Abstract
Primary central nervous system phaeohyphomycosis is a fatal fungal infection due mainly to the neurotropic melanized fungi Cladophialophora bantiana, Rhinocladiella mackenziei, and Exophiala dermatitidis. Despite the combination of surgery with antifungal treatment, the prognosis continues to be poor, with mortality rates ranging from 50 to 70%. Therefore, a search for a more-appropriate therapeutic approach is urgently needed. Our in vitro studies showed that with the combination of amphotericin B and flucytosine against these species, the median fractional inhibitory concentration (FIC) indices for strains ranged from 0.25 to 0.38, indicating synergy. By use of Bliss independence analysis, a significant degree of synergy was confirmed for all strains, with the sum ΔE ranging from 90.2 to 698.61%. No antagonism was observed. These results indicate that amphotericin B, in combination with flucytosine, may have a role in the treatment of primary cerebral infections caused by melanized fungi belonging to the order Chaetothyriales. Further in vivo studies and clinical investigations to elucidate and confirm these observations are warranted.
INTRODUCTION
Cerebral phaeohyphomycosis is a rare but frequently fatal fungal infection due mainly to neurotropic black fungi belonging to the ascomycete order Chaetothyriales: Cladophialophora bantiana, Rhinocladiella mackenziei, and Exophiala dermatitidis (1–5). Other opportunistic pathogens from this group of environmental fungi (i.e., Cladophialophora modesta, Exophiala asiatica, Fonsecaea monophora, and Fonsecaea pugnacius) are being encountered as causal agents of this infection (6, 7).
The infection may occur in immunosuppressed patients following the inhalation of conidia; however, a high proportion of primary cerebral infections are reported in apparently immunocompetent individuals without any obvious predisposing factors (2, 4). If the infection remains untreated, mortality can be as high as 100% within weeks, months, or years (4).
The optimal therapeutic regimen for the treatment of cerebral phaeohyphomycosis is not known. Therapy with amphotericin B alone (a standard or lipid preparation) may not be adequate (8–10), while in vivo studies and single cases suggest that voriconazole and posaconazole may provide better outcomes (8, 11). Moreover, combinations of a triazole with an echinocandin and/or flucytosine have shown better efficacy than monotherapy (12–15), but the results are not yet conclusive. When possible, complete surgical removal of brain lesions combined with systemic antifungal therapy is recommended (13, 16). For those who are treated, mortality is lower than for those without treatment, but the prognosis continues to be poor, with a case fatality rate as high as 70% (11, 17–19).
Considering the poor clinical outcomes, the development of a more appropriate therapeutic approach is required. From a clinical perspective, the combination of amphotericin B and flucytosine is generally associated with improved survival among patients with systemic fungal infections (20, 21), including cryptococcal meningitis (22–24). However, data on the clinical use of this combination for patients with cerebral phaeohyphomycosis are scarce. In this study, we therefore investigated the in vitro antifungal activity of amphotericin B in combination with flucytosine against a collection of black fungi obtained from patients with primary brain infections.
MATERIALS AND METHODS
Fungal isolates.
A collection of 12 clinical isolates consisting of 5 strains of C. bantiana, 4 strains of R. mackenziei, and 3 strains of E. dermatitidis originating from both human and animal brain abscesses were used (see Table 1). All strains were obtained from the reference collection of the CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands, and were handled under biosafety laboratory regulations (for biosafety levels 2 and 3, as appropriate). The identities of the organisms were confirmed by sequencing of the internal transcribed spacer regions of ribosomal DNA (rDNA), as described previously (25, 26).
TABLE 1.
