Abstract
In this study, we tested five compounds belonging to a novel series of piperazine arylideneimidazolones for the ability to inhibit the AcrAB-TolC efflux pump. The biphenylmethylene derivative (BM-19) and the fluorenylmethylene derivative (BM-38) were found to possess the strongest efflux pump inhibitor (EPI) activities in the AcrAB-TolC-overproducing Escherichia coli strain 3-AG100, whereas BM-9, BM-27, and BM-36 had no activity at concentrations of up to 50 μM in a Nile red efflux assay. MIC microdilution assays demonstrated that BM-19 at 1/4 MIC (intrinsic MIC, 200 μM) was able to reduce the MICs of levofloxacin, oxacillin, linezolid, and clarithromycin 8-fold. BM-38 at 1/4 MIC (intrinsic MIC, 100 μM) was able to reduce only the MICs of oxacillin and linezolid (2-fold). Both compounds markedly reduced the MIC of rifampin (BM-19, 32-fold; and BM-38, 4-fold), which is suggestive of permeabilization of the outer membrane as an additional mechanism of action. Nitrocefin hydrolysis assays demonstrated that in addition to their EPI activity, both compounds were in fact weak permeabilizers of the outer membrane. Moreover, it was found that BM-19, BM-27, BM-36, and BM-38 acted as near-infrared-emitting fluorescent membrane probes, which allowed for their use in a combined influx and efflux assay and thus for tracking of the transport of an EPI across the outer membrane by an efflux pump in real time. The EPIs BM-38 and BM-19 displayed the most rapid influx of all compounds, whereas BM-27, which did not act as an EPI, showed the slowest influx.
INTRODUCTION
The Escherichia coli AcrAB-TolC efflux pump is the best-characterized resistance-nodulation-cell division (RND) pump (1) and is capable of extruding a wide variety of structurally diverse compounds, encompassing many clinically administered antibiotics (e.g., beta-lactams, fluoroquinolones, and tetracyclines) (2). It is constitutively expressed under physiological conditions, and upon exposure to antibiotics, mutations in local or global regulator genes can occur, hence leading to overexpression of this efflux pump and to a multidrug resistance (MDR) phenotype (3).
To combat MDR, efflux pump inhibitors (EPIs) are an attractive option, and several EPIs that act against the AcrAB-TolC efflux pump have already been described in the literature (4–16), among which arylpiperazine and arylmorpholine derivatives constitute some of the largest systematically examined compound classes.
In this study, we set out to test five compounds belonging to a novel series of piperazine derivatives of arylideneimidazolones for the ability to inhibit the AcrAB-TolC efflux pump. Moreover, since they displayed several structural features reminiscent of fluorescent charge transfer complexes, we opted to test all of them in a fluorescence spectral scan of whole cells to establish whether these compounds could be used in membrane transport assays.
MATERIALS AND METHODS
Bacterial strains and culture media.
For the fluorescence and MIC assays described below, E. coli strain 3-AG100 (a multidrug-resistant mutant [gyrA mar] with acrB overexpression; obtained from E. coli K-12 strain AG100 after repeated exposure to a fluoroquinolone) (3) and the acrB deletion strain 3-AG100 ΔacrB (17) were used. The Pseudomonas aeruginosa PAO1 derivatives PA1426 (oprD::ΩTc; overproduces MexAB-OprM and was selected after exposure to carbenicillin) and PA1425 (oprD::ΩTc oprM::ΩHg) (18) were a generous gift from Thilo Köhler (Geneva, Switzerland) and were used in MIC assays to test if the novel compounds could also potentiate the activity of antibiotics in nonfermenting bacteria.
The acrB deletion strain KUN9180 ΔacrB was generated from the extended-spectrum beta-lactamase (ESBL)-expressing E. coli strain KUN9180 (a generous gift from Yasufumi Matsumura, Kyoto, Japan) by use of a Quick & Easy E. coli gene deletion kit (Red/ET recombination) from Gene Bridges (Heidelberg, Germany) according to the manufacturer's instructions.
The strains were cultivated in either LB broth (1% tryptone, 0.5% yeast extract, and 1% NaCl) (for fluorescence assays) or Mueller-Hinton broth (for MIC microdilution assays). Details are given below.
Synthesis of piperazine arylideneimidazolones.
The piperazine arylideneimidazolones BM-9, BM-19, BM-27, BM-36, and BM-38 (Table 1) were synthesized according to the detailed information given in the supplemental material.
TABLE 1.
Basic properties of piperazine arylideneimidazolones
a Measured in an E. coli 3-AG100 cell suspension (OD600 = 0.25). —, no excitation or emission maximum is given because the fluorescence intensity upon 3-AG100 dye loading was found to be very low, and no marked difference between the deenergized and energized states could be detected.
Briefly, the final compounds were obtained with a 3- or 4-step synthesis route including (i) Knoevenagel condensation of 2-thiohydatoin with appropriate aromatic aldehydes, (ii) S-methylation processing, and (iii) condensation with suitable piperazines [1-(2-hydroxyethyl)piperazine (BM-9 and BM-27) or 1-acetylpiperazine (acetyl-protected forms of BM-19, BM-36, and BM-38)]. The deacetylation of the protected compounds BM-19, BM-36, and BM-38 was accomplished by acid hydrolysis. All five compounds were converted into hydrochlorides by saturation with gaseous HCl. The following commercial chemical compounds were used for the syntheses: 2-thiohydantoin (Sigma-Aldrich, Poznań, Poland), biphenyl-4-carboxaldehyde (Sigma-Aldrich, Poznań, Poland), fluorene-2-carboxaldehyde (Sigma-Aldrich, Poznań, Poland), naphthalene-2-carboxaldehyde (Sigma-Aldrich, Poznań, Poland), methyl iodide (Sigma-Aldrich, Poznań, Poland), 1-(2-hydroxyethyl)piperazine (Sigma-Aldrich, Poznań, Poland), and 1-acetylpiperazine (Alfa-Aesar, Chemat, Gdańsk, Poland). The purity and identity of the synthesized compounds were evaluated by the use of 1H nuclear magnetic resonance (1H-NMR), infrared (IR) spectroscopy, elemental analysis, thin-layer chromatography (TLC), and melting point measurements.
The synthesis and characteristics of compound BM-9 and its intermediates were described previously (19–23). Chemical procedures for and characteristics of BM-19, BM-27, BM-36, and BM-38 are presented in the supplemental material.
Other chemicals.
