans. Proc Natl Acad Sci U S A 100:14327–14332. doi: 10.1073/pnas.2332326100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sokol-Anderson M, Sligh JE Jr, Elberg S, Brajtburg J, Kobayashi GS, Medoff G. 1988. Role of cell defense against oxidative damage in the resistance of Candida albicans to the killing effect of amphotericin B. Antimicrob Agents Chemother 32:702–705. doi: 10.1128/AAC.32.5.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sokol-Anderson ML, Brajtburg J, Medoff G. 1986. Amphotericin B-induced oxidative damage and killing of Candida albicans. J Infect Dis 154:76–83. doi: 10.1093/infdis/154.1.76. [DOI] [PubMed] [Google Scholar]
- 8.Belenky P, Camacho D, Collins JJ. 2013. Fungicidal drugs induce a common oxidative-damage cellular death pathway. Cell Rep 3:350–358. doi: 10.1016/j.celrep.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mesa-Arango AC, Trevijano-Contador N, Roman E, Sanchez-Fresneda R, Casas C, Herrero E, Arguelles JC, Pla J, Cuenca-Estrella M, Zaragoza O. 2014. The production of reactive oxygen species is a universal action mechanism of amphotericin B against pathogenic yeasts and contributes to the fungicidal effect of this drug. Antimicrob Agents Chemother 58:6627–6638. doi: 10.1128/AAC.03570-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen RH, Astvad KM, Silva LV, Sanglard D, Jorgensen R, Nielsen KF, Mathiasen EG, Doroudian G, Perlin DS, Arendrup MC. 2015. Stepwise emergence of azole, echinocandin and amphotericin B multidrug resistance in vivo in Candida albicans orchestrated by multiple genetic alterations. J Antimicrob Chemother 70:2551–2555. doi: 10.1093/jac/dkv140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young LY, Hull CM, Heitman J. 2003. Disruption of ergosterol biosynthesis confers resistance to amphotericin B in Candida lusitaniae. Antimicrob Agents Chemother 47:2717–2724. doi: 10.1128/AAC.47.9.2717-2724.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woods RA, Bard M, Jackson IE, Drutz DJ. 1974. Resistance to polyene antibiotics and correlated sterol changes in two isolates of Candida tropicalis from a patient with an amphotericin B-resistant funguria. J Infect Dis 129:53–58. doi: 10.1093/infdis/129.1.53. [DOI] [PubMed] [Google Scholar]
- 13.Kelly SL, Lamb DC, Taylor M, Corran AJ, Baldwin BC, Powderly WG. 1994. Resistance to amphotericin B associated with defective sterol delta 8→7 isomerase in a Cryptococcus neoformans strain from an AIDS patient. FEMS Microbiol Lett 122:39–42. doi: 10.1111/j.1574-6968.1994.tb07140.x. [DOI] [PubMed] [Google Scholar]
- 14.Vandeputte P, Tronchin G, Berges T, Hennequin C, Chabasse D, Bouchara JP. 2007. Reduced susceptibility to polyenes associated with a missense mutation in the ERG6 gene in a clinical isolate of Candida glabrata with pseudohyphal growth. Antimicrob Agents Chemother 51:982–990. doi: 10.1128/AAC.01510-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joseph-Horne T, Loeffler RS, Hollomon DW, Kelly SL. 1996. Amphotericin B resistant isolates of Cryptococcus neoformans without alteration in sterol biosynthesis. J Med Vet Mycol 34:223–225. doi: 10.1080/02681219680000381. [DOI] [PubMed] [Google Scholar]
- 16.Joseph-Horne T, Manning N, Holoman D, Kelly S. 1996. Nonsterol related resistance in Ustilago maydis to the polyene antifungals, amphotericin B and nystatin. Phytochemistry 42:637–639. doi: 10.1016/0031-9422(96)00037-4. [DOI] [PubMed] [Google Scholar]
- 17.Blum G, Perkhofer S, Haas H, Schrettl M, Wurzner R, Dierich MP, Lass-Florl C. 2008. Potential basis for amphotericin B resistance in Aspergillus terreus. Antimicrob Agents Chemother 52:1553–1555. doi: 10.1128/AAC.01280-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seo K, Akiyoshi H, Ohnishi Y. 1999. Alteration of cell wall composition leads to amphotericin B resistance in Aspergillus flavus. Microbiol Immunol 43:1017–1025. doi: 10.1111/j.1348-0421.1999.tb01231.x. [DOI] [PubMed] [Google Scholar]