FIC indices based on 10% growth endpoints and Bliss independence results for melanized fungi causing primary cerebral infections
| Fungal species | Biosafety level | Strain no. | Source | Origin | MIC (μg/ml)a |
FIC index | Sum ΔE | Result | |
|---|---|---|---|---|---|---|---|---|---|
| AmB | 5-FC | ||||||||
| Cladophialophora bantiana | 3 | CBS 101251 | Human, brain abscess | USA | 0.25 | 2 | 0.5 | 90.2 | Synergy |
| CBS 101158 | Human, brain abscess | Japan | 0.25 | 64 | 0.25 | 149.58 | Synergy | ||
| CBS 102586 | Human, brain abscess | Brazil | 0.25 | 2 | 0.25 | 189.69 | Synergy | ||
| CBS 98496 | Human, brain abscess | South Africa | 0.5 | 4 | 0.5 | 140.16 | Synergy | ||
| CBS 100436 | Cat, brain abscess | California, USA | 0.5 | 2 | 0.25 | 114.23 | Synergy | ||
| Rhinocladiella mackenziei | 3 | CBS 65093 | Human, brain abscess | Saudi Arabia | 8 | 16 | 0.5 | 396.42 | Synergy |
| CBS 36892 | Human, brain abscess | Israel | 8 | 16 | 0.25 | 527.31 | Synergy | ||
| CBS 102589 | Human, brain abscess | Egypt | 8 | 32 | 0.25 | 293.89 | Synergy | ||
| CBS 109634 | Human, brain abscess | Oman | 2 | 32 | 0.5 | 419.4 | Synergy | ||
| Exophiala dermatitidis | 2 | CBS 120473 | Human, brain abscess | USA | 0.5 | 32 | 0.125 | 698.61 | Synergy |
| CBS 57976 | Human, brain abscess | Japan | 1 | 32 | 0.25 | 498.65 | Synergy | ||
| CBS 57876 | Human, brain abscess | Japan | 0.5 | 64 | 0.25 | 450.11 | Synergy | ||
AmB, amphotericin B; 5-FC, flucytosine.
Stock cultures were grown on malt extract agar (MEA; Difco, Leeuwarden, The Netherlands) at 25°C for 1 to 3 weeks before the preparation of the inoculum. Paecilomyces variotii (ATCC 22319), Candida parapsilosis (ATCC 22019), and Candida krusei (ATCC 6258) were used as quality controls in all experiments.
Preparation of the inoculum.
All isolates were subcultured on MEA at 25°C. Then conidial suspensions were harvested and were suspended in normal saline containing 0.025% Tween 20. Supernatants were adjusted spectrophotometrically at 530-nm wavelengths to optical densities (ODs) that ranged from 0.15 to 0.17 (68 to 71% transmission) for C. bantiana and R. mackenziei and from 0.09 to 0.13 (80 to 83% transmission) for E. dermatitidis.
Antifungal agents.
Amphotericin B and flucytosine (Sigma-Aldrich, St. Louis, MO, USA) were obtained as standard pure powders, and serial dilutions were prepared according to the Clinical and Laboratory Standards Institute (CLSI) broth microdilution guidelines (27).
Susceptibility and drug interaction testing.
Antifungal susceptibility testing and drug interaction testing were performed by using the broth microdilution checkerboard (2-dimensional, 8-by-12) method, utilizing XTT dye {2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide}, as described previously (28–30). XTT (Sigma-Aldrich, St. Louis, MO) was dissolved in normal saline at a concentration of 0.5 mg/ml. Menadione (Sigma-Aldrich) was initially dissolved in absolute ethanol at a concentration of 10 mg/ml and was subsequently added to the XTT solutions at a concentration of 6.25 μM for each solution. The final concentrations of the antifungal agents ranged from 0.125 to 8 mg/liter for amphotericin B and from 0.125 to 128 mg/liter for flucytosine. Aliquots (50 μl) of each drug at a concentration four times the targeted final concentration were dispensed into the wells of U-shaped 96-well microtiter plates (Costar, Corning, NY). Trays were maintained for a period of <1 month at −70°C until the day of testing. After the microtitration trays were defrosted, 100 μl of the inoculum was added to each well, corresponding to a final concentration of 0.5 × 104 to 4 × 104 CFU/ml for each isolate. The microtiter plates were incubated at 35 to 37°C for 72 h. If no growth was observed, or if growth was inadequate, the incubation was extended to 14 days. Subsequently, 50 μl of the XTT-menadione solution was added to each well, as described previously (30–32). The microtitration plates were further incubated at 35 to 37°C for 2 h in order to allow the conversion of XTT to its formazan derivative. XTT conversion was measured as the OD with a microtitration plate spectrophotometric reader (Anthos HTIII; Anthos Labtec Instruments, Salzburg, Austria) at 450 nm/630 nm (30–32). For each well, the XTT conversion was calculated after subtraction of the background OD, which was the OD of a simultaneously incubated well with 200 μl of medium and 50 μl of the XTT-menadione solution but no inoculum. Percentages of fungal growth were calculated for each well by dividing the XTT conversion in each well by the XTT conversion in the drug-free growth control well. All experiments on each strain were performed using three independent replicates on different days.
MIC determination.
The MICs of amphotericin B and flucytosine were defined as the lowest concentrations that completely inhibited growth relative to the growth in the drug-free well, as assessed by visual inspection. Because the MIC value for amphotericin B is considered the lowest drug concentration corresponding to <10% growth and the flucytosine MIC is the lowest drug concentration corresponding to 50% growth inhibition, 10%, 25%, and 50% growth endpoints were calculated as MIC endpoints for the amphotericin B–flucytosine combination (27).
