Abstract
Benznidazole (BNZ) is the first-line drug for the treatment of Chagas disease. The drug is available in the form of immediate-release tablets for 100-mg (adult) and 12.5-mg (pediatric) doses. The drug is administered two or three times daily for 60 days. The high frequency of daily administrations and the long period of treatment are factors that significantly contribute to the abandonment of therapy, affecting therapeutic success. Accordingly, this study aimed to evaluate the preclinical pharmacokinetics of BNZ administered as extended-release tablets (200-mg dose) formulated with different types of polymers (hydroxypropyl methylcellulose K4M and K100M), compared to the tablets currently available. The studies were conducted with rabbits, and BNZ quantification was performed in plasma and urine by ultraperformance liquid chromatography methods previously validated. The bioavailability of BNZ was adequate in the administration of extended-release tablets; however, with the administration of the pediatric tablet, the bioavailability was lower than with other tablets, which showed that the clinical use of this formulation should be monitored. The pharmacokinetic parameters demonstrated that the extended-release tablets prolonged drug release from the pharmaceutical matrix and provided an increase in the maintenance of the drug concentration in vivo, which would allow the frequency of administration to be reduced. Thus, a relative bioavailability study in humans will be planned for implementation of a new product for the treatment of Chagas disease.
INTRODUCTION
Chagas disease is caused by the protozoan parasite Trypanosoma cruzi. The parasite is transmitted by hemipteran insects of the subfamily Triatominae (Reduviidae) when a person comes in contact with its contaminated feces/urine through either a scratch in the skin (including the bite), the eyes, or mouth (1, 2). The transmission can also occur by the oral route through contaminated food (3, 4), congenital transmission (5), organ transplantation (6), transfusion of infected blood (7), and laboratory accidents (8).
Chagas disease manifests in two phases: acute and chronic. The acute phase lasts for 2 months with a high parasitemia and may be oligosymptomatic or asymptomatic (9). In the chronic phase, the parasites are hidden in target tissues, especially cardiac tissue and muscles of the digestive system (10). Chagas disease may be more severe in children <5 years of age, immunosuppressed patients (mainly with HIV/AIDS), the elderly, and individuals infected with a large number of parasites (9, 11).
Recently, the World Health Organization (WHO) updated the epidemiological data on Chagas disease from the 21 Latin American countries. This report estimated that 5,742,167 people were infected with T. cruzi in the region where it is considered endemic. The 3 countries with the largest estimated numbers of infected people were Argentina, Brazil, and Mexico (1,505,235; 1,156,821, and 876,458, respectively) (12). Due to the immigration of infected persons, cases of the disease have been registered in North America (13), Australia, New Zealand (14), Japan, and Europe (15).
Chagas is a neglected disease due to its pattern of affecting poor communities in large regions, which have little political priority, leading to barriers to control, poor investment in treatment and diagnosis, morbidity, and consequent mortality (16). Only two drugs are available for Chagas disease treatment: nifurtimox and benznidazole (BNZ). BNZ is the only drug widely available (17), and it is highly effective in the acute phase, while in the chronic phase, it is recommended to reduce cardiac symptoms (18). Moreover, BNZ treatment is recommended in congenital cases and reactive infections among all children and for patients up to 18 years old in the indeterminate chronic phase. The BNZ posology (i.e., dosage system) for patients >12 years old is 5 to 7 mg/kg body weight/day, and that for patients <12 years old is 5 to 7.5 mg/kg body weight/day, divided into 2 to 3 daily doses, for 60 days (19). Until 2011, BNZ was only available as a 100-mg tablet, manufactured by Brazilian Pharmaceutical Laboratory of the State of Pernambuco (LAFEPE). Between 2011 and 2012, a 12.5-mg pediatric tablet was developed through the efforts of the Drugs for Neglected Diseases Initiative (DNDi) and LAFEPE. This new pediatric form aimed to minimize the risk of continued use of extemporaneous formulations of tablets for the pediatric population and to avoid imprecise drug administration (20). The 12.5-mg pediatric tablet was registered in Brazil (Brazilian Health Surveillance Agency, ANVISA) in 2011 and is currently present on the WHO Model List of Essential Medicines for Children (21). Thus, the treatment of children would be more accurate and safe, resulting in improved treatment of Chagas disease in pediatric patients.
Along the same line, the Technology Laboratory of Medicine of the Federal University of Pernambuco (Recife, Brazil) developed extended-release tablets of BNZ to improve treatment compliance by reducing the number of daily administrations and to increase the residence time of the drug in the body. These tablets were developed using hydroxypropyl methylcellulose (HPMC) for modulation of the drug release rate with different degrees of viscosity (K4M and K100M polymers). In in vitro dissolution assays (unpublished data), the release rates (85% dissolution) were 24 h for tablets composed of HPMC K4M polymer and 72 h for tablets composed of the HPMC K100M polymer, compared to the tablets available on the market (immediate-release system) with a release rate of 60 min. Thus, these new tablets with prolonged release of BNZ showed promising results and prospects for in vivo testing.
