Abstract
The in vitro antibacterial activities of ceftazidime-avibactam and comparator agents were evaluated using reference broth microdilution methods against 1,743 Pseudomonas aeruginosa isolates collected in 2014 from 69 U.S. medical centers, representing each of the nine census regions. Ceftazidime-avibactam demonstrated potent activity against P. aeruginosa, including many isolates not susceptible to ceftazidime, meropenem, and piperacillin-tazobactam. In each of the nine census regions, ceftazidime-avibactam demonstrated the highest percentage of susceptible isolates.
TEXT
Increasing occurrence of antimicrobial resistance in Gram-negative bacilli, including Enterobacteriaceae and Pseudomonas aeruginosa, has complicated the treatment of serious nosocomial infections. β-Lactam antibacterials, which were once highly effective against these pathogens, have been compromised by isolates that harbor resistance due to the production of extended-spectrum β-lactamases (ESBLs) and carbapenemases (1–4), along with other resistance mechanisms, including efflux and porin loss (5, 6) and the recent emergence of plasmid-mediated resistance to colistin (7). The spread of β-lactamases is particularly problematic due to the potential for additional mutations that can broaden their spectrum of hydrolysis, as well as their ability to disseminate to other pathogens. Ceftazidime-avibactam is the combination of the established third-generation cephalosporin ceftazidime and the novel non–β-lactam β-lactamase inhibitor avibactam. Avibactam inhibits a broad range of serine β-lactamases, including Ambler class A (ESBL and Klebsiella pneumoniae carbapenemase), class C (AmpC), and some class D (OXA-48) enzymes. When used in combination with ceftazidime, avibactam restores the activity of ceftazidime against a number of clinically relevant β-lactamase–producing Gram-negative pathogens that cause serious infections (8). We evaluated the in vitro antibacterial activities and susceptibility patterns of ceftazidime-avibactam and comparator compounds against P. aeruginosa surveillance isolates obtained in 2014 from a variety of infection types (skin and soft tissue, urinary tract, intra-abdominal, and others) from each of the nine census regions within the United States.
A total of 1,743 P. aeruginosa isolates collected in 2014 from 69 medical centers within the nine U.S. census regions were included in the International Network for Optimal Resistance Monitoring (INFORM) surveillance program. Broth microdilution susceptibility testing for ceftazidime-avibactam, ceftazidime, cefepime, meropenem, piperacillin-tazobactam, ciprofloxacin, levofloxacin, amikacin, gentamicin, and colistin was performed according to Clinical and Laboratory Standards Institute (CLSI) guidelines (9) using validated MIC panels produced by Thermo Fisher Scientific (Cleveland, OH). Susceptibility interpretive criteria for comparator compounds included both CLSI (10) and EUCAST (European Committee on Antimicrobial Susceptibility Testing) (11) breakpoint criteria, when available. The recently approved U.S. Food and Drug Administration (FDA) breakpoint interpretative criteria were applied for ceftazidime-avibactam (12). Quality-control (QC) testing included the following reference bacterial strains: Escherichia coli ATCC 25922, E. coli ATCC 35218, Klebsiella pneumoniae ATCC 700603, and P. aeruginosa ATCC 27853. All QC results for ceftazidime-avibactam and comparator compounds were within published ranges (10). Ceftazidime-nonsusceptible and meropenem-nonsusceptible isolates displayed intermediate or resistant MIC values (≥16 or ≥4 μg/ml, respectively) according to published breakpoint criteria (10, 11).