Definitions for drug interaction modeling.
In order to assess the nature of in vitro interactions between amphotericin B and flucytosine, the data obtained as described above were analyzed using two different models. These models were nonparametric approaches of the following two zero-interaction theories: the Loewe additivity (LA) and Bliss independence (BI) theories (33–36). The fractional inhibitory concentration (FIC) index is defined as follows: ∑FIC = FICA + FICB = (CAcomb/MICAalone) + (CBcomb/MICBalone), where MICAalone and MICBalone are the MICs of the drugs A and B when acting alone, and CAcomb and CBcomb are the concentrations of the drugs A and B at the isoeffective combinations, respectively (34). To determine the synergistic and antagonistic interactions among all ∑FICs calculated for each isolate and replicate, the FIC index was determined as the ∑FICmin (the lowest ∑FIC) or the ∑FICmax (the highest ∑FIC) (34). Ten percent endpoints of fungal growth were used to assess pharmacodynamic interactions at different concentrations. Drug interactions were defined as synergistic if the FIC index was <0.5, as antagonistic if the FIC index was >4, and as noninteractive if the FIC index was between 0.5 and 4 (37).
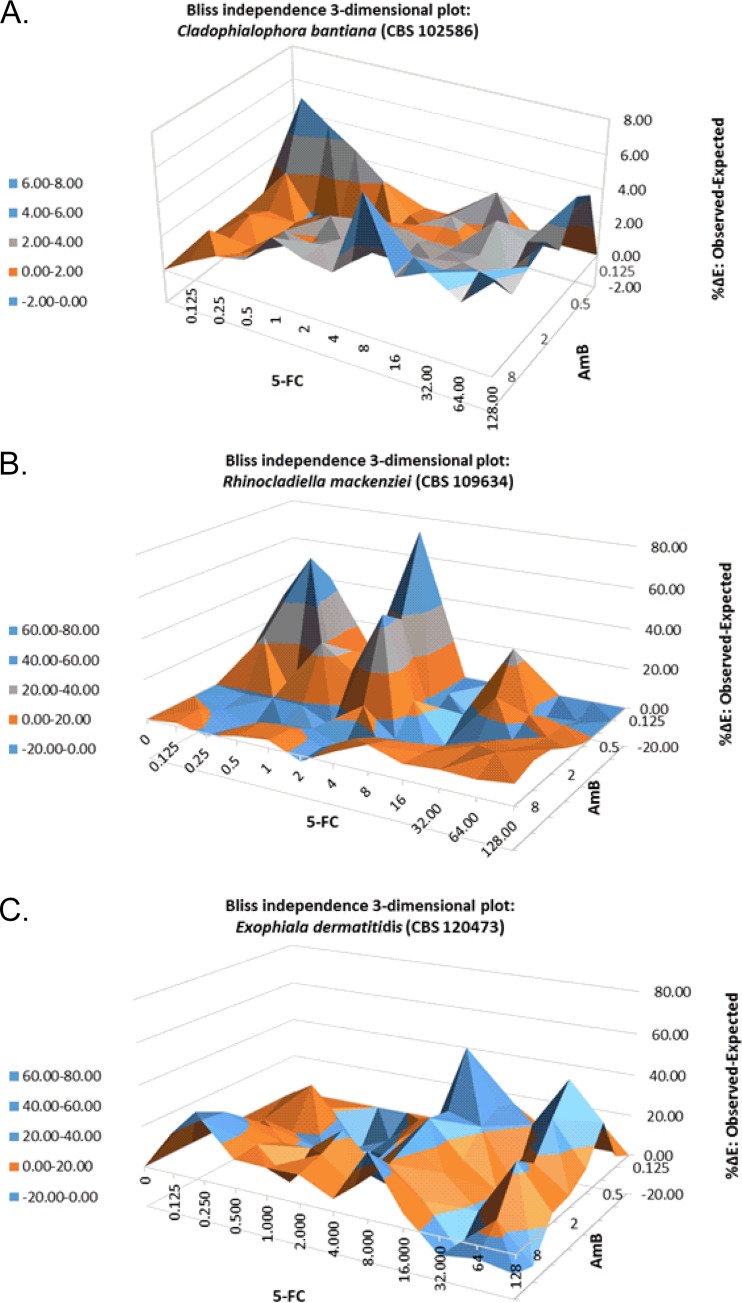
The BI parameter was described by the equation Iind = IA + IB − (IA × IB), where Iind is the predicted percentage of inhibition of a noninteractive theoretical combination, calculated with the experimental percentages of inhibition (IA, IB) of each drug acting alone (36). In the 3-dimensional plots, peaks above and below the zero plane indicate synergistic and antagonistic combinations, respectively, whereas the zero plane itself indicates no statistically significant interactions. The average sum of the three replicates of all Bliss interactions was used as a measure of the pharmacodynamic interactions for each strain. Drug interactions were considered synergistic if ΔE was greater than zero (positive ΔE), indifferent if ΔE was zero, and antagonistic if ΔE was less than zero (negative ΔE).