Carbonyl cyanide m-chlorophenylhydrazone (CCCP), phenyl-arginine-β-naphthylamide (PAβN), and Nile red were obtained from Sigma-Aldrich (Taufkirchen, Germany). Nitrocefin was ordered from Cayman Chemical (Ann Arbor, MI).
Susceptibility testing.
The MICs of a range of antimicrobial agents in the presence and absence of the various compounds were determined in a 96-well microtiter plate by using strains incubated overnight at 37°C at 100 μl/well, using a final inoculum of 5 × 105 CFU/ml, by a standard Mueller-Hinton broth microdilution procedure in accordance with the CLSI M07-A9 guidelines (24). MIC testing was done in triplicate. Custom 96-well microtiter plates containing selected antimicrobials at increasing concentrations were purchased from Merlin Diagnostics (Bornheim, Germany).
Nitrocefin hydrolysis assays.
To determine whether BM-19 and BM-38 are capable of permeabilizing the outer membrane of E. coli, ESBL strain KUN9180 ΔacrB was cultivated in LB broth, centrifuged for 8 min at room temperature (RT) and 4,000 rpm, washed twice in phosphate-buffered saline (PBS), and then resuspended in PBS containing 0.4% glucose, with or without 1 mM MgCl2, until an optical density at 600 nm (OD600) of 0.5 was reached. Thereafter, nitrocefin (final concentration, 32 μg/ml) was added to the bacterial suspension in the absence or presence of BM-19, BM-38, or PAβN (final concentration, 50 μM), and nitrocefin hydrolysis was monitored spectrophotometrically (increase in absorbance at 490 nm) by using an Infinite 200Pro (Tecan, Crailsheim, Germany) 96-well plate reader.
Nile red efflux assay in the absence and presence of the piperazine arylideneimidazolone EPIs.
The protocol for the Nile red efflux assay has been published previously (25), and all procedures were carried out accordingly. Briefly, the cells were cultivated overnight in LB broth to deenergize them. After a washing step, the cells were resuspended in potassium phosphate buffer, and 15 min after the addition of 5 μM CCCP, the desired piperazine arylideneimidazolone was added at a standard concentration of 50 μM to screen for activity in the preliminary experiments. Nile red efflux was initiated by addition of glucose. If an effect on Nile red efflux was observed, the compounds were retested at different concentration ranges from threshold activity to complete abolishment of dye efflux. All assays were carried out at least in triplicate. Since the piperazine arylideneimidazolone efflux experiments demonstrated that these compounds were properly retained within the cell envelope in the deenergized state, we did not add them to the cell suspension after the washing step. In the upper concentration range, quenching phenomena of various degrees were observed and were compensated for by adjusting the preenergization fluorescence intensity to 100 relative fluorescence units. Prior to the experiments, it was established that no compound displayed any considerable autofluorescence with the bacterial cells by using the Nile red excitation and emission wavelengths.
Preparation of bacterial cells for fluorescence assays with the piperazine arylideneimidazolones.
A single bead of the 3-AG100 −80°C frozen stock (maintained in Cryobank vials from Mast Diagnostica GmbH, Reinfeld, Germany) was added directly to 20 ml of LB broth in an Erlenmeyer flask and grown on a shaker (200 rpm, 37°C) for 16 to 18 h. Thereafter, a 10-ml portion of the culture was centrifuged at 4,000 rpm for 5 min at room temperature. The pellet was resuspended in 20 mM potassium phosphate buffer (pH 7.0) containing 1 mM MgCl2 (PPB). After another centrifugation and resuspension step, the cells were adjusted to an OD600 of 0.25 in PPB.
Fluorescence spectral scans of the piperazine arylideneimidazolones in the presence of strain 3-AG100.
A 3-AG100 cell suspension was prepared as described above, and 2 ml was transferred to a glass cuvette in a PerkinElmer (Waltham, MA) LS55 spectrofluorimeter. CCCP (final concentration, 10 μM) and the respective piperazine arylideneimidazolone (final concentration, 10 μM) were added and allowed to rest for 15 min at room temperature. The mixture was stirred with a magnetic stirrer.
A fluorescence spectral scan was performed using excitation wavelengths of 200 to 420 nm, with 10-nm steps. The emission spectrum was recorded from 200 to 900 nm. Thereafter, the spectral scan was repeated for the energized state after addition of glucose (final concentration, 50 mM) to the cell suspension.
Combined real-time influx and efflux assay of BM-19, BM-27, BM-36, and BM-38.
A cell suspension was prepared as described above, and 2 ml was transferred to a glass cuvette in a PerkinElmer LS55 spectrofluorimeter. The mixture was stirred with a magnetic stirrer.
The fluorescence intensity was recorded over a time course of 2,000 s, using the wavelength settings given in Table 1. CCCP was added to a final concentration of 5 μM at 50 s. BM-19, BM-27, BM-36, or BM-38 was added to a final concentration of 5 μM at 500 s. At 1,500 s, efflux of the respective dye was triggered by the addition of glucose (final concentration, 50 mM). All assays were carried out at least in triplicate.
Modified real-time efflux assay of BM-19, BM-27, BM-36, and BM-38.
Alternatively, the piperazine arylideneimidazolone real-time efflux assay was performed as described previously for the fluorescent membrane probe 1,2′-dinaphthylamine (1,2′-DNA) (26). Briefly, after loading of the deenergized cells with the dye, the cells were centrifuged and washed once in PPB to remove CCCP and the dye from the extracellular PPB. Thereafter, the fluorescence intensity was recorded and glucose was added for energization as described above.
Cytotoxicity assays.
Human liver carcinoma HepG2 cell viability after 72 h of incubation with the piperazine arylideneimidazolones and the reference cytostatic drug doxorubicin was determined by performing the CellTiter 96 AQueous nonradioactive cell proliferation assay (Promega GmbH, Mannheim, Germany).
Cells were seeded in a 96-well plate at 75 cells/μl in a total volume of 200 μl of medium 24 h prior to the experiment. On the next day, the cell culture medium was removed, and fresh medium was placed in the microplate wells. The piperazine arylideneimidazolones were then added to final concentrations of 0.03 to 100 μM for BM-9 and 0.3 to 100 μM for the other compounds, whereas doxorubicin was tested at the concentration range of 0.01 to 10 μM. The dimethyl sulfoxide (DMSO) concentration used was 1%.
After the incubation period, the medium containing the piperazine arylideneimidazolones or doxorubicin was removed, and 120 μl of fresh medium containing 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) dye–phenazine methosulfate solution was added to each well. After 4 h of further incubation, the absorbance at 490 nm was measured directly from 96-well assay plates. Wells containing no cells were used as blank samples, whereas wells containing 1% DMSO were taken as nontreated controls (fully viable cells).