In this study, we evaluated the preclinical pharmacokinetics (PK) of BNZ administered as the commercially available adult (100-mg) and pediatric (12.5-mg) tablets, two types of extended-release tablets (200 mg), and intravenously (i.v.) in healthy white rabbits. In addition, we determined the absolute (F) and relative (RBA) bioavailability of the new BNZ tablets. The pharmacokinetic parameters were statistically compared between all groups to determine differences in the kinetic disposition of BNZ from tablets in different administered forms.
MATERIALS AND METHODS
Ethics statement.
This study was approved by the Research Ethics Committee of the School of Pharmaceutical Sciences of the São Paulo State University (Araraquara, Brazil) (process 01/2014).
Benznidazole tablets.
BNZ immediate-release tablets in the adult (100-mg [lot 13030214]) and pediatric (12.5 mg [lot 13030261]) forms were manufactured and provided by LAFEPE. BNZ extended-release tablets (200 mg) were formulated by the wet granulation process, using as excipients lactose, hydroxypropyl methylcellulose (HPMC), polyvinylpyrrolidone K30 in hydroalcoholic solution, and magnesium stearate. HPMC polymers with different degrees of viscosity were used: HPMC K4M (4,000 mPa · s in 2% aqueous solution at 20°C) and HPMC K100M (100,000 mPa · s in 2% aqueous solution at 20°C). All quality control testing, characterization of these formulations, and dissolution assays (data not shown) were previously performed by the Laboratory of Medicine Technology of the Federal University of Pernambuco.
Benznidazole administration.
Oral administration of the BNZ tablets in rabbits was performed using a pill dispenser with a fixed volume of water (10 ml) in the initial portion of the esophagus. The tablets were administered in full form (not broken) and single dose.
In i.v. administration, BNZ (Sigma-Aldrich, lot MKBL3727V) was dissolved in dimethyl sulfoxide (DMSO). The DMSO volume used (0.3 ml) did not exceed the limit suggested by Neervannan (22) for occurrence of toxic effects. The BNZ solution was administered in the marginal auricular vein of the rabbits in a single 10-mg dose.
Experimental animals.
Thirty-five healthy male New Zealand White rabbits (weighing 2.5 to 3.5 kg) were used. The animals were randomly divided into five groups (n = 7 in each group) and individually housed in metabolic cages with free access to food and water. Before administration, the rabbits were fasted for 12 h. Food was offered to the animals 4 h after oral administration to prevent food interactions.
The blood samples (100 μl) were collected in heparinized tubes at predetermined intervals for each group. For the pediatric dosage form (12.5-mg tablet), the blood collection times were 0.25 to 6.0 h postdose. For the adult dosage form (100-mg tablet), the blood collection times were 0.25 to 24 h postdose. For extended-release dosage forms (ER-K4M and ER-K100M [200-mg tablets]), the blood collection times were 0.50 to 36 h postdose. With i.v. administration (10 mg), the blood collection times were 0.25 to 8.0 h postdose.
Heparinized blood (50 μl) was separated by centrifugation at 6,000 × g for 5 min, and plasma was stored (at −20°C) until chromatographic analysis.
Urine samples were collected using metabolic cages. The urine volume was measured for each collection period (6 h), and aliquots of urine (500 μl) were filtered through nitrocellulose filters and stored (−20°C) until chromatographic analysis.
Plasma analysis.
A Waters Acquity ultra-high-performance liquid chromatography (UHPLC) system equipped with a UV-visible (UV-Vis) detector was employed. The chromatographic separation was performed on an Acquity HSS SB C18 column (2.1 by 100 mm, 1.8 μm) preceded by an Acquity HSS VanGuard (2.1 by 5 mm; 1.8 μm) guard column at 45°C. The mobile phase was a 65:35 (vol/vol) water-acetonitrile mixture, and elution was in the isocratic mode, with detection at 324 nm. A flow rate of 0.55 ml/min was used, and 1 μl of sample was injected into the chromatographic system. The run time was 2 min.
Prior to the chromatographic analysis, all samples were treated according to the following procedure. Fifty-microliter plasma samples were deproteinized by the addition of 50 μl of acetonitrile (containing 5 μg/ml benzocaine as an internal standard), vortexed for 1 min, and centrifuged at 13,000 × g for 10 min. A 50-μl volume of supernatant was withdrawn and filtered with polytetrafluoroethylene (PTFE) syringe filters (0.22 μm, 4 mm) in a maximum recovery vial (Waters). The final extract was stored at 25°C in the sample manager compartment until chromatographic analysis. The linear concentration range was 0.15 to 20 μg/ml. These chromatographic conditions were used in previous studies of BNZ pharmacokinetics in rats (23).
Urine analysis.
Urine samples (500 μl) were added 1.5 ml ethyl acetate (containing 50 μg/ml benzocaine as internal standard), vortexed for 30 s, and centrifuged at 2,000 × g for 5 min. After phase separation, 1.2 ml organic phase was removed and dried using a sample concentrator under vacuum. The final extract was resuspended in 50 μl acetonitrile, filtered with PTFE syringe filters (0.22 μm, 4 mm) and injected (1 μl) into the chromatographic system. The chromatographic conditions were the same as those used in the analysis of plasma samples. The linear concentration range was 0.078 to 20 μg/ml.