Data analysis.
All data analyses were performed by using the GraphPad Prism software package (version 5.0 for Windows; GraphPad Software, San Diego, CA). The FIC and BI indices among the different groups were compared by analysis of variance (ANOVA) followed by a posttest for linear trend. The correlation between the mean FIC indices and the sum ΔE was determined by Spearman's correlation coefficient (r); a P value of ≤0.05 (by a two-tailed test) was considered significant.
RESULTS
The MICs for the isolates used in the current study and the results of the FIC index model are summarized in Table 1. For the combination of amphotericin B and flucytosine, the median FIC indices were 0.25 for C. bantiana (∑FIC ranging from 0.25 to 0.5), 0.38 for R. mackenziei (∑FIC ranging from 0.25 to 0.5), and 0.25 for E. dermatitidis (∑FIC ranging from 0.125 to 0.25), indicating synergy for all strains. In addition, a mean FIC value of >4 for all replicates was not obtained with any of the isolates tested, indicating that no antagonism was found.
Table 1 and Fig. 1 show the results of Bliss independence drug interaction analysis for the in vitro interactions of amphotericin B and flucytosine. The amphotericin B–flucytosine combination resulted in a synergistic interaction for all strains. The degree of synergy was highest among the E. dermatitidis strains (sum ΔE, 450.11% to 698.61%), followed by R. mackenziei (sum ΔE, 293.89% to 527.31%) and C. bantiana (sum ΔE, 90.2% to 189.69%), respectively.
FIG 1.
Interaction surfaces obtained from response surface analysis of the Bliss independence no-interaction model for the in vitro combination of amphotericin B (AmB) and flucytosine (5-FC) against a Cladophialophora bantiana strain (CBS 102586) (AmB MIC, 0.25 μg/ml; 5-FC MIC, 2 μg/ml), a Rhinocladiella mackenziei strain (CBS 109634) (AmB MIC, 2 μg/ml; 5-FC MIC, 32 μg/ml), and an Exophiala dermatitidis strain (CBS 120473) (AmB MIC, 0.5 μg/ml; 5-FC MIC, 32 μg/ml). The x and y axes represent the efficacies of AmB and 5-FC, respectively. The z axis is ΔE, expressed as a percentage. The zero plane represents Bliss independent interactions, whereas values above the zero plane represent statistically significantly synergistic (positive ΔE) interactions. The magnitude of interactions is directly related to ΔE. The different tones in the 3-dimensional plots represent different percentile bands of synergy.
DISCUSSION
Overall, our results show that the amphotericin B–flucytosine combination has consistent synergistic effects against C. bantiana, R. mackenziei, and E. dermatitidis. The results of FIC analysis were supported by response surface analysis using the Bliss independence no-interaction model for the isolates tested. Both models were shown to correlate well with the in vivo results of combination therapy in experimental invasive fungal infections, such as invasive pulmonary aspergillosis (32, 38). Therefore, these results could help to support the combination of amphotericin B and flucytosine against infections caused by neurotropic species of melanized fungi. On the other hand, the Bliss independence theory was derived from the probability that two drugs do not interact with each other and therefore will act independently (38, 39).
C. bantiana causes severe infections, mainly in immunocompetent hosts worldwide, with a general preference for warm and humid climates. The species causes cerebral abscesses almost exclusively, with a high mortality rate (up to 70%) (1, 5, 7, 16). R. mackenziei causes cerebral infections mostly in debilitated patients, with a mortality rate of almost 100% if infections remain untreated; even in patients treated with surgery and antifungal therapy, mortality is almost 65%. This fungus is restricted to the Middle East, the Persian Gulf, Somalia, and Pakistan (2, 40, 41). E. dermatitidis is one of the most common clinically significant human pathogens in the black-yeast genus Exophiala, causing disseminated infection with a marked predilection for the central nervous system (CNS). Infections by this fungus are reported mainly from East Asia, although several cases in other geographical regions worldwide have been reported (42, 43). This fungus seems to be able to affect young, otherwise healthy patients (5, 42, 44, 45). E. dermatitidis cerebral infection is generally associated with a high mortality rate (about 50%) (17).