RESULTS
Antimicrobial activity of piperazine arylideneimidazolones in E. coli and P. aeruginosa strains overproducing or lacking a major RND efflux pump.
The MICs of the various compounds for E. coli and P. aeruginosa strains are given in Table 2. BM-38 was found to be the compound with the lowest MIC for the AcrAB-TolC-overproducing E. coli strain 3-AG100 (100 μM), whereas BM-9 and BM-27 displayed MICs of >1,600 μM. Both P. aeruginosa strains displayed the same MIC (400 μM) for BM-19 and BM-38.
TABLE 2.
MICs of piperazine arylideneimidazolones
| Strain | Compound MIC (μM)a |
||||
|---|---|---|---|---|---|
| BM-9 | BM-19 | BM-27 | BM-36 | BM-38 | |
| 3-AG100 | >1,600 | 200 | >1,600 | 800 | 100 |
| 3-AG100 ΔacrB | >1,600 | 100 | >1,600 | 400 | 50 |
| PA1425 | ND | 400 | ND | ND | 400 |
| PA1426 | ND | 400 | ND | ND | 400 |
ND, not done.
BM-19 and BM-38 are weak permeabilizers of the outer membrane in an acrB-deficient E. coli strain.
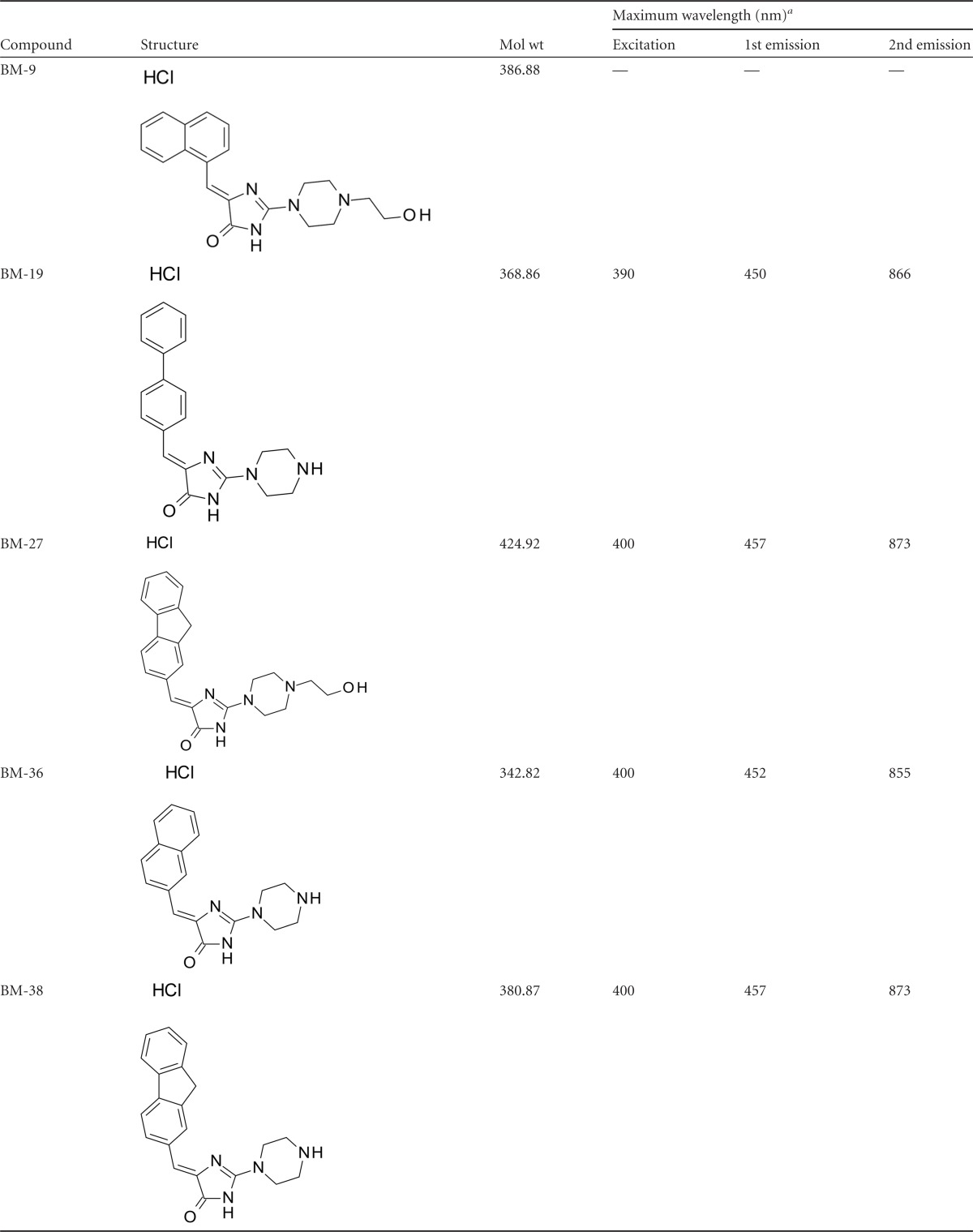
BM-19 and BM-38 (50 μM) were found to weakly permeabilize the outer membrane of E. coli ESBL strain KUN9180 ΔacrB as determined by a nitrocefin hydrolysis assay with an absence of MgCl2 in the cell suspension (Fig. 1A). BM-19's effect was slightly more pronounced than that of BM-38. PAβN, however, was found to markedly enhance nitrocefin hydrolysis in a MgCl2-free cell suspension, as described previously (13). When the experiments were carried out with medium containing 1 mM MgCl2, the nitrocefin hydrolysis curves for all three EPIs approached the no-EPI control curve (Fig. 1B).
FIG 1.
(A) Impacts of PAβN, BM-19, and BM-38 on outer membrane permeabilization of E. coli KUN9180 ΔacrB as measured by nitrocefin hydrolysis (OD490) in the absence of MgCl2. (B) Impacts of PAβN, BM-19, and BM-38 on outer membrane permeabilization of E. coli KUN9180 ΔacrB as measured by nitrocefin hydrolysis (OD490) in the presence of 1 mM MgCl2.
BM-19 and BM-38 inhibit Nile red efflux in the acrAB-overexpressing strain 3-AG100.