- 19.Sarinova M, Ticha E, Obernauerova M, Gbelska Y. 2007. Impact of mitochondrial function on yeast susceptibility to antifungal compounds. Folia Microbiol (Praha) 52:223–229. doi: 10.1007/BF02931302. [DOI] [PubMed] [Google Scholar]
- 20.Nett J, Lincoln L, Marchillo K, Massey R, Holoyda K, Hoff B, VanHandel M, Andes D. 2007. Putative role of beta-1,3 glucans in Candida albicans biofilm resistance. Antimicrob Agents Chemother 51:510–520. doi: 10.1128/AAC.01056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forastiero A, Mesa-Arango AC, Alastruey-Izquierdo A, Alcazar-Fuoli L, Bernal-Martinez L, Pelaez T, Lopez JF, Grimalt JO, Gomez-Lopez A, Cuesta I, Zaragoza O, Mellado E. 2013. Candida tropicalis antifungal cross-resistance is related to different azole target (Erg11p) modifications. Antimicrob Agents Chemother 57:4769–4781. doi: 10.1128/AAC.00477-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun J, Liu D. 2003. Geometric models for calculating cell biovolume and surface area for phytoplankton. J Plankton Res 25:1331–1346. doi: 10.1093/plankt/fbg096. [DOI] [Google Scholar]
- 23.Meikle PJ, Hoogenraad NJ, Bonig I, Clarke AE, Stone BA. 1994. A (1→3,1→4)-beta-glucan-specific monoclonal antibody and its use in the quantitation and immunocytochemical location of (1→3,1→4)-beta-glucans. Plant J 5:1–9. doi: 10.1046/j.1365-313X.1994.5010001.x. [DOI] [PubMed] [Google Scholar]
- 24.Rueda C, Cuenca-Estrella M, Zaragoza O. 2014. Paradoxical growth of Candida albicans in the presence of caspofungin is associated with multiple cell wall rearrangements and decreased virulence. Antimicrob Agents Chemother 58:1071–1083. doi: 10.1128/AAC.00946-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fradin C, Jouault T, Mallet A, Mallet JM, Camus D, Sinay P, Poulain D. 1996. Beta-1,2-linked oligomannosides inhibit Candida albicans binding to murine macrophage. J Leukoc Biol 60:81–87. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki T, Ohno N, Ohshima Y, Yadomae T. 1998. Soluble mannan and beta-glucan inhibit the uptake of Malassezia furfur by human monocytic cell line, THP-1. FEMS Immunol Med Microbiol 21:223–230. [DOI] [PubMed] [Google Scholar]
- 27.Gantner BN, Simmons RM, Underhill DM. 2005. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J 24:1277–1286. doi: 10.1038/sj.emboj.7600594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gow NA, Netea MG, Munro CA, Ferwerda G, Bates S, Mora-Montes HM, Walker L, Jansen T, Jacobs L, Tsoni V, Brown GD, Odds FC, Van der Meer JW, Brown AJ, Kullberg BJ. 2007. Immune recognition of Candida albicans beta-glucan by dectin-1. J Infect Dis 196:1565–1571. doi: 10.1086/523110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ram AF, Klis FM. 2006. Identification of fungal cell wall mutants using susceptibility assays based on Calcofluor white and Congo red. Nat Protoc 1:2253–2256. doi: 10.1038/nprot.2006.397. [DOI] [PubMed] [Google Scholar]
- 30.Martin H, Rodriguez-Pachon JM, Ruiz C, Nombela C, Molina M. 2000. Regulatory mechanisms for modulation of signaling through the cell integrity Slt2-mediated pathway in Saccharomyces cerevisiae. J Biol Chem 275:1511–1519. doi: 10.1074/jbc.275.2.1511. [DOI] [PubMed] [Google Scholar]
- 31.Roman E, Nombela C, Pla J. 2005. The Sho1 adaptor protein links oxidative stress to morphogenesis and cell wall biosynthesis in the fungal pathogen Candida albicans. Mol Cell Biol 25:10611–10627. doi: 10.1128/MCB.25.23.10611-10627.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scorzoni L, de Lucas MP, Mesa-Arango AC, Fusco-Almeida AM, Lozano E, Cuenca-Estrella M, Mendes-Giannini MJ, Zaragoza O. 2013. Antifungal efficacy during Candida krusei infection in non-conventional models correlates with the yeast in vitro susceptibility profile. PLoS One 8:e60047. doi: 10.1371/journal.pone.0060047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wheeler RT, Fink GR. 2006. A drug-sensitive genetic network masks fungi from the immune system. PLoS Pathog 2:e35. doi: 10.1371/journal.ppat.0020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffmann JA. 1995. Innate immunity of insects. Curr Opin Immunol 7:4–10. doi: 10.1016/0952-7915(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 35.Mesa-Arango AC, Forastiero A, Bernal-Martinez L, Cuenca-Estrella M, Mellado E, Zaragoza O. 2013. The non-mammalian host Galleria mellonella can be used to study the virulence of the fungal pathogen Candida tropicalis and the efficacy of antifungal drugs during infection by this pathogenic yeast. Med Mycol 51:461–472. doi: 10.3109/13693786.2012.737031. [DOI] [PubMed] [Google Scholar]