Evidence to support treatment choices for cerebral phaeohyphomycosis caused by these fungi is scarce at present, and patients have died in most cases despite a combination of surgery and antifungal therapy (2–4, 46). On the other hand, the use of a potent antifungal with increased efficacy does not guarantee the therapeutic outcome, since treatment failures might occur, possibly because of poor penetration into the CNS (47). Few studies have reported data on the efficacy of antifungal combination therapy against invasive fungal infections caused by neurotropic melanized fungi (12, 48, 49). Most studies investigating combinations of azoles with echinocandins or polyenes and/or combinations of echinocandins with polyenes have shown a synergistic or additive interaction in vitro and in vivo (12, 14, 48, 49). One study, using a murine model, tested double or triple combinations of amphotericin B, micafungin, voriconazole, flucytosine, and posaconazole in the treatment of disseminated infections caused by C. bantiana (12). Combination therapy with three of the drugs (posaconazole, micafungin, and flucytosine) appeared to be a promising option for the treatment of C. bantiana infections (12). In another study, Sun et al. investigated the in vitro interactions of the following combinations against E. dermatitidis strains: caspofungin with itraconazole, voriconazole, amphotericin B, or fluconazole; terbinafine with itraconazole; and fluconazole with amphotericin B (49). Combinations of caspofungin with voriconazole, amphotericin B, or itraconazole showed synergistic activity against E. dermatitidis (49).
Of note, combination therapy with amphotericin B and flucytosine is the recommended first-line treatment for disseminated cryptococcal meningitis, a fungal infection of the CNS, in both immunocompetent and immunosuppressed patients (22–24). Our results therefore suggest that a combination of amphotericin B and flucytosine may have a promising role in the treatment of primary cerebral phaeohyphomycosis due to neurotropic species of melanized fungi and possibly other emerging pathogens from this group of environmental fungi. In vivo studies and in vitro-in vivo correlation investigations to validate and confirm these observations are warranted.
ACKNOWLEDGMENTS
S.S. has received a research grant from Astellas Pharma B.V. P.E.V. has served as a consultant to, and has received research grants from, Astellas, Basilea, Gilead Sciences, Merck, and Pfizer. The other authors declare no conflict of interest.
This study was supported, in part, by the Department of Medical Microbiology, Radboudumc, Nijmegen, The Netherlands, and the CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands. The work of Wanqing Liao was supported by grant no. 2013CB531601 from the 973 Program of China, that of Weihua Pan by grant no. 2013ZX10004612 from National Science and Technology Major Projects of China, that of Wanqing Liao by grant no. 14DZ2272900 from the Shanghai Science and Technology Committee, and that of Shuwen Deng by grant no. 81260236 of the National Natural Science Foundation of China.
REFERENCES
- 1.Chakrabarti A, Kaur H, Rudramurthy SM, Appannanavar SB, Patel A, Mukherjee KK, Ghosh A, Ray U. 2016. Brain abscess due to Cladophialophora bantiana: a review of 124 cases. Med Mycol 54:111–119. doi: 10.1093/mmy/myv091. [DOI] [PubMed] [Google Scholar]
- 2.Li DM, de Hoog GS. 2009. Cerebral phaeohyphomycosis—a cure at what lengths? Lancet Infect Dis 9:376–383. doi: 10.1016/S1473-3099(09)70131-8. [DOI] [PubMed] [Google Scholar]
- 3.De Hoog GS, Queiroz-Telles F, Haase G, Fernandez-Zeppenfeldt G, Attili Angelis D, Gerrits Van Den Ende AH, Matos T, Peltroche-Llacsahuanga H, Pizzirani-Kleiner AA, Rainer J, Richard-Yegres N, Vicente V, Yegres F. 2000. Black fungi: clinical and pathogenic approaches. Med Mycol 38(Suppl 1):S243–S250. [PubMed] [Google Scholar]
- 4.Kantarcioglu AS, de Hoog GS. 2004. Infections of the central nervous system by melanized fungi: a review of cases presented between 1999 and 2004. Mycoses 47:4–13. doi: 10.1046/j.1439-0507.2003.00956.x. [DOI] [PubMed] [Google Scholar]