These bioanalytical methods (plasma and urine) were validated based on the U.S. Food and Drug Administration's Guidance for Industry (24) and Brazilian Health Surveillance Agency (ANVISA) resolutions 899/2003 (25) and 27/2012 (26).
Pharmacokinetic parameters.
The elimination half-life (t1/2) was determined by elimination phase of the graph of log plasma concentration versus time. The absorption half-life (t1/2a) was determined by the method of residuals. The elimination (kel) and absorption (ka) constants were calculated by the formula 0.693/t1/2 or t1/2a. ka was used to calculate the mean absorption time (MAT) by the formula 1/ka. The area under the curve from 0 to the last quantifiable concentration (AUC0–t) was calculated by the trapezoidal method, and the area under the curve from 0 to infinity (AUC0–∞) was calculated by the formula AUC0–t + (Cn/kel), where Cn was the last quantifiable plasma BNZ concentration. The area under the moment curve (AUMC) was calculated by the statistical moments method and used to determine the mean residence time (MRT): MRT = AUMC/AUC0–∞. The clearance (Cl) and the distribution volume (Vz) were determined by the equations Cl = dose/AUC0–∞ and Vz = Cl/kel, corrected by bioavailability for each oral administration group. The maximum plasma drug concentration (Cmax) was obtained directly from the experimental data, as was the time of the occurrence of Cmax (tmax). F and RBA were evaluated by the equations (AUC0–∞ tablet × dosei.v.)/(AUC0–∞ i.v. × dosetablet) and (AUC0–∞ test × dosereference)/(AUC0–∞ reference × dosetest), respectively.
Urine analysis was performed to calculate the fraction of dose excreted (fe) in urine as unchanged BNZ and the renal clearance (ClR) by dividing the total amount of unchanged BNZ excreted in the urine by the AUC0–t.
The formulas described were inserted in Excel software for the calculation of pharmacokinetic parameters, and values were confirmed by PhoenixWinNonlin software.
Statistical analysis.
The data for pharmacokinetic parameters are expressed as the mean and 95% confidence interval (95% CI). The groups were compared by the nonparametric Mann-Whitney test (GraphPad Instat software, version 3.06).
RESULTS AND DISCUSSION
Validation of bioanalytical methods.
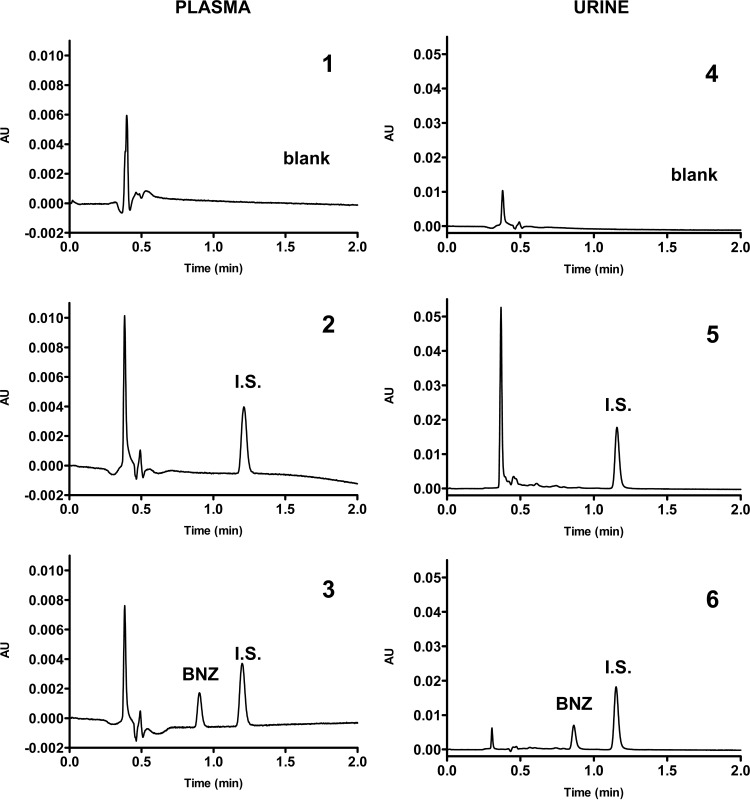
The bioanalytical methods for quantification of BNZ in plasma and urine by UHPLC were validated according to the aforementioned guidelines. The selectivity methods proved adequate by showing that substances in the plasma and urine blank did not interfere with the retention times of BNZ and benzocaine as the internal standard Figure 1 illustrates the chromatograms of plasma (panel 1) and urine (panel 4) blank and zero samples (panels 2 and 5) and plasma (panel 3) and urine (panel 6) samples spiked with BNZ (2.5 μg/ml in plasma and 0.625 μg/ml in urine).
FIG 1.
Chromatograms of plasma and urine samples from rabbits showing selectivity of bioanalytical methods for quantification of BNZ. Panels: 1, plasma blank; 2, zero sample of plasma; 3, BNZ at 2.5 μg/ml in plasma; 4, urine blank; 5, zero sample of urine; 6, BNZ at 0.625 μg/ml in urine. I.S., internal standard (benzocaine); AU, absorbance units.