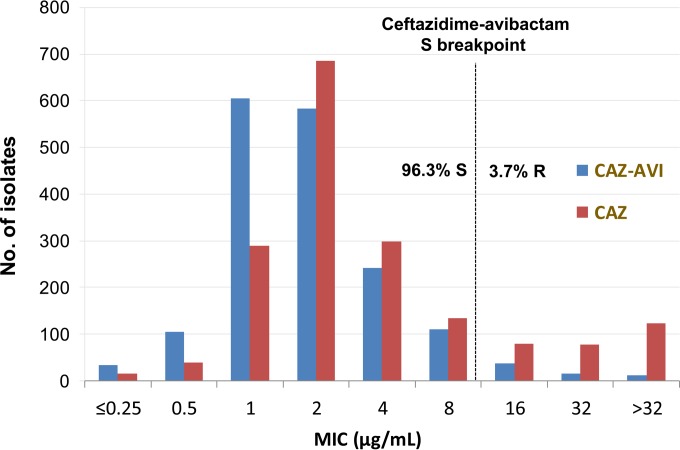
Table 1 lists ceftazidime-avibactam and comparator compound susceptibility testing results against 1,743 P. aeruginosa isolates collected in 2014, including the number of isolates tested, the MIC50, the MIC90, and the percentages of susceptible, intermediate, and resistant isolates by U.S. census region, categorized according to CLSI, EUCAST, and/or FDA breakpoint interpretive criteria. Ceftazidime-avibactam activity (MIC50/90, 2/8 μg/ml; 96.3% susceptible at ≤8 μg/ml) against all 1,743 P. aeruginosa isolates was enhanced over that with ceftazidime when tested alone (MIC50/90, 2/32 μg/ml; 84.0% susceptible at ≤8 μg/ml; Fig. 1; Table 1) and was more active than the other β-lactam comparators, including cefepime, meropenem, and piperacillin-tazobactam (86.5%, 83.0%, and 83.0% susceptible, respectively).
TABLE 1.
Activities of ceftazidime-avibactam and comparator antimicrobial agents against contemporary Pseudomonas aeruginosa isolates by census region
| Region and antimicrobial agent (no. tested) | MIC50 | MIC90 | CLSIa |
EUCASTa |
||||
|---|---|---|---|---|---|---|---|---|
| %S | %I | %R | %S | %I | %R | |||
| All regions (1,743) | ||||||||
| Ceftazidime-avibactam | 2 | 8 | 96.3 | 3.7b | ||||
| Ceftazidime | 2 | 32 | 84.0 | 4.5 | 11.5 | 84.0 | 16.0 | |
| Cefepime | 2 | 16 | 86.5 | 8.0 | 5.5 | 86.5 | 13.5 | |
| Piperacillin-tazobactam | 4 | 64 | 83.0 | 8.7 | 8.3 | 83.0 | 17.0 | |
| Meropenem | 0.5 | 8 | 83.0 | 6.1 | 10.9 | 83.0 | 11.6 | 5.3 |
| Ciprofloxacin | 0.12 | >4 | 77.9 | 4.9 | 17.2 | 72.0 | 5.9 | 22.1 |
| Levofloxacin | 0.5 | >4 | 75.2 | 6.4 | 18.5 | 65.3 | 9.8 | 24.8 |
| Gentamicin | ≤1 | 8 | 88.0 | 3.7 | 8.3 | 88.0 | 12.0 | |
| Amikacin | 2 | 8 | 96.7 | 1.2 | 2.1 | 93.2 | 3.5 | 3.3 |
| Colistin | 2 | 2 | 99.0 | 1.0 | 0.0 | 100.0 | 0.0 | |
| Region 1, New England (119) | ||||||||
| Ceftazidime-avibactam | 1 | 4 | 100.0 | 0.0b | ||||
| Ceftazidime | 2 | 32 | 84.0 | 5.9 | 10.1 | 84.0 | 16.0 | |
| Cefepime | 2 | 16 | 85.7 | 12.6 | 1.7 | 85.7 | 14.3 | |
| Piperacillin-tazobactam | 4 | 64 | 86.6 | 8.4 | 5.0 | 86.6 | - | 13.4 |
| Meropenem | 0.25 | 4 | 85.7 | 5.0 | 9.2 | 85.7 | 12.6 | 1.7 |
| Ciprofloxacin | 0.12 | >4 | 75.6 | 5.9 | 18.5 | 72.3 | 3.4 | 24.4 |
