Abstract
The treatment of dermatophytoses, including onychomycosis, has come a long way over the past few decades with the introduction of oral antifungals (e.g., terbinafine and itraconazole). However, with these advancements in oral therapies come several undesirable effects, such as kidney and liver toxicity, along with drug-drug interactions. Consequently, there is a need for new topical agents that are effective against dermatophytosis. ME1111 is a topical antifungal under development. In this study, the in vivo efficacy of ME1111 was compared to that of ciclopirox in the topical treatment of dermatophytosis caused by Trichophyton mentagrophytes using a guinea pig model. Animals were treated with the topical antifungals starting at 3 days postinfection, with each agent being applied once daily for seven consecutive days. After the treatment period, the clinical and mycological efficacies were evaluated. The data showed that both antifungals demonstrated significant clinical and mycological efficacies; however, ME1111 showed clinical efficacy superior to that of ciclopirox (46.9% and 25.0%, respectively, with a P value of <0.001). The potent efficacy of ME1111 could be attributed to its properties, such as low keratin binding.
INTRODUCTION
Dermatophytoses, such as onychomycosis, are infections of the hair, nail, and skin caused principally by the Trichophyton, Epidermophyton, and Microsporum fungal genera. Two of the most common of these etiological agents of skin mycoses are Trichophyton rubrum and Trichophyton mentagrophytes. Though fungal skin infections caused by these organisms may not be life threatening, they are extremely persistent and result in considerable discomfort, affecting patients' quality of life. Current oral therapies (e.g., terbinafine and itraconazole) are associated with a number of adverse events and drug-drug interactions, making them less desirable. Aside from oral therapies, most topical therapies have been proven to be inadequate, due to their inability to penetrate the tough layers of the nail plate. Even with these advances, however, complete cure is often unattainable, as up to 15 to 20% of patients experience relapses (1). Therefore, the development of safer and more effective antifungal agents is needed.
2-(3,5-Dimethyl-1H-pyrazol-1-yl)-5-methylphenol (ME1111) is a member of a new class of compounds that has shown strong fungicidal properties in nonclinical studies and is a novel selective inhibitor of succinate dehydrogenase of Trichophyton species (2). ME1111 is a novel antidermatophytic drug discovered by Meiji Seika Pharma Co., Ltd. (Tokyo, Japan) through an optimization process directed at selecting compounds with (i) potent antidermatophyte activity, (ii) favorable physicochemical and nail permeation properties, and (iii) a small molecular size. Further research demonstrated that ME1111 is a first-in-class, low-molecular-weight compound with antifungal activity, mediated by the inhibition of succinate dehydrogenase (complex II), a critical enzyme involved in mitochondrial respiratory electron transfer (3).
ME1111 demonstrated potent in vitro efficacy against dermatophyte strains. Susceptibility testing showed that against T. rubrum, T. mentagrophytes, Trichophyton tonsurans, and Epidermophyton floccosum ME1111 demonstrated a range of MICs of <0.06 to 1 μg/ml. ME1111 has also shown efficacy against dermatophytes for which terbinafine or itraconazole MICs are elevated (4). Additionally, ME1111 has fungicidal activity against T. rubrum and T. mentagrophytes. In this study, we compared the clinical and mycological efficacies of a 10% ME1111 solution with those of ciclopirox (8%) for the topical treatment of T. mentagrophytes dermatophytosis in a guinea pig model.
MATERIALS AND METHODS
Laboratory animals.
Guinea pigs were chosen as the test subjects because, like humans, they are susceptible to dermatophytosis and their large body surface provides a sufficient area in which to perform experiments to determine clinical and mycological efficacies. All procedures in the protocol were in compliance with the guidelines of the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals (5), and the Office of Laboratory Welfare. The protocol for animal infection was approved by the Institutional Animal Care and Use Committee (IACUC) at the Case Western Reserve University School of Medicine, Cleveland, OH (protocol approval number 2011-0140). According to the protocol, male albino guinea pigs (Harlan-Sprague-Dawley, San Diego, CA) with a body weight of 450 to 500 g were housed in the Animal Resource Center. The environmental controls for the animal room were set to maintain a temperature of 16 to 22°C, a relative humidity of 30 to 70%, and a 12-h light and 12-h dark cycle. Experimental animals underwent an acclimation period for a minimum of 5 days prior to use. All procedures were performed while the animals were under general anesthesia, and all efforts were made to minimize animal suffering.
Fungal strain.
