Abstract
Despite a dearth of new agents currently being developed to combat multidrug-resistant Gram-negative pathogens, the combination of ceftolozane and tazobactam was recently approved by the Food and Drug Administration to treat complicated intra-abdominal and urinary tract infections. To characterize the activity of the combination product, time-kill studies were conducted against 4 strains of Escherichia coli that differed in the type of β-lactamase they expressed. The four investigational strains included 2805 (no β-lactamase), 2890 (AmpC β-lactamase), 2842 (CMY-10 β-lactamase), and 2807 (CTX-M-15 β-lactamase), with MICs to ceftolozane of 0.25, 4, 8, and >128 mg/liter with no tazobactam, and MICs of 0.25, 1, 4, and 8 mg/liter with 4 mg/liter tazobactam, respectively. All four strains were exposed to a 6 by 5 array of ceftolozane (0, 1, 4, 16, 64, and 256 mg/liter) and tazobactam (0, 1, 4, 16, and 64 mg/liter) over 48 h using starting inocula of 106 and 108 CFU/ml. While ceftolozane-tazobactam achieved bactericidal activity against all 4 strains, the concentrations of ceftolozane and tazobactam required for a ≥3-log reduction varied between the two starting inocula and the 4 strains. At both inocula, the Hill plots (R2 > 0.882) of ceftolozane revealed significantly higher 50% effective concentrations (EC50s) at tazobactam concentrations of ≤4 mg/liter than those at concentrations of ≥16 mg/liter (P < 0.01). Moreover, the EC50s at 108 CFU/ml were 2.81 to 66.5 times greater than the EC50s at 106 CFU/ml (median, 10.7-fold increase; P = 0.002). These promising results indicate that ceftolozane-tazobactam achieves bactericidal activity against a wide range of β-lactamase-producing E. coli strains.
INTRODUCTION
The rising prevalence of multidrug-resistant (MDR) Gram-negative organisms has forced urgent efforts to expand the therapeutic armamentarium against these problematic pathogens. Recent endeavors to develop new cephalosporin compounds exhibiting promising antipseudomonal activity led to the discovery of ceftolozane (ceftolozane, previously designated CXA-101 or FR264205) (1). This novel agent has been credited with enhanced stability to popular chromosomally mediated cephalosporin resistance mechanisms (including hyperexpression of AmpC β-lactamase enzymes and efflux pumps) (2), with a low propensity for cross-resistance to other cephalosporins to arise (3).
Antimicrobial inactivation arising from transferable plasmid-mediated β-lactamase hydrolysis presents the principal basis of β-lactam resistance among Enterobacteriaceae. More than 850 structurally diverse β-lactamase enzymes have been identified to date and classified as groups A to D based on their amino acid sequence homology (4). Worryingly, the rapid emergence of extended-spectrum-β-lactamase-producing Escherichia coli strains in recent times has been implicated in community-onset infections as a significant cause of morbidity, mortality, and health care-related costs. Despite ceftolozane's intrinsic benefits over other cephalosporins, the inability of the compound to overcome extended-spectrum β-lactamases (ESBLs) has been documented (5–7). To ameliorate this deficiency, the addition of the β-lactamase inhibitor tazobactam to ceftolozane extends the spectrum of activity of ceftolozane (8). The resulting ceftolozane-tazobactam combination was found to display remarkable activity against a range of MDR Gram-negative species (7) and was subsequently approved by the Food and Drug Administration to treat complicated intra-abdominal and urinary tract infections in adults (9).
Although the activity of ceftolozane-tazobactam against E. coli has been characterized in prior in vitro studies (10–12), a systematic analysis of the combination's performance against strains producing different β-lactamases has yet to be investigated at multiple levels of bacterial burden. Therefore, integrating antimicrobial pharmacokinetics (PK) and pharmacodynamics (PD) to effectively evaluate the bacteriologic response to ceftolozane-tazobactam is warranted (13). Our objective was to utilize time-kill studies to characterize the bacterial killing effects of ceftolozane and tazobactam alone and in combination against different β-lactamase-producing E. coli strains.
MATERIALS AND METHODS
Bacterial strains.
The four isogenic strains of E. coli employed for this study were engineered by Merck & Co. to differentially express a single β-lactamase; these included (i) 2805 (wild type, no β-lactamase), (ii) 2890 (AmpC β-lactamase), (iii) 2842 (CMY-10 β-lactamase), and (iv) 2807 (CTX-M-15 β-lactamase) (Table 1). β-Lactamase expression was modulated by assembly of the enzyme open reading frame per published GenBank sequences (strain 2890, Pseudomonas aeruginosa AmpC and ampR [5′ region], GenBank accession no. X54719.1; strain 2842, E. coli K998298 ESBL precursor [blaCMY-10], GenBank no. AF381617.1; strain 2807, E. coli strain 405/06 plasmid pKC405 β-lactamase CTX-M-15 [blaCTX-M-15] and insertion sequence IS26 TnpA, Genbank no. GQ274933.1), insertion into a pBR322 cloning vector (GenBank no. J0749), and replacement of the blaTEM-1 open reading frame in the β-lactamase-deficient E. coli DH10B parent strain. The native blaTEM-1 promoter was included in the modified pBR322 plasmid to regulate the expression of the desired β-lactamase. Prior to experiments, subculture of 2805 was performed on tryptic soy agar (TSA) with 5% sheep blood (BD Biosciences, Franklin Lakes, NJ), while 2890, 2842, and 2807 were subcultured in the presence of 25 μg/ml tetracycline to maintain the selection of plasmids.
TABLE 1.