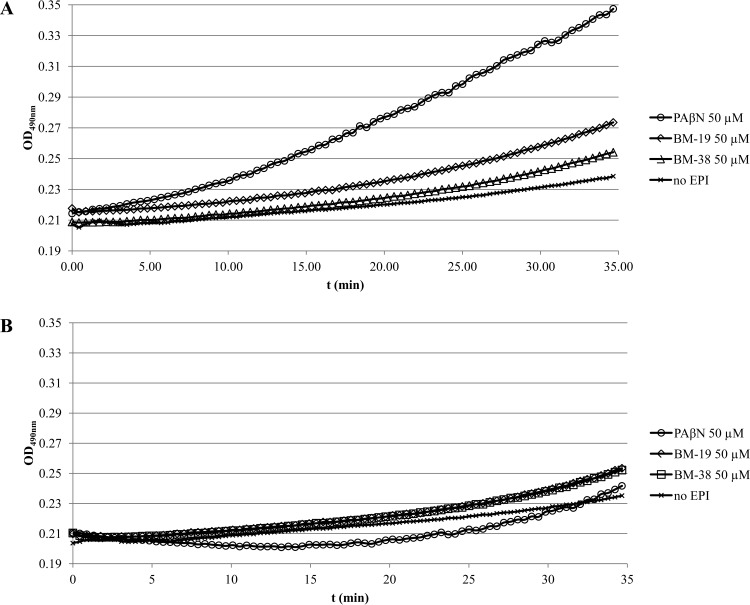
BM-9, BM-27, and BM-36 had no impact on Nile red efflux at concentrations of up to 50 μM. However, it was found that both BM-19 and BM-38 inhibited Nile red efflux in a dose-dependent manner (Fig. 2A and B). The first effects were seen at 25 μM, and efflux was completely abolished at 100 μM.
FIG 2.
(A) Dose-dependent inhibition of real-time Nile red efflux by BM-38 in the acrAB-overexpressing strain 3-AG100. Energization was initiated with 50 mM glucose at 50 s. The preenergization fluorescence intensity was adjusted to 100 relative fluorescence units. (B) Dose-dependent inhibition of real-time Nile red efflux by BM-19 in the acrAB-overexpressing strain 3-AG100. Energization was initiated with 50 mM glucose at 50 s. The preenergization fluorescence intensity was adjusted to 100 relative fluorescence units.
BM-19 and BM-38 potentiate the activity of various antibiotics in E. coli but have very poor activity in P. aeruginosa.
Since BM-9, BM-27, and BM-36 had no impact on E. coli Nile red efflux at concentrations of up to 50 μM (see above), we opted to examine only BM-19 and BM-38 for the ability to sensitize our E. coli 3-AG100 strain to various antibiotics. All results are given in Table 3. BM-38 was found to possess the best antimicrobial activity (MIC for 3-AG100, 100 μM), as described above, but displayed only relatively weak potentiating activity. At 1/4 MIC, BM-38 reduced the MIC of rifampin 4-fold and the MICs of oxacillin and linezolid 2-fold, but it had no effect on the MICs of the other tested antibiotics, even when the concentration of BM-38 was raised to 1/2 MIC.
TABLE 3.
Effects of the EPIs BM-19 and BM-38 on various antibiotic MICs
| Strain | MIC (mg/liter)a |
||||||
|---|---|---|---|---|---|---|---|
| Levofloxacin | Tetracycline | Chloramphenicol | Rifampin | Oxacillin | Linezolid | Clarithromycin | |
| 3-AG100 without EPI | 1 | 4 | 16 | 16 | 512 | 512 | 128 |
| 3-AG100 + 25 μM BM-19 | 1 | 4 | 16 | 16 | 512 | 256 | 128 |
| 3-AG100 + 50 μM BM-19 (1/4 MIC) | 0.13 | 1 | 2 | 0.5 | 64 | 64 | 16 |
| 3-AG100 + 25 μM BM-38 (1/4 MIC) | 1 | 4 | 8 | 4 | 256 | 256 | 128 |
| 3-AG100 + 50 μM BM-38 | 1 | 2 | 8 | 2 | 256 | 256 | 256 |
| 3-AG100 ΔacrB without EPI | 0.03 | 0.25 | 1 | 8 | 1 | 16 | 1 |
| 3-AG100 ΔacrB + 25 μM BM-19 | 0.03 | 0.13 | 1 | 1 | 1 | 16 | 0.5 |
| 3-AG100 ΔacrB + 50 μM BM-19 | 0.03 | ≤0.06 | 1 | 0.13 | ≤0.5 | 8 | 1 |
| 3-AG100 ΔacrB + 25 μM BM-38 | 0.03 | ≤0.06 | 1 | 0.13 | ≤0.5 | 8 | ≤0.5 |
| PA1426 without EPI | 1 | 256 | 128 | 32 | 2,048 | 2,048 | 256 |
| PA1426 + 100 μM BM-19 (1/4 MIC) | 2 | 256 | 128 | 16 | 2,048 | 2,048 | 256 |
| PA1426 + 100 μM BM-38 (1/4 MIC) | 2 | 256 | 128 | 16 | 2,048 | 2,048 | 256 |
| PA1425 without EPI | 0.06 | 16 | 2 | 16 | 64 | 64 | 16 |
| PA1425 + 100 μM BM-19 (1/4 MIC) | 0.06 | 16 | 2 | 16 | 128 | 32 | 8 |
| PA1425 + 100 μM BM-38 (1/4 MIC) | 0.06 | 16 | 2 | 16 | 64 | 32 | 8 |
MICs which differed at least 4-fold in the presence of BM-19 or BM-38 from the MIC of the AcrAB-TolC-overproducing strain 3-AG100 in the absence of these compounds are given in bold.
However, BM-19, which possessed less antimicrobial activity (MIC for 3-AG100, 200 μM), was found at 1/4 MIC to potentiate the antimicrobial activity of all of the tested antibiotics—it reduced the MIC of rifampin 32-fold, the MICs of levofloxacin, chloramphenicol, oxacillin, linezolid, and clarithromycin 8-fold, and the MIC of tetracycline 4-fold.
The acrB deletion strain 3-AG100 ΔacrB demonstrated dose-dependent MIC reductions with both piperazine arylideneimidazolones and most tested antibiotics, except for levofloxacin and chloramphenicol.
Finally, we carried out potentiating assays with our two P. aeruginosa strains, but we found very poor activity (2-fold MIC reductions for rifampin in PA1426 and 2-fold MIC reductions for linezolid and clarithromycin in PA1425).
Piperazine arylideneimidazolones act as fluorescent membrane probes and are extruded by the AcrAB-TolC efflux pump of 3-AG100.