| Levofloxacin | 0.5 | >4 | 73.9 | 6.7 | 19.3 | 68.1 | 5.9 | 26.1 |
| Gentamicin | ≤1 | >8 | 87.4 | 0.8 | 11.8 | 87.4 | 12.6 | |
| Amikacin | 2 | 8 | 97.5 | 0.8 | 1.7 | 93.3 | 4.2 | 2.5 |
| Colistin | 2 | 2 | 99.2 | 0.8 | 0.0 | 100.0 | 0.0 | |
| Region 2, Mid-Atlantic (199) | ||||||||
| Ceftazidime-avibactam | 2 | 8 | 96.0 | 4.0b | ||||
| Ceftazidime | 2 | 32 | 80.9 | 7.5 | 11.6 | 80.9 | 19.1 | |
| Cefepime | 2 | 16 | 84.9 | 10.1 | 5.0 | 84.9 | 15.1 | |
| Piperacillin-tazobactam | 8 | >64 | 77.9 | 11.6 | 10.6 | 77.9 | 22.1 | |
| Meropenem | 0.5 | 8 | 79.9 | 8.5 | 11.6 | 79.9 | 14.6 | 5.5 |
| Ciprofloxacin | 0.12 | >4 | 83.9 | 2.5 | 13.6 | 75.9 | 8.0 | 16.1 |
| Levofloxacin | 0.5 | >4 | 79.9 | 6.0 | 14.1 | 69.8 | 10.1 | 20.1 |
| Gentamicin | ≤1 | 4 | 91.0 | 4.5 | 4.5 | 91.0 | 9.0 | |
| Amikacin | 2 | 8 | 99.0 | 0.0 | 1.0 | 96.0 | 3.0 | 1.0 |
| Colistin | 2 | 2 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | |
| Region 3, East North Central (299) | ||||||||
| Ceftazidime-avibactam | 2 | 4 | 97.3 | 2.7b | ||||
| Ceftazidime | 2 | 16 | 88.6 | 3.0 | 8.4 | 88.6 | 11.4 | |
| Cefepime | 2 | 16 | 90.0 | 6.7 | 3.3 | 90.0 | 10.0 | |
| Piperacillin-tazobactam | 4 | 32 | 86.6 | 7.4 | 6.0 | 86.6 | 13.4 | |
| Meropenem | 0.5 | 8 | 83.3 | 6.4 | 10.4 | 83.3 | 11.4 | 5.4 |
| Ciprofloxacin | 0.12 | >4 | 78.6 | 5.7 | 15.7 | 74.2 | 4.3 | 21.4 |
| Levofloxacin | 0.5 | >4 | 77.9 | 4.7 | 17.4 | 67.6 | 10.4 | 22.1 |
| Gentamicin | ≤1 | 8 | 88.3 | 5.4 | 6.4 | 88.3 | 11.7 | |
| Amikacin | 2 | 8 | 97.7 | 0.3 | 2.0 | 93.6 | 4.0 | 2.3 |
| Colistin | 2 | 2 | 98.7 | 1.3 | 0.0 | 100.0 | 0.0 | |
| Region 4, West North Central (191) | ||||||||
| Ceftazidime-avibactam | 1 | 4 | 99.0 | 1.0b | ||||
| Ceftazidime | 2 | 8 | 91.6 | 3.7 | 4.7 | 91.6 | 8.4 | |
| Cefepime | 2 | 8 | 93.7 | 4.7 | 1.6 | 93.7 | 6.3 | |
| Piperacillin-tazobactam | 4 | 16 | 93.2 | 3.7 | 3.1 | 93.2 | 6.8 | |
| Meropenem | 0.25 | 2 | 94.8 | 1.6 | 3.7 | 94.8 | 2.6 | 2.6 |
| Ciprofloxacin | 0.12 | 4 | 85.3 | 4.2 | 10.5 | 80.6 | 4.7 | 14.7 |
| Levofloxacin | 0.5 | >4 | 83.2 | 5.2 | 11.5 | 73.3 | 9.9 | 16.8 |
| Gentamicin | ≤1 | 4 | 95.3 | 2.6 | 2.1 | 95.3 | 4.7 | |
| Amikacin | 2 | 8 | 99.5 | 0.5 | 0.0 | 97.9 | 1.6 | 0.5 |
| Colistin | 2 | 2 | 99.0 | 1.0 | 0.0 | 100.0 | 0.0 | |