β-Lactamase production and MICs of each strain to ceftolozane in the absence and presence of tazobactam
| Strain | β-Lactamase |
Ceftolozane MIC with tazobactam concn of (mg/liter): |
|||||
|---|---|---|---|---|---|---|---|
| Type | Ambler class (4) | 0 | 1 | 4 | 16 | 64 | |
| 2805 | None | 0.25 | |||||
| 2890 | AmpC | C | 4 | 4 | 1 | 0.5 | 0.5 |
| 2842 | CMY-10 | C | 8 | 4 | 4 | 1 | 0.5 |
| 2807 | CTX-M-15 | A | >128 | >128 | 8 | 2 | 1 |
Antibiotics, susceptibility testing, and medium.
Analytical-grade ceftolozane and tazobactam powders were obtained from Merck & Co. (Kenilworth, NJ). Fresh stock solutions were prepared immediately prior to experiments in Milli-Q water. Standard broth microdilution methods were adopted from the Clinical and Laboratory Standards Institute (CLSI) (14) for the determination of MICs. The MIC experiments were performed in quadruplicate, while time-kill experiments were performed in duplicate; all studies were conducted using cation-adjusted Mueller-Hinton broth (CaMHB, at 25.0 mg/liter Ca2+ and 12.5 mg/liter Mg2+; Difco, Detroit, MI).
Time-kill experiments.
Time-kill experiments were performed for ceftolozane and tazobactam alone and in combination, using previously described methods (15). Briefly, fresh bacterial colonies from overnight growth were added to CaMHB to provide a bacterial suspension, which was diluted with CaMHB to achieve the desired starting inoculum of ∼106 or ∼108 CFU/ml in a 50-ml Falcon tube. A 6 by 5 array of ceftolozane (0, 1, 4, 16, 64, and 256 mg/liter) and tazobactam (0, 1, 4, 16, and 64 mg/liter) concentrations was tested as monotherapy and in combination over a period of 48 h. Thus, 30 treatment regimens were examined in total against each strain at both starting inocula. The choice of studied concentrations was based on clinically achievable targets discerned from free-drug PK profiles in healthy volunteers (16).
Samples were withdrawn at 1, 2, 4, 8, 24, 26, 28, 32, and 48 h after dosing. Colony counts were performed by plating 50-μl aliquots of each diluted sample onto tryptic soy agar (TSA) plates containing 5% sheep blood (Becton Dickinson, Franklin Lakes, NJ) using an automated spiral dispenser (Whitley automatic spiral plater; Don Whitley Scientific Limited, West Yorkshire, England). Plates were incubated at 37°C for 24 h, and viable bacterial counts were determined (log10 CFU/ml) using a laser bacteria colony counter (ProtoCOL version 2.05.02; Synbiosis, Cambridge, England). Bactericidal activity (99.9% kill) was associated with a ≥3.0-log10 CFU/ml decrease in bacterial density compared to the initial inoculum at any time. Experiments were conducted over 48 h (as opposed to the traditional 24-h standard) to provide insight into the pharmacodynamics of each combination beyond 24 h, and also to better discriminate between the killing activities of the different ceftolozane and tazobactam concentrations. As the degradation of ceftolozane and tazobactam at 35°C was of concern, concentrations of ceftolozane and tazobactam were measured in the absence of bacteria over 48 h to calculate the rate of degradation for each agent.
Pharmacodynamic analyses.
The bacterial killing effect (E) of mono- and combination therapies was quantified as the log ratio change in bacterial density (CFU/ml) at 48 h versus preantibiotic exposure at 0 h (see equation 1) (17). Point-based analyses were performed on plots of E versus ceftolozane concentrations. Taking into consideration the general mechanism of action of β-lactam–β-lactam inhibitor combinations (whereby inhibitors are understood to bind to inactivating β-lactamase enzymes, thus allowing β-lactam agents to exert their action), we attributed the majority of the killing activity exerted by the ceftolozane-tazobactam combination to the effect of ceftolozane. Consequently, using nonlinear regression, concentration-effect relationships were fit to Hill-type models for each strain at each fixed tazobactam concentration, according to equation 2, where E0 is the measured effect in the absence of ceftolozane, Emax is the maximal effect, C is the concentration of ceftolozane, EC50 is the concentration at which there is a 50% maximal effect, and H is the Hill constant. Statistical analyses of Emax and EC50 parameters were conducted to determine the effect of increasing tazobactam concentrations, using a nonparametric Friedman two-way analysis of variance (ANOVA) with pairwise comparisons (P < 0.05). Differences in parameter estimates at 106 versus 108 CFU/ml were determined using hypothesis testing (P < 0.05, F-test). All PD analyses and statistical evaluations were performed using Systat (version 13.00.05; Systat Software, IL).
| (1) |
| (2) |
RESULTS
MICs and degradation rates.
The ceftolozane MICs of each strain without tazobactam and in combination with 4 mg/liter tazobactam are presented in Table 1. In the absence of tazobactam, the wild-type non-β-lactamase-producing strain 2805 exhibited the lowest MIC, at 0.25 mg/liter. Strains 2890 and 2842 producing class C β-lactamases exhibited MICs of 4 and 8 mg/liter, respectively, while high resistance to ceftolozane was displayed by the CTX-M-15-producing strain, 2807, with an MIC of >128 mg/liter. In the presence of 4 mg/liter tazobactam (per CLSI guidelines [14]), the MICs of all β-lactamase-producing strains were reduced to 2- to 16-fold.
After 48 h of incubation at 35°C, the degradation rate constants of ceftolozane ranged from 0.00466 to 0.00679 h−1 (half-life range, 102 to 148 h; R2 ≥ 0.938). Tazobactam concentrations were constant for the duration of the 48-h experiments.
Time-kill experiments.