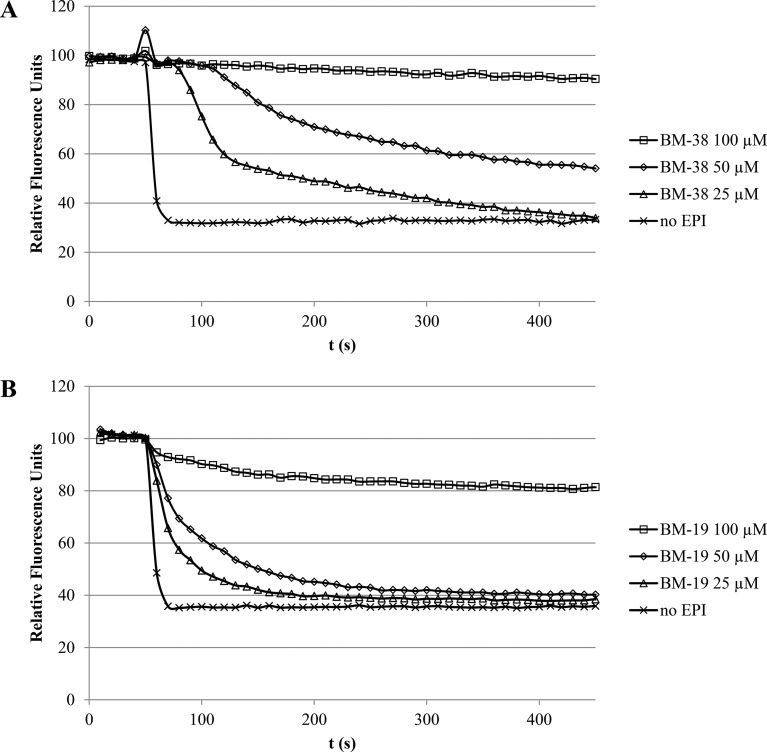
With the exception of BM-9 (which also displayed only a weak fluorescence signal upon dye loading), every compound produced different spectra in the deenergized and energized states for whole cells and possessed a second emission maximum in the near-infrared range. The BM-27 spectral scans are depicted in Fig. 3.
FIG 3.
Emission spectra of BM-27 (10 μM) in a bacterial suspension of 3-AG100 (OD600 = 0.25) in the deenergized and energized states. The excitation wavelength was 400 nm.
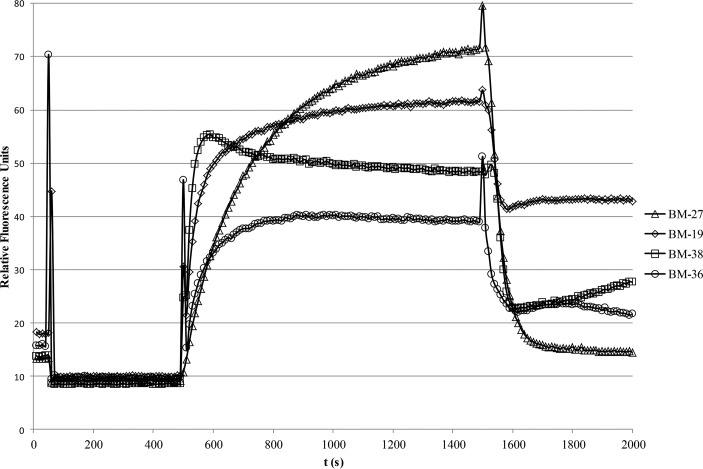
The optimal excitation and emission wavelengths for the individual dyes are given in Table 1. Using these data, combined influx and efflux assays could be performed. Optimization experiments determined that a CCCP concentration of 5 to 10 μM and a dye concentration of 5 to 10 μM gave the highest ratio between fluorescence intensities in the dye-loaded deenergized state and the energized state (data not shown). Of all the compounds tested, BM-27 was found to be the dye which showed the most dramatic drop in fluorescence intensity after energization of the cells (Fig. 4). The other compounds demonstrated more moderate but still clearly visible drops in fluorescence intensity after the onset of efflux.
FIG 4.
Combined real-time influx and efflux assays using the different piperazine arylideneimidazolones as fluorescent membrane probes and the acrAB-overexpressing strain 3-AG100. CCCP (5 μM) was added to deenergized cells at 50 s, 5 μM dye was added at 500 s, and efflux was triggered by addition of 50 mM glucose at 1,500 s. Identical conditions were used for all dyes (OD600 of 0.25, near-infrared emission maximum as given in Table 1, and slit width of 10 nm).
The influx rates differed considerably between the dyes, in the following order (from fast influx to slow influx): BM-38 > BM-19 > BM-36 > BM-27.
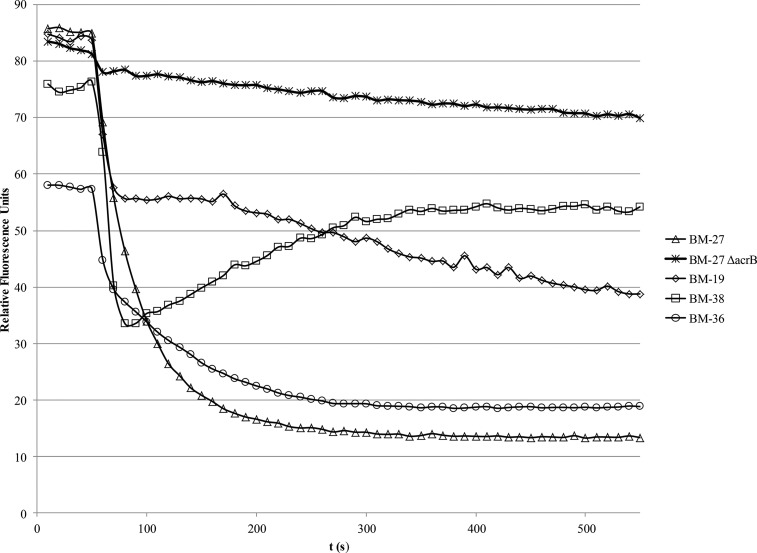
Using CCCP concentrations of around 5 μM for the combined influx and efflux assays, efflux could easily be triggered by the addition of 50 mM glucose. By use of a more classical efflux assay in which most of the CCCP was removed by a washing step before energization, it could be demonstrated that the dyes were easily retained in the cell envelope, as demonstrated previously with the dyes Nile red (25) and 1,2′-DNA (26). The washing step led to a moderate acceleration of dye efflux (Fig. 5) compared with that in the combined influx and efflux assay, in which small amounts of CCCP were still present in the buffer. Dye efflux was almost completely abolished in the ΔacrB strain.