| Region 5, South Atlantic (266) | ||||||||
| Ceftazidime-avibactam | 2 | 8 | 95.1 | 4.9b | ||||
| Ceftazidime | 2 | 32 | 82.0 | 5.3 | 12.8 | 82.0 | 18.0 | |
| Cefepime | 4 | 16 | 83.5 | 7.5 | 9.0 | 83.5 | 16.5 | |
| Piperacillin-tazobactam | 4 | 64 | 83.1 | 7.5 | 9.4 | 83.1 | 16.9 | |
| Meropenem | 0.5 | 8 | 82.3 | 5.6 | 12.0 | 82.3 | 12.8 | 4.9 |
| Ciprofloxacin | 0.25 | 4 | 77.8 | 8.3 | 13.9 | 71.8 | 6.0 | 22.2 |
| Levofloxacin | 0.5 | >4 | 74.8 | 7.9 | 17.3 | 62.8 | 12.0 | 25.2 |
| Gentamicin | 2 | >8 | 85.0 | 3.8 | 11.3 | 85.0 | 15.0 | |
| Amikacin | 4 | 16 | 91.7 | 3.0 | 5.3 | 86.1 | 5.6 | 8.3 |
| Colistin | 2 | 2 | 98.1 | 1.9 | 0.0 | 100.0 | 0.0 | |
| Region 6, East South Central (153) | ||||||||
| Ceftazidime-avibactam | 2 | 8 | 95.4 | 4.6b | ||||
| Ceftazidime | 4 | 32 | 79.1 | 4.6 | 16.3 | 79.1 | 20.9 | |
| Cefepime | 2 | 16 | 81.0 | 13.1 | 5.9 | 81.0 | 19.0 | |
| Piperacillin-tazobactam | 4 | >64 | 75.2 | 9.2 | 15.7 | 75.2 | 24.8 | |
| Meropenem | 0.5 | 8 | 78.4 | 7.2 | 14.4 | 78.4 | 15.7 | 5.9 |
| Ciprofloxacin | 0.12 | >4 | 77.8 | 3.9 | 18.3 | 69.9 | 7.8 | 22.2 |
| Levofloxacin | 0.5 | >4 | 71.9 | 9.2 | 19.0 | 63.4 | 8.5 | 28.1 |
| Gentamicin | ≤1 | >8 | 85.6 | 2.0 | 12.4 | 85.6 | 14.4 | |
| Amikacin | 2 | 8 | 98.7 | 0.7 | 0.7 | 95.4 | 3.3 | 1.3 |
| Colistin | 2 | 2 | 98.7 | 1.3 | 0.0 | 100.0 | 0.0 | |
| Region 7, West South Central (150) | ||||||||
| Ceftazidime-avibactam | 2 | 4 | 96.7 | 3.3b | ||||
| Ceftazidime | 2 | 32 | 82.7 | 5.3 | 12.0 | 82.7 | 17.3 | |
| Cefepime | 2 | 16 | 86.7 | 8.0 | 5.3 | 86.7 | 13.3 | |
| Piperacillin-tazobactam | 4 | 64 | 81.3 | 10.7 | 8.0 | 81.3 | 18.7 | |
| Meropenem | 0.5 | 8 | 80.7 | 6.7 | 12.7 | 80.7 | 12.7 | 6.7 |
| Ciprofloxacin | 0.25 | >4 | 68.0 | 4.0 | 28.0 | 62.7 | 5.3 | 32.0 |
| Levofloxacin | 0.5 | >4 | 66.7 | 4.0 | 29.3 | 56.7 | 10.0 | 33.3 |
| Gentamicin | ≤1 | 8 | 88.7 | 2.0 | 9.3 | 88.7 | 11.3 | |
| Amikacin | 2 | 8 | 96.7 | 2.0 | 1.3 | 94.7 | 2.0 | 3.3 |
| Colistin | 2 | 2 | 98.0 | 2.0 | 0.0 | 100.0 | 0.0 | |
| Region 8, Mountain (166) | ||||||||
| Ceftazidime-avibactam | 2 | 8 | 97.0 | 3.0b | ||||
| Ceftazidime | 2 | 32 | 81.9 | 4.2 | 13.9 | 81.9 | 18.1 | |
| Cefepime | 2 | 16 | 87.3 | 6.6 | 6.0 | 87.3 | 12.7 | |
| Piperacillin-tazobactam | 4 | 64 | 79.5 | 13.3 | 7.2 | 79.5 | 20.5 | |