Time-kill profiles illustrating the change in bacterial density of the four E. coli strains following exposure to ceftolozane alone and in combination with tazobactam are presented according to fixed tazobactam concentrations at both 106 and 108 CFU/ml in Fig. 1 and 2, respectively).
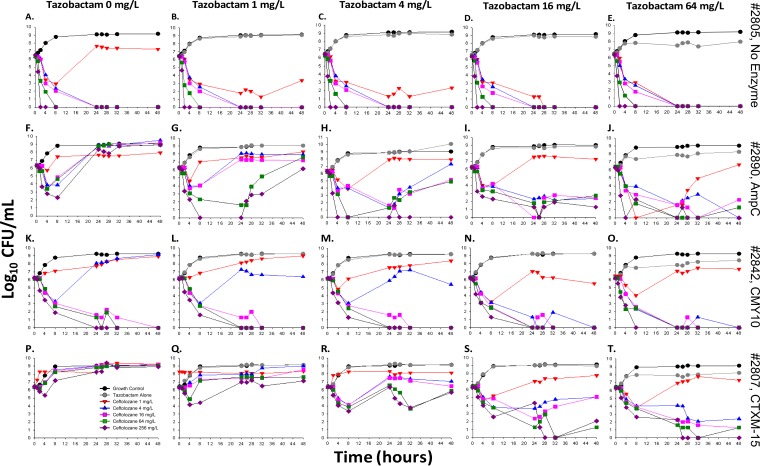
FIG 1.
Change in bacterial burdens of strains 2805 (A to E), 2890 (F to J), 2842 (K to O), and 2807 (P to T) at a low inoculum (∼106 CFU/ml) over 48 h, following treatment with ceftolozane (0 to 256 mg/liter) at fixed tazobactam concentrations (0 to 64 mg/liter). The colored curves on each graph represent different ceftolozane concentrations, including the untreated growth control (black), tazobactam alone (gray), 1 mg/liter (red), 4 mg/liter (blue), 16 mg/liter (pink), 64 mg/liter (green), and 256 mg/liter (purple).
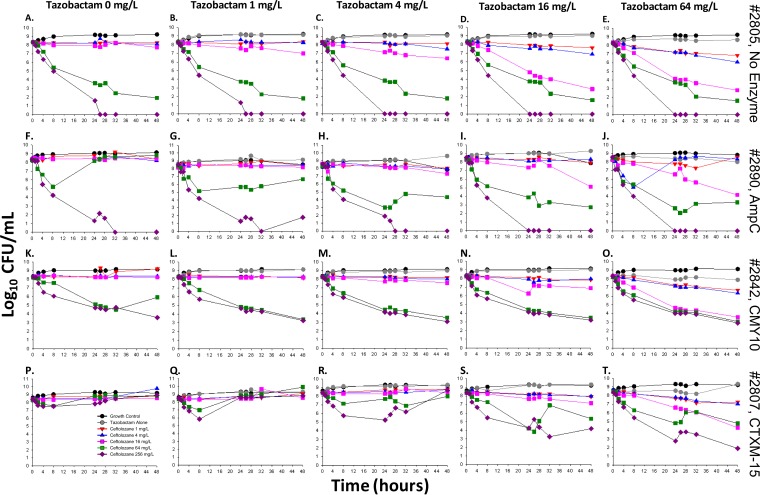
FIG 2.
Change in bacterial burdens of strains 2805 (A to E), 2890 (F to J), 2842 (K to O), and 2807 (P to T) at a high inoculum (∼108 CFU/ml) over 48 h, following treatment with ceftolozane (0 to 256 mg/liter) at fixed tazobactam concentrations (0 to 64 mg/liter). The colored curves on each graph represent different ceftolozane concentrations, which includes the untreated growth control (black), tazobactam alone (gray), 1 mg/liter (red), 4 mg/liter (blue), 16 mg/liter (pink), 64 mg/liter (green), and 256 mg/liter (purple).
Strain 2805 (no β-lactamase).
For the susceptible strain 2805 at 106 CFU/ml, complete bactericidal activity reaching undetectable limits of eradication was achieved within 24 h in response to monotherapy with 4 mg/liter ceftolozane (−6.48 log10 CFU/ml, Fig. 1A). No improvement in activity was gained at higher concentrations or in combination with tazobactam (Fig. 1B to E). At 108 CFU/ml, bactericidal activity was attained at low tazobactam concentrations (0 to 4 mg/liter) in combination with ceftolozane at ≥64 mg/liter (−6.43 to −8.33 log10 CFU/ml; Fig. 2A to E), or at higher tazobactam concentrations of ≥16 mg/liter in combination with ≥16 mg/liter ceftolozane (−5.32 to −8.23 log10 CFU/ml; Fig. 2D and E).
Strain 2890 (AmpC).
Rapid activity was demonstrated with ceftolozane monotherapy at concentrations of 4 to 256 mg/liter against 2890 at 106 CFU/ml, with a decrease of −2.39 to −3.53 log10 CFU/ml after 4 h, followed by regrowth, whereby bacterial counts mimicked those of the untreated control within 24 h (Fig. 1F). A concentration-dependent trend toward a greater level of ceftolozane killing was observed in combination with increasing tazobactam concentrations. After 48 h, sustained bactericidal activity was attained with combinations of ≥4 mg/liter ceftolozane and ≥16 mg/liter tazobactam (−3.93 to −6.33 log10 CFU/ml, Fig. 1I and J). At 108 CFU/ml, both monotherapy and combination regimens containing ≤4 mg/liter ceftolozane were mostly inactive (below −0.5 log10 CFU/ml), while bacterial counts were reduced to undetectable limits with all regimens containing ceftolozane concentrations of 256 mg/liter (Fig. 2F to J). Bactericidal activity at 48 h was noted for 64 mg/liter ceftolozane in combination with ≥4 mg/liter tazobactam (−4.01, −5.65, and −5.18 log10 CFU/ml, Fig. 2H to J, respectively). The arm with 16 mg/liter ceftolozane was capable of achieving bactericidal activity at 48 h when in the presence of 16 and 64 mg/liter tazobactam (−3.12 and −4.09 log10 CFU/ml, respectively).