The linearity tests met the acceptance criteria, with precision and accuracy being in accordance with the established limits. The results of precision and accuracy were adequate according to the aforementioned acceptance criteria for analyses of quality controls. The lower limits of quantification (LLOQ) were 0.1562 μg/ml for plasma and 0.039 μg/ml for urine, given the criteria of precision and accuracy. The recovery test also showed precision and accuracy according to the acceptance criteria, demonstrating that sample processing was suitable for the analysis of BNZ in plasma and urine. The stability of BNZ was evaluated under several conditions, including short-term room temperature, long-term frozen, and postprocessed. BNZ was considered stable if the measured analyte concentration of stored samples was between 85 and 115% of the measured concentration of a fresh sample. The confidence limits for the bioanalytical methods for quantification of BNZ in plasma and urine are given in Table 1.
TABLE 1.
Parameters of the bioanalytical methods for quantification of BNZ in plasma and urine
| Parametera | Result for: |
|
|---|---|---|
| Plasma | Urine | |
| Linearity (n = 10) | ||
| Concn range, μg/ml | 0.1562–20 | 0.0390–20 |
| Correlation coefficient | r = 0.9995 | r = 0.9998 |
| Equation | y = 1.5626x − 0.1968 | y = 0.8445x − 0.0174 |
| LLOQ, μg/ml | 0.1562 | 0.0390 |
| Precision, RSD | ||
| Intra-assay | 8.18 | 10.1 |
| Interassay | 10.3 | 10.6 |
| Accuracy | ||
| Intra-assay | 93.8 | 97.3 |
| Interassay | 101.6 | 100.2 |
| Recovery, % | 65.5 | 82.0 |
| Stability, RSD/accuracy | ||
| Short term (24 h at room temp) | 3.85/94.2 | 6.5/96.5 |
| Long term (14 days at 20°C) | 5.5/107.3 | 10.0/101.3 |
| Postpreparative (24 h at 25°C) | 3.5/92.4 | 7.4/103.4 |
LLOQ, lower limit of quantitation; RSD, relative standard deviation.
Pharmacokinetic evaluation.
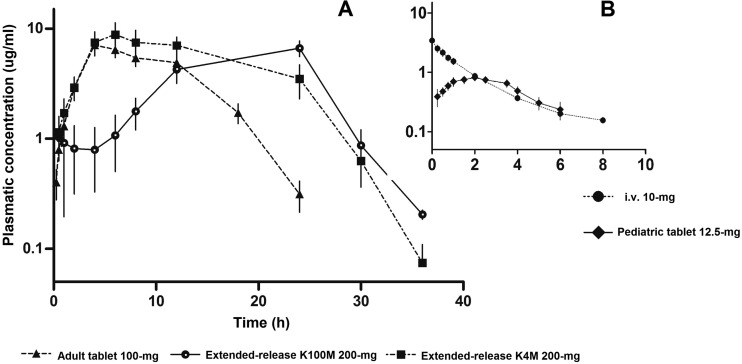
The PK profile of BNZ administered as extended-release tablets is shown in Fig. 2 in comparison to the PK profile of the adult immediate-release tablet (Fig. 2A) and the PK profile of BNZ administered as pediatric immediate-release tablet in comparison to the PK profile of i.v. administration (Fig. 2B).
FIG 2.
Pharmacokinetic profile after administration of BNZ in tablets and i.v. in rabbits. Values are shown as the mean ± 95% CI. n = 35 total (n = 7 in each group).
The pharmacokinetic parameters for all groups were tabulated, and statistical comparison is shown in Table 2. A one-compartment model was used for the determination of pharmacokinetic parameters of the BNZ tablet groups, and a two-compartment model was used for the i.v. group.
TABLE 2.
