LETTER
In several bacterial species, chromosomal blaCTX-M-15 or blaCTX-M-2 has been identified (1–5). The prevalence of chromosomal blaCTX-M-14 among Escherichia coli strains producing CTX-M-14-type extended-spectrum β-lactamase (ESBL) remains unclear. We preliminarily analyzed the location of blaCTX-M-14 in E. coli isolates possessing this gene by inverse PCR and conventional sequencing analysis, as described previously (5) and detected at least five chromosomal locations of blaCTX-M-14 (AB780367, AB780368, AB914799, AB915399, and AB915400). Multiple copies of the insertion sequence have become an obstacle to evaluation of blaCTX-M-14 location by whole-genome sequencing using the next-generation sequencer. Therefore, we evaluated the location of blaCTX-M-14 in 74 E. coli isolates possessing blaCTX-M-14, including 16 isolates (JO isolates) obtained from nursing home residents in the Kinki region of Japan and 58 isolates (KC isolates) obtained from asymptomatic healthy individuals living in a Thai rural community by pulsed-field gel electrophoresis (PFGE) using S1 nuclease (S1-PFGE) and Southern blot hybridization (6–8).
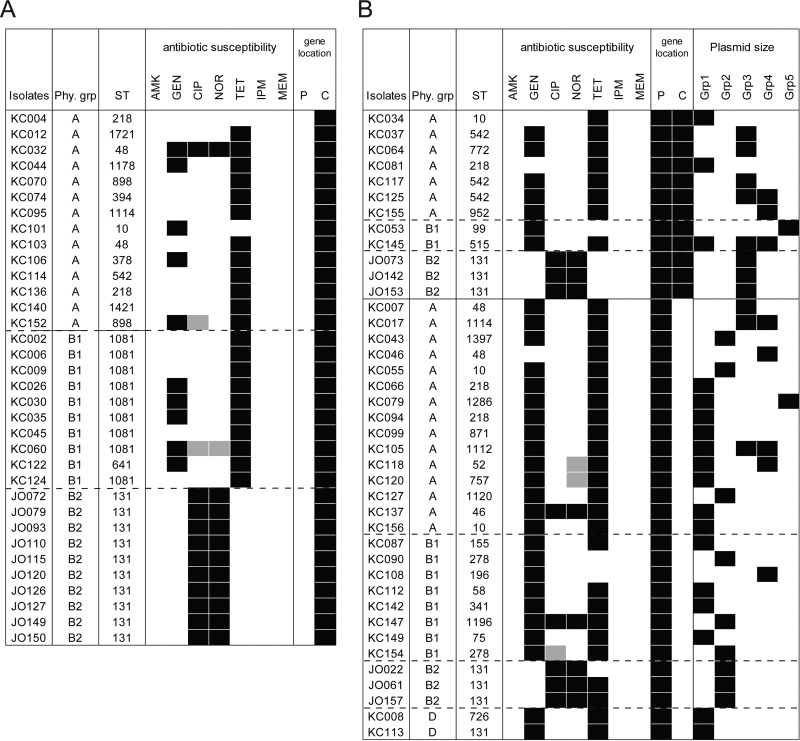
Locations of blaCTX-M-14 of the E. coli isolates examined were summarized in Fig. 1 along with genetic information of the E. coli isolates. Chromosomal blaCTX-M-14 was detected in 46 (62.2%) of the 74 E. coli isolates examined. Among the 58 KC isolates and the 16 JO isolates, 33 (56.9%) and 13 (81.3%) possessed chromosomal blaCTX-M-14, respectively. Nine (15.5%) and three (18.8%) of the 33 KC and 13 JO isolates, respectively, possessed both chromosomal and plasmid-mediated blaCTX-M-14. As negative control, we used a Klebsiella pneumoniae clinical strain which possesses blaTEM and blaSHV but not blaCTX-M (9). One to three bands were detected in the examined E. coli isolates by the Southern blotting; however, no band was detected in the negative control (data not shown). Our preliminary observation using KC002 (AB914799), KC012 (AB915399), KC140 (AB915400), and JO72 (AB780367 and AB780368) was consistent with results obtained by S1-PFGE and Southern blotting hybridization. These results strongly suggested that our evaluation of blaCTX-M-14 location in the examined E. coli isolates was appropriately performed. No statistical significance was observed by the χ2 test among isolate group, phylogenetic group, location of blaCTX-M-14, and prevalence of chromosomal blaCTX-M-14.
FIG 1.
Summary of blaCTX-M-14 location in the E. coli isolates producing CTX-M-14-type ESBL. Information on isolates possessing blaCTX-M-14 only on the chromosome (A) and on plasmids, with or without the gene on the chromosome (B), is summarized. Black squares indicate resistance to antibiotic, an identified location of blaCTX-M-14, and classification of the detected plasmids harboring blaCTX-M-14. Gray squares indicate intermediate sensitivity to antibiotic. The location of blaCTX-M-14 is indicated as C (chromosomal) or P (plasmid). Plasmids were classified by size: group 1, 104.5 kb or shorter; group 2, from 104.5 to 150 kb; group 3, from 150 to 250 kb; group 4, from 250 to 310 kb; group 5, 310 kb or longer. Phy. grp, phylogenetic group; ST, sequence type.