- 36.Eisman B, Alonso-Monge R, Roman E, Arana D, Nombela C, Pla J. 2006. The Cek1 and Hog1 mitogen-activated protein kinases play complementary roles in cell wall biogenesis and chlamydospore formation in the fungal pathogen Candida albicans. Eukaryot Cell 5:347–358. doi: 10.1128/EC.5.2.347-358.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roman E, Cottier F, Ernst JF, Pla J. 2009. Msb2 signaling mucin controls activation of Cek1 mitogen-activated protein kinase in Candida albicans. Eukaryot Cell 8:1235–1249. doi: 10.1128/EC.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Navarro-Garcia F, Eisman B, Fiuza SM, Nombela C, Pla J. 2005. The MAP kinase Mkc1p is activated under different stress conditions in Candida albicans. Microbiology 151:2737–2749. doi: 10.1099/mic.0.28038-0. [DOI] [PubMed] [Google Scholar]
- 39.Navarro-Garcia F, Sanchez M, Pla J, Nombela C. 1995. Functional characterization of the MKC1 gene of Candida albicans, which encodes a mitogen-activated protein kinase homolog related to cell integrity. Mol Cell Biol 15:2197–2206. doi: 10.1128/MCB.15.4.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vertut-Doi A, Ohnishi SI, Bolard J. 1994. The endocytic process in CHO cells, a toxic pathway of the polyene antibiotic amphotericin B. Antimicrob Agents Chemother 38:2373–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kagan S, Ickowicz D, Shmuel M, Altschuler Y, Sionov E, Pitusi M, Weiss A, Farber S, Domb AJ, Polacheck I. 2012. Toxicity mechanisms of amphotericin B and its neutralization by conjugation with arabinogalactan. Antimicrob Agents Chemother 56:5603–5611. doi: 10.1128/AAC.00612-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zumbuehl A, Jeannerat D, Martin SE, Sohrmann M, Stano P, Vigassy T, Clark DD, Hussey SL, Peter M, Peterson BR, Pretsch E, Walde P, Carreira EM. 2004. An amphotericin B-fluorescein conjugate as a powerful probe for biochemical studies of the membrane. Angew Chem Int Ed Engl 43:5181–5185. doi: 10.1002/anie.200460489. [DOI] [PubMed] [Google Scholar]
- 43.Mesa-Arango AC, Scorzoni L, Zaragoza O. 2012. It only takes one to do many jobs: amphotericin B as antifungal and immunomodulatory drug. Front Microbiol 3:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Defontaine A, Bouchara JP, Declerk P, Planchenault C, Chabasse D, Hallet JN. 1999. In-vitro resistance to azoles associated with mitochondrial DNA deficiency in Candida glabrata. J Med Microbiol 48:663–670. doi: 10.1099/00222615-48-7-663. [DOI] [PubMed] [Google Scholar]
- 45.Brun S, Berges T, Poupard P, Vauzelle-Moreau C, Renier G, Chabasse D, Bouchara JP. 2004. Mechanisms of azole resistance in petite mutants of Candida glabrata. Antimicrob Agents Chemother 48:1788–1796. doi: 10.1128/AAC.48.5.1788-1796.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wagener J, Malireddi RK, Lenardon MD, Koberle M, Vautier S, MacCallum DM, Biedermann T, Schaller M, Netea MG, Kanneganti TD, Brown GD, Brown AJ, Gow NA. 2014. Fungal chitin dampens inflammation through IL-10 induction mediated by NOD2 and TLR9 activation. PLoS Pathog 10:e1004050. doi: 10.1371/journal.ppat.1004050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Netea MG, Gow NA, Munro CA, Bates S, Collins C, Ferwerda G, Hobson RP, Bertram G, Hughes HB, Jansen T, Jacobs L, Buurman ET, Gijzen K, Williams DL, Torensma R, McKinnon A, MacCallum DM, Odds FC, Van der Meer JW, Brown AJ, Kullberg BJ. 2006. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Invest 116:1642–1650. doi: 10.1172/JCI27114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. 2003. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med 197:1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown GD, Gordon S. 2001. Immune recognition. A new receptor for beta-glucans. Nature 413:36–37. [DOI] [PubMed] [Google Scholar]