- 5.Horre R, De Hoog GS. 1999. Primary cerebral infections by melanized fungi: a review. Stud Mycol 43:176–193. [Google Scholar]
- 6.Surash S, Tyagi A, De Hoog GS, Zeng JS, Barton RC, Hobson RP. 2005. Cerebral phaeohyphomycosis caused by Fonsecaea monophora. Med Mycol 43:465–472. doi: 10.1080/13693780500220373. [DOI] [PubMed] [Google Scholar]
- 7.Revankar SG, Sutton DA, Rinaldi MG. 2004. Primary central nervous system phaeohyphomycosis: a review of 101 cases. Clin Infect Dis 38:206–216. doi: 10.1086/380635. [DOI] [PubMed] [Google Scholar]
- 8.Al-Abdely HM, Najvar LK, Bocanegra R, Graybill JR. 2005. Antifungal therapy of experimental cerebral phaeohyphomycosis due to Cladophialophora bantiana. Antimicrob Agents Chemother 49:1701–1707. doi: 10.1128/AAC.49.5.1701-1707.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Abdely HM, Najvar L, Bocanegra R, Fothergill A, Loebenberg D, Rinaldi MG, Graybill JR. 2000. SCH 56592, amphotericin B, or itraconazole therapy of experimental murine cerebral phaeohyphomycosis due to Ramichloridium obovoideum (“Ramichloridium mackenziei”). Antimicrob Agents Chemother 44:1159–1162. doi: 10.1128/AAC.44.5.1159-1162.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Revankar SG. 2011. Cladophialophora bantiana brain abscess in an immunocompetent patient. Can J Infect Dis Med Microbiol 22:149–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Abdely HM, Alkhunaizi AM, Al-Tawfiq JA, Hassounah M, Rinaldi MG, Sutton DA. 2005. Successful therapy of cerebral phaeohyphomycosis due to Ramichloridium mackenziei with the new triazole posaconazole. Med Mycol 43:91–95. doi: 10.1080/13693780400011104. [DOI] [PubMed] [Google Scholar]
- 12.Mariné M, Pastor FJ, Guarro J. 2009. Combined antifungal therapy in a murine model of disseminated infection by Cladophialophora bantiana. Med Mycol 47:45–49. doi: 10.1080/13693780802526840. [DOI] [PubMed] [Google Scholar]
- 13.Chowdhary A, Meis JF, Guarro J, de Hoog GS, Kathuria S, Arendrup MC, Arikan-Akdagli S, Akova M, Boekhout T, Caira M, Guinea J, Chakrabarti A, Dannaoui E, van Diepeningen A, Freiberger T, Groll AH, Hope WW, Johnson E, Lackner M, Lagrou K, Lanternier F, Lass-Florl C, Lortholary O, Meletiadis J, Munoz P, Pagano L, Petrikkos G, Richardson MD, Roilides E, Skiada A, Tortorano AM, Ullmann AJ, Verweij PE, Cornely OA, Cuenca-Estrella M; European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group, European Confederation of Medical Mycology. 2014. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of systemic phaeohyphomycosis: diseases caused by black fungi. Clin Microbiol Infect 20(Suppl 3):S47–S75. doi: 10.1111/1469-0691.12515. [DOI] [PubMed] [Google Scholar]
- 14.Trinh JV, Steinbach WJ, Schell WA, Kurtzberg J, Giles SS, Perfect JR. 2003. Cerebral phaeohyphomycosis in an immunodeficient child treated medically with combination antifungal therapy. Med Mycol 41:339–345. doi: 10.1080/369378031000137369. [DOI] [PubMed] [Google Scholar]
- 15.Levin TP, Baty DE, Fekete T, Truant AL, Suh B. 2004. Cladophialophora bantiana brain abscess in a solid-organ transplant recipient: case report and review of the literature. J Clin Microbiol 42:4374–4378. doi: 10.1128/JCM.42.9.4374-4378.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garzoni C, Markham L, Bijlenga P, Garbino J. 2008. Cladophialophora bantiana: a rare cause of fungal brain abscess. Clinical aspects and new therapeutic options. Med Mycol 46:481–486. doi: 10.1080/13693780801914906. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto T, Matsuda T, McGinnis MR, Ajello L. 1993. Clinical and mycological spectra of Wangiella dermatitidis infections. Mycoses 36:145–155. [DOI] [PubMed] [Google Scholar]
- 18.Delfino D, De Hoog S, Polonelli L, Benecchi M, Fanti F, Galatioto S, Manti G, Cusumano V. 2006. Survival of a neglected case of brain abscess caused by Cladophialophora bantiana. Med Mycol 44:651–654. doi: 10.1080/13693780600637690. [DOI] [PubMed] [Google Scholar]