The guinea pig model has been used to evaluate the efficacies of various approved antifungals for the treatment of dermatophytosis. We selected T. mentagrophytes to infect the animals since this is a zoophilic fungus and it is able to infect the animals' skin, resulting in an inflammatory reaction of the skin and hair root invasion. In contrast, T. rubrum is an anthrophilic fungus and fails to cause infection. Moreover, T. mentagrophytes is responsible for human cutaneous infections, making it clinically relevant. Importantly, the strain of T. mentagrophytes that we used has repeatedly and consistently produced dermatophytosis with dramatic clinical features in our guinea pig model. To prepare the challenge inocula, petri dishes containing potato dextrose agar (PDA; Difco Laboratories, Detroit, MI) were seeded with T. mentagrophytes and incubated at 30°C for 5 to 7 days. At the end of this growth period, conidia were scraped from the plates with sterile cell scrapers (BD Falcon; BD Biosciences, Bedford, MA) in normal sterile saline (0.85% NaCl). A suspension of 1 × 107 conidia/100 μl was prepared fresh and used to challenge the animals in this study.
Antifungal agents.
ME1111 solution and its alcohol-based placebo solution were provided by Meiji Seika Pharma Co., Ltd. (Tokyo, Japan), while 8% ciclopirox (Penlac nail lacquer) was provided by the Center for Medical Mycology, Case Western Reserve University.
Animal inoculation and antifungal therapy.
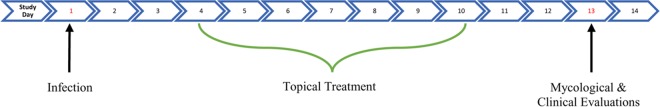
All animal challenge procedures were done while the animals were under general anesthesia. Each guinea pig was anesthetized with a 0.2-ml cocktail of ketamine, xylazine, and acepromazine (3:3:1; vol/vol/vol) administered intramuscularly. Using an electric shaver, an area 2 cm to the left of the midline on the animals' backs was clipped. A disposable razor was then used to obtain a closer shave of the area. Using a stencil and marking pen, a 2.5-cm by 2.5-cm2 outline was drawn on the shaved guinea pig skin. The marked areas were then abraded using sterile fine-grit sandpaper. A conidial suspension containing 1 × 107 T. mentagrophytes conidia in 100 μl of sterile normal saline was applied and rubbed thoroughly on the abraded skin using a sterile pipette tip. Infected guinea pigs were randomized into the following four groups (5 animals per group): a group receiving ME1111 solution, a group receiving ME1111 placebo solution, a group receiving 8% ciclopirox, and an infected untreated control group. Treatment with the test compounds began at 72 h postchallenge and continued once daily for the next 7 days (see the treatment schedule in Fig. 1). Each guinea pig in a treatment group received 0.2 ml applied topically to the infected area once daily using a sterile pipette tip. Infected untreated control guinea pigs did not receive any form of treatment.
FIG 1.
Study schedule.
Clinical and mycological evaluation of treatment efficacy.
Treated and control guinea pigs were examined daily throughout the course of the experiment. Clinical and mycological endpoints were used to determine the efficacies of the drugs tested. As can be seen in Fig. 1, clinical and mycological assessments were performed on study day 13, with the clinical assessment being carried out first. The hair samples were then uprooted and inoculated onto PDA plates for mycological assessment.
Clinical evaluation.
The infected area marked on the back of each animal was divided into four quadrants. The clinical assessment of local changes of the infected skin in each quadrant was scored on a scale of from 0 to 5, as described in reference 6. Specifically, 0 indicated no lesions, 1 indicated few slightly erythematous places on the skin, 2 indicated well-defined redness and swelling with bristling hairs, 3 indicated large areas of marked redness, incrustation, scaling, bald patches, and ulceration in places, 4 indicated partial damage to the integument and the loss of hair, and 5 indicated extensive damage to the integument and a complete loss of hair at the infection site. Scores from the quadrants were summed for each animal and used to determine the clinical efficacy of the different treatments. The clinical efficacy for each treatment group was expressed as a percentage relative to the result for the infected untreated control group using the following formula: 100 − [T × (100/K)], where T is the total score for the test group and K is the total score for the infected untreated control group. The total score for any treatment group signifies the average clinical score for animals in the same group.
Mycological evaluation.