Strain 2842 (CMY-10).
Concentration-dependent enhancement of ceftolozane activity in combination with tazobactam was similarly observed for strain 2842 at 106 CFU/ml (Fig. 1K to O). Accordingly, at ≤4 mg/liter tazobactam, activity was negligible in combination with 1 mg/liter ceftolozane, while bactericidal activity was noted within 8 h of exposure to 4 mg/liter ceftolozane (−2.94 to 3.23 log10 CFU/ml) and was followed by rapid regrowth by 24 h (Fig. 1K to M). However, bacteria were driven below detectable limits by all monotherapy and combination regimens containing ≥16 mg/liter ceftolozane (−6.18 log10 CFU/ml; Fig. 1K to O). At 108 CFU/ml, substantial activity at ≤16 mg/liter tazobactam was seen in combination with ≥64 mg/liter ceftolozane (−2.28 to −5.18 log10 CFU/ml; Fig. 2K to N). At the highest tazobactam concentration of 64 mg/liter, bactericidal activity was achieved with ≥16 mg/liter ceftolozane (−4.74 to −5.39 log10 CFU/ml; Fig. 2O).
Strain 2807 (CTX-M-15).
Strain 2807 was resistant to all ceftolozane monotherapy regimens at both inocula, with bacterial counts mirroring the control strain after 48 h. Interestingly, tazobactam maintained the ability to enhance activity in a concentration-dependent manner (Fig. 1 and 2P to T, 106 and 108 CFU/ml, respectively). Against both inocula, ceftolozane concentrations of ≥64 mg/liter achieved bactericidal activity at 48 h at a tazobactam concentration of 16 mg/liter (Fig. 1S and 2S, at or above −4.24 and −3.07 log10 CFU/ml, respectively). In the presence of 64 mg/liter tazobactam, ceftolozane concentrations of ≥4 mg/liter achieved bactericidal activity at 48 h against the 106 CFU/ml inoculum (Fig. 1T, at or above −3.97 log10 CFU/ml), whereas ceftolozane concentrations of ≥16 mg/liter were required for bactericidal activity against the 108 CFU/ml inoculum (Fig. 2T, at or above −3.66 log10 CFU/ml).
PK/PD analyses.
PK/PD analyses of time-kill data were performed to determine the target concentrations of ceftolozane and tazobactam associated with optimal activity. PD relationships were fit to a sigmoidal Hill-type function (equation 2), from which PD parameters were estimated (R2 > 0.882, Table 2). The ceftolozane-tazobactam PD relationship was primarily defined by Emax and EC50 parameters, which provide a measure of the maximal capacity of efficacy and half-maximal potency, respectively. Across all strains at both inocula, no significant trends were noted in Emax values with increasing tazobactam concentrations (P > 0.2, ANOVA). However, in the presence of tazobactam, a clear tendency toward lower EC50 values was demonstrated for all strains at both inocula (Table 2). Overall, pairwise comparisons revealed that all EC50 differences at tazobactam concentrations of 0 to 4 mg/liter versus 16 and 64 mg/liter were statistically significant (P < 0.01, ANOVA). The changes in EC50 as tazobactam concentrations increased from 0 to 4 mg/liter were not statistically significant, nor was the difference in EC50s at tazobactam concentrations of 16 versus 64 mg/liter (P > 0.1, ANOVA). Hypothesis testing revealed that the EC50s at 108 CFU/ml were 2.81 to 66.5 times greater than those at 106 CFU/ml (median, 10.7-fold increase; P = 0.002, F-test); however, no significant differences in Emax were observed between the two inocula (P = 0.531, F-test).
TABLE 2.
