Abstract
Despite the enormous disease burden associated with dengue virus infections, a licensed antiviral drug is lacking. Here, we show that the paracetamol (acetaminophen) metabolite AM404 inhibits dengue virus replication. Moreover, we find that mutations in NS4B that were previously found to confer resistance to the antiviral compounds NITD-618 and SDM25N also render dengue virus insensitive to AM404. Our work provides further support for NS4B as a direct or indirect target for antiviral drug development.
TEXT
Dengue virus (DENV) is a major health concern. The virus was initially estimated to cause 50 million to 100 million infections each year (1, 2), but more recent estimates suggest even greater numbers (390 million infections, of which 96 million manifest clinically) (3). Dengue is a mosquito-transmitted infection that was once considered a tropical disease, but the virus is spreading rapidly across the globe and is now endemic in 128 countries, with up to 4 billion people at risk of infection (1, 4–7). No specific drug or licensed vaccine is available for DENV infection, leaving vector control the only option to prevent transmission, although this approach is threatened by the emergence of insecticide resistance (8–10). A specific antiviral therapeutic agent would be an important tool to inhibit virus replication and transmission and to reduce the global burden of DENV.
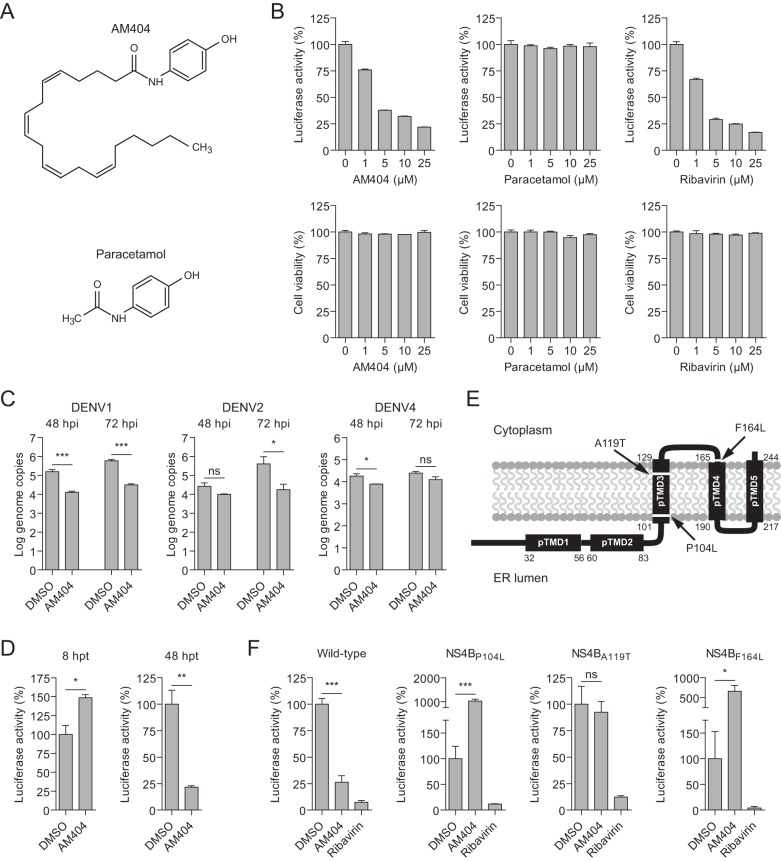
To identify new inhibitors of DENV replication, we screened the NIH Clinical Collection, a library of small molecules with a history of use in humans, using a replicon-based assay in HeLa cells (11). In this DENV serotype 2 (DENV2) (strain New Guinea C [NGC])-based replicon (RepDVPacLuc), the structural genes are replaced by a puromycin resistance gene and a firefly luciferase (FLuc) reporter gene, which can be assessed as a readout for virus replication (11, 12). AM404 (PubChem identification no. 6604822) was one of the compounds that, at a concentration of 10 μM, reduced FLuc activity in HeLa DENV2 replicon cells by >50%, relative to the dimethyl sulfoxide (DMSO) control, without affecting cell viability by >20%. AM404, also known as N-arachidonoylphenolamine, is an active metabolite of paracetamol (acetaminophen) (Fig. 1A) and is suggested to be responsible for all or part of its analgesic activity (13, 14).
