Abstract
Treatment of solid-organ transplant (SOT) patients with ganciclovir (GCV)-valganciclovir (VGCV) according to the manufacturer's recommendations may result in over- or underexposure. Bayesian prediction based on a population pharmacokinetics model may optimize GCV-VGCV dosing, achieving the area under the curve (AUC) therapeutic target. We conducted a two-arm, randomized, open-label, 40% superiority trial in adult SOT patients receiving GCV-VGCV as prophylaxis or treatment of cytomegalovirus infection. Group A was treated according to the manufacturer's recommendations. For group B, the dosing was adjusted based on target exposures using a Bayesian prediction model (NONMEM). Fifty-three patients were recruited (27 in group A and 26 in group B). About 88.6% of patients in group B and 22.2% in group A reached target AUC, achieving the 40% superiority margin (P < 0.001; 95% confidence interval [CI] difference, 47 to 86%). The time to reach target AUC was significantly longer in group A than in group B (55.9 ± 8.2 versus 15.8 ± 2.3 days, P < 0.001). A shorter time to viral clearance was observed in group B than in group A (12.5 versus 17.6 days; P = 0.125). The incidences of relapse (group A, 66.67%, and group B, 9.01%) and late-onset infection (group A, 36.7%, and group B, 7.7%) were higher in group A. Neutropenia and anemia were related to GCV overexposure. GCV-VCGV dose adjustment based on a population pharmacokinetics Bayesian prediction model optimizes GCV-VGCV exposure. (This study has been registered at ClinicalTrials.gov under registration no. NCT01446445.)
INTRODUCTION
Human cytomegalovirus (CMV) is a common viral pathogen affecting solid-organ transplant (SOT) recipients, in whom it causes significant morbidity. CMV infection may be clinically manifested as either an acute viral syndrome or a tissue-invasive disease (1–5). Without antiviral drug prophylaxis, most CMV infections occur during the first 3 months posttransplant, when patients are receiving intensive immunosuppressive regimens for the prevention of graft rejection. SOT patients at the highest risk of developing CMV infection are seronegative recipients of organs from seropositive donors (CMV D+/R−) and those receiving T-cell-depleting agents (6).
The “gold standard” therapy for the prevention and treatment of CMV disease in SOT patients is intravenous ganciclovir (i.v. GCV) and/or oral valganciclovir (VGCV) at doses that should be adjusted according to renal function. Insufficient antiviral dosing may result in a lack of clinical efficacy and the development and selection of resistant viral strains, whereas overdosing may increase toxicity (7). According to Wiltshire et al. (8), exposure values of 40 to 50 μg · h/ml, as measured by the area under the concentration-time curve (AUC) at steady state, result in a low incidence of breakthrough viremia during prophylactic treatment, whereas lower AUCs are associated with up to 8-fold-higher viral replication rates.
However, it is well known that both GCV and VGCV display high pharmacokinetic (PK) interindividual variability (7, 8). Our group developed a population pharmacokinetics (PPK) modeling approach to GCV-VGCV dosing in SOT patients with CMV infection. Interpatient variability was explained by differences in serum creatinine clearance (CLCR) (52.03%), whereas body weight explained only 4% of it and thus was not included in our current PPK model (9). Also, we examined the adequacy of the manufacturer's recommended dosing schedule to achieve target AUC values and found that potential dosing refinements were clearly possible. In this pharmacokinetic study, we hypothesized that by applying our previously developed PPK model as a tool for Bayesian prediction, we could optimize GCV-VGCV treatment and more rapidly achieve target AUC values as well as maintain them throughout the treatment period. Thus, the primary endpoint of this study was a pharmacological parameter assessed as the percentage of patients achieving the target therapeutic exposure.
MATERIALS AND METHODS
Study design.
This was a two-arm, randomized, open-label, single-center trial with adult SOT recipients treated with GCV-VGCV as either prophylaxis or treatment for CMV infection (viremia or disease) (EudraCT no. 2010-021433-32; ClinicalTrials.gov registration no. NCT01446445). The trial was designed as a superiority study based on the percentage of defined target AUC values (40 to 50 μg · h/ml) achieved in each group with a superiority margin of 40%.
Patients were randomly allocated into two groups depending on the dosing adjustment strategy to be applied (1:1). Group A received GCV-VGCV according to the manufacturer's dosing recommendations based on Cockcroft-Gault-calculated CLCR and body weight according to the criteria showed in Table 1. Doses were adjusted based on the individual CLCR at each time point. Group B received initial doses of GCV-VGCV as calculated using our previously developed PPK model (Table 2) (9). Subsequent doses were then adjusted applying a Bayesian prediction model to ongoing measurements of drug concentrations and renal function.
TABLE 1.
Dosing adjustments based on CLCR in the solid-organ transplant population of oral valganciclovir and i.v. ganciclovir
| Oral valganciclovir |
i.v. ganciclovir |
|||
|---|---|---|---|---|
| CLCR (ml/min) | Treatment dose | Prophylaxis dose | CLCR (ml/min) | Treatment dose |
| ≥60 | 900 mg/12 h | 900 mg/24 h | ≥70 | 5 mg/kg/12 h |
| 40–60 | 450 mg/12 h | 450 mg/24 h | 50–70 | 2.5 mg/kg/12 h |
| 25–40 | 450 mg/24 h | 450 mg/48 h | 25–50 | 2.5 mg/kg/24 h |
| 10–25 | 450 mg/48 h | 450 mg twice per week | 10–25 | 1.25 mg/kg/24 h |
TABLE 2.