Hill plot PD parameter estimates describing the effect of ceftolozane in the presence of increasing tazobactam concentrations (0 to 64 mg/liter) at low and high inocula
| Strain | Tazobactam concn (mg/liter) | Low inoculum of ceftolozane (SE) |
High inoculum of ceftolozane (SE) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| E0 | Emax | Ha | EC50 | R2 | E0 | Emax | Ha | EC50 | R2 | ||
| 2805 | 0 | 2.68 | 9.16 | 10.0 | 1.14 | 1.00 | 0.194 (0.343) | 8.64 (0.765) | 2.46 (0.737) | 39.3 (7.58) | 0.991 |
| 1 | 2.62 | 9.10 | 10.0 | 0.948 | 1.00 | 0.291 (0.275) | 8.84 (0.643) | 1.91 (0.388) | 33.7 (5.36) | 0.995 | |
| 4 | 2.35 | 8.83 | 10.0 | 0.903 | 1.00 | 0.104 (0.451) | 8.94 (1.23) | 1.49 (0.509) | 32.8 (9.27) | 0.987 | |
| 16 | 2.48 | 8.96 | 9.53 | 0.156 | 1.00 | 0.552 (0.645) | 9.03 (1.26) | 1.00 (0.325) | 10.2 (3.47) | 0.984 | |
| 64 | 1.83 | 8.31 | 10.0 | 0.136 | 1.00 | 0.0450 (0.668) | 8.25 (1.25) | 1.00 (0.357) | 9.04 (3.37) | 0.980 | |
| 2890 | 0 | —b | — | — | — | — | 0.629 (0.456) | 10 (4.78) | 1.00 (0.735) | 52.1 (74.3) | 0.993 |
| 1 | 2.61 (0.734) | 2.20 (1.12) | 1.00 (1.25) | 4.44 (5.65) | 0.882 | 0.780 (0.292) | 7.32 (0.505) | 1.32 (0.271) | 68.4 (1.709) | 0.938 | |
| 4 | 2.40 (1.56) | 9.47 (5.25) | 1.00 (1.36) | 23.5 (34.7) | 0.923 | 0.497 (0.772) | 10.0 (5.56) | 1.00 (0.922) | 66.0 (7.37) | 0.970 | |
| 16 | 2.51 (0.751) | 6.69 (0.87) | 3.23 (2.72) | 1.45 (0.53) | 0.981 | 0.622 (0.554) | 7.11 (1.13) | 1.43 (0.689) | 16.1 (5.53) | 0.981 | |
| 64 | 1.96 (1.60) | 6.98 (2.07) | 5.10 (5.98) | 1.27 (1.41) | 0.952 | 0.137 (0.581) | 5.81 (0.861) | 3.38 (5.64) | 12.2 (6.04) | 0.970 | |
| 2842 | 0 | 2.93 (0.109) | 9.12 (0.140) | 10.0 (27.1) | 8.52 (1.48) | 1.00 | 0.727 (0.297) | 7.37 (2.87) | 1.02 (0.478) | 88.3 (75.6) | 0.989 |
| 1 | 2.95 (0.100) | 9.15 (0.143) | 4.57 (3.153) | 4.81 (0.620) | 1.00 | 0.179 (0.301) | 5.28 (0.603) | 4.64 (4.41) | 28.8 (17.3) | 0.985 | |
| 4 | 2.70 (0.296) | 8.95 (0.435) | 2.96 (1.45) | 4.67 (0.466) | 0.987 | 0.039 (0.308) | 5.33 (0.637) | 2.85 (1.28) | 28.7 (8.93) | 0.983 | |
| 16 | 3.10 (0.119) | 9.29 (0.100) | 10.0 (0.361) | 1.04 (0.001) | 1.00 | 0.0451 (0.382) | 5.16 (0.806) | 2.54 (1.93) | 23.1 (8.56) | 0.974 | |
| 64 | 2.26 (0.003) | 8.44 (0.108) | 10.0 (0.112) | 0.84 (0.013) | 1.00 | −0.929 (0.458) | 4.52 (0.678) | 1.76 (0.867) | 6.82 (2.51) | 0.973 | |
| 2807 | 0 | — | — | — | — | — | — | — | — | — | — |
| 1 | 2.57 (0.424) | 2.00 (4.17) | 1.46 (1.95) | 34.9 (11.0) | 0.986 | — | — | — | — | — | |
| 4 | 3.17 (0.511) | 4.16 (0.748) | 0.633 (0.196) | 2.34 (0.956) | 0.995 | — | — | — | — | — | |
| 16 | 2.49 (0.551) | 7.27 (0.675) | 3.25 (1.36) | 1.28 0.421) | 0.912 | 0.0530 (0.473) | 5.02 (1.71) | 1.00 (0.627) | 31.8 (25.0) | 0.952 | |
| 64 | 1.82 (0.738) | 7.46 (0.876) | 2.24 (2.57) | 1.65 (0.642) | 0.982 | 1.61 (2.05) | 5.08 (2.67) | 1.00 (1.19) | 1.10 (1.14) | 0.913 | |
Hill's constants were constrained between the limits of 1 and 10.
—, no parameter estimates calculated because there was negligible activity that was not modeled well by a Hill-type function.
DISCUSSION
The introduction of ceftolozane-tazobactam has attracted much interest, with in vitro data providing evidence that this combination may present a promising addition to the therapeutic armamentarium against a range of Gram-negative pathogens, including β-lactamase-expressing strains (1–3, 5–8, 18–20). It is well recognized that appropriate antimicrobial dosing strategies necessitate a solid understanding of PK/PD principles to understand the relationship between the concentration-time profile and antimicrobial activity, allowing the design of regimens that maximize bacterial eradication and minimize the development of resistance. In the present study, the in vitro activities of an array of ceftolozane and tazobactam concentrations were tested alone and in combination in a dynamic fashion over 48 h against four strains of E. coli expressing different β-lactamases. Our study design incorporated a wide range of concentrations to ensure that all relevant PD parameters were captured for mathematical modeling purposes in the future.
The ability of inhibitors to inactivate specific β-lactamase enzymes varies markedly within and between classes; for tazobactam, irreversible β-lactamase inhibition (“suicide inhibition”) has mainly been demonstrated against pathogens expressing class A β-lactamases, while reduced activity has been reported for those expressing classes B, C, and D (21). In the present study, however, the time-kill data provide evidence that ceftolozane activity was enhanced by the addition of tazobactam in a concentration-dependent manner, which was observed against all β-lactamase-producing strains at both inocula, irrespective of β-lactamase expression (Fig. 1 and 2). This trend was substantiated by analyses of PD parameters, which revealed significantly reduced EC50 values for all strains, indicating that the potency of ceftolozane increased at both inocula in the presence of increasing tazobactam concentrations. Characteristic differences noted in the time-kill profiles obtained for each strain serve to reinforce the varied extent of inactivation that tazobactam may pose on different β-lactamases (21). Notably, a 10.7-fold median increase in EC50 was observed at the inoculum of 108 CFU/ml compared to EC50 values at 106 CFU/ml; similar trends have been observed for other β-lactam agents against β-lactamase-producing strains in the presence of a high bacterial load (22–24). Mechanistic explanations for the differential PD observed between the two inocula have been hypothesized, including the possible release of larger quantities of β-lactamase enzymes from lysed bacteria (25), cell-to-cell communication, or the decreased presence of active penicillin-binding proteins due to the reduction in bacterial cell wall synthesis at higher bacterial densities (26, 27). Although the clinical implications of these observations have yet to be understood, consideration may be given to the administration of elevated ceftolozane doses when employed for the treatment of infections that entail a higher bacterial burden.