FIG 5.
Real-time efflux assays using the different piperazine arylideneimidazolones as fluorescent membrane probes and the acrAB-overexpressing strain 3-AG100. After dye loading for 30 min, the deenergized cells were washed once in PPB, and efflux was triggered by addition of 50 mM glucose at 50 s. Identical conditions were used for all dyes (OD600 of 0.25, near-infrared emission maximum as given in Table 1, and slit width of 10 nm). The 3-AG100 ΔacrB strain demonstrated that BM-27 efflux in the 3-AG100 strain is mediated mainly by the AcrAB-TolC pump.
Cytotoxicity of piperazine arylideneimidazolones.
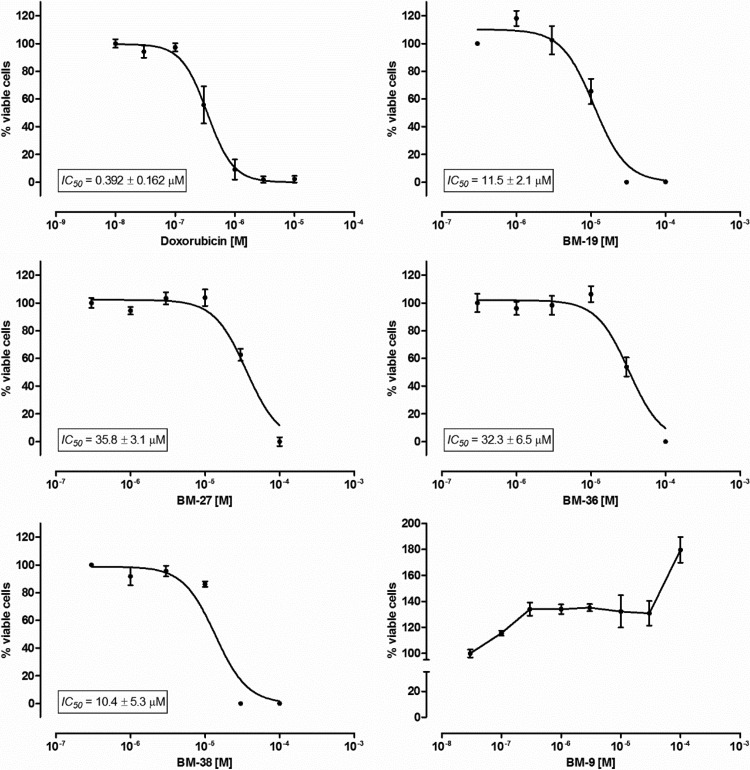
The evaluated compounds showed moderate to weak antiproliferative activity toward the human liver carcinoma cell line HepG2 (Fig. 6). The 50% inhibitory concentrations (IC50s) determined for the compounds were between 25-fold and 90-fold higher than the IC50 for the reference cytotoxic drug doxorubicin. Nevertheless, cytotoxicity was demonstrated for BM-19 and BM-38 at concentrations used for efflux pump inhibition (25 to 50 μM).
FIG 6.
HepG2 cell viability after 72 h of incubation with the indicated compounds, using the CellTiter 96 AQueous nonradioactive cell proliferation assay.
One of the tested compounds, BM-9, dose-dependently stimulated the growth of the evaluated cell line.
DISCUSSION
The novel piperazine arylideneimidazolones BM-19 and BM-38 were found to act as EPIs in a Nile red efflux assay using an AcrAB-TolC-overproducing E. coli strain. In line with these findings, potentiating activity was observed with certain antibiotics in an MIC microdilution assay.
However, BM-38's activity as an EPI was overshadowed by its relatively potent intrinsic antimicrobial activity (MIC of 100 μM, in contrast to 200 μM for BM-19). In a comparison of both compounds at 1/4 the respective intrinsic MICs, BM-19 (which displayed less antimicrobial activity than BM-38) showed all the characteristics of a broad-spectrum EPI, namely, it reduced the MICs of almost all tested antibiotics 4- to 8-fold, coming close to but not reaching the MICs of the ΔacrB strain. Rifampin was the only exception, since BM-19 reduced its MIC 32-fold, and thus below the MIC of the ΔacrB strain, suggesting that this compound might possess additional mechanisms of action (e.g., permeabilization of the outer membrane). A similar but somewhat weaker effect was found with BM-38 and rifampin. To determine if the potentiation of rifampin activity could be due to permeabilization of the outer membrane (an effect already described for the model EPI PAβN [13]), we carried out standard nitrocefin hydrolysis assays and indeed showed that both BM-19 and BM-38 are permeabilizers in MgCl2-free medium, albeit much weaker than PAβN, and that the outer membrane can withstand this action by addition of 1 mM MgCl2.
Besides the good potentiation of rifampin activity, BM-38 at 1/4 the intrinsic MIC affected only the MICs of oxacillin and linezolid (2-fold) in 3-AG100. Even raising the concentration to 1/2 the intrinsic MIC (and thus to the same maximum concentration used in the BM-19 assay) only moderately increased the antimicrobial activity of oxacillin and linezolid and failed to demonstrate any activity with levofloxacin and clarithromycin. Like the model piperazine EPI 1-(1-naphthylmethyl)-piperazine (NMP), our novel piperazine arylideneimidazolones had very poor EPI activity in P. aeruginosa, probably due to the different outer membrane structure of this species, with greatly reduced influx rates.
Considering the structural properties of the five arylideneimidazolones investigated, BM-19, BM-36, and BM-38 represent an amphiphilic group, with an unsubstituted positive ionizable piperazine nitrogen at position 2 of imidazolone, placed opposite to the bulky double-aromatic–methylidene system at position 5. Both BM-19 and BM-38 display a more extended aromatic area than the closely fused naphthalene rings of BM-36. However, a difference in the flexibility of the five aromatic moieties of both active compounds is seen. In the case of BM-19, the single bond between both phenyl rings of the biphenyl gives some rotary freedom, which might facilitate a good fit within the AcrB binding pocket. The fluorene aromatic moiety of BM-38 includes an additional methylene bridge between the two phenyl rings, which blocks the freedom of mutual rotation of the rings in comparison to that of BM-19. Thus, the five-aromatic area of compound BM-38 creates a spatial hindrance of the fused triple rings that might significantly affect the fit within the AcrB binding pocket.
We therefore hypothesize that the more rigid structure of BM-38 may make it more difficult for this compound to interact with different target sites within the AcrB binding pocket and might thus affect the transport of fewer substrates.