Initial doses based on the PPK model in the solid organ transplant population of oral valganciclovir and iv ganciclovir
| Oral valganciclovir |
i.v. ganciclovir |
|||
|---|---|---|---|---|
| CLCR (ml/min) | Treatment dose | Prophylaxis dose | CLCR (ml/min) | Treatment dose |
| 100 | 1,000 mg/12 h | 1,000 mg/24 h | 100 | 10 mg/kg/12 h |
| 95 | 950 mg/12 h | 950 mg/24 h | 95 | 9.5 mg/kg/12 h |
| 90 | 900 mg/12 h | 900 mg/24 h | 90 | 9 mg/kg/12 h |
| 85 | 850 mg/12 h | 850 mg/24 h | 85 | 8.5 mg/kg/12 h |
| 80 | 800 mg/12 h | 800 mg/24 h | 80 | 8 mg/kg/12 h |
| 75 | 750 mg/12 h | 750 mg/24 h | 75 | 7.5 mg/kg/12 h |
| 70 | 700 mg/12 h | 700 mg/24 h | 70 | 7 mg/kg/12 h |
| 65 | 650 mg/12 h | 650 mg/24 h | 65 | 6.5 mg/kg/12 h |
| 60 | 600 mg/12 h | 600 mg/24 h | 60 | 6.0 mg/kg/12 h |
| 55 | 550 mg/12 h | 550 mg/24 h | 55 | 5.5 mg/kg/12 h |
| 50 | 500 mg/12 h | 500 mg/24 h | 50 | 5.0 mg/kg/12 h |
| 45 | 450 mg/12 ha | 450 mg/24 ha | 45 | 4.5 mg/kg/12 h |
| 40 | 400 mg/12 h | 400 mg/24 h | 40 | 4.0 mg/kg/12 h |
| 35 | 350 mg/24 h | 350 mg/48 h | 35 | 3.5 mg/kg/12 h |
| 30 | 300 mg/24 h | 300 mg/48 h | 30 | 3.0 mg/kg/12 h |
| 25 | 250 mg/24 h | 250 mg/48 h | 25 | 2.5 mg/kg/24 h |
| 20 | 200 mg/48 h | 200 mg/72 h | 20 | 2.0 mg/kg/24 h |
Solution was used when doses did not fit the tablet presentation.
Kidney, liver, or heart transplant recipients were eligible to participate in the study if they were ≥18 years of age and were treated with GCV or VGCV as either prophylaxis or treatment of CMV infection, according to standard clinical practice. Patients were excluded if they had a calculated CLCR below 10 ml/min using the Cockcroft-Gault equation, had a history of hypersensitivity to GCV-VGCV, or were receiving concomitant treatment with other anti-CMV agents. Patients under prophylaxis received oral VGCV for 90 days, whereas those with CMV infection were treated with GCV-VGCV until two consecutive negative CMV viral load tests were obtained, performed at least 1 week apart.
Patients with CMV infection included patients with CMV viremia (evidence of CMV replication regardless of symptoms) and those with CMV disease. CMV disease included both viral syndrome and tissue invasive disease. Identification of the viral syndrome caused by CMV required the following: (i) viral load of >1,000 copies/ml, (ii) temperature of >38°C with no other source to account for it, and (iii) one of the following findings: leukocyte count of <4,000/mm3, atypical lymphocytes of >3%, elevation of transaminases, and platelet count of <100,000/mm. Tissue-invasive disease required histopathological evidence of CMV, with or without virus culture of the tissue.
Oral VGCV was administered as tablets (Valcyte, 450 mg; F. Hoffmann-La Roche Ltd., Basel, Switzerland) and/or solution (Valcyte, 50-mg/ml suspension; F. Hoffmann-La Roche Ltd.). Solution was used when treatment dose did not fit the tablet presentation. Patients with CMV infection could receive either oral VGCV or i.v. GCV according to standard clinical practice. For those under i.v. treatment, sequential treatment with i.v. GCV (Cymevene; F. Hoffmann-La Roche Ltd.) for a maximum of 5 days, followed by oral VGCV, was administered.
The study was conducted in accordance with the Declaration of Helsinki and good clinical practice (GCP) guidelines. The study protocol was approved by the Bellvitge Hospital's Ethics Research Committee. Written informed consent was obtained from each patient prior to randomization.
Study endpoints.
The primary study endpoint was defined as the percentage of patients achieving target AUC values between 40 and 50 μg · h/ml. In all patients included in the study, AUC values were estimated at steady state in one or more scenarios: (i) 3 days after treatment onset, (ii) 3 days after any dose adjustment, (iii) after any change in CLCR of >10 ml/min, or (iv) after any change in drug administration route. Exposures on days 30, 60, and 90, if the treatment was ongoing, and the time needed to achieve target AUC values were also assessed.
The secondary study endpoints were measurements of time to viral clearance (time to first negative viral load result), recurrence of CMV infection (6 months follow-up), and incidence of late-onset CMV infection (infection occurring after the discontinuation of prophylaxis). Determinations of viral loads in patients with CMV infection were performed until day 45. Hematological and biochemistry laboratory parameters were measured at baseline and every time PK samples were collected, as well as on days 30, 60 and 90, if the treatment was ongoing. Assessments of safety and tolerability were also performed.
Plasma ganciclovir concentration measurements.
Blood samples were collected 0.5 to 1.5, 4 to 5, and 6 to 8 h after dose according to a limited-sampling strategy previously developed by our group (10). Plasma GCV concentrations were determined by a validated ultrahigh-performance liquid chromatography assay coupled with UV detection (11).
GCV dose, plasma GCV concentrations, and CLCR in every patient were used for a maximum a posteriori (MAP) Bayesian probability estimate of GCV systemic exposure with a PPK model previously developed and implemented in the nonlinear mixed-effects modeling (NONMEM) computer program, version 7.2 (Icon Development, Ellicott City, MD).
Briefly, in the PPK model the PK disposition of GCV was best described by a two-compartment open linear model with first-order absorption process and elimination from the central compartment. Renal function given by CLCR was the most influential covariate in clearance (CL). The final pharmacokinetic parameters were as follows: GCV CL was 7.49 × (CLCR/57) liters/h (57 was the mean population value of CLCR); the central and peripheral distribution volumes were 31.9 liters and 32.0 liters, respectively; intercompartmental clearance was 10.2 liter/h; the first-order absorption rate constant was 0.895 h−1; bioavailability was 0.825; and lag time was 0.382 h (9).
Viral load monitoring.
CMV DNA replication analysis was conducted with the Abbott real-time CMV amplification reagent kit (Abbott Molecular, Des Plaines, IL) using 500 μl of plasma-EDTA (quantification limit, 20 to 107 copies/ml). DNA was obtained 24 h after sample collection using magnetic DNA extraction on an m24sp instrument. Patients who tested positive (viral load higher than 1,000 copies/ml) received i.v. GCV or oral VGCV until two consecutive negative CMV viral load tests were obtained, performed at least 1 week apart.
Safety monitoring.
Hematological adverse events were assessed at every sampling time point as present or absent. Anemia was defined as a hemoglobin concentration of <100 g/liter, neutropenia as a neutrophil cell count of <1 × 109 cells/liter, and thrombocytopenia as a platelet count of <50 × 109 cells/liter (12).