FIG 1.
AM404 inhibits DENV genomic RNA replication. (A) Molecular structures of paracetamol (acetaminophen) and its metabolite AM404. (B) Luciferase activity (top) and cell viability (lower) of HeLa cells containing a stably replicating subgenomic DENV2 replicon (RepDVPacLuc). The cells were treated for 2 days with the indicated compounds before luciferase activity and cell viability were assessed. (C) Viral RNA accumulation, at 48 and 72 hpi, in the culture supernatant of HeLa cells treated with AM404 (20 μM) or DMSO and infected with wild-type DENV1 (strain 16007), DENV2 (strain NGC), or DENV4 (strain H241). (D) Luciferase activity, at 8 and 48 hpt, in HeLa cells treated with AM404 (10 μM) or DMSO and transfected with DENV2 replicon RNA (RepDVRLuc). (E) Schematic diagram of the resistance mutations P104L, A119T, and F164L (arrows) in NS4B. The DENV2 NS4B membrane topology was determined by Miller et al. (34). pTMD1 to pTMD5, predicted transmembrane domains. ER, endoplasmic reticulum. A recent NMR study of recombinant NS4B (residues 1 to 125) identified two helical structures overlapping pTMD1 and pTMD2 (α2, residues 36 to 57; α3, residues 61 to 82), which were proposed to be transmembrane regions (35). In addition, the study confirmed the presence of a transmembrane helix overlapping pTMD3 (α5, residues 105 to 124). Based on the predicted structure, the A119T and P104L substitutions are located close to the phospholipid head groups. (F) Luciferase activity, at 48 hpt, in HeLa cells transfected with wild-type or mutant DENV2 replicon RNA (RepDVRLuc) and treated with AM404 (10 μM), ribavirin (10 μM), or DMSO. All compounds were dissolved in DMSO, which was used as a negative control in all experiments. Ribavirin was included as a positive control. Luciferase activity and cell viability in panels B, D, and F are expressed as percentages of the DMSO-treated control values. Bars and error bars represent the means and standard errors of the means, respectively, of three independent samples. Statistical significance in panels C, D, and F was assessed with unpaired t tests. ***, P < 0.001; **, P < 0.01; *, P < 0.05; ns, not significant.
To confirm the results from our screen, we analyzed an independent batch of AM404 (Tocris Bioscience; purity, 99.5% by HPLC) for antiviral activity on HeLa DENV2 replicon cells and found that AM404, but not paracetamol, reduced FLuc activity in a dose-dependent manner (50% effective concentration [EC50], 3.6 μM [95% confidence interval [CI], 3.0 to 4.2 μM]) (Fig. 1B). As expected (15–17), the nucleoside analogue ribavirin (Sigma-Aldrich), which was used as a positive control, also inhibited virus replication in a dose-dependent manner (EC50, 2.2 μM [95% CI, 1.8 to 2.6 μM]) (Fig. 1B). Importantly, none of the compounds affected cell viability, as assessed by a colorimetric assay for cell metabolic activity (Fig. 1B). We next analyzed whether AM404 also inhibits replication of wild-type DENV. Because it has been reported that antiviral compounds can be serotype specific (18), we tested DENV2 strain NGC, DENV serotype 1 (DENV1) strain 16007 (19), and DENV serotype 4 (DENV4) strain H241 (20). HeLa cells were infected with these viruses at a multiplicity of infection (MOI) of 0.01 times the 50% cell culture infective dose (CCID50) per cell and treated with AM404 or DMSO. Virus accumulation was measured in the supernatant by quantitative reverse transcription (qRT)-PCR, at 48 and 72 h postinfection (hpi) (for primer sequences and methods, see Table S1 in the supplemental material). As expected on the basis of our replicon data, AM404 treatment resulted in 3- and 25-fold reductions in viral RNA accumulation of DENV2 at 48 and 72 hpi, respectively (Fig. 1C). Similarly, AM404 reduced DENV1 RNA accumulation 16- and 19-fold at these time points, but we observed only mild decreases in viral RNA production for DENV4-infected cells (∼2-fold reductions at both time points) (Fig. 1C).