Susceptibility of the E. coli isolates examined to amikacin (AMK), gentamicin (GEN), ciprofloxacin (CIP), norfloxacin (NOR), tetracycline (TET), imipenem (IPM), and meropenem (MEM) was also examined. Antibiotic resistance profiles of the JO and the KC isolates were not noticeably different among each of the isolate groups, regardless of blaCTX-M-14 location.
Our results showed that 46 of the 74 examined isolates (62.2%) possessed at least one copy of chromosomal blaCTX-M-14. In this study, we could not determine all sequences surrounding chromosomal blaCTX-M-14 in the E. coli isolates possessing chromosomal blaCTX-M-14. In addition, the copy number and stability of chromosomal blaCTX-M-14 in the E. coli isolates remain unclear. Nevertheless, taking into account that the chromosomal location of blaCTX-M-14 transposition units was detected even in the KC isolates from asymptomatic healthy individuals living in a rural area in Thailand, the chromosomal location of the blaCTX-M-14 transposition unit might be one factor contributing to the worldwide spread of E. coli strains producing CTX-M-14-type ESBL.
ACKNOWLEDGMENTS
This work was supported in part by KAKENHI (22406015) and Japan Agency for Medical Research and Development/Japan International Cooperation Agency, Science and Technology Research Partnership for Sustainable Development (AMED/JICA, SATREPS).
Funding Statement
This study is also supported by the Japan Agency for Medical Research and Development/Japan International Cooperation Agency, Science and Technology Research Partnership for Sustainable Development (AMED/JICA, SATREPS).
REFERENCES
- 1.Coelho A, Gonzalez-Lopez JJ, Miro E, Alonso-Tarres C, Mirelis B, Larrosa MN, Bartolome RM, Andreu A, Navarro F, Johnson JR, Prats G. 2010. Characterisation of the CTX-M-15-encoding gene in Klebsiella pneumoniae strains from the Barcelona metropolitan area: plasmid diversity and chromosomal integration. Int J Antimicrob Agents 36:73–78. doi: 10.1016/j.ijantimicag.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Harada S, Ishii Y, Saga T, Kouyama Y, Tateda K, Yamaguchi K. 2012. Chromosomal integration and location on IncT plasmids of the blaCTX-M-2 gene in Proteus mirabilis clinical isolates. Antimicrob Agents Chemother 56:1093–1096. doi: 10.1128/AAC.00258-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fabre L, Delaune A, Espie E, Nygard K, Pardos M, Polomack L, Guesnier F, Galimand M, Lassen J, Weill FX. 2009. Chromosomal integration of the extended-spectrum beta-lactamase gene blaCTX-M-15 in Salmonella enterica serotype Concord isolates from internationally adopted children. Antimicrob Agents Chemother 53:1808–1816. doi: 10.1128/AAC.00451-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahrouki S, Belhadj O, Chihi H, Mohamed BM, Celenza G, Amicosante G, Perilli M. 2012. Chromosomal blaCTX-M-(1)(5) associated with ISEcp1 in Proteus mirabilis and Morganella morganii isolated at the Military Hospital of Tunis, Tunisia. J Med Microbiol 61:1286–1289. doi: 10.1099/jmm.0.039487-0. [DOI] [PubMed] [Google Scholar]
- 5.Hirai I, Fukui N, Taguchi M, Yamauchi K, Nakamura T, Okano S, Yamamoto Y. 2013. Detection of chromosomal blaCTX-M-15 in Escherichia coli O25b-B2-ST131 isolates from the Kinki region of Japan. Int J Antimicrob Agents 42:500–506. doi: 10.1016/j.ijantimicag.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Luvsansharav UO, Hirai I, Niki M, Nakata A, Yoshinaga A, Yamamoto A, Yamamoto M, Toyoshima H, Kawakami F, Matsuura N, Yamamoto Y. 2013. Fecal carriage of CTX-M beta-lactamase-producing Enterobacteriaceae in nursing homes in the Kinki region of Japan. Infect Drug Resist 6:67–70. doi: 10.2147/IDR.S43868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luvsansharav UO, Hirai I, Nakata A, Imura K, Yamauchi K, Niki M, Komalamisra C, Kusolsuk T, Yamamoto Y. 2012. Prevalence of and risk factors associated with faecal carriage of CTX-M beta-lactamase-producing Enterobacteriaceae in rural Thai communities. J Antimicrob Chemother 67:1769–1774. doi: 10.1093/jac/dks118. [DOI] [PubMed] [Google Scholar]
- 8.Luvsansharav UO, Hirai I, Niki M, Sasaki T, Makimoto K, Komalamisra C, Maipanich W, Kusolsuk T, Sa-Nguankiat S, Pubampen S, Yamamoto Y. 2011. Analysis of risk factors for a high prevalence of extended-spectrum β-lactamase-producing Enterobacteriaceae in asymptomatic individuals in rural Thailand. J Med Microbiol 60:619–624. doi: 10.1099/jmm.0.026955-0. [DOI] [PubMed] [Google Scholar]
- 9.Nakama R, Shingaki A, Miyazato H, Higa R, Nagamoto C, Hamamoto K, Ueda S, Hachiman T, Touma Y, Miyagi K, Kawahara R, Toyosato T, Hirai I. Current status of extended spectrum β-lactamase-producing Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis in Okinawa prefecture, Japan. J Infect Chemother, in press. [DOI] [PubMed] [Google Scholar]