Sample size calculation and statistical methods.
The study was designed as a superiority trial. The primary endpoint was defined as a 40% or higher superiority margin in the number of patients reaching the AUC target. Thus, the percentages of patients with AUC achieved assessed by median AUC values for each patient were compared between group B (Bayesian prediction dose adjustment group) and group A (treated per the manufacturer's dosing recommendations). A sample size of 27 patients per group provided 80% statistical power to claim superiority of one group over the other (P < 0.05). The percentages of patients with median AUC values, within each patient, that reached the therapeutic target were compared between both by a chi-square test. Superiority was declared when statistically significant differences between proportions were found and the lower bound of the one-sided 95% confidence interval (CI) for the difference between groups was above the superiority margin of 40% (group B versus group A), assuming a 20% incidence of the primary endpoint in group A. Although our previous study showed that AUC values obtained with i.v. GCV and oral VGCV were comparable (13), the analysis of the primary endpoint was performed separately.
The primary endpoint was also evaluated using isolated GCV determinations for every patient and by different CLCR cutoffs. Differences between groups in the time to achieve target AUC values were assessed using a Kaplan-Meier survival analysis.
Statistical analysis of secondary endpoints included a t test for normally distributed data, a chi-square test for categorical variables, and a Kaplan-Meier survival analysis for time to viral clearance comparisons. The ability to predict relapse and late-onset infection based on dosing strategy after discontinuation of prophylactic treatment was assessed by an analysis of area under the ROC (receiver-operator curve). All statistical analyses were performed using SPSS v19.0, with a significance level of 0.05.
Statistical analyses were performed using the intention-to-treat (ITT) population for the primary endpoint. Analysis of secondary safety endpoints included all randomized patients who had received at least one dose of study medication and had at least one AUC determination and one safety assessment. The per-protocol population analysis for the efficacy endpoint included only those patients who completed treatment and the 90-day follow-up period.
RESULTS
Baseline characteristics.
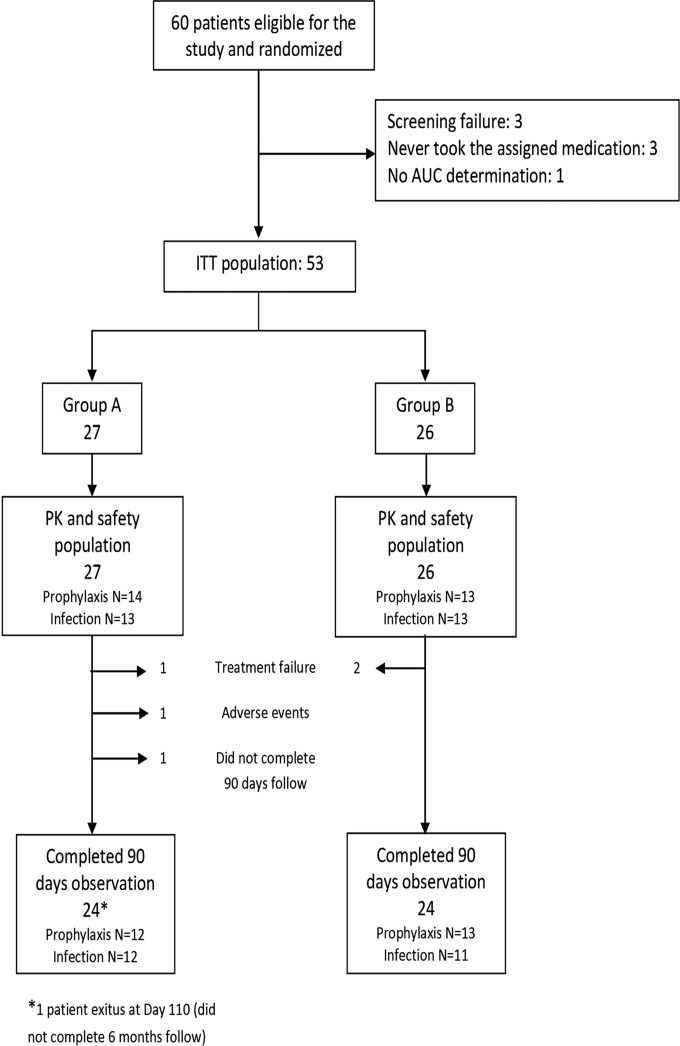
A total of 60 SOT patients eligible to receive GCV or VGC for either prophylaxis or CMV infection were enrolled. The ITT population included 53 patients: 27 were assigned to group A and 26 to group B. Study patient allocation and disposition are shown in Fig. 1. There were no significant differences in main demographic or baseline characteristics between groups (Table 3). The baseline viral data characteristics of patients with CMV infection are shown in Table 4. Only three patients received sequential treatment with i.v. GCV followed by oral VGCV.
FIG 1.
Study patient allocation and disposition.
TABLE 3.