Interestingly, despite previous suggestions of improved stability of ceftolozane against AmpC β-lactamase from Pseudomonas aeruginosa (2, 7, 28), the exposure of 2890 to ceftolozane monotherapy resulted in extensive regrowth, even at extremely high concentrations (256 mg/liter) far in excess of the MIC (Fig. 1F). Owing to the relatively stable ceftolozane concentrations used during the time-killing experiments, regrowth was likely characterized by the amplification of ceftolozane-resistant subpopulations. Indeed, the phenomenon of heteroresistance to colistin (29), vancomycin (30), and carbapenems (31) has been reported and defined as the presence of a resistant subpopulation of bacteria within a susceptible strain based on MICs. Although heteroresistance has yet to be detected in E. coli, it certainly provides a plausible explanation for the regrowth observed here (Fig. 1F). Importantly, for strain 2890, the addition of 4 mg/liter tazobactam to regimens with ≥4 mg/liter ceftolozane resulted in bactericidal activity by 24 h at 106 CFU/ml (Fig. 1H), although regrowth at a concentration of 4 mg/liter ceftolozane was observed by 48 h. Increasing the dose of tazobactam further to 16 mg/liter resulted in sustained killing at the ceftolozane concentration of 4 mg/liter up to 48 h (Fig. 1I). Similarly, the addition of ≥16 mg/liter tazobactam to regimens with ≥64 mg/liter ceftolozane achieved bactericidal activity by 48 h in the ceftolozane-resistant strain 2807 at both inocula. Ceftolozane and tazobactam concentrations of 64 mg/liter and 16 mg/liter reflect achievable maximum concentration of drug (Cmax) values obtained in healthy volunteers receiving the equivalent of 1.0-g and 0.5-g doses of ceftolozane and tazobactam, respectively, which were the doses investigated in phase III clinical trials (16, 32, 33). Taken together, these results highlight the utility of tazobactam to increase the susceptibility of β-lactamase-producing E. coli strains to ceftolozane therapy at both inocula.
Although the %T>threshold has been identified as the PK/PD index most predictive of the activity of ceftolozane-tazobactam, our results show a clear concentration dependence, with enhanced killing at elevated ceftolozane concentrations. The %T>threshold was first proposed by VanScoy et al. (12) as the PK/PD index that best described ceftolozane-tazobactam killing in E. coli strains expressing the CTX-M-15 β-lactamase (12). Further work with E. coli and Klebsiella pneumoniae strains expressing various β-lactamases led to the unifying conclusion that the percentage of time above the ceftolozane-tazobactam MIC × 0.5 is predictive of the combination's efficacy, regardless of the β-lactamase expression profile of the pathogen (11). An in vivo study utilizing a neutropenic mouse model corroborated %T>threshold as the ideal PK/PD index, and the authors were able to identify the % time above the MIC (%T>MIC) targets required to achieve specific magnitudes of bacterial killing (34). In the present study, the concentration dependence observed in the time-kill experiments may partially be ascribed to the elevated bacterial burden (108 CFU/ml) that exceeded the bacterial load utilized in previous in vitro (106 CFU/ml) and in vivo (106.2 to 107.1 CFU/ml) studies (11, 12, 34). The static concentrations used in the current investigation may also account for some of the discordance with the results from prior studies. However, previous investigations evaluating the PK/PD of β-lactam–β-lactamase inhibitor combinations have asserted that stoichiometric inhibition of β-lactamase enzymes is determined by exposure of the inhibitor over time, which is best predicted by the area under the concentration-time curve (AUC) (35). A prior study utilizing piperacillin and tazobactam also found that dose fractionating the administration of both agents while maintaining the same drug exposure did not alter the killing of a TEM-producing strain of E. coli (36). The activity of ceftolozane-tazobactam may therefore not be completely described by time-dependent killing, and a hybrid index that accounts for the AUC or Cmax may improve the predictive capability of the %T>threshold index.
In summary, we systematically described the concentration-effect relationship of ceftolozane and tazobactam alone and in combination, revealing the ability of ceftolozane-tazobactam to achieve potent bactericidal activity against E. coli strains expressing different types of β-lactamase enzymes. While these results are promising, our time-kill studies utilized static concentrations of ceftolozane-tazobactam, making the extrapolation of our results into the clinical setting difficult. To the best of our knowledge, only two studies, by VanScoy et al. (10, 37), have looked at ceftolozane-tazobactam in a hollow-fiber infection model. Further studies evaluating the performance of the combination regimen against other pathogens containing β-lactamase enzymes are needed to completely understand the niche of ceftolozane-tazobactam among other β-lactams.
ACKNOWLEDGMENTS
This study was funded by Merck and Co.
We thank Christa McPhee for excellent technical assistance.
Funding Statement
Merck (Merck & Co., Inc.) provided funding to the University at Buffalo.