- 19.Lyons MK, Blair JE, Leslie KO. 2005. Successful treatment with voriconazole of fungal cerebral abscess due to Cladophialophora bantiana. Clin Neurol Neurosurg 107:532–534. doi: 10.1016/j.clineuro.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Mukherjee PK, Sheehan DJ, Hitchcock CA, Ghannoum MA. 2005. Combination treatment of invasive fungal infections. Clin Microbiol Rev 18:163–194. doi: 10.1128/CMR.18.1.163-194.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis RE, Kontoyiannis DP. 2001. Rationale for combination antifungal therapy. Pharmacotherapy 21:149S–164S. doi: 10.1592/phco.21.12.149S.34505. [DOI] [PubMed] [Google Scholar]
- 22.Day JN, Chau TT, Wolbers M, Mai PP, Dung NT, Mai NH, Phu NH, Nghia HD, Phong ND, Thai CQ, Thai LH, Chuong LV, Sinh DX, Duong VA, Hoang TN, Diep PT, Campbell JI, Sieu TP, Baker SG, Chau NV, Hien TT, Lalloo DG, Farrar JJ. 2013. Combination antifungal therapy for cryptococcal meningitis. N Engl J Med 368:1291–1302. doi: 10.1056/NEJMoa1110404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennett JE, Dismukes WE, Duma RJ, Medoff G, Sande MA, Gallis H, Leonard J, Fields BT, Bradshaw M, Haywood H, McGee ZA, Cate TR, Cobbs CG, Warner JF, Alling DW. 1979. A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptococcal meningitis. N Engl J Med 301:126–131. doi: 10.1056/NEJM197907193010303. [DOI] [PubMed] [Google Scholar]
- 24.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen MH, Pappas PG, Powderly WG, Singh N, Sobel JD, Sorrell TC. 2010. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Hoog GS, Guarro J, Gene J, Figueras MJ. 2009. Atlas of clinical fungi: the ultimate benchtool for diagnostics, a pilot version of the 3rd ed. CD-ROM. Centraalbureau voor Schimmelcultures, Baarn, The Netherlands. [Google Scholar]
- 26.Seyedmousavi S, Netea MG, Mouton JW, Melchers WJ, Verweij PE, de Hoog GS. 2014. Black yeasts and their filamentous relatives: principles of pathogenesis and host defense. Clin Microbiol Rev 27:527–542. doi: 10.1128/CMR.00093-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd ed CLSI document M38-A2, vol 28, no.16 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 28.Antachopoulos C, Meletiadis J, Sein T, Roilides E, Walsh TJ. 2007. Use of high inoculum for early metabolic signalling and rapid susceptibility testing of Aspergillus species. J Antimicrob Chemother 59:230–237. doi: 10.1093/jac/dkl488. [DOI] [PubMed] [Google Scholar]
- 29.Antachopoulos C, Meletiadis J, Sein T, Roilides E, Walsh TJ. 2007. Concentration-dependent effects of caspofungin on the metabolic activity of Aspergillus species. Antimicrob Agents Chemother 51:881–887. doi: 10.1128/AAC.01160-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meletiadis J, Mouton JW, Meis JF, Bouman BA, Donnelly JP, Verweij PE. 2001. Colorimetric assay for antifungal susceptibility testing of Aspergillus species. J Clin Microbiol 39:3402–3408. doi: 10.1128/JCM.39.9.3402-3408.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meletiadis J, Mouton JW, Meis JF, Bouman BA, Donnelly PJ, Verweij PE. 2001. Comparison of spectrophotometric and visual readings of NCCLS method and evaluation of a colorimetric method based on reduction of a soluble tetrazolium salt, 2,3-bis [2-methoxy-4-nitro-5-[(sulfenylamino) carbonyl]-2H-tetrazolium-hydroxide], for antifungal susceptibility testing of Aspergillus species. J Clin Microbiol 39:4256–4263. doi: 10.1128/JCM.39.12.4256-4263.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seyedmousavi S, Meletiadis J, Melchers WJ, Rijs AJ, Mouton JW, Verweij PE. 2013. In vitro interaction of voriconazole and anidulafungin against triazole-resistant Aspergillus fumigatus. Antimicrob Agents Chemother 57:796–803. doi: 10.1128/AAC.00980-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prichard MN, Prichard LE, Shipman C Jr. 1993. Strategic design and three-dimensional analysis of antiviral drug combinations. Antimicrob Agents Chemother 37:540–545. doi: 10.1128/AAC.37.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hindler J. 1995. Antimicrobial susceptibility testing, p 5.18.11–15.18.20. In Isenberg HD. (ed), Clinical microbiology procedures handbook. American Society for Microbiology, Washington DC. [Google Scholar]