Pharmacokinetic parameters of oral and intravenous BNZ in rabbitsa
| Unitb | Result for BNZ administration, mean (95% CI)c |
||||
|---|---|---|---|---|---|
| Immediate-release tablet |
i.v. (10 mg) | Extended-release tabletd |
|||
| Adult (100 mg) | Pediatric (12.5 mg) | ER-K4M (200 mg) | ER-K100M (200 mg) | ||
| kel, h−1 | 0.1955 (0.1283–0.2628) | 0.4351 (0.3366–0.5335)* | 0.2792 (0.1562–0.4023) | 0.2076 (0.1231–0.2921)† | 0.2309 (0.1464–0.3155)† |
| t1/2, h | 4.02 (2.50–5.53) | 1.66 (1.34–1.99)* | 3.07 (1.65–4.50) | 3.92 (2.45–5.41)† | 3.56 (1.96–5.17)† |
| ka, h−1 | 0.3476 (0.2971–0.3982) | 1.1472 (0.8660–1.4280)* | 0.3277 (0.2463–0.4091)† | 0.2468 (0.1736–0.3201)*,† | |
| t1/2a, h | 2.03 (1.73–2.35) | 0.63 (0.51–0.75)* | 2.24 (1.70–2.79)† | 3.19 (1.83–4.54)*,† | |
| MRT, h | 9.64 (7.88–11.42) | 3.41 (3.03–3.80)* | 3.3 (2.20–4.40)* | 12.8 (9.25–16.34)†,‡ | 19.1 (16.0–22.3)*,†,‡,§ |
| MAT, h | 2.94 (2.49–3.39) | 0.91 (0.74–1.09)* | 3.24 (2.45–5.41)† | 4.60 (2.64–6.56)*,† | |
| tmax, h | 6.0 (3.4–8.6) | 2.3 (1.6–2.9)* | 11.1 (2.9–19.4)† | 18.8 (12.9–24.8)*,† | |
| Cmax, μg/ml | 8.39 (6.11–10.67) | 0.84 (0.62–1.07)* | 10.26 (6.81–13.7)† | 7.15 (5.00–9.31)† | |
| Vz, ml/kg | 3,165 (1,394–4,936) | 1,295 (870–1,721)* | 2,338 (1,110–3,567) | 2,985 (516–5,454) | 3,190 (2,078–4,303)† |
| Cl, ml/h · kg | 515 (406–624) | 555 (336–775) | 511 (390–632) | 483 (246–720) | 668 (497–838) |
| ClR, ml/h · kg | 9.88 (5.63–14.14) | 0.46 (0–0.95)* | 1.22 (0.05–2.39)* | 8.28 (0–17.01)† | 15.68 (10.62–20.74)†,‡ |
| fe, % | 1.83 (0.96–2.71) | 0.08 (0–0.18)* | 0.21 (0–0.42)* | 1.45 (0.27–2.64)† | 2.43 (1.64–3.23)†,‡ |
Results are shown as means with 95% CIs in parentheses. n = 35 rabbits total (n=7 in each group).
kel, elimination constant; t1/2, elimination half-life; ka, absorption constant; t1/2a, absorption half-life; MRT, mean residence time; MAT, mean absorption time; Cmax, maximum plasma concentration; tmax, time to Cmax; Vz, distribution volume; Cl, total clearance; ClR, renal clearance; fe, fraction of drug excreted as unchanged in the urine.
Statistical analysis (Mann-Whitney test): *, P < 0.05 compared to the adult tablet group; †, P < 0.05 compared to the pediatric tablet group; ‡, P < 0.05 compared to the i.v. group; §, P < 0.05 compared to the ER-K4M group.
ER-K4M, extended-release tablet composed of type K4M HPMC polymer; ER-K100M, extended-release tablet composed of type K100M HPMC polymer.
Comparison between the groups and interpretation of pharmacokinetic parameters.
There was no statistical difference in Cmax values between the adult (8.39 μg/ml), ER-K4M (10.26-μg/ml), and ER-K100M (7.15-μg/ml) tablets, although the dose of extended-release tablets (200 mg) was 2-fold the dose of the immediate-release adult tablet (100 mg). It is important to consider this factor in clinical use, because even though the dose was doubled, the Cmax levels were close in comparison with the immediate-release adult tablet that is already on the market. According to literature data, plasma concentrations equal to or greater than 20 μg/ml in humans increase the risk of adverse effects, mainly dermal manifestations (27).
The ClR and fe parameters for adult, ER-K4M, and ER-K100M tablets were higher than those for the pediatric (12.5 mg) and i.v. (10 mg) administrations, which were given in smaller doses. It is possible to infer that the difference in the ClR and fe parameters are related to a possible saturation of the biotransformation system and consequent increase in renal excretion and elimination of the drug unchanged in the urine. It is known that BNZ is mainly metabolized by the hepatic cytochrome P450 (CYP450) enzymes (CYP3A4). Studies in HepG2 cells (human hepatocyte cell line) and rats demonstrated that BNZ induces the expression and activity of CYP3A4, P glycoprotein (P-gp), multidrug-resistance-associated protein 2 (Mrp-2), and glutathione S-transferase (GST [hepatic and intestinal]) in a concentration-dependent manner (28). The findings of this study raise the hypothesis that in this animal model (rabbits), similar doses of the adult, ER-K4M, and ER-K100M tablets can lead to saturation of the animal biotransformation system and increase renal excretion of unchanged BNZ. For confirmation of this hypothesis, specific research is required with this animal model to identify and describe this phenomenon.
In studies with mice, it was also observed that with the dose of 78 mg/kg body weight, there is saturation of the biotransformation system and thus consequent changes in the pharmacokinetic parameters (29). In humans, it has been reported that there is linearity in the pharmacokinetic parameters in the dose range of 4 to 30 mg/kg in a phase I study (single dose) (30). Studies with patients who received BNZ for 30 days (7 mg/kg/day twice daily) demonstrated that the Cmax after the first morning dose decreases with time (approximately 20% on average after 25 days of treatment), suggesting an increase in metabolism or excretion of the drug with chronic use (31).
Therefore, the saturation of the biotransformation system by BNZ in humans can be dependent on the frequency of administration and plasma concentration oscillation (high concentration levels). These changes in ClR and fe may not occur with the administration of extended-release tablets assessed in future human studies.