Demographic and baseline characteristics for all ITT patients
| Characteristicf | Value for: |
P value | |
|---|---|---|---|
| Group A (n = 27) | Group B (n = 26) | ||
| Type of treatment (no.) | 1.000c | ||
| Prophylaxis | 14 | 13 | |
| Infection | 13 | 13 | |
| Sex | 0.218c | ||
| No. (%) female/no. (%) male | 9 (33.3)/18 (66.7) | 13 (50)/13 (50) | |
| Age (yrs) | 0.586d | ||
| Mean ± SD | 52.6 ± 19.6 | 55.1 ± 14.7 | |
| Wt (kg) | 0.939d | ||
| Mean ± SD | 70.1 ± 13.6 | 70.4 ± 16.4 | |
| Transplanted organ, no. | 0.852c | ||
| Kidney | 23 | 25 | |
| Liver | 2 | 0 | |
| Heart | 2 | 1 | |
| HLA-A mismatches, no. | 0.601c | ||
| No mismatch | 1 | 3 | |
| 1 or 2 mismatches | 22 | 22 | |
| Missing | 4 | 1 | |
| HLA-B mismatches, no. | 0.573c | ||
| No mismatch | 0 | 0 | |
| 1 or 2 mismatches | 23 | 25 | |
| Missing | 4 | 1 | |
| HLA-DR mismatches, no. | 0.341c | ||
| No mismatch | 3 | 3 | |
| 1 or 2 mismatches | 20 | 22 | |
| Missing | 4 | 1 | |
| Donor/recipient CMV IgG serostatus at time of transplantation, no. (%)e | 0.241c | ||
| D+/R− | 10 (37.0) | 4 (16) | |
| D+/R+ | 15 (55.6) | 16 (64)a | |
| D−/R+ | 2 (7.4) | 4 (16) | |
| D−/R− | 0 | 1 (3.8) | |
| Induction immunosuppression, no. (%)e | 0.779c | ||
| ATG | 9 (33.3) | 10 (38.5) | |
| Basiliximab | 17 (63) | 16 (61.5) | |
| None | 1 (3.7) | 0 | |
| CMV risk, no. (%)b,e | 0.570c | ||
| High/low | 18 (66.7)/9 (33.3) | 14 (56)/11 (44)a | |
| Rejection episode, no. (%)e | 1.000c | ||
| Yes/no | 3 (12)/22 (88) | 2 (8.7)/21 (91.3) | |
| Maintenance immunosuppressione | 0.795c | ||
| CsA+ MMF + corticoids | 2 (7.5) | 4 (15.4) | |
| TAC + MMF + corticoids | 26 (92.5) | 19 (73.1) | |
| CsA + MMF | 0 | 1 (3.8) | |
| TAC + MMF | 0 | 1 (3.8) | |
| CsA+ corticoids | 0 | 1 (3.8) | |
| Serum creatinine (μmol/liter) | 157.8 | 144.8 | 0.560d |
| CLCR (ml/min) | 55.8 | 54.3 | 0.823d |
| Hemoglobin (g/liter) | 108.5 | 102 | 0.070d |
| Leukocytes (×109 cells/liter) | 6.84 | 7.15 | 0.741d |
| Lymphocytes (×109 cells/liter) | 1.23 | 1.33 | 0.749d |
| Neutrophils (×109 cells/liter) | 4.9 | 5.1 | 0.818d |
| Platelets (×109 cells/liter) | 207 | 239 | 0.126d |
For one of the CMV IgG-seropositive recipients, the donor's IgG serostatus was missing.
Patients classified according to donor and recipient IgG serostatus and induction therapy: high risk (D+/R− with any induction therapy or D−/R+ and D+/R+ treated with antithymocyte globulin) and low risk (D−/R− or D−/R+ and D+/R+ treated with basiliximab or no induction therapy).
Chi-square.
t test.
Risk factor associated with therapeutic efficacy.
ATG, antithymocyte globulin; TAC, tacrolimus; MMF, mycophenolate mofetil; CsA, cyclosporine.
TABLE 4.
Baseline viral data characteristics of per-protocol-treated patients for CMV infection (viremia or disease)
| Characteristic | Value for: |
P value | |
|---|---|---|---|
| Group A (n = 12)a | Group B (n = 11)b | ||
| Previous anti-CMV strategy, no. (%) | |||
| Prophylactic | 5 (58.3) | 1 (9.1) | 0.378c |
| Preemptive | 7 (41.7) | 10 (90.9) | |
| Median baseline viral load (copies/ml) | 3,542 | 3,392 | 0.611d |
| Patients with previous prophylactic treatment | 33,774 | 4,809 | 0.739d |
| Patients under preemptive treatment | 3,406 | 3,379 | 0.696d |
In group A (13 ITT patients), 1 patient was excluded because the 90-day protocol follow-up was not reach due to the treatment failure (dosing increase).
In group B (13 ITT patients), 2 patients were excluded. One patient was excluded from the PK analysis because foscarnet was initiated as an add-on treatment, and the other patient did not reach the 90-day follow-up (dosing increase).
Chi-square.
Mann-Whitney test.
Evaluation of systemic exposure.
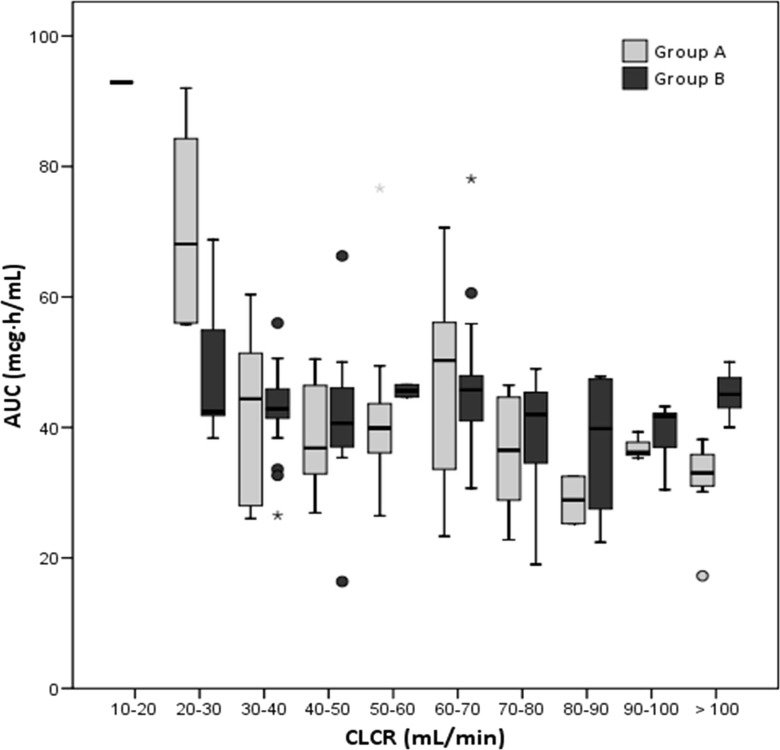
A total of 155 AUC determinations from 53 patients were analyzed. We excluded data after intravenous administration from the statistical analysis (5 AUC determinations not shown). Median AUC values by CLCR cutoff interval are shown in Fig. 2. Analysis of median systemic exposure during the study period within each patient showed that 88.64% (23/26) of patients treated following the Bayesian prediction based on the PPK model (group B) achieved the target AUC values, compared to only 22.2% (6/27) of patients treated by following the manufacturer's dosing recommendations (group A), thus fulfilling the 40% superiority margin in target AUC values established as a primary endpoint (P < 0.001; 95% CI for the difference, 47 to 86%). Median AUC values were 38.2 μg · h/ml in group A and 42.7 μg · h/ml in group B.
FIG 2.