Although BM-19 appears to be a broad-spectrum EPI, it is definitely not an “ideal EPI,” such as NMP (6), which would reduce the substrate MICs only in an AcrAB-TolC-overproducing strain, not in the respective efflux-deficient strain.
In contrast, both BM-19 and BM-38 were found to markedly potentiate the activity of the tested antibiotics in the ΔacrB strain, suggesting the occurrence of additional mechanisms, such as—albeit moderate—permeabilization of the outer membrane.
Moreover, we serendipitously discovered that most of the piperazine arylideneimidazolones are also environment-sensitive fluorescent membrane probes as well as substrates of the AcrAB-TolC efflux pump. Some of them, especially BM-27, can thus be used excellently for real-time efflux assays, in which cells are loaded with a dye in the deenergized state and efflux is triggered upon energization of the cells. Like 1,2′-DNA (26), the piperazine arylideneimidazolones are capable of emission in the near-infrared range of the electromagnetic spectrum, where the signal-to-noise ratio for biological samples is typically high due to low autofluorescence. In contrast to the highly hydrophobic dyes 1,2′-DNA and Nile red, however, this new group of dyes is readily water soluble, and thus no organic solvents which may adversely affect dye efflux have to be used. Moreover, hydrophobicity may pose problems with adhesion to reaction vessels, thus rendering use in a reproducible influx assay almost impossible. Due to their amphiphilic nature, the piperazine arylideneimidazolones are membrane probes which can readily be used in combined influx and efflux assays.
Interestingly, compounds such as BM-19 and BM-38 can play multiple roles in assessing and modifying transport of substrates across the outer membrane. First, they can be used as EPIs to block the transport of other substrates (as described above using the Nile red efflux assay). Second, their own transport across the outer membrane by an RND efflux pump can be monitored in real time due to their fluorescent membrane probe characteristics. Third, these compounds exert intrinsic antimicrobial activity and thus allow one to relate their MICs to influx and efflux characteristics in a given strain. Researchers typically compare MIC ratios established for RND pump overproducers versus an RND pump-deficient strain to assess whether a given substrate is a good or poor substrate of an RND efflux pump. However, without knowledge of influx rates, MIC ratios tell us nothing about the corresponding efflux rates. In fact, a compound may be an excellent substrate of an RND efflux pump, but due to its high influx rate, the pump may simply be overwhelmed by substrate accumulation, leading to no or very little difference in MICs, as demonstrated previously in a study on beta-lactam efflux (27).
Based on the MIC ratio between the AcrAB-TolC-overproducing 3-AG100 strain (MIC = 100 μM) and the ΔacrB strain (MIC = 50 μM)—which is only 2—one would conclude that BM-38 is a poor substrate of this pump. However, the real-time efflux assay clearly demonstrated that this compound is in fact an excellent substrate. As explained above, the influx of BM-38 seems to be quite fast (although this compound seemed to be a weaker permeabilizer than BM-19 in the nitrocefin assays) relative to the efflux rates, and thus even a highly active efflux pump may not be capable of pumping out enough substrate to markedly increase the MIC. In fact, since piperazine arylideneimidazolone efflux assays can easily be combined with an influx assay, it was found that influx in deenergized cells occurs quite rapidly with BM-38 (more than 90% dye accumulation reached about 100 s into an experiment), whereas it takes much longer (up to 1,000 s) with BM-27, which is not an EPI. Hence, overwhelming the efflux pump by rapid influx of a substrate may be a good strategy to outcompete at least a subset of xenobiotics that use similar routes and recognition sites across the pump protein.
Unfortunately, as described previously (25, 26), the concentration-signal intensity relationship for substrates used in fluorescence-based real-time efflux assays is not linear, so Michaelis-Menten kinetics cannot easily be derived to establish whether EPIs inhibit a pump protein in a competitive manner or not. However, it might be possible to determine what causes the nonlinearity observed (e.g., self-quenching) and to compensate for this phenomenon by using a numerical model.
To conclude, although BM-19 and BM-38 are cytotoxic to eukaryotic cells at the concentrations used for EPI activity (Fig. 6), they are interesting lead structures for synthesis of less cytotoxic compounds in the future. Moreover, the unique combination of fluorescent, EPI, and antimicrobial properties makes them interesting basic research tools for elucidating molecular mechanisms of efflux pump inhibition, e.g., by random or site-directed mutagenesis of substrate binding pockets and subsequent fluorescent substrate/EPI transport assays.
Supplementary Material
ACKNOWLEDGMENTS
We thank Beata Mastek and Karolina Wyrzuc for participation in the chemical syntheses.
This work was supported in part by the Innovative Medicines Initiative (IMI) joint undertaking project Translocation (http://www.translocation.eu; funded by contributions from the European Union's Seventh Framework Program and EFPIA companies) and by a grant (to W.V.K.) from the German Federal Ministry of Education and Research (grant BMBF 01KI9951). The chemical syntheses were partly supported by Polish statutory research program K/ZDS/005593 and by the European Cooperation in Science and Technology (COST) grant 501/N-COST/2009/0 (COST BM0701).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01995-15.