Mean residence time (MRT), mean absorption time (MAT), and the time of occurrence of Cmax (tmax) of BNZ for ER-K100M administration were statistically greater than those in all other groups, demonstrating that the extended-release system with the HPMC K100M polymer promoted prolonged drug release, causing a greater drug absorption time and higher maintenance of the plasma drug concentration compared to the other tablets tested. These facts can be considered beneficial for Chagas disease treatment, because this type of tablet can contribute to higher antiparasitic activity.
The pediatric tablet showed faster release and promoted faster absorption than the adult tablet, and it also showed no difference compared to i.v. administration, reinforcing the idea that the drug absorption is much faster in the pediatric formulation, as can be observed in the briefer MAT and tmax values. These findings are in accordance with the MRT values observed with the pediatric tablet compared to the others. This may be related to the physicochemical characteristics (size, weight, and hardness) of the tablets and the proportion of excipients (20) in each formulation, leading to different ranges of disintegration and dissolution in vivo.
The Vz value found for the pediatric tablet was statistically lower than those for the adult and ER-K100M tablets, which may are related to the lower dose in the pediatric tablet. At higher doses, an eventual saturation of the excretion system may occur, as mentioned above, which allows greater distribution of the drug in the body.
The elimination half-life (t1/2) for the pediatric tablet (1.66 h) was statistically shorter than those for the other groups, reinforcing that the elimination of the drug was faster with the administration of this dose. The different dosages between the groups can be a factor to be considered, since there can be a biotransformation system saturation at high doses in this animal model.
Oral bioavailability evaluation.
Table 3 presents the mean values of oral F and RBA for each tablet. RBA was calculated based on the adult tablet (100 mg) as the reference.
TABLE 3.
Results from the oral bioavailability study of BNZ tabletsa
| Parameterb | Result for BNZ administration |
||||
|---|---|---|---|---|---|
| Immediate-release tablet |
i.v. | Extended-release tablet |
|||
| Adultc | Pediatric | ER-K4M | ER-K100M | ||
| Dose,d mg/kg | 42.2 (36,125–48,381) | 4.60 (4,324–4,882) | 3.07 (2,962–3,177) | 62.9 (58,194–67,794) | 68.6 (63,534–73,822) |
| AUC0–∞, μg/ml · h | 86.6 (62.4–110.8) | 3.6 (2.3–4.8) | 6.3 (5.0–7.6) | 153.9 (102.5–205.2) | 111.0 (77.3–144.7) |
| F, % | 99.8 (63.1–151.9) | 37.7 (25.9–51.0) | 118.9 (78.5–170.8) | 78.8 (36.8–139.4) | |
| RBA, % | 37.8 (21.6–61.4) | 119.2 (74.0–171.9) | 78.9 (59.5–102.8) | ||
Results are shown as means with 95% CIs in parentheses. n = 35 rabbits total (n = 7 in each group).
AUC0—∞, area under the concentration-time curve extrapolated to infinity; F, oral absolute bioavailability; RBA, oral relative bioavailability.
Used as the reference group in the RBA calculation.
Corrected dose by weight of the animals.
The bioavailability study demonstrated that the adult tablet, which is currently available for Chagas disease treatment, showed excellent oral absolute bioavailability (F), close to 100%. These results are similar to those found in humans, where Raaflaub and Ziegler showed that absorption of the drug occurred completely after the administration of 100-mg tablets (32). On the other hand, the pediatric tablet (12.5 mg) did not show a value close to 100%. F was 37.7%, and RBA was 37.8%, showing that a little more than one-third of the dose administered reached the bloodstream. It is noteworthy that the pediatric tablet is now available for Chagas disease treatment in children, so pharmacokinetic studies should be performed with these patients to evaluate whether F in children is enough to achieve clinical effects.
For extended-release tablets, the bioavailability values were good according to bioequivalence guidelines, which state that RBA should be within the range of 80 to 125% (33). The ER-K100M tablet was close to 80% RBA, which may be related to a long drug release time and the fast gastrointestinal motility of rabbits, which may lead to a fraction of the administered dose being excreted via the digestive tract (feces) without absorption. It is known that gastrointestinal motility and intestinal transit are different for rabbits compared to humans (34), and thus the F found in this study for this type of tablet may be different when used in clinical trials (human), since the time in the gastrointestinal tract will be longer and will enable longer drug release.
Conclusion.
The pharmacokinetic profile of BNZ in the administration of extended-release tablets allowed the selection of the most advantageous formulation for development. The extended-release K100M tablet exhibited a promising reduction in the frequency of drug administration and enabled a longer residence time in the body. Therefore, a clinical study of relative bioavailability will be conducted in the future with the objective of implementing this new formulation in the treatment of Chagas disease.
The bioavailability of the pediatric tablet was low in comparison with those of the other formulations tested. Considering that this is the first pharmacokinetic study of this formulation, its bioavailability indicates that clinical use of this tablet requires the monitoring of pediatric patients regarding efficacy and plasma drug concentrations.