Average estimated systemic exposure of GCV following administration of oral VGC by CLCR cutoff values.
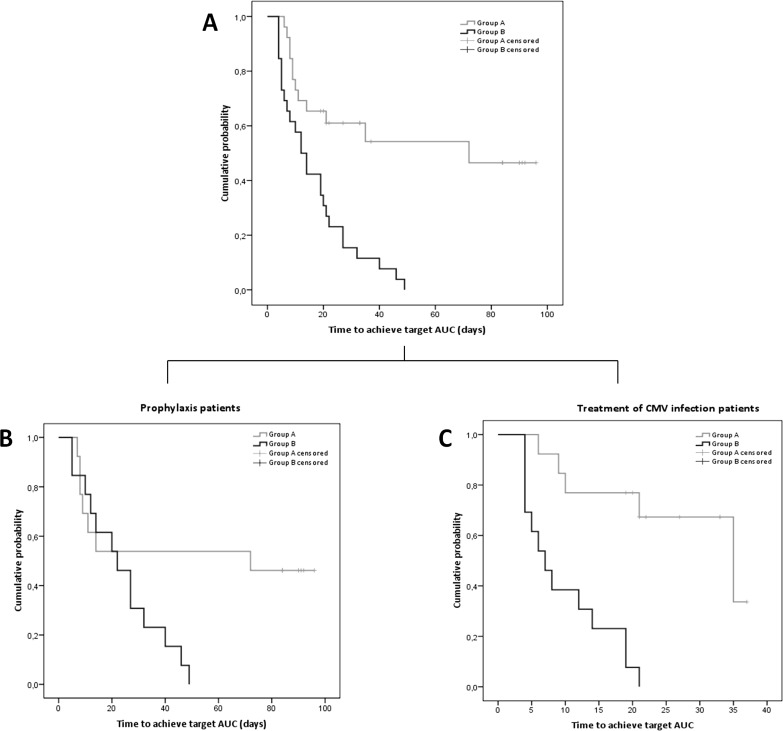
The time required to reach target AUC values was longer in group A than in group B (log rank statistics = 15.48, P < 0.001, 56.7 days [95% CI = 40.24 to 73.07], versus 16.8 days [95% CI = 11.67 to 21.87]) (Fig. 3A). Differences remained statistically significant (P < 0.001) when patients on prophylaxis or treatment of CMV infection were considered separately, even though the time to target AUC was longer in prophylaxis-treated individuals, as patients were visited weekly. The times between samples and AUC determinations were longer in patients receiving prophylaxis than in those receiving treatment for CMV infection (Fig. 3B and C).
FIG 3.
Rate of first achievement of AUC target according to randomization group over the follow-up period (Kaplan-Meier analysis using log rank statistics) for all patients included (A), for patients on prophylaxis (B), and for patients on treatment of CMV infection (C). Time scale is based on days under GCV-VGCV treatment.
Considering all AUC determinations, the Bayesian prediction approach resulted in a higher proportion of AUC values falling within the therapeutic range (19.2% for group A, versus 65.9% for group B; P < 0.001; 95% CI for the difference, 33 to 60%) and fulfilling the 40% superiority margin. The proportions of on-target AUC values by CLCR and prophylactic versus treatment of CMV infection in each group are shown in Table 5. Differences in AUC values between groups were statistically significant for patients with CLCRs of <30 ml/min (P = 0.008; 95% CI for the difference, 38 to 105%) or >60 ml/min (P = <0.001; 95% CI for the difference, 43 to 77%). No significant differences were observed in patients with CLCRs between 30 and 50 ml/min (Table 5).
TABLE 5.
Proportions of on-target AUC values by group, CLCR cutoff value, and type of treatment
| Classification variable for statistical analysis | % measurement achieving the target AUC (40–50 μg · h/ml) |
% difference (95% CI) | P value | |
|---|---|---|---|---|
| Group A ( = 73) | Group B ( = 82) | |||
| All patients included | 19.2 | 65.9 | 47.0 (33–60) | <0.001 |
| Patients with indicated CLCR cutoff value | ||||
| <30 ml/min | 0 | 71.4 | 71.4 (38–105) | 0.008 |
| 30–40 ml/min | 37.5 | 64.7 | 27.2 (−13–68) | 0.467 |
| 40–50 ml/min | 36.4 | 57.1 | 20.8 (−21–63) | 0.582 |
| 50–60 ml/min | 36.7 | 100 | 64.6 (35–92) | 0.252 |
| >60 ml/min | 8.6 | 68.3 | 59.7 (43–77) | <0.001 |
| Type of treatment | ||||
| Prophylaxis | 21.3 | 62.9 | 41.6 (27–56) | <0.001 |
| Treatment of CMV disease | 15.4 | 71.4 | 56.0 (34–78) | <0.001 |
In some patients in group B, the dose of GCV or VGCV was adjusted due to subtherapeutic (24.7%) or supratherapeutic (9.41%) AUC values. Subsequent AUC values fell within target exposure limits in all cases.
Clinical outcome analysis. (i) Patients on treatment for CMV infection.
The time to viral clearance was shorter in patients in group B than in patients assigned to group A (group A, 17.6 days, and group B, 12.5 days), reflecting a 40.8% reduction (log rank statistics = 2.34; P = not significant [NS]). A Kaplan-Meier curve for time to viral clearance is shown in Fig. 4. The median two-exponential decay of viral load for each group until day 45 is displayed in Fig. 5.
FIG 4.
Kaplan-Meier curves showing cumulative probability of viral clearance in patients treated with either oral VGCV or i.v. GCV.
FIG 5.
Plots of individual viral load decays observed until day 45 for patients treated according to the manufacturer's recommendation (left) and for patients with dose adjusted by Bayesian approach (right). The gray line represents the limit of quantification of the analytical method. (Relapse data are not shown.)