- 35.Meletiadis J, Verweij PE, te Dorsthorst DT, Meis JF, Mouton JW. 2005. Assessing in vitro combinations of antifungal drugs against yeasts and filamentous fungi: comparison of different drug interaction models. Med Mycol 43:133–152. doi: 10.1080/13693780410001731547. [DOI] [PubMed] [Google Scholar]
- 36.Meletiadis J, Meis JFGM, Mouton JW, Verweij PE. 2002. Methodological issues related to antifungal drug interaction modelling for filamentous fungi. Rev Med Microbiol 13:101–117. [Google Scholar]
- 37.Vitale RG, Afeltra J, de Hoog GS, Rijs AJ, Verweij PE. 2003. In vitro activity of amphotericin B and itraconazole in combination with flucytosine, sulfadiazine and quinolones against Exophiala spinifera. J Antimicrob Chemother 51:1297–1300. doi: 10.1093/jac/dkg218. [DOI] [PubMed] [Google Scholar]
- 38.Meletiadis J, te Dorsthorst DT, Verweij PE. 2006. The concentration-dependent nature of in vitro amphotericin B-itraconazole interaction against Aspergillus fumigatus: isobolographic and response surface analysis of complex pharmacodynamic interactions. Int J Antimicrob Agents 28:439–449. doi: 10.1016/j.ijantimicag.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Tam VH, Schilling AN, Lewis RE, Melnick DA, Boucher AN. 2004. Novel approach to characterization of combined pharmacodynamic effects of antimicrobial agents. Antimicrob Agents Chemother 48:4315–4321. doi: 10.1128/AAC.48.11.4315-4321.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taj-Aldeen SJ, Almaslamani M, Alkhalf A, Al Bozom I, Romanelli AM, Wickes BL, Fothergill AW, Sutton DA. 2010. Cerebral phaeohyphomycosis due to Rhinocladiella mackenziei (formerly Ramichloridium mackenziei): a taxonomic update and review of the literature. Med Mycol 48:546–556. doi: 10.3109/13693780903383914. [DOI] [PubMed] [Google Scholar]
- 41.Al-Tawfiq JA, Boukhamseen A. 2011. Cerebral phaeohyphomycosis due to Rhinocladiella mackenziei (formerly Ramichloridium mackenziei): case presentation and literature review. J Infect Public Health 4:96–102. doi: 10.1016/j.jiph.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Chang X, Li R, Yu J, Bao X, Qin J. 2009. Phaeohyphomycosis of the central nervous system caused by Exophiala dermatitidis in a 3-year-old immunocompetent host. J Child Neurol 24:342–345. doi: 10.1177/0883073808323524. [DOI] [PubMed] [Google Scholar]
- 43.Zeng JS, Sutton DA, Fothergill AW, Rinaldi MG, Harrak MJ, de Hoog GS. 2007. Spectrum of clinically relevant Exophiala species in the United States. J Clin Microbiol 45:3713–3720. doi: 10.1128/JCM.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hiruma M, Kawada A, Ohata H, Ohnishi Y, Takahashi H, Yamazaki M, Ishibashi A, Hatsuse K, Kakihara M, Yoshida M. 1993. Systemic phaeohyphomycosis caused by Exophiala dermatitidis. Mycoses 36:1–7. [DOI] [PubMed] [Google Scholar]
- 45.Chang CL, Kim DS, Park DJ, Kim HJ, Lee CH, Shin JH. 2000. Acute cerebral phaeohyphomycosis due to Wangiella dermatitidis accompanied by cerebrospinal fluid eosinophilia. J Clin Microbiol 38:1965–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Revankar SG, Sutton DA. 2010. Melanized fungi in human disease. Clin Microbiol Rev 23:884–928. doi: 10.1128/CMR.00019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lewis RE. 2011. Current concepts in antifungal pharmacology. Mayo Clinic Proc 86:805–817. doi: 10.4065/mcp.2011.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polak A. 1987. Combination therapy of experimental candidiasis, cryptococcosis, aspergillosis and wangiellosis in mice. Chemotherapy 33:381–395. doi: 10.1159/000238524. [DOI] [PubMed] [Google Scholar]
- 49.Sun Y, Liu W, Wan Z, Wang X, Li R. 2011. Antifungal activity of antifungal drugs, as well as drug combinations against Exophiala dermatitidis. Mycopathologia 171:111–117. doi: 10.1007/s11046-010-9358-6. [DOI] [PubMed] [Google Scholar]