- 50.Cheng SC, van de Veerdonk FL, Lenardon M, Stoffels M, Plantinga T, Smeekens S, Rizzetto L, Mukaremera L, Preechasuth K, Cavalieri D, Kanneganti TD, van der Meer JW, Kullberg BJ, Joosten LA, Gow NA, Netea MG. 2011. The dectin-1/inflammasome pathway is responsible for the induction of protective T-helper 17 responses that discriminate between yeasts and hyphae of Candida albicans. J Leukoc Biol 90:357–366. doi: 10.1189/jlb.1210702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smits GJ, Kapteyn JC, van den Ende H, Klis FM. 1999. Cell wall dynamics in yeast. Curr Opin Microbiol 2:348–352. doi: 10.1016/S1369-5274(99)80061-7. [DOI] [PubMed] [Google Scholar]
- 52.Ernst JF, Pla J. 2011. Signaling the glycoshield: maintenance of the Candida albicans cell wall. Int J Med Microbiol 301:378–383. doi: 10.1016/j.ijmm.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Alonso-Monge R, Roman E, Arana DM, Pla J, Nombela C. 2009. Fungi sensing environmental stress. Clin Microbiol Infect 15(Suppl 1):S17–S19. [DOI] [PubMed] [Google Scholar]
- 54.Gale EF, Ingram J, Kerridge D, Notario V, Wayman F. 1980. Reduction of amphotericin resistance in stationary phase cultures of Candida albicans by treatment with enzymes. J Gen Microbiol 117:383–391. [DOI] [PubMed] [Google Scholar]
- 55.Hapala I, Klobucnikova V, Mazanova K, Kohut P. 2005. Two mutants selectively resistant to polyenes reveal distinct mechanisms of antifungal activity by nystatin and amphotericin B. Biochem Soc Trans 33:1206–1209. [DOI] [PubMed] [Google Scholar]
- 56.Sarinova M, Strakova V, Balkova K, Gbelska Y. 2007. Decreased susceptibility to antifungals in respiratory-deficient Kluyveromyces lactis mutants. Folia Microbiol (Praha) 52:484–490. doi: 10.1007/BF02932108. [DOI] [PubMed] [Google Scholar]
- 57.Brown GD, Gordon S. 2005. Immune recognition of fungal beta-glucans. Cell Microbiol 7:471–479. doi: 10.1111/j.1462-5822.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- 58.Leung MY, Liu C, Koon JC, Fung KP. 2006. Polysaccharide biological response modifiers. Immunol Lett 105:101–114. doi: 10.1016/j.imlet.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 59.Lacchini AH, Davies AJ, Mackintosh D, Walker AJ. 2006. Beta-1, 3-glucan modulates PKC signalling in Lymnaea stagnalis defence cells: a role for PKC in H2O2 production and downstream ERK activation. J Exp Biol 209:4829–4840. doi: 10.1242/jeb.02561. [DOI] [PubMed] [Google Scholar]
- 60.Yoon TJ, Koppula S, Lee KH. 2013. The effects of beta-glucans on cancer metastasis. Anticancer Agents Med Chem 13:699–708. doi: 10.2174/1871520611313050004. [DOI] [PubMed] [Google Scholar]
- 61.Torosantucci A, Bromuro C, Chiani P, De Bernardis F, Berti F, Galli C, Norelli F, Bellucci C, Polonelli L, Costantino P, Rappuoli R, Cassone A. 2005. A novel glyco-conjugate vaccine against fungal pathogens. J Exp Med 202:597–606. doi: 10.1084/jem.20050749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lavigne LM, Albina JE, Reichner JS. 2006. Beta-glucan is a fungal determinant for adhesion-dependent human neutrophil functions. J Immunol 177:8667–8675. [DOI] [PubMed] [Google Scholar]
- 63.Noss I, Doekes G, Thorne PS, Heederik DJ, Wouters IM. 2013. Comparison of the potency of a variety of beta-glucans to induce cytokine production in human whole blood. Innate Immun 19:10–19. doi: 10.1177/1753425912447129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ooi VE, Liu F. 2000. Immunomodulation and anti-cancer activity of polysaccharide-protein complexes. Curr Med Chem 7:715–729. doi: 10.2174/0929867003374705. [DOI] [PubMed] [Google Scholar]
- 65.Fidan I, Kalkanci A, Yesilyurt E, Erdal B. 2014. In vitro effects of Candida albicans and Aspergillus fumigatus on dendritic cells and the role of beta glucan in this effect. Adv Clin Exp Med 23:17–24. doi: 10.17219/acem/37016. [DOI] [PubMed] [Google Scholar]