REFERENCES
- 1.Takeda S, Nakai T, Wakai Y, Ikeda F, Hatano K. 2007. In vitro and in vivo activities of a new cephalosporin, FR264205, against Pseudomonas aeruginosa. Antimicrob Agents Chemother 51:826–830. doi: 10.1128/AAC.00860-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takeda S, Ishii Y, Hatano K, Tateda K, Yamaguchi K. 2007. Stability of FR264205 against AmpC beta-lactamase of Pseudomonas aeruginosa. Int J Antimicrob Agents 30:443–445. doi: 10.1016/j.ijantimicag.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Zamorano L, Juan C, Fernandez-Olmos A, Ge Y, Canton R, Oliver A. 2009. Activity of the new cephalosporin CXA-101 (FR264205) against Pseudomonas aeruginosa isolates from chronically-infected cystic fibrosis patients. Clin Microbiol Infect 16:1482–1487. [DOI] [PubMed] [Google Scholar]
- 4.Ambler RP, Meadway RJ. 1969. Chemical structure of bacterial penicillinases. Nature 222:24–26. doi: 10.1038/222024a0. [DOI] [PubMed] [Google Scholar]
- 5.Moya B, Zamorano L, Juan C, Perez JL, Ge Y, Oliver A. 2010. Activity of a new cephalosporin, CXA-101 (FR264205), against beta-lactam-resistant Pseudomonas aeruginosa mutants selected in vitro and after antipseudomonal treatment of intensive care unit patients. Antimicrob Agents Chemother 54:1213–1217. doi: 10.1128/AAC.01104-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giske CG, Ge J, Nordmann P. 2009. Activity of cephalosporin CXA-101 (FR264205) and comparators against extended-spectrum-{beta}-lactamase-producing Pseudomonas aeruginosa. J Antimicrob Chemother 64:430–431. doi: 10.1093/jac/dkp193. [DOI] [PubMed] [Google Scholar]
- 7.Livermore DM, Mushtaq S, Ge Y, Warner M. 2009. Activity of cephalosporin CXA-101 (FR264205) against Pseudomonas aeruginosa and Burkholderia cepacia group strains and isolates. Int J Antimicrob Agents 34:402–406. doi: 10.1016/j.ijantimicag.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Livermore DM, Mushtaq S, Ge Y. 2010. Chequerboard titration of cephalosporin CXA-101 (FR264205) and tazobactam versus beta-lactamase-producing Enterobacteriaceae. J Antimicrob Chemother 65:1972–1974. doi: 10.1093/jac/dkq248. [DOI] [PubMed] [Google Scholar]
- 9.Cubist Pharmaceuticals. 2014. Zerbaxa (ceftolozane/tazobactam) for injection, for intravenous use. Cubist Pharmaceuticals, Lexington, MA: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206829lbl.pdf. [Google Scholar]
- 10.VanScoy B, Mendes RE, Castanheira M, McCauley J, Bhavnani SM, Forrest A, Jones RN, Okusanya OO, Friedrich LV, Steenbergen J, Ambrose PG. 2013. Relationship between ceftolozane-tazobactam exposure and drug resistance amplification in a hollow-fiber infection model. Antimicrob Agents Chemother 57:4134–4138. doi: 10.1128/AAC.00461-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.VanScoy B, Mendes RE, McCauley J, Bhavnani SM, Bulik CC, Okusanya OO, Forrest A, Jones RN, Friedrich LV, Steenbergen JN, Ambrose PG. 2013. Pharmacological basis of β-lactamase inhibitor therapeutics: tazobactam in combination with ceftolozane. Antimicrob Agents Chemother 57:5924–5930. doi: 10.1128/AAC.00656-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.VanScoy B, Mendes RE, Nicasio AM, Castanheira M, Bulik CC, Okusanya OO, Bhavnani SM, Forrest A, Jones RN, Friedrich LV, Steenbergen JN, Ambrose PG. 2013. Pharmacokinetics-pharmacodynamics of tazobactam in combination with ceftolozane in an in vitro infection model. Antimicrob Agents Chemother 57:2809–2814. doi: 10.1128/AAC.02513-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gloede J, Scheerans C, Derendorf H, Kloft C. 2010. In vitro pharmacodynamic models to determine the effect of antibacterial drugs. J Antimicrob Chemother 65:186–201. doi: 10.1093/jac/dkp434. [DOI] [PubMed] [Google Scholar]
- 14.Clinical Laboratory and Standards Institute (CLSI). 2011. Performance standards for antimicrobial susceptibility testing; 21st informational supplement. CLSI document M100-S21. Clinical Laboratory and Standards Institute, Wayne, PA. [Google Scholar]
- 15.Tsuji BT, von Eiff C, Kelchlin PA, Forrest A, Smith PF. 2008. Attenuated vancomycin bactericidal activity against Staphylococcus aureus hemB mutants expressing the small-colony-variant phenotype. Antimicrob Agents Chemother 52:1533–1537. doi: 10.1128/AAC.01254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge Y, Whitehouse MJ, Friedland I, Talbot GH. 2010. Pharmacokinetics and safety of CXA-101, a new antipseudomonal cephalosporin, in healthy adult male and female subjects receiving single- and multiple-dose intravenous infusions. Antimicrob Agents Chemother 54:3427–3431. doi: 10.1128/AAC.01753-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuji BT, Harigaya Y, Lesse AJ, Sakoulas G, Mylotte JM. 2009. Loss of vancomycin bactericidal activity against accessory gene regulator (agr) dysfunctional Staphylococcus aureus under conditions of high bacterial density. Diagn Microbiol Infect Dis 64:220–224. doi: 10.1016/j.diagmicrobio.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 18.Riera E, Macia MD, Mena A, Mulet X, Perez JL, Ge Y, Oliver A. 2010. Anti-biofilm and resistance suppression activities of CXA-101 against chronic respiratory infection phenotypes of Pseudomonas aeruginosa strain PAO1. J Antimicrob Chemother 65:1399–1404. doi: 10.1093/jac/dkq143. [DOI] [PubMed] [Google Scholar]