As we used a subgenomic replicon in the initial screen, our results imply that AM404 inhibits a postentry stage of the DENV replication cycle. To better define which stage of the viral life cycle is targeted by AM404, we analyzed luciferase activity at 8 and 48 h posttransfection (hpt) of RNA of a replicon in which the structural genes are replaced by a Renilla luciferase (RLuc) reporter gene (RepDVRLuc) (11). At 8 hpt, viral RNA has not yet been replicated, and, as a consequence, RLuc can be produced only by translation of the transfected RNA (21). At 48 hpt, in contrast, the viral RNA is replicating and Rluc may be derived from progeny viral RNA (21). AM404 strongly reduced RLuc activity at 48 hpt but not at 8 hpt, indicating that AM404 inhibits viral RNA replication rather than translation of the viral genome (Fig. 1D).
The identification of resistance mutations in viral genomes can be a first step toward defining the mechanism of action of novel antiviral drugs. Therefore, we aimed at generating resistant replicons by prolonged passage of HeLa DENV2 replicon cells in the presence of both puromycin and AM404, an approach that we previously used to generate replicons that are resistant to SDM25N (11). Thus far, however, we have been unsuccessful in generating novel AM404-resistant replicons. As an alternative, we tested DENV mutants that escaped the antiviral activity of other compounds for cross-resistance to AM404. Specifically, we tested mutant RepDVRLuc replicons carrying the following amino acid substitutions in the viral NS4B protein: a proline-to-leucine substitution at position 104 (P104L), an alanine-to-threonine substitution at position 119 (A119T), and a phenylalanine-to-leucine substitution at position 164 (F164L) (Fig. 1E). These mutations were previously found to render DENV insensitive to the structurally unrelated antiviral compounds SDM25N (P104L and F164L) (11) and NITD-618 (P104L and A119T) (22). Surprisingly, all three amino acid substitutions rendered DENV insensitive to AM404, whereas the mutants remained sensitive to ribavirin (Fig. 1F). Unexpectedly, AM404 treatment increased RLuc levels of the P104L and F164L mutants but not the A119T mutant (Fig. 1F). Similar but less pronounced increases in replication were previously observed for these mutants upon SDM25N treatment (11). The mechanism for the increased replication of the mutants in the presence of AM404 remains to be understood. A possible scenario is that AM404 exerts two opposing effects on DENV replication, i.e., stimulatory and inhibitory. In wild-type DENV infections, the inhibitory effect may be stronger than the stimulatory effect, whereas the inhibitory effect may be lost in infections with escape mutants, resulting in AM404-dependent increases in replication.
A range of biological activities have been attributed to paracetamol/AM404; it is an agonist of the TRPV1 receptor, an inhibitor of cellular anandamide reuptake, an indirect activator of cannabinoid receptors, an inhibitor of the cyclooxygenases Cox-1 and Cox-2 and prostaglandin production, an inhibitor of NFAT activity, and an inhibitor of IκB kinase beta phosphorylation and activation (13, 14, 23–25). Given these pleiotropic effects, it will not be trivial to define the mechanism by which AM404 inhibits virus replication. We note, however, that the NIH Clinical Collection contains a number of Cox-2 and nonselective Cox inhibitors, none of which reduced FLuc activities in HeLa DENV2 replicon cells more than 2-fold at a concentration of 10 μM (see Table S2 in the supplemental material).