| Meropenem | 0.5 | 8 | 77.7 | 9.6 | 12.7 | 77.7 | 15.7 | 6.6 |
| Ciprofloxacin | 0.25 | >4 | 75.3 | 1.8 | 22.9 | 68.1 | 7.2 | 24.7 |
| Levofloxacin | 0.5 | >4 | 70.5 | 6.0 | 23.5 | 62.7 | 7.8 | 29.5 |
| Gentamicin | ≤1 | 8 | 89.8 | 4.8 | 5.4 | 89.8 | 10.2 | |
| Amikacin | 2 | 8 | 99.4 | 0.6 | 0.0 | 98.2 | 1.2 | 0.6 |
| Colistin | 2 | 2 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | |
| Region 9, Pacific (200) | ||||||||
| Ceftazidime-avibactam | 2 | 8 | 91.5 | 8.5b | ||||
| Ceftazidime | 2 | >32 | 82.0 | 2.5 | 15.5 | 82.0 | 18.0 | |
| Cefepime | 2 | 16 | 84.0 | 6.0 | 10.0 | 84.0 | 16.0 | |
| Piperacillin-tazobactam | 4 | >64 | 81.0 | 8.5 | 10.5 | 81.0 | 19.0 | |
| Meropenem | 0.25 | 8 | 83.5 | 4.5 | 12.0 | 83.5 | 8.5 | 8.0 |
| Ciprofloxacin | 0.25 | >4 | 74.5 | 6.0 | 19.5 | 68.5 | 6.0 | 25.5 |
| Levofloxacin | 0.5 | >4 | 72.5 | 8.0 | 19.5 | 62.0 | 10.5 | 27.5 |
| Gentamicin | 2 | >8 | 81.5 | 5.0 | 13.5 | 81.5 | 18.5 | |
| Amikacin | 2 | 16 | 93.0 | 2.5 | 4.5 | 88.0 | 5.0 | 7.0 |
| Colistin | 1 | 2 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | |
FIG 1.
Ceftazidime-avibactam and ceftazidime MIC distributions for Pseudomonas aeruginosa (n = 1,743; United States, 2014). CAZ-AVI, ceftazidime-avibactam; CAZ, ceftazidime.
The addition of avibactam to ceftazidime increased the percentages of susceptible P. aeruginosa isolates across each of the census regions from 7.4% (West North Central region) to 16.3% (east South Central region) over those of ceftazidime tested alone (Table 1). The greatest restorations of ceftazidime activity by avibactam against P. aeruginosa were observed in the New England and East South Central regions (16.0% and 16.3%, respectively, susceptibility increases over ceftazidime tested alone). Additionally, ceftazidime-avibactam consistently demonstrated the highest percentages of susceptible isolates in each of the nine census regions (91.5% to 100.0% susceptible; Table 1) when compared with those of cefepime, meropenem, and piperacillin-tazobactam (81.0% to 93.7%, 77.7% to 94.8%, and 75.2% to 93.2% susceptible, respectively). Only colistin (98.0% to 100% susceptible) demonstrated higher susceptibility percentages (CLSI and EUCAST interpretive criteria).