- 66.Lowman DW, Greene RR, Bearden DW, Kruppa MD, Pottier M, Monteiro MA, Soldatov DV, Ensley HE, Cheng SC, Netea MG, Williams DL. 2014. Novel structural features in Candida albicans hyphal glucan provide a basis for differential innate immune recognition of hyphae versus yeast. J Biol Chem 289:3432–3443. doi: 10.1074/jbc.M113.529131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klippel N, Cui S, Groebe L, Bilitewski U. 2010. Deletion of the Candida albicans histidine kinase gene CHK1 improves recognition by phagocytes through an increased exposure of cell wall beta-1,3-glucans. Microbiology 156:3432–3444. doi: 10.1099/mic.0.040006-0. [DOI] [PubMed] [Google Scholar]
- 68.Galan-Diez M, Arana DM, Serrano-Gomez D, Kremer L, Casasnovas JM, Ortega M, Cuesta-Dominguez A, Corbi AL, Pla J, Fernandez-Ruiz E. 2010. Candida albicans beta-glucan exposure is controlled by the fungal CEK1-mediated mitogen-activated protein kinase pathway that modulates immune responses triggered through dectin-1. Infect Immun 78:1426–1436. doi: 10.1128/IAI.00989-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wheeler RT, Kombe D, Agarwala SD, Fink GR. 2008. Dynamic, morphotype-specific Candida albicans beta-glucan exposure during infection and drug treatment. PLoS Pathog 4:e1000227. doi: 10.1371/journal.ppat.1000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rex JH, Cooper CR Jr, Merz WG, Galgiani JN, Anaissie EJ. 1995. Detection of amphotericin B-resistant Candida isolates in a broth-based system. Antimicrob Agents Chemother 39:906–909. doi: 10.1128/AAC.39.4.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cotter G, Doyle S, Kavanagh K. 2000. Development of an insect model for the in vivo pathogenicity testing of yeasts. FEMS Immunol Med Microbiol 27:163–169. doi: 10.1111/j.1574-695X.2000.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 72.Trevijano-Contador N, Herrero-Fernandez I, Garcia-Barbazan I, Scorzoni L, Rueda C, Rossi SA, Garcia-Rodas R, Zaragoza O. 2015. Cryptococcus neoformans induces antimicrobial responses and behaves as a facultative intracellular pathogen in the non mammalian model Galleria mellonella. Virulence 6:66–74. doi: 10.4161/21505594.2014.986412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hohl TM, Feldmesser M, Perlin DS, Pamer EG. 2008. Caspofungin modulates inflammatory responses to Aspergillus fumigatus through stage-specific effects on fungal beta-glucan exposure. J Infect Dis 198:176–185. doi: 10.1086/589304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ratcliffe NA. 1985. Invertebrate immunity—a primer for the non-specialist. Immunol Lett 10:253–270. doi: 10.1016/0165-2478(85)90100-2. [DOI] [PubMed] [Google Scholar]
- 75.Whitten MM, Tew IF, Lee BL, Ratcliffe NA. 2004. A novel role for an insect apolipoprotein (apolipophorin III) in beta-1,3-glucan pattern recognition and cellular encapsulation reactions. J Immunol 172:2177–2185. doi: 10.4049/jimmunol.172.4.2177. [DOI] [PubMed] [Google Scholar]
- 76.Casadevall A, Pirofski LA. 2003. The damage-response framework of microbial pathogenesis. Nat Rev Microbiol 1:17–24. doi: 10.1038/nrmicro732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Graybill JR, Montalbo E, Kirkpatrick WR, Luther MF, Revankar SG, Patterson TF. 1998. Fluconazole versus Candida albicans: a complex relationship. Antimicrob Agents Chemother 42:2938–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vincent BM, Lancaster AK, Scherz-Shouval R, Whitesell L, Lindquist S. 2013. Fitness trade-offs restrict the evolution of resistance to amphotericin B. PLoS Biol 11:e1001692. doi: 10.1371/journal.pbio.1001692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barchiesi F, Calabrese D, Sanglard D, Falconi Di Francesco L, Caselli F, Giannini D, Giacometti A, Gavaudan S, Scalise G. 2000. Experimental induction of fluconazole resistance in Candida tropicalis ATCC 750. Antimicrob Agents Chemother 44:1578–1584. doi: 10.1128/AAC.44.6.1578-1584.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]