In conclusion, we have shown that the paracetamol metabolite AM404 has anti-DENV activity and that mutations in NS4B render the virus insensitive to AM404. Successful antiviral therapies may target either the virus itself or host factors that are required for virus replication. Viral enzymes, such as viral proteases and polymerases, are attractive drug targets because they are essential for virus replication and are not expressed by noninfected host cells (26–28). However, recent examples indicate that host proteins can be successful drug targets as well (29–31). Although we have not formally excluded the possibility that AM404 acts directly on NS4B, we deem it likely that AM404 targets a cellular pathway that DENV needs for efficient replication.
Several other flavivirus NS4B inhibitors have recently been reported, including SDM25N (11), NITD-618 (22), spiropyrazolopyridone compound 14a (18), and CCG-3394 and CCG-4088 (32). All of these compounds inhibit viral RNA accumulation, with EC50s ranging from 42 nM for compound 14a to 0.4 to 1.9 μM for the other compounds (33). A direct interaction between compound 14a and NS4B has been demonstrated (18); the NS4B-targeting activities of the other compounds have been deduced from the emergence of resistance mutations in NS4B (11, 22, 32). The pleiotropic effects of AM404 and the relatively high EC50 will be hurdles for further drug development. However, AM404 (and other NS4B inhibitors) may be useful experimental tools to identify host factors needed for DENV replication, providing insights into the viral life cycle. Moreover, these host factors may represent novel targets for antiviral drug development. The observation that the same mutations in NS4B provide resistance to multiple, structurally unrelated compounds (AM404, NITD-618, and SDM25N) suggests that these drugs target the same host factors or pathways. However, the mechanistic bases for their antiviral activity and for NS4B-mediated escape await elucidation.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jolanda Smit (University Medical Center Groningen, Groningen, The Netherlands) for kindly providing DENV2 strain NGC, Beate Kümmerer (University of Bonn Medical Center, Bonn, Germany) for providing DENV1 strain 16007 and DENV4 strain H241, and Andrew Davidson (University of Bristol, Bristol, United Kingdom) for providing the RepDVPacLuc replicon plasmid. The NIH Clinical Collection was kindly provided through the National Institutes of Health Molecular Libraries Roadmap Initiative. We thank Frank van Kuppeveld, Martin Feiters, and members of the van Rij laboratory for fruitful discussions.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02462-15.
REFERENCES
- 1.World Health Organization. 2009. Dengue: guidelines for diagnosis, treatment, prevention and control, new edition. WHO Press, Geneva, Switzerland. [PubMed] [Google Scholar]
- 2.Guzman A, Isturiz RE. 2010. Update on the global spread of dengue. Int J Antimicrob Agents 36(Suppl 1):S40–S42. doi: 10.1016/j.ijantimicag.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, Moyes CL, Farlow AW, Scott TW, Hay SI. 2012. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis 6:e1760. doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franco C, Hynes NA, Bouri N, Henderson DA. 2010. The dengue threat to the United States. Biosecur Bioterror 8:273–276. doi: 10.1089/bsp.2010.0032. [DOI] [PubMed] [Google Scholar]
- 6.La Ruche G, Souares Y, Armengaud A, Peloux-Petiot F, Delaunay P, Despres P, Lenglet A, Jourdain F, Leparc-Goffart I, Charlet F, Ollier L, Mantey K, Mollet T, Fournier JP, Torrents R, Leitmeyer K, Hilairet P, Zeller H, Van Bortel W, Dejour-Salamanca D, Grandadam M, Gastellu-Etchegorry M. 2010. First two autochthonous dengue virus infections in metropolitan France, September 2010. Euro Surveill 15(39):pii=19676 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19676. [PubMed] [Google Scholar]