Susceptibilities were lowest for ceftazidime, cefepime, and piperacillin-tazobactam against P. aeruginosa (79.1%, 81.0%, and 75.2% susceptible, respectively) in the East South Central region (95.4% susceptible to ceftazidime-avibactam) and for meropenem (77.7%) in the Mountain region (97.0% susceptible to ceftazidime-avibactam). Susceptibilities to ceftazidime-avibactam were lowest for P. aeruginosa in the Pacific region (91.5% susceptible); however, ceftazidime-avibactam remained more active than the ceftazidime, cefepime, meropenem, and piperacillin-tazobactam, fluoroquinolone (ciprofloxacin and levofloxacin), and aminoglycoside (gentamicin) comparators, with the exceptions of amikacin and colistin (93.0% and 100.0% susceptible, respectively; Table 1). Susceptibilities of P. aeruginosa to ceftazidime-avibactam were highest in the New England and West North Central regions (100.0% and 99.0% susceptible, respectively). Similarly, susceptibilities to ceftazidime, cefepime, meropenem, and piperacillin-tazobactam were also highest (91.6% to 94.8% susceptible) in the West North Central region.
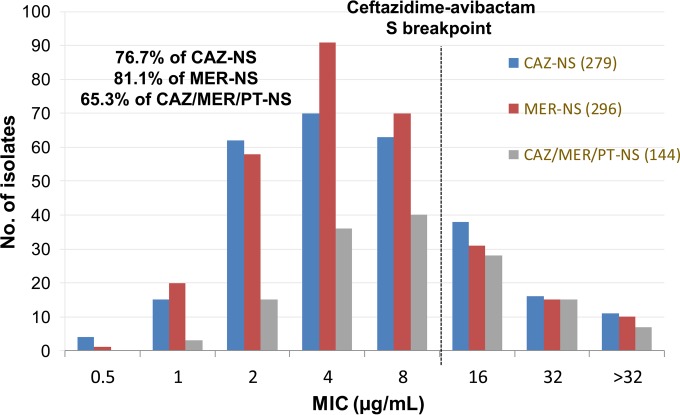
In vitro ceftazidime-avibactam activities were evaluated against resistant subgroups of P. aeruginosa (Fig. 2; Table 2), including ceftazidime-nonsusceptible (n = 279) and meropenem-nonsusceptible (n = 296) isolates. Ceftazidime-avibactam activity against ceftazidime-nonsusceptible P. aeruginosa (76.7% susceptible to ceftazidime-avibactam) was significantly greater than those for cefepime, meropenem, and piperacillin-tazobactam (31.9%, 40.1%, and 15.4% susceptible, respectively); moreover, ceftazidime-avibactam retained this improved in vitro potency against meropenem-nonsusceptible P. aeruginosa (81.1% ceftazidime-avibactam susceptible) isolates (40.5% to 46.3% susceptible to ceftazidime, cefepime, and piperacillin-tazobactam). It is interesting that, of the 144 P. aeruginosa isolates (8.3%) that were not susceptible to ceftazidime, meropenem, and piperacillin-tazobactam, 94 (65.3%) were susceptible to ceftazidime-avibactam (Fig. 2) at the FDA-approved breakpoint (12). In other studies, ceftazidime-avibactam resistance in P. aeruginosa isolates was primarily the result of decreased membrane permeability and upregulated efflux mechanisms and less frequently the result of a loss of outer membrane porins or the production of class B or D β-lactamases (13, 14).
FIG 2.
Ceftazidime-avibactam MIC distributions for resistant subsets of Pseudomonas aeruginosa isolates (Unites States, 2014). CAZ-NS, ceftazidime nonsusceptible; MER-NS, meropenem nonsusceptible; CAZ/MER/PT-NS, ceftazidime/meropenem/piperacillin-tazobactam nonsusceptible.
TABLE 2.