The hair root invasion test was used to assess the mycological cure rate resulting from treatment with the test antifungals. Hair samples were removed with sterile forceps from the four quadrants. Ten uprooted hairs from each quadrant were planted onto the surface of PDA plates divided into quadrants corresponding to the quadrants on the skin of the animals. The plates were incubated at 30°C for 2 to 4 days. Following incubation, the number of hairs exhibiting fungal growth at the hair root was counted using a stereomicroscope. Counts from the quadrants were summed for each animal and used to determine the mycological efficacy of the different treatments. The effectiveness of a compound in reducing the number of fungus-positive hair samples per treatment group was expressed as a percentage relative to the result for the infected untreated group using the following formula: 100 − [T × (100/K)], where T is the total number of positive hairs in the test group and K is the total number of positive hairs in the infected untreated control group. The total score for any group denotes the average count of fungus-positive hairs obtained from the animals in the same group.
Statistical analysis.
A one-way analysis of variance with a post hoc Bonferroni test was used to determine whether there were statistically significant differences among the mean clinical scores and the mean fungus-positive hair counts of the treatment groups. A P value of <0.05 was considered significant.
RESULTS
Clinical assessment.
Infected guinea pigs were monitored daily for signs of infection. By study day 4 (72 h postinoculation), the animals showed scaling and redness of the infected area. As expected, the infected untreated control guinea pigs showed hair loss and ulcerated, scaly skin at the time of evaluation (study day 13). Table 1 shows the clinical efficacy of each test compound. The percent efficacies for the ME1111 solution and placebo solution were 46.9% and 10.4%, respectively. The percent efficacy for the comparator (8% ciclopirox) was 25.0%, which was significantly lower than that observed for the ME1111 solution (P < 0.001). Animals in all treatment groups were significantly improved in comparison to the animals in the infected untreated control group, with the P value for the group treated with the ME1111 solution being <0.001. Furthermore, the clinical efficacy of ME1111 was significantly better than that of the placebo and the ciclopirox treatment (P < 0.001).
TABLE 1.
Summary of clinical and mycological efficacies of test articles
| Treatment | Clinical efficacy |
Mycological efficacy |
||||
|---|---|---|---|---|---|---|
| % efficacy | Mean clinical score ± SD | P value compared to no treatment | % efficacy | Mean clinical score ± SD | P value compared to no treatment | |
| 10% ME1111 solution | 46.9 | 2.55 ± 0.51b | <0.001 | 83.7 | 0.95 ± 1.15a | <0.001 |
| Placebo solution | 10.4 | 4.30 ± 0.92 | 0.225 | 28.9 | 4.15 ± 2.13 | 0.039 |
| 8% ciclopirox lacquer | 25.0 | 3.60 ± 0.60 | <0.001 | 79.4 | 1.20 ± 1.01 | <0.001 |
| None (control) | 4.80 ± 0.41 | NAc | 5.83 ± 2.28 | NA | ||
P value of <0.001 compared to treatment with placebo solution.
P value of <0.001 compared to treatment with ciclopirox.
NA, not applicable.
Mycological assessment.
Table 1 shows the mycological efficacy of each test compound. The results for the guinea pigs in the infected untreated control group were as expected, having the highest average number of fungus-positive hairs. Percent efficacies for the ME1111 solution and placebo solution were 83.7% and 28.9%, respectively. The percent efficacy for 8% ciclopirox was 79.4%. The mycological efficacy for all treatment groups was significantly better than that for the infected untreated control group (P < 0.05 for all comparisons). Furthermore, the mycological efficacy of ME1111 was significantly better than that of the placebo (P < 0.001).
DISCUSSION
In this study, we assessed the clinical and mycological efficacies of a potential antifungal agent, ME1111, in the topical treatment of T. mentagrophytes dermatophytosis using a guinea pig model. In addition, 8% ciclopirox was used as a comparator along with an infected untreated control.
The data gathered from this study show that all treatments demonstrated statistically significantly better clinical and mycological efficacies than those achieved with the infected untreated control. Although all treatments showed similar clinical and mycological efficacies, the ME1111 treatment showed a significantly better clinical efficacy than the ME1111 placebo and 8% ciclopirox treatments, demonstrated by the persistence of a small number of slightly erythematous areas of the skin and a few bristling hairs. Statistical analysis of the clinical scores supported these observations. The lower efficacy of ciclopirox is in line with the clinical experience with this agent, where it showed modest efficacy in the treatment of nail infections (6% to 9%) and had a treatment failure rate of 61% to 64% after 48 weeks of use (7). The improved efficacy of ME1111 over that of 8% ciclopirox is most likely due to its physiochemical properties, such as its low molecular weight and low affinity to keratin (3). Ciclopirox is a topical antimycotic agent belonging to the chemical class of hydroxypyridones and is not related to azoles (8). Unlike currently available agents, ME1111 targets the succinate dehydrogenase (complex II) of dermatophyte species. Azoles (e.g., itraconazole) and allylamines (e.g., terbinafine) target different steps in the ergosterol biosynthetic pathway, while ciclopirox relies on its high affinity for trivalent metal cations, resulting in the inhibition of metal-dependent enzymes that are responsible for the degradation of peroxides within the fungal cell (9). Unlike invasive fungal infections, the pharmacokinetic/pharmacodynamic (PK/PD) relationship for superficial dermatophyte infection is less well understood. Drug concentrations in the infected tissue (e.g., skin for tinea pedis and the nail plate/bed for onychomycosis) are likely important components of a PK/PD relationship, and further study of ME1111 PKs/PDs is necessary.