- 7.Gjenero-Margan I, Aleraj B, Krajcar D, Lesnikar V, Klobucar A, Pem-Novosel I, Kurecic-Filipovic S, Komparak S, Martic R, Duricic S, Betica-Radic L, Okmadzic J, Vilibic-Cavlek T, Babic-Erceg A, Turkovic B, Avsic-Zupanc T, Radic I, Ljubic M, Sarac K, Benic N, Mlinaric-Galinovic G. 2011. Autochthonous dengue fever in Croatia, August–September 2010. Euro Surveill 16(9):pii=19805 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19805. [PubMed] [Google Scholar]
- 8.Hemingway J. 2014. The role of vector control in stopping the transmission of malaria: threats and opportunities. Philos Trans R Soc Lond B Biol Sci 369:20130431. doi: 10.1098/rstb.2013.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemingway J, Beaty BJ, Rowland M, Scott TW, Sharp BL. 2006. The Innovative Vector Control Consortium: improved control of mosquito-borne diseases. Trends Parasitol 22:308–312. doi: 10.1016/j.pt.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Hemingway J, Hawkes NJ, McCarroll L, Ranson H. 2004. The molecular basis of insecticide resistance in mosquitoes. Insect Biochem Mol Biol 34:653–665. doi: 10.1016/j.ibmb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 11.van Cleef KW, Overheul GJ, Thomassen MC, Kaptein SJ, Davidson AD, Jacobs M, Neyts J, van Kuppeveld FJ, van Rij RP. 2013. Identification of a new dengue virus inhibitor that targets the viral NS4B protein and restricts genomic RNA replication. Antiviral Res 99:165–171. doi: 10.1016/j.antiviral.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Kaptein SJ, De Burghgraeve T, Froeyen M, Pastorino B, Alen MM, Mondotte JA, Herdewijn P, Jacobs M, de Lamballerie X, Schols D, Gamarnik AV, Sztaricskai F, Neyts J. 2010. A derivate of the antibiotic doxorubicin is a selective inhibitor of dengue and yellow fever virus replication in vitro. Antimicrob Agents Chemother 54:5269–5280. doi: 10.1128/AAC.00686-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogestatt ED, Jonsson BA, Ermund A, Andersson DA, Bjork H, Alexander JP, Cravatt BF, Basbaum AI, Zygmunt PM. 2005. Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. J Biol Chem 280:31405–31412. doi: 10.1074/jbc.M501489200. [DOI] [PubMed] [Google Scholar]
- 14.Mallet C, Barriere DA, Ermund A, Jonsson BA, Eschalier A, Zygmunt PM, Hogestatt ED. 2010. TRPV1 in brain is involved in acetaminophen-induced antinociception. PLoS One 5(9):e12748. doi: 10.1371/journal.pone.0012748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takhampunya R, Ubol S, Houng HS, Cameron CE, Padmanabhan R. 2006. Inhibition of dengue virus replication by mycophenolic acid and ribavirin. J Gen Virol 87:1947–1952. doi: 10.1099/vir.0.81655-0. [DOI] [PubMed] [Google Scholar]
- 16.Diamond MS, Zachariah M, Harris E. 2002. Mycophenolic acid inhibits dengue virus infection by preventing replication of viral RNA. Virology 304:211–221. doi: 10.1006/viro.2002.1685. [DOI] [PubMed] [Google Scholar]
- 17.Huang YH, Lei HY, Liu HS, Lin YS, Liu CC, Yeh TM. 2000. Dengue virus infects human endothelial cells and induces IL-6 and IL-8 production. Am J Trop Med Hyg 63:71–75. [DOI] [PubMed] [Google Scholar]