REFERENCES
- 1.Ruggerone P, Murakami S, Pos KM, Vargiu AV. 2013. RND efflux pumps: structural information translated into function and inhibition mechanisms. Curr Top Med Chem 13:3079–3100. doi: 10.2174/15680266113136660220. [DOI] [PubMed] [Google Scholar]
- 2.Nikaido H, Pages JM. 2012. Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol Rev 36:340–363. doi: 10.1111/j.1574-6976.2011.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kern WV, Oethinger M, Jellen-Ritter AS, Levy SB. 2000. Non-target gene mutations in the development of fluoroquinolone resistance in Escherichia coli. Antimicrob Agents Chemother 44:814–820. doi: 10.1128/AAC.44.4.814-820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohnert JA, Schuster S, Kern WV. 2013. Pimozide inhibits the AcrAB-TolC efflux pump in Escherichia coli. Open Microbiol J 7:83–86. doi: 10.2174/1874285801307010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohnert JA, Szymaniak-Vits M, Schuster S, Kern WV. 2011. Efflux inhibition by selective serotonin reuptake inhibitors in Escherichia coli. J Antimicrob Chemother 66:2057–2060. doi: 10.1093/jac/dkr258. [DOI] [PubMed] [Google Scholar]
- 6.Bohnert JA, Kern WV. 2005. Selected arylpiperazines are capable of reversing multidrug resistance in Escherichia coli overexpressing RND efflux pumps. Antimicrob Agents Chemother 49:849–852. doi: 10.1128/AAC.49.2.849-852.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whalen KE, Poulson-Ellestad KL, Deering RW, Rowley DC, Mincer TJ. 2015. Enhancement of antibiotic activity against multidrug-resistant bacteria by the efflux pump inhibitor 3,4-dibromopyrrole-2,5-dione isolated from a Pseudoalteromonas sp. J Nat Prod 78:402–412. doi: 10.1021/np500775e. [DOI] [PubMed] [Google Scholar]
- 8.Opperman TJ, Kwasny SM, Kim HS, Nguyen ST, Houseweart C, D'Souza S, Walker GC, Peet NP, Nikaido H, Bowlin TL. 2014. Characterization of a novel pyranopyridine inhibitor of the AcrAB efflux pump of Escherichia coli. Antimicrob Agents Chemother 58:722–733. doi: 10.1128/AAC.01866-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aparna V, Dineshkumar K, Mohanalakshmi N, Velmurugan D, Hopper W. 2014. Identification of natural compound inhibitors for multidrug efflux pumps of Escherichia coli and Pseudomonas aeruginosa using in silico high-throughput virtual screening and in vitro validation. PLoS One 9:e101840. doi: 10.1371/journal.pone.0101840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garvey MI, Rahman MM, Gibbons S, Piddock LJ. 2011. Medicinal plant extracts with efflux inhibitory activity against Gram-negative bacteria. Int J Antimicrob Agents 37:145–151. doi: 10.1016/j.ijantimicag.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 11.Zeng B, Wang H, Zou L, Zhang A, Yang X, Guan Z. 2010. Evaluation and target validation of indole derivatives as inhibitors of the AcrAB-TolC efflux pump. Biosci Biotechnol Biochem 74:2237–2241. doi: 10.1271/bbb.100433. [DOI] [PubMed] [Google Scholar]
- 12.Piddock LJ, Garvey MI, Rahman MM, Gibbons S. 2010. Natural and synthetic compounds such as trimethoprim behave as inhibitors of efflux in Gram-negative bacteria. J Antimicrob Chemother 65:1215–1223. doi: 10.1093/jac/dkq079. [DOI] [PubMed] [Google Scholar]
- 13.Lomovskaya O, Warren MS, Lee A, Galazzo J, Fronko R, Lee M, Blais J, Cho D, Chamberland S, Renau T, Leger R, Hecker S, Watkins W, Hoshino K, Ishida H, Lee VJ. 2001. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob Agents Chemother 45:105–116. doi: 10.1128/AAC.45.1.105-116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey AM, Paulsen IT, Piddock LJ. 2008. RamA confers multidrug resistance in Salmonella enterica via increased expression of acrB, which is inhibited by chlorpromazine. Antimicrob Agents Chemother 52:3604–3611. doi: 10.1128/AAC.00661-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorarensen A, Presley-Bodnar AL, Marotti KR, Boyle TP, Heckaman CL, Bohanon MJ, Tomich PK, Zurenko GE, Sweeney MT, Yagi BH. 2001. 3-Arylpiperidines as potentiators of existing antibacterial agents. Bioorg Med Chem Lett 11:1903–1906. doi: 10.1016/S0960-894X(01)00330-4. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen ST, Kwasny SM, Ding X, Cardinale SC, McCarthy CT, Kim HS, Nikaido H, Peet NP, Williams JD, Bowlin TL, Opperman TJ. 2015. Structure-activity relationships of a novel pyranopyridine series of Gram-negative bacterial efflux pump inhibitors. Bioorg Med Chem 23:2024–2034. doi: 10.1016/j.bmc.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuster S, Kohler S, Buck A, Dambacher C, König A, Bohnert JA, Kern WV. 2014. Random mutagenesis of the multidrug transporter AcrB from Escherichia coli for identification of putative target residues of efflux pump inhibitors. Antimicrob Agents Chemother 58:6870–6878. doi: 10.1128/AAC.03775-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohler T, Michea-Hamzehpour M, Epp SF, Pechere JC. 1999. Carbapenem activities against Pseudomonas aeruginosa: respective contributions of OprD and efflux systems. Antimicrob Agents Chemother 43:424–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szymanska E, Kiec-Kononowicz K, Bialecka A, Kasprowicz A. 2002. Antimicrobial activity of 5-arylidene aromatic derivatives of hydantoin. Part 2. Farmaco 57:39–44. doi: 10.1016/S0014-827X(01)01172-7. [DOI] [PubMed] [Google Scholar]
- 20.Szymanska E, Kiec-Kononowicz K. 2002. Antimycobacterial activity of 5-arylidene aromatic derivatives of hydantoin. Farmaco 57:355–362. doi: 10.1016/S0014-827X(01)01194-6. [DOI] [PubMed] [Google Scholar]
- 21.Shalaby AFA, Daboun HA, Boghdadi SS. 1974. Reactions with 4-thiohydantoin. Preparation of 5-arylidene-4-thiohydantoin, their reactions towards Grignard reagents and alkylating agents. Z Naturforsch B 29:99–103. [Google Scholar]
- 22.Kiec-Kononowicz K, Szymanska E, Motyl M, Holzer W, Bialecka A, Kasprowicz A. 1998. Synthesis, spectral and antimicrobial properties of 5-chloroarylidene aromatic derivatives of imidazoline-4-one. Pharmazie 53:680–684. [PubMed] [Google Scholar]
- 23.Handzlik J, Spengler G, Mastek B, Dela A, Molnar J, Amaral L, Kiec-Kononowicz K. 2012. 5-Arylidene(thio)hydantoin derivatives as modulators of cancer efflux pump. Acta Pol Pharm 69:149–156. [PubMed] [Google Scholar]
- 24.CLSI. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard–ninth edition (M07-A9). CLSI, Wayne, PA. [Google Scholar]
- 25.Bohnert JA, Karamian B, Nikaido H. 2010. Optimized Nile red efflux assay of AcrAB-TolC multidrug efflux system shows competition between substrates. Antimicrob Agents Chemother 54:3770–3775. doi: 10.1128/AAC.00620-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bohnert JA, Schuster S, Szymaniak-Vits M, Kern WV. 2011. Determination of real-time efflux phenotypes in Escherichia coli AcrB binding pocket phenylalanine mutants using a 1,2′-dinaphthylamine efflux assay. PLoS One 6:e21196. doi: 10.1371/journal.pone.0021196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim SP, Nikaido H. 2010. Kinetic parameters of efflux of penicillins by the multidrug efflux transporter AcrAB-TolC of Escherichia coli. Antimicrob Agents Chemother 54:1800–1806. doi: 10.1128/AAC.01714-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.