ACKNOWLEDGMENTS
We thank São Paulo Research Foundation (FAPESP; process 13/25882-6 and process 14/13192-8), Coordination for the Improvement of Higher Education Personnel (CAPES), National Council for Scientific and Technological Development (CNPq; process 130607/2014-2 and process 134389/2015-8), and PADC—Programa de Apoio ao Desenvolvimento Científico da Faculdade de Ciências Farmacêuticas (UNESP) for providing financial support during the course of this study.
A. Leyva helped with editing the English in the manuscript.
REFERENCES
- 1.Chagas C. 1909. Nova tripanozomiaze humana: estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Mem Inst Oswaldo Cruz 1:159–218. doi: 10.1590/S0074-02761909000200008. [DOI] [Google Scholar]
- 2.Coura JR, Dias JC. 2009. Epidemiology, control and surveillance of Chagas disease: 100 years after its discovery. Mem Inst Oswaldo Cruz 104(Suppl 1):31–40. [DOI] [PubMed] [Google Scholar]
- 3.Shikanai-Yasuda MA, Carvalho MA. 2012. Oral transmission of Chagas disease. Clin Infect Dis 54:845–852. doi: 10.1093/cid/cir956. [DOI] [PubMed] [Google Scholar]
- 4.Nóbrega AA, Garcia MH, Tatto E, Obara MT, Costa E, Sobel J, Araujo WN. 2009. Oral transmission of Chagas disease by consumption of açaí palm fruit, Brazil. Emerg Infect Dis 15:653–655. doi: 10.3201/eid1504.081450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliveira I, Torrico F, Muñoz J, Gascon J. 2010. Congenital transmission of Chagas disease: a clinical approach. Expert Rev Anti Infect Ther 8:945–956. doi: 10.1586/eri.10.74. [DOI] [PubMed] [Google Scholar]
- 6.Chin-Hong PV, Schwartz BS, Bern C, Montgomery SP, Kontak S, Kubak B, Morris MI, Nowicki M, Wright C, Ison MG. 2011. Screening and treatment of Chagas disease in organ transplant recipients in the United States: recommendations from the Chagas in Transplant Working Group. Am J Transplant 11:672–680. doi: 10.1111/j.1600-6143.2011.03444.x. [DOI] [PubMed] [Google Scholar]
- 7.Fearon MA, Scalia V, Huang M, Dines I, Ndao M, Lagacé-Wiens P. 2013. A case of vertical transmission of Chagas disease contracted via blood transfusion in Canada. Can J Infect Dis Med Microbiol 24:32–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herwaldt BL. 2001. Laboratory-acquired parasitic infections from accidental exposures. Clin Microbiol Rev 14:659–688. doi: 10.1128/CMR.14.3.659-688.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrade DV, Gollob KJ, Dutra WO. 2014. Acute Chagas disease: new global challenges for an old neglected disease. PLoS Negl Trop Dis 8:e3010. doi: 10.1371/journal.pntd.0003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coura JR, Borges-Pereira J. 2011. Chronic phase of Chagas disease: why should it be treated? A comprehensive review. Mem Inst Oswaldo Cruz 106:641–645. doi: 10.1590/S0074-02762011000600001. [DOI] [PubMed] [Google Scholar]
- 11.Hernández C, Cucunubá Z, Parra E, Toro G, Zambrano P, Ramírez JD. 2014. Chagas disease (Trypanosoma cruzi) and HIV co-infection in Colombia. Int J Infect Dis 26:146–148. doi: 10.1016/j.ijid.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. 2015. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec 90:33–44. http://www.who.int/wer/2015/wer9006.pdf?ua=1. [PubMed] [Google Scholar]
- 13.Hotez PJ, Dumonteil E, Cravioto MB, Bottazzi ME, Tapia-Conyer R, Meymandi S, Karunakara U, Ribeiro I, Cohen RM, Pecoul B. 2013. An unfolding tragedy of Chagas disease in North America. PLoS Negl Trop Dis 7:e2300. doi: 10.1371/journal.pntd.0002300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson Y, Pinto A, Pett S. 2014. Chagas disease in Australia and New Zealand: risks and needs for public health interventions. Trop Med Int Health 19:212–218. doi: 10.1111/tmi.12235. [DOI] [PubMed] [Google Scholar]
- 15.Carlier Y. 2011. Globalization of Chagas disease (American trypanosomiasis): the situation in Europe and Belgium. Bull Mem Acad R Med Belg 166:347–357. [PubMed] [Google Scholar]