- 19.Bulik CC, Christensen H, Nicolau DP. 2010. In vitro potency of CXA-101, a novel cephalosporin, against Pseudomonas aeruginosa displaying various resistance phenotypes, including multidrug resistance. Antimicrob Agents Chemother 54:557–559. doi: 10.1128/AAC.00912-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Titelman E, Karlsson IM, Ge Y, Giske CG. 2011. In vitro activity of CXA-101 plus tazobactam (CXA-201) against CTX-M-14- and CTX-M-15-producing Escherichia coli and Klebsiella pneumoniae. Diagn Microbiol Infect Dis 70:137–141. doi: 10.1016/j.diagmicrobio.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Drawz SM, Bonomo RA. 2010. Three decades of beta-lactamase inhibitors. Clin Microbiol Rev 23:160–201. doi: 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.López-Cerero L, Picón E, Morillo C, Hernández JR, Docobo F, Pachón J, Rodríguez-Baño J, Pascual A. 2010. Comparative assessment of inoculum effects on the antimicrobial activity of amoxycillin-clavulanate and piperacillin-tazobactam with extended-spectrum beta-lactamase-producing and extended-spectrum beta-lactamase-non-producing Escherichia coli isolates. Clin Microbiol Infect 16:132–136. doi: 10.1111/j.1469-0691.2009.02893.x. [DOI] [PubMed] [Google Scholar]
- 23.Nannini EC, Stryjewski ME, Singh KV, Rude TH, Corey GR, Fowler VG Jr, Murray BE. 2010. Determination of an inoculum effect with various cephalosporins among clinical isolates of methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother 54:2206–2208. doi: 10.1128/AAC.01325-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robin F, Krebs M, Delmas J, Gibold L, Mirande C, Bonnet R. 2011. In vitro efficiency of the piperacillin/tazobactam combination against inhibitor-resistant TEM- and complex mutant TEM-producing clinical strains of Escherichia coli. J Antimicrob Chemother 66:1052–1056. doi: 10.1093/jac/dkr045. [DOI] [PubMed] [Google Scholar]
- 25.Craig WA, Bhavnani SM, Ambrose PG. 2004. The inoculum effect: fact or artifact? Diagn Microbiol Infect Dis 50:229–230. doi: 10.1016/j.diagmicrobio.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Anderl JN, Zahller J, Roe F, Stewart PS. 2003. Role of nutrient limitation and stationary-phase existence in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother 47:1251–1256. doi: 10.1128/AAC.47.4.1251-1256.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waxman DJ, Strominger JL. 1983. Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Annu Rev Biochem 52:825–869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]
- 28.Bulik CC, Tessier PR, Keel RA, Sutherland CA, Nicolau DP. 2012. In vivo comparison of CXA-101 (FR264205) with and without tazobactam versus piperacillin-tazobactam using human simulated exposures against phenotypically diverse Gram-negative organisms. Antimicrob Agents Chemother 56:544–549. doi: 10.1128/AAC.01752-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Rayner CR, Nation RL, Owen RJ, Spelman D, Tan KE, Liolios L. 2006. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 50:2946–2950. doi: 10.1128/AAC.00103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khatib R, Jose J, Musta A, Sharma M, Fakih MG, Johnson LB, Riederer K, Shemes S. 2011. Relevance of vancomycin-intermediate susceptibility and heteroresistance in methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother 66:1594–1599. doi: 10.1093/jac/dkr169. [DOI] [PubMed] [Google Scholar]
- 31.Ikonomidis A, Neou E, Gogou V, Vrioni G, Tsakris A, Pournaras S. 2009. Heteroresistance to meropenem in carbapenem-susceptible Acinetobacter baumannii. J Clin Microbiol 47:4055–4059. doi: 10.1128/JCM.00959-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller B, Hershberger E, Benziger D, Trinh M, Friedland I. 2012. Pharmacokinetics and safety of intravenous ceftolozane-tazobactam in healthy adult subjects following single and multiple ascending doses. Antimicrob Agents Chemother 56:3086–3091. doi: 10.1128/AAC.06349-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagenlehner FM, Umeh O, Steenbergen J, Yuan G, Darouiche RO. 2015. Ceftolozane-tazobactam compared with levofloxacin in the treatment of complicated urinary-tract infections, including pyelonephritis: a randomised, double-blind, phase 3 trial (ASPECT-cUTI). Lancet 385:1949–1956. doi: 10.1016/S0140-6736(14)62220-0. [DOI] [PubMed] [Google Scholar]
- 34.Craig WA, Andes DR. 2013. In vivo activities of ceftolozane, a new cephalosporin, with and without tazobactam against Pseudomonas aeruginosa and Enterobacteriaceae, including strains with extended-spectrum β-lactamases, in the thighs of neutropenic mice. Antimicrob Agents Chemother 57:1577–1582. doi: 10.1128/AAC.01590-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Livermore DM. 1993. Determinants of the activity of beta-lactamase inhibitor combinations. J Antimicrob Chemother 31(Suppl A):9–21. [DOI] [PubMed] [Google Scholar]
- 36.Strayer AH, Gilbert DH, Pivarnik P, Medeiros AA, Zinner SH, Dudley MN. 1994. Pharmacodynamics of piperacillin alone and in combination with tazobactam against piperacillin-resistant and -susceptible organisms in an in vitro model of infection. Antimicrob Agents Chemother 38:2351–2356. doi: 10.1128/AAC.38.10.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.VanScoy BD, Mendes RE, Castanheira M, McCauley J, Bhavnani SM, Jones RN, Friedrich LV, Steenbergen JN, Ambrose PG. 2014. Relationship between ceftolozane-tazobactam exposure and selection for Pseudomonas aeruginosa resistance in a hollow-fiber infection model. Antimicrob Agents Chemother 58:6024–6031. doi: 10.1128/AAC.02310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]