Activities of ceftazidime-avibactam and comparator antimicrobial agents against two resistant subgroups of Pseudomonas aeruginosa
| Region and antimicrobial agent (no. tested) | MIC50 | MIC90 | CLSIa |
EUCASTa |
||||
|---|---|---|---|---|---|---|---|---|
| %S | %I | %R | %S | %I | %R | |||
| Ceftazidime nonsusceptible (279) | ||||||||
| Ceftazidime-avibactam | 4 | 16 | 76.7 | 23.3b | ||||
| Cefepime | 16 | >16 | 31.9 | 36.2 | 31.9 | 31.9 | 68.1 | |
| Piperacillin-tazobactam | 64 | >64 | 15.4 | 36.6 | 48.0 | 15.4 | 84.6 | |
| Meropenem | 4 | 32 | 40.1 | 16.1 | 43.7 | 40.1 | 26.5 | |
| Meropenem nonsusceptible (296) | ||||||||
| Ceftazidime-avibactam | 4 | 16 | 81.1 | 18.9b | ||||
| Ceftazidime | 16 | >32 | 43.6 | 13.5 | 42.9 | 43.6 | 56.4 | |
| Cefepime | 16 | >16 | 46.3 | 29.4 | 24.3 | 46.3 | 53.7 | |
| Piperacillin-tazobactam | 32 | >64 | 40.5 | 27.4 | 32.1 | 40.5 | 59.5 | |
The high level of in vitro activity observed for ceftazidime-avibactam against P. aeruginosa was corroborated by an additional study that showed similar activity (67.4% susceptible to ceftazidime-avibactam) against 396 P. aeruginosa isolates, obtained between 2011 and 2014, with nonsusceptibility to ceftazidime, cefepime, meropenem, and piperacillin-tazobactam (15).
In each of the census regions, ceftazidime-avibactam consistently demonstrated higher susceptibility rates than comparators commonly used as first-line agents for the treatment of P. aeruginosa infections. The potent in vitro activity observed in this surveillance study for ceftazidime-avibactam against P. aeruginosa isolates, including significant activity against isolates that are not susceptible to ceftazidime, meropenem, and piperacillin-tazobactam, highlight the potential clinical utility of this antibacterial combination against serious difficult-to-treat P. aeruginosa infection. These in vitro surveillance results also reinforce and support existing clinical data regarding ceftazidime-avibactam activity against P. aeruginosa.
ACKNOWLEDGMENTS
We thank all participating laboratories of the International Network for Optimal Resistance Monitoring (INFORM) program for providing bacterial isolates.
This study was performed at JMI Laboratories and was supported by an educational/research grant from Forest/Cerexa (a subsidiary of Allergan Pharmaceuticals, Irvine, CA). JMI Laboratories also received compensation fees for services in relation to preparing the manuscript, which was funded by this sponsor.
All authors are employees of JMI Laboratories, which received research and educational grants in 2014-2015 from Achaogen, Actavis, Actelion, Allergan, American Proficiency Institute (API), AmpliPhi, Anacor, Astellas, AstraZeneca, Basilea, Bayer, BD, Cardeas, Cellceutix, CEM-102 Pharmaceuticals, Cempra, Cerexa, Cidara, Cormedix, Cubist, Debiopharm, Dipexium, Dong Wha, Durata, Enteris, Exela, Forest Research Institute, Furiex, Genentech, GSK, Helperby, ICPD, Janssen, Lannett, Longitude, Medpace, Meiji Seika Kasha, Melinta, Merck, Motif, Nabriva, Novartis, Paratek, Pfizer, Pocared, PTC Therapeutics, Rempex, Roche, Salvat, Scynexis, Seachaid, Shionogi, Tetraphase, The Medicines Company, Theravance, Thermo Fisher, VenatoRX, Vertex, Wockhardt, Zavante, and some other corporations. Some JMI employees are advisors/consultants for Allergan, Astellas, Cubist, Pfizer, Cempra, and Theravance. Regarding speakers' bureaus and stock options, the authors have nothing to declare.
