Abstract
Parthenin and parthenolide are natural products that are closely related in structure to artemisinin, which is also a sesquiterpene lactone (SQL) and one of the most important antimalarial drugs available. Parthenin, like artemisinin, has an effect on Plasmodium blood stage development. We extended the evaluation of parthenin as a potential therapeutic for the transmissible stages of Plasmodium falciparum as it transitions between human and mosquito, with the aim of gaining potential mechanistic insight into the inhibitory activity of this compound. We posited that if parthenin targets different biological pathways in the parasite, this in turn could pave the way for the development of druggable compounds that could prevent the spread of artemisinin-resistant parasites. We examined parthenin's effect on male gamete activation and the ookinete-to-oocyst transition in the mosquito as well as on stage V gametocytes that are present in peripheral blood. Parthenin arrested parasite development for each of the stages tested. The broad inhibitory properties of parthenin on the evaluated parasite stages may suggest different mechanisms of action between parthenin and artemisinin. Parthenin's cytotoxicity notwithstanding, its demonstrated activity in this study suggests that structurally related SQLs with a better safety profile deserve further exploration. We used our battery of assays to test parthenolide, which has a more compelling safety profile. Parthenolide demonstrated activity nearly identical to that of parthenin against P. falciparum, highlighting its potential as a possible transmission-blocking drug scaffold. We discuss the context of the evidence with respect to the next steps toward expanding the current antimalarial arsenal.
INTRODUCTION
An estimated 198 million cases of malaria and 584,000 deaths, mostly among young children, were reported worldwide in 2013 (1). However, as a result of concerted control efforts since 2000, including antimalarial drugs, mortality has declined by 47%, resulting in an estimated 4.3 million lives saved (1). Artemisinin, delivered with a partnered drug, is one of the fastest acting antimalarial therapies available (2, 3). However, antimalarial resistance in Plasmodium falciparum has been described recently in Southeast Asia (4). This discovery emphasizes the need for novel compounds that are effective against resistant strains, presumably because they act on the parasite differently from artemisinin. Artemisinin was originally isolated from Artemisia annua, or sweet wormwood, a member of the Asteraceae family. We hypothesized that natural product compounds derived from related family members might retain potent activities against Plasmodium while targeting different biological pathways.
Parthenin is a sesquiterpene lactone (SQL) derived from Parthenium hysterophorus, which is an invasive, flowering annual weed in the Asteraceae family; it grows to 2 m in height, with small white flowers, and has spread throughout much of the world (5). P. hysterophorus causes millions of dollars in damage yearly from diminished agricultural productivity (6, 7) and also causes allergic contact dermatitis. These negative effects have been attributed to parthenin, a compound that is found throughout the plant tissue and is also secreted to inhibit the growth of nearby plants (8–11). Further studies on parthenin have revealed that it has a wide array of potentially useful biological effects and that it has shown activity as a potential herbicide (10, 12, 13), pesticide (14, 15), antiparasitic (16, 17), and anticancer compound (18–21). Parthenin may act through a variety of mechanisms, including directly damaging DNA, inhibiting oxidative phosphorylation, inducing formation of nitric oxide, and promoting apoptotic signaling (18, 19, 22–25). General cytotoxicity of parthenin remains a concern, but its activity varies greatly among different cell lines, as illustrated by 50% inhibitory concentrations (IC50s) of 0.061 μM and 594 μM against Jurkat cells and HeLa cells, respectively (26). Less toxic, synthetic parthenin derivatives show promise when tested across a number of human cell lines (17, 19, 26). As mentioned above, parthenin belongs to a class of compounds (SQLs) that also includes artemisinin. Artemisinin is one of a number of compounds that save lives by effectively treating the asexual blood stage form of Plasmodium. However, medicines that treat not only the asexual stages but also the transmissible forms of the parasite will be essential in preventing the spread of new cases of malaria, a critical hurdle that must be overcome if we are to achieve disease eradication (27).