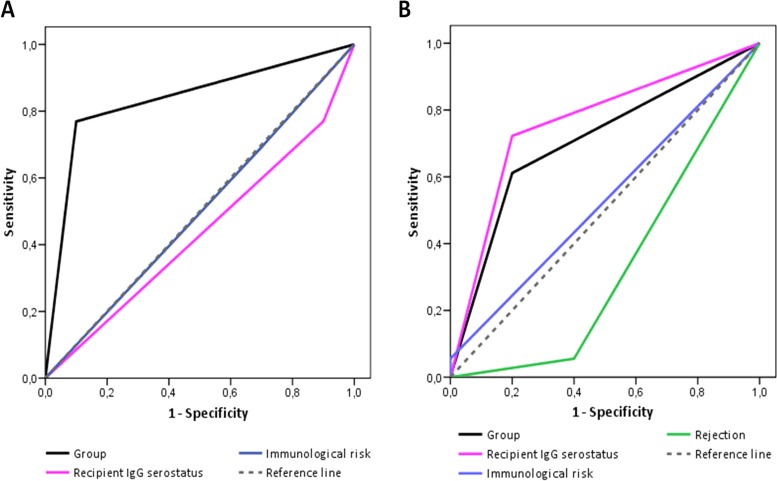
The proportion of patients with CMV recurrence was higher in group A than in group B (66.67% [8/12] versus 9.01% [1/11]), and 85% of patients who relapsed had received GCV-VGCV according to the manufacturer's dosing recommendations (group A). No differences were found in time to recurrence between groups. The ability to predict relapse based on dosing strategy, recipient and donor CMV serostatus, and immunological risk assessed by a sensitivity/specificity ROC analysis revealed the dosing strategy as the most accurate variable predicting the viral relapse (P = 0.007; AUC, 0.835; 95% CI, 0.66 to 1.02; sensitivity, 100%; specificity, 91.7%) (Fig. 6A).
FIG 6.
Receiver-operator curve (ROC) of dose adjustment based on target AUC, recipient IgG serostatus, and immunological risk for predicting relapse (A) and of dose adjustment based on AUC target, recipient IgG serostatus, episodes of rejection, and immunological risk for predicting late CMV disease (B).
(ii) Patients on CMV prophylaxis.
There were no incident cases of CMV viremia during the prophylactic period. The incidence of late-onset infection during the 3 months following discontinuation of prophylaxis was lower in group B than in group A (7.7% [1/13] versus 36.7% [4/11]). However, the accuracy of our dose adjustment strategy predicting late-onset CMV infection was not statistically significant (P = 0.168; AUC, 0.706; 95% CI, 0.45 to 0.96; sensitivity, 100%; specificity, 40%) (Fig. 6B).
Adverse events.
About 32.7% of patients reported at least one hematological adverse event during treatment. Neutropenia occurred in 7.5% of patients (7.4% in group A [2 patients] and 7.7% in group B [2 patients]). Two episodes of neutropenia, out of 27 cases in which the AUC values were supratherapeutic (7.4%), one in each group, were related to GCV overexposure. In the other two patients, toxicity was associated with concomitant treatment with co-trimoxazole. The difference in the occurrence of anemia was associated with GCV overexposure. In those patients with AUC values higher than 50 μg · h/ml, the incidence of anemia was 51.9% (14/27 determinations), versus 26.6% (31/128 determinations) in those patients whose values were under or within the defined target AUC ranges (P = 0.010).
Dosage.
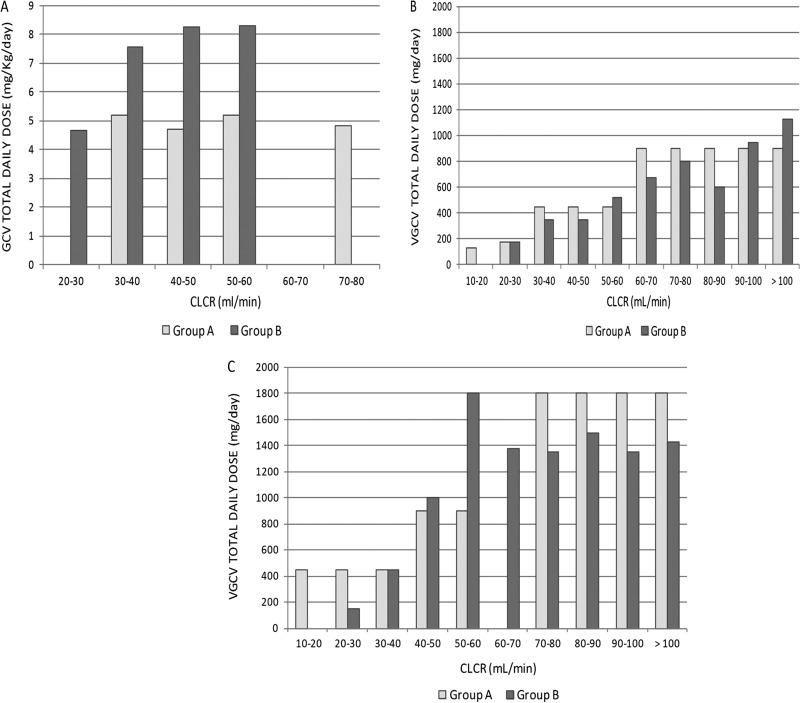
Patients in group B received higher doses of either i.v. GCV or oral VGCV than patients in group A. The median daily dose of oral VGCV for patients under prophylaxis was lower in group B than in group A. Dosage distributions are shown in Fig. 7.
FIG 7.
Dosage distributions considering the randomization group, route of administrations, type of treatment, and CLCR cutoffs. (A) i.v. GCV dosage; (B) oral VGCV dosage in patients on prophylaxis; (C) oral VGCV dosage in patients on treatment for CMV infection.
DISCUSSION
This is the first prospective, randomized, controlled superiority trial with SOT patients comparing conventional GCV-VGCV dosing for either CMV prophylaxis or infection treatment to a Bayesian prediction model approach in an attempt to optimize GCV exposure. Herein, we show that the proportion of patients achieving therapeutic target AUC values was higher after GCV-VGCV dose adjustment using a Bayesian prediction model approach than simply following the manufacturer's dosing recommendations. A preestablished superiority margin of 40% in the number of patients reaching a therapeutic target AUC value, the study primary endpoint, was fulfilled. The time to target AUC values was also shorter in patients dosed according to the Bayesian prediction model approach. These results are important since therapeutic exposure to GCV-VGCV has been previously correlated with treatment efficacy (8); thus, the achievement of therapeutic drug exposure is especially advisable in the treatment of CMV infection in order to rapidly control and inhibit viral replication.