Through the use of the guinea pig model, our group was able to demonstrate the clinical superiority of ME1111 to the placebo solution and ciclopirox. This finding is in agreement with the findings of an evaluation of the susceptibility of dermatophytes to ME1111 in vitro using the Clinical and Laboratory Standard Institutes reference method (M38-A2). The in vitro data showed that ME1111 had potent activity against 16 dermatophyte strains tested with elevated terbinafine or itraconazole MICs. Furthermore, the data showed that the MICs of ME1111 were equivalent to those of ciclopirox, with MIC90 values being in the range of 0.125 to 0.5 μg/ml (4).
In conclusion, this study shows that both ME1111 and ciclopirox have similar mycological efficacies. However, ME1111 demonstrated clinical efficacy superior to that of ciclopirox. This superiority may be due to the potent antifungal activity and the difference in keratin binding between the two drugs (which is low for ME1111 and very high for ciclopirox) (10). These findings warrant further investigation of ME1111 and its antifungal activity.
ACKNOWLEDGMENT
This work was supported by a grant from Meiji Seika Pharma Co., Ltd.
REFERENCES
- 1.Tosti A, Piraccini BM, Stinchi C, Colombo MD. 1998. Relapses of onychomycosis after successful treatment with systemic antifungals: a three-year follow-up. Dermatology 197:162–166. doi: 10.1159/000017990. [DOI] [PubMed] [Google Scholar]
- 2.Takahata S, Kubota N, Takei-Masuda N, Yamada T, Maeda M, Alshahni MM, Abe S, Tabata Y, Maebashi K. 2016. Mechanism of action of ME1111, a novel antifungal agent for topical treatment of onychomycosis. Antimicrob Agents Chemother 60:873–880. doi: 10.1128/AAC.01790-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabata Y, Takei-Masuda N, Kubota N, Takahata S, Ohyama M, Kaneda K, Lida M, Maebashi K. 2016. Characterization of antifungal activity and nail penetration of ME1111, a new antifungal agent for topical treatment of onychomycosis. Antimicrob Agents Chemother 60:1035–1039. doi: 10.1128/AAC.01739-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghannoum M, Isham N, Long L. 2015. In vitro antifungal activity of ME1111, a new topical agent for onychomycosis, against clinical isolates of dermatophytes. Antimicrob Agents Chemother 59:5154–5158. doi: 10.1128/AAC.00992-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 6.Ghannoum M, Long L, Pfister WR. 2009. Determination of the efficacy of terbinafine hydrochloride nail solution in the topical treatment of dermatophytosis in a guinea pig model. Mycoses 52:35–43. doi: 10.1111/j.1439-0507.2008.01540.x. [DOI] [PubMed] [Google Scholar]
- 7.Westerberg DP, Voyack MJ. 2013. Onychomycosis: current trends in diagnosis and treatment. Am Fam Physician 88:762–770. [PubMed] [Google Scholar]
- 8.Subissi A, Monti D, Togni G, Mailland F. 2010. Ciclopirox: recent nonclinical and clinical data relevant to its use as a topical antimycotic agent. Drugs 70:2133–2152. doi: 10.2165/11538110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Gupta AK, Skinner AR. 2003. Ciclopirox for the treatment of superficial fungal infections: a review. Int J Dermatol 42(Suppl 1):3–9. doi: 10.1046/j.1365-4362.2003.01712.x. [DOI] [PubMed] [Google Scholar]
- 10.Takei-Masuda N, Nagira Y, Kaneda K, Chikada T, Nomoto M, Tabata Y, Maebashi K. 2015. Potent antifungal activity of ME1111 against Trichophyton species in the presence of keratin, abstr F-742 Abstr 55th Intersci Conf Antimicrob Agents Chemother American Society for Microbiology, Washington, DC. [Google Scholar]