Funding Statement
This study was performed at JMI Laboratories and was supported by an educational/research grant from Forest/Cerexa (a subsidiary of Allergan Pharmaceuticals, Irvine, CA). JMI Laboratories also received compensation fees for services relating to preparing the manuscript, which was funded by this sponsor.
REFERENCES
- 1.Castanheira M, Farrell DE, Krause KM, Jones RN, Sader HS. 2014. Contemporary diversity of β-lactamases among Enterobacteriaceae in the nine United States census regions and ceftazidime-avibactam activity tested against isolates producing the most prevalent β-lactamase groups. Antimicrob Agents Chemother 58:833–888. doi: 10.1128/AAC.01896-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villegas MV, Lolans K, Correa A, Kattan NJ, Lopez JA, Quinn JP. 2007. First identification of Pseudomonas aeruginosa isolates producing a KPC-type carbapenem-hydrolyzing β-lactamase. Antimicrob Agents Chemother 51:1553–1555. doi: 10.1128/AAC.01405-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bush K. 2010. Bench-to-bedside review: the role of β-lactamases in antibiotic-resistant Gram-negative infections. Critical Care 14:224–231. doi: 10.1186/cc8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poirel L, Nordmann P, Lagrutta E, Cleary T, Munoz-Price SL. 2010. Emergence of KPC-producing Pseudomonas aeruginosa in the United States. Antimicrob Agents Chemother 54:3072. doi: 10.1128/AAC.00513-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livermore DM. 2002. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin Infect Dis 34:634–640. doi: 10.1086/338782. [DOI] [PubMed] [Google Scholar]
- 6.Breidenstein EBM, de la Fuente-Nunez C, Hancock REW. 2011. Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol 19:419–426. doi: 10.1016/j.tim.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J. 2015. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biology study. Lancet Infect Dis doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 8.Zhanel GG, Lawson CD, Adam H, Schweizer F, Zelenitsky S, Lagace-Wiens PRS, Denisuik A, Tubinstein E, Gin AS, Hoban DJ, Lynch JP, Karlowsky JA. 2013. Ceftazidime-avibactam: a novel cephalosporin/β-lactamase inhibitor combination. Drugs 73:159–177. doi: 10.1007/s40265-013-0013-7. [DOI] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—10th ed CLSI M07-A10. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing; 25th informational supplement. CLSI M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 11.EUCAST. 2015. Breakpoint tables for interpretation of MICs and zone diameters, version 6.0. http://www.eucast.org/clinical_breakpoints/ Accessed 13 January 2016.
- 12.US Food and Drug Administration. 2015. Avycaz (ceftazidime-avibactam) package insert. NDA 206494. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206494s000lbl.pdf Accessed October 27, 2015. [Google Scholar]
- 13.Winkler ML, Papp-Wallace KM, Hujer AM, Domitrovic TN, Hujer KM, Hurless KN, Tuhoy M, Hall G, Bonomo RA. 2015. Unexpected challenges in treating multidrug-resistant Gram-negative bacteria: resistance to ceftazidime-avibactam in archived isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother 59:1020–1029. doi: 10.1128/AAC.04238-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Esabrook M, Jacoby GA, Nichols WW, Testa RT, Bush K. 2015. In vitro susceptibility of characterized β-lactamase-producing strains tested with avibactam combinations. Antimicrob Agents Chemother 59:1789–1793. doi: 10.1128/AAC.04191-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sader HS, Castanheira M, Farrell DJ, Flamm RK, Jones RN. 2015. Ceftazidime-avibactam activity when tested against ceftazidime-nonsusceptible Citrobacter spp., Enterobacter spp., Serratia marcescens, and Pseudomonas aeruginosa from United States medical centers (2011-2014). Diagn Microbiol Infect Dis 83:389–394. doi: 10.1016/j.diagmicrobio.2015.06.008. [DOI] [PubMed] [Google Scholar]