- 18.Wang QY, Dong H, Zou B, Karuna R, Wan KF, Zou J, Susila A, Yip A, Shan C, Yeo KL, Xu H, Ding M, Chan WL, Gu F, Seah PG, Liu W, Lakshminarayana SB, Kang C, Lescar J, Blasco F, Smith PW, Shi PY. 2015. Discovery of dengue virus NS4B inhibitors. J Virol 89:8233–8244. doi: 10.1128/JVI.00855-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang CY, Butrapet S, Pierro DJ, Chang GJ, Hunt AR, Bhamarapravati N, Gubler DJ, Kinney RM. 2000. Chimeric dengue type 2 (vaccine strain PDK-53)/dengue type 1 virus as a potential candidate dengue type 1 virus vaccine. J Virol 74:3020–3028. doi: 10.1128/JVI.74.7.3020-3028.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halstead SB, Diwan AR, Marchette NJ, Palumbo NE, Srisukonth L. 1984. Selection of attenuated dengue 4 viruses by serial passage in primary kidney cells. I. Attributes of uncloned virus at different passage levels. Am J Trop Med Hyg 33:654–665. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez DE, De Lella Ezcurra AL, Fucito S, Gamarnik AV. 2005. Role of RNA structures present at the 3′UTR of dengue virus on translation, RNA synthesis, and viral replication. Virology 339:200–212. doi: 10.1016/j.virol.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Xie X, Wang QY, Xu HY, Qing M, Kramer L, Yuan Z, Shi PY. 2011. Inhibition of dengue virus by targeting viral NS4B protein. J Virol 85:11183–11195. doi: 10.1128/JVI.05468-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ottani A, Leone S, Sandrini M, Ferrari A, Bertolini A. 2006. The analgesic activity of paracetamol is prevented by the blockade of cannabinoid CB1 receptors. Eur J Pharmacol 531:280–281. doi: 10.1016/j.ejphar.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Bertolini A, Ferrari A, Ottani A, Guerzoni S, Tacchi R, Leone S. 2006. Paracetamol: new vistas of an old drug. CNS Drug Rev 12:250–275. doi: 10.1111/j.1527-3458.2006.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caballero FJ, Soler-Torronteras R, Lara-Chica M, Garcia V, Fiebich BL, Munoz E, Calzado MA. 2015. AM404 inhibits NFAT and NF-κB signaling pathways and impairs migration and invasiveness of neuroblastoma cells. Eur J Pharmacol 746:221–232. doi: 10.1016/j.ejphar.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 26.Feeney ER, Chung RT. 2014. Antiviral treatment of hepatitis C. BMJ 348:g3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Looney D, Ma A, Johns S. 2015. HIV therapy: the state of art. Curr Top Microbiol Immunol 389:1–29. [DOI] [PubMed] [Google Scholar]
- 28.Webster DP, Klenerman P, Dusheiko GM. 2015. Hepatitis C. Lancet 385:1124–1135. doi: 10.1016/S0140-6736(14)62401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lou Z, Sun Y, Rao Z. 2014. Current progress in antiviral strategies. Trends Pharmacol Sci 35:86–102. doi: 10.1016/j.tips.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sayce AC, Miller JL, Zitzmann N. 2010. Targeting a host process as an antiviral approach against dengue virus. Trends Microbiol 18:323–330. doi: 10.1016/j.tim.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Lim SP, Wang QY, Noble CG, Chen YL, Dong H, Zou B, Yokokawa F, Nilar S, Smith P, Beer D, Lescar J, Shi PY. 2013. Ten years of dengue drug discovery: progress and prospects. Antiviral Res 100:500–519. doi: 10.1016/j.antiviral.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Patkar CG, Larsen M, Owston M, Smith JL, Kuhn RJ. 2009. Identification of inhibitors of yellow fever virus replication using a replicon-based high-throughput assay. Antimicrob Agents Chemother 53:4103–4114. doi: 10.1128/AAC.00074-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie X, Zou J, Wang QY, Shi PY. 2015. Targeting dengue virus NS4B protein for drug discovery. Antiviral Res 118:39–45. doi: 10.1016/j.antiviral.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Miller S, Sparacio S, Bartenschlager R. 2006. Subcellular localization and membrane topology of the dengue virus type 2 non-structural protein 4B. J Biol Chem 281:8854–8863. doi: 10.1074/jbc.M512697200. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Kim YM, Zou J, Wang QY, Gayen S, Wong YL, Lee le T, Xie X, Huang Q, Lescar J, Shi PY, Kang C. 2015. Secondary structure and membrane topology of dengue virus NS4B N-terminal 125 amino acids. Biochim Biophys Acta 1848:3150–3157. doi: 10.1016/j.bbamem.2015.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.