A previously reported study by our group showed both under- and overexposure to GCV in patients following manufacturer's dosing recommendations (13). Based on our own PPK model simulations, and using the manufacturer's initial dose recommendations, GCV-VGCV dosing results in overexposure in patients with CLCR values below 30 ml/min and underexposure in patients with CLCR values over 60 ml/min. The current study confirms that the manufacturer's label dosing algorithm (14, 15) is appropriate for only approximately one-fifth of patients, most of whom fall within CLCR values between 30 and 50 ml/min. To date, there are limited data concerning GCV dose optimization (16), and some authors suggested testing the adequacy of drug level in patients with critical CMV infection in which GCV resistance is suspected (6).
Clinical outcome analysis of patients treated for CMV infection following such an approach also showed a trend toward reduced time to viral clearance. However, since our study had a primarily pharmacokinetic focus, it was therefore not powered to assess clinical efficacy.
Besides dosing adequacy, other factors that may result in longer treatment duration are a high baseline viral load and a high net state of immunosuppression (6). According to Asberg et al. (17), the baseline viral load can be a significant predictor of viral clearance. In our study, the baseline viral load was lower than 200,000 copies/ml in all patients, as the majority had received preemptive therapy, and no differences between groups were observed.
With regard to CMV recurrence, our study showed a rate of 28%, which is in line with earlier reports showing rates of 25% to 30% (18–20). However, a trend toward higher recurrence rates was observed in patients treated according to the manufacturer's dosing recommendations (group A). Even though response to antiviral therapy is likely dependent on a complex interaction between the virus and the host immunity (6, 17, 21), our results suggest that therapeutic drug exposure may help improve the control of viral replication.
In our study, the incidence of hematological adverse events was similar to that previously reported (32.7) (8). There were no differences between groups in the incidence of neutropenia, and 14.8% of patients that presented AUC values of >50 μg · h/ml developed neutropenia. The small sample size did not allow achieving statistical significance, but Wiltshire et al. (8) reported a weak tendency to increase the incidence of neutropenia with higher GCV exposure. A significantly superior incidence of anemia was observed in those patients with AUC values higher than 50 μg · h/ml who presented CLCR values below 30 ml/min. In fact, patients with worse renal function (CLCR, <30 ml/min) do not fit the target AUC when dosed using the manufacturer recommendations.
In conclusion, this PK study achieved the primary endpoint, with 88.4% of patients reaching target exposure when dosed according to a PPK model using a Bayesian prediction approach, with a superiority margin of 40%. GCV-VGCV treatment following the manufacturer's dosing recommendations resulted in only 18.5% of patients falling within the therapeutic range. Applying our PPK model, using a Bayesian prediction model approach, constitutes a significant step forward in optimizing GCV dosing in the management of CMV infection and may allow for individualized anti-CMV therapy. It was mainly for those patients with CLCR values below 30 ml/min and over 60 ml/min that CGV-VGCV dosage did not achieve the optimal drug exposure. Our study was an application of therapeutic drug monitoring, but further dose tailoring studies, focused on clinical efficacy as a primary endpoint, may show improved clinical outcomes. Further powered randomized dose tailoring clinical trials are warranted to assess the clinical benefits of PPK-based dosage of GCV-VGCV in the prevention and management of CMV infections in SOT recipients.
ACKNOWLEDGMENTS
We gratefully acknowledge Gema Cerezo for technical assistance with this study.
N. Lloberas is a researcher from ISCIII Miguel Servet (CP06/00067) and REDinREN RD12/0021/003. This study was supported by grants from Instituto de Salud Carlos III and Ministerio de Sanidad y Consumo (PI12/01564), MSPSI (EC10-144), and Fondo Europeo de Desarrollo Regional (FEDER).
We have no financial or personal conflicts of interest related to this article.
A. Padullés performed research, analyzed data and wrote the manuscript; H. Colom, J. M. Grinyó, and N. Lloberas designed the research, performed research, analyzed data, wrote the manuscript, and contributed new reagents and analytical tools; O. Bestard, J. Torras, and J. M. Cruzado performed research and wrote the manuscript; E. Melilli, N. Sabé, L. Lladó, and N. Manito performed research; R. Rigo and J. Niubó performed research and contributed new reagents and analytical tools, and A. Caldés analyzed data.
Funding Statement
This work was supported by Spanish Public Funding sources: Instituto de Salud Carlos III (ISCIII) provided funding to Nuria Lloberas under grant number PI12/01564 (FIS) and grant number EC10-144 (MSP clinical trial); and REDin REN.
REFERENCES
- 1.de la Torre-Cisneros J, Fariñas MC, Castón JJ, Aguado JM, Cantisán S, Carratalá J, Cervera C, Cisneros JM, Cordero E, Crespo-Leiro MG, Fortún J, Frauca E, Gavaldá J, Gil-Vernet S, Gurguí M, Len O, Lumbreras C, Marcos MÁ, Martín-Dávila P, Monforte V, Montejo M, Moreno A, Muñoz P, Navarro D, Pahissa A, Pérez JL, Rodriguez-Bernot A, Rumbao J, San Juan R, Santos F, Varo E, Zurbano F, GESITRA-SEIMC/REIPI . 2011. GESITRA-SEIMC/REIPI recommendations for the management of cytomegalovirus infection in solid-organ transplant patients. Enferm Infecc Microbiol Clin 29:735–758. doi: 10.1016/j.eimc.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 2.Cervera C, Fernández-Ruiz M, Valledor A, Linares L, Antón A, Ángeles Marcos M, Sanclemente G, Hoyo I, Cofán F, Ricart MJ, Pérez-Villa F, Navasa M, Pumarola T, Moreno A. 2011. Epidemiology and risk factors for late infection in solid organ transplant recipients. Transpl Infect Dis 13:598–607. doi: 10.1111/j.1399-3062.2011.00646.x. [DOI] [PubMed] [Google Scholar]