- 16.Viotti R, Alarcón de Noya B, Araujo-Jorge T, Grijalva MJ, Guhl F, López MC, Ramsey JM, Ribeiro I, Schijman AG, Sosa-Estani S, Torrico F, Gascon J. 2014. Towards a paradigm shift in the treatment of chronic Chagas disease. Antimicrob Agents Chemother 58:635–639. doi: 10.1128/AAC.01662-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinazo MJ, Muñoz J, Posada E, López-Chejade P, Gállego M, Ayala E, del Cacho E, Soy D, Gascon J. 2010. Tolerance of benznidazole in treatment of Chagas' disease in adults. Antimicrob Agents Chemother 54:4896–4899. doi: 10.1128/AAC.00537-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia S, Ramos CO, Senra JF, Vilas-Boas F, Rodrigues MM, Campos-de-Carvalho AC, Ribeiro-Dos-Santos R, Soares MB. 2005. Treatment with benznidazole during the chronic phase of experimental Chagas' disease decreases cardiac alterations. Antimicrob Agents Chemother 49:1521–1528. doi: 10.1128/AAC.49.4.1521-1528.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coura JR. 2009. Present situation and new strategies for Chagas disease chemotherapy: a proposal. Mem Inst Oswaldo Cruz 104:549–554. [DOI] [PubMed] [Google Scholar]
- 20.Drugs for Neglected Disease Initiative. 2012. Proposal for the inclusion of benznidazol pediatric dosage form as treatment for Chagas disease in children younger than 2 years old in the World Health Organization Model List of Essential Medicines for Children. Drugs for Neglected Disease Initiative (DNDi), Rio de Janeiro, Brazil: http://www.who.int/selection_medicines/committees/expert/19/applications/Benznidazole_6552_C_NF.pdf Accessed 10 March 2015. [Google Scholar]
- 21.World Health Organization. 2013. Model list of essential medicines for children. World Health Organization, Geneva, Switzerland: http://www.who.int/medicines/publications/essentialmedicines/4th_EMLc_FINAL_web_8Jul13.pdf Accessed 10 July 2015. [Google Scholar]
- 22.Neervannan S. 2006. Preclinical formulations for discovery and toxicology: physicochemical challenges. Expert Opin Drug Metab Toxicol 2:715–731. doi: 10.1517/17425255.2.5.715. [DOI] [PubMed] [Google Scholar]
- 23.Davanço MG, de Campos ML, Peccinini RG. 2015. Rapid and sensitive ultra-high-pressure liquid chromatography method for quantification of antichagasic benznidazole in plasma: application in a preclinical pharmacokinetic study. Biomed Chromatogr 29:1008–1015. doi: 10.1002/bmc.3386. [DOI] [PubMed] [Google Scholar]
- 24.Food and Drug Administration. May 2001. Guidance for industry: bioanalytical method validation. Food and Drug Administration, Center for Drug Evaluation and Research, Silver Spring, MD. [Google Scholar]
- 25.Agência Nacional de Vigilância Sanitária. 2003. Resolution no. 899, May 29, 2003. Guia para validação de métodos analíticos e bioanalíticos. ANVISA, São Paulo, Brazil: http://portal.anvisa.gov.br/wps/wcm/connect/4983b0004745975da005f43fbc4c6735/RE_899_2003_Determina+a+publica%C3%A7%C3%A3o+do+Guia+para+valida%C3%A7%C3%A3o+de+m%C3%A9todos+anal%C3%ADticos+e+bioanal%C3%ADticos.pdf?MOD=AJPERES Accessed 10 January 2014. [Google Scholar]
- 26.Agência Nacional de Vigilância Sanitária. 2012. Resolution RDC—no. 27, May 17, 2012. Guia para validação de método bioanalíticos. ANVISA, São Paulo, Brazil: Accessed 10 January 2014. [Google Scholar]
- 27.Pinazo MJ, Guerrero L, Posada E, Rodríguez E, Soy D, Gascon J. 2013. Benznidazole-related adverse drug reactions and their relationship to serum drug concentrations in patients with chronic Chagas disease. Antimicrob Agents Chemother 57:390–395. doi: 10.1128/AAC.01401-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perdomo VG, Rigalli JP, Villanueva SS, Ruiz ML, Luquita MG, Echenique CG, Catania VA. 2013. Modulation of biotransformation systems and ABC transporters by benznidazole in rats. Antimicrob Agents Chemother 57:4894–4902. doi: 10.1128/AAC.02531-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Workman P, White RA, Walton MI, Owen LN, Twentyman PR. 1984. Preclinical pharmacokinetics of benznidazole. Br J Cancer 50:291–303. doi: 10.1038/bjc.1984.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts JT, Bleehen NM, Lee FY, Workman P, Walton MI. 1984. A phase I study of the combination of benznidazole and CCNU in man. Int J Radiat Oncol Biol Phys 10:1745–1748. doi: 10.1016/0360-3016(84)90541-8. [DOI] [PubMed] [Google Scholar]
- 31.Raaflaub J. 1980. Multiple-dose kinetics of the trypanosomicide benznidazole in man. Arzneimittelforschung 30:2192–2194. [PubMed] [Google Scholar]
- 32.Raaflaub J, Ziegler WH. 1979. Single-dose pharmacokinetics of the trypanosomide benznidazole in man. Arzneimittelforschung 29:1611–1614. [PubMed] [Google Scholar]
- 33.Food and Drug Administration. March 2014. Guidance for industry: bioavailability and bioequivalence studies submitted in NDAs or INDs—General considerations. Food and Drug Administration, Center for Drug Evaluation and Research, Silver Spring, MD. [Google Scholar]
- 34.Kararli TT. 1995. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos 16:351–380. doi: 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]