- 3.Cervera C, Gurguí M, Lumbreras C. 2011. Risk factors for cytomegalovirus in solid organ transplant recipients. Enferm Infecc Microbiol Clin 29:11–17. doi: 10.1016/S0213-005X(11)70051-9. [DOI] [PubMed] [Google Scholar]
- 4.Charpentier B, Rostaing L, Berthoux F, Lang P, Civati G, Touraine JL, Squifflet JP, Vialtel P, Abramowicz D, Mourad G, Wolf P, Cassuto E, Moulin B, Rifle G, Pruna A, Merville P, Mignon F, Legendre C, Le Pogamp P, Lebranchu Y, Toupance O, Hurault De Ligny B, Touchard G, Olmer M, Purgus R, Pouteil-Noble C, Glotz D, Bourbigot B, Leski M, Wauters JP, Kessler M. 2003. A three arm study comparing immediate tacrolimus therapy with antithymocyte globulin induction therapy followed by tacrolimus or cyclosporine A in adult renal transplant recipients. Transplantation 75:844–851. doi: 10.1097/01.TP.0000056635.59888.EF. [DOI] [PubMed] [Google Scholar]
- 5.Tolkoff-Rubin NE, Rubin RH. 1999. The impact of cytomegalovirus infection on graft function and patient outcome. Graft 2:S101–S103. [Google Scholar]
- 6.Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Danziger-Isakov L, Humar A, Transplantation Society International CMV Consensus Group . 2013. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 96:333–360. doi: 10.1097/TP.0b013e31829df29d. [DOI] [PubMed] [Google Scholar]
- 7.Paya C, Humar A, Dominguez E, Washburn K, Blumberg E, Alexander B, Freeman R, Heaton N, Pescovitz MD, Valganciclovir Solid Organ Transplant Study Group. 2004. Efficacy and safety of valganciclovir versus oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant 4:611–620. doi: 10.1111/j.1600-6143.2004.00382.x. [DOI] [PubMed] [Google Scholar]
- 8.Wiltshire H, Paya CV, Pescovitz MD, Humar A, Dominguez E, Washburn K, Blumberg E, Alexander B, Freeman R, Heaton N, Zuideveld KP, Valganciclovir Solid Organ Transplant Study Group. 2005. Pharmacodynamics of oral ganciclovir and valganciclovir in solid organ transplant recipients. Transplantation 79:1477–1483. doi: 10.1097/01.TP.0000164512.99703.AD. [DOI] [PubMed] [Google Scholar]
- 9.Caldés A, Colom H, Armendariz Y, Garrido MJ, Troconiz IF, Gil-Vernet S, Lloberas N, Pou L, Peraire C, Grinyó JM. 2009. Population pharmacokinetics of ganciclovir after intravenous ganciclovir and oral valganciclovir administration in solid organ transplant patients infected with cytomegalovirus. Antimicrob Agents Chemother 53:4816–4824. doi: 10.1128/AAC.00085-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padullés Caldés A, Colom H, Caldes A, Cerezo G, Torras J, Grinyó JM, Lloberas N. 2014. Optimal sparse sampling for estimating ganciclovir/valganciclovir AUC in solid organ transplant patients using NONMEN. Ther Drug Monit 36:371–377. doi: 10.1097/FTD.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 11.Padullés A, Colom H, Armendariz Y, Cerezo G, Caldes A, Pou L, Torras J, Grinyó JM, Lloberas N. 2012. Determination of ganciclovir in human plasma by ultra performance liquid chromatography-UV detection. Clin Biochem 45:309–314. doi: 10.1016/j.clinbiochem.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 12.US Department of Health and Human Services. 2010. Common terminology criteria for adverse events (CTCAE), v4.03. US Department of Health and Human Services, Washington, DC. [Google Scholar]
- 13.Caldés A, Gil-Vernet S, Armendariz Y, Colom H, Pou L, Niubó J, Lladó L, Torras J, Manito N, Rufí G, Grinyó JM. 2010. Sequential treatment of cytomegalovirus infection or disease with a short course of intravenous ganciclovir followed by oral valganciclovir: efficacy, safety, and pharmacokinetics. Transpl Infect Dis 12:204–212. [DOI] [PubMed] [Google Scholar]
- 14.F Hoffmann-La Roche Ltd. 2013. Ganciclovir product information. F Hoffmann-La Roche Ltd, Basel, Switzerland. [Google Scholar]
- 15.F Hoffmann-La Roche Ltd. 2014. Valganciclovir product information. F Hoffmann-La Roche Ltd, Basel, Switzerland. [Google Scholar]
- 16.Bedino G, Esposito P, Bosio F, Corradetti V, Valsania T, Rocca C, Pattonieri EF, Gregorini M, Rampino T, Dal Canton A. 2013. The role of therapeutic drug monitoring in the treatment of cytomegalovirus disease in kidney transplantation. Int Urol Nephrol 45:1809–1813. doi: 10.1007/s11255-012-0293-y. [DOI] [PubMed] [Google Scholar]
- 17.Asberg A, Humar A, Rollag H, Jardine AG, Mouas H, Pescovitz MD, Sgarabotto D, Tuncer M, Noronha IL, Hartmann Ap, VICTOR Study Group . 2007. Oral valganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant 7:2106–2113. doi: 10.1111/j.1600-6143.2007.01910.x. [DOI] [PubMed] [Google Scholar]
- 18.Falagas ME, Snydman DR, Griffith J, Werner BG, Freeman R, Rohrer R. 1997. Clinical and epidemiological predictors of recurrent cytomegalovirus disease in orthotopic liver transplant recipients. Boston Center for Liver Transplantation CMVIG Study Group. Clin Infect Dis 25:314–317. [DOI] [PubMed] [Google Scholar]
- 19.Humar A, Kumar D, Raboud J, Caliendo AM, Moussa G, Levy G, Mazzulli T. 2002. Interactions between cytomegalovirus, human herpesvirus-6, and the recurrence of hepatitis C after liver transplantation. Am J Transplant 2:461–466. doi: 10.1034/j.1600-6143.2002.20511.x. [DOI] [PubMed] [Google Scholar]
- 20.Asberg A, Humar A, Jardine AG, Rollag H, Pescovitz MD, Mouas H, Bignamini A, Töz H, Dittmer I, Montejo M, Hartmann A, VICTOR Study Group . 2009. Long-term outcomes of CMV disease treatment with valganciclovir versus iv ganciclovir in solid organ transplant recipients. Am J Transplant 9:1205–1213. doi: 10.1111/j.1600-6143.2009.02617.x. [DOI] [PubMed] [Google Scholar]
- 21.Bestard O, Lucia M, Crespo E, Van Liempt B, Palacio D, Melilli E, Torras J, Llaudó I, Cerezo G, Taco O, Gil-Vernet S, Grinyó JM, Cruzado JM. 2013. Pretransplant immediately early-1-specific T cell responses provide protection for CMV infection after kidney transplantation. Am J Transplant 13:1793–1805. doi: 10.1111/ajt.12256. [DOI] [PubMed] [Google Scholar]