Fully mature, P. falciparum stage V gametocytes (0.1 to 2% of the total parasite biomass) are the sexual, transmissible stages of the parasite (28) and are the only stages that can continue the parasite life cycle in the mosquito following uptake in a blood meal. As the gametocytes enter the mosquito, the male and female gametes emerge from the red blood cells, and the male gametes undergo three rounds of rapid DNA replication, after which the male gamete sends forth its DNA in flagellated packets in a process called exflagellation. After the female macrogamete is fertilized, the developing zygote elongates into a mature, motile ookinete. Within approximately 24 h the ookinete has successfully crossed the midgut peritrophic matrix and traversed a midgut epithelial cell, transforming into a sessile oocyst just beneath the midgut basal lamina (29). After 10 to 20 days of development (depending on Plasmodium species and temperature), these oocysts rupture, releasing thousands of sporozoites into the hemocoel, which in turn attach to and invade the salivary glands, where they await their opportunity to infect a new host during a subsequent blood meal.
Evidence of parthenin's potential activity against these stages comes from studies of the sugar-feeding preferences of Anopheles gambiae in Kenya. It was observed that this species was preferentially attracted to P. hysterophorus (30) even though mosquitoes feeding on this plant had shortened life spans (31). The authors also observed that mosquitoes infected with Plasmodium feed more frequently on this plant (31, 32), and, most importantly, they found that when mosquitoes were provided P. hysterophorus clippings before and after feeding on gametocytemic blood, the mosquitoes had lower oocyst burdens than mosquitoes that were not exposed to the plant (33). Based on these initial studies, we explored the inhibitory properties of parthenin on Plasmodium oocyst formation as well as on the sequential developmental steps during gametocyte-to-oocyst transition.
Given parthenin's poor safety profile, we also extended our analysis to another SQL to explore whether antimalarial activity is unique to a subset of SQLs or if it is generally conserved among related but structurally diverse compounds. Parthenolide is derived from Tanacetum parthenium and exhibits properties similar to those of parthenin (see Fig. S1 in the supplemental material). Parthenolide was selected because it has been more thoroughly evaluated as a potential therapeutic, is safer for nontarget cells, and shows acceptable pharmacological properties (34). Parthenolide's effects on specific biological pathways are also well documented and have demonstrated both anticancer and anti-inflammatory properties by inducing apoptotic signaling and by targeting the NF-κB pathway, respectively (34–39). Although artemisinin, parthenin, and parthenolide are derived from members of the same plant family, parthenin and parthenolide do not contain the endoperoxide bridge essential for artemisinin's activity. This endoperoxide bridge is hypothesized to act as a prodrug, requiring cleavage of the bridge to induce reactive oxygen species (17, 40). We therefore predicted that comparable activities against blood and mosquito stages are a result of different modes of action of the two compounds, which is important, given the observed parasite resistance to artemisinin-combined therapy. Here, we report on the potent transmission-blocking activity of parthenin and parthenolide against P. falciparum gametocytes as well as gametes and ookinetes developing inside the A. gambiae midgut.
MATERIALS AND METHODS
Preparation of parthenin stocks.
Parthenin was isolated from whole plant tissue using methanol extraction and purified by high performance liquid chromatography (HPLC) (41). The extract was dissolved into acetone and then diluted 1:10 into water to give a stock solution of 5 mg/ml. The purity of parthenin (98.7%) was reconfirmed by HPLC after it was dissolved into the acetone-water mixture (see Fig. S2 in the supplemental material).
Mosquito rearing and standard membrane feeding assay (SMFA).
A. gambiae Keele mosquitoes were maintained at the Johns Hopkins Malaria Research Institute Insectary. Mosquitoes were kept at 26°C and 70% relative humidity with 12-h/12-h light-dark cycles and sustained on cotton soaked in 10% sucrose.
P. falciparum NF54 gametocytes (chloroquine and artemisinin sensitive) were cultured in RPMI 1640 medium containing HEPES and glutamine, supplemented with 10% human serum and hypoxanthine. The P. falciparum NF54 strain was diluted to 0.5% mixed-stage asexual parasites and 4% hematocrit in complete culture medium in six-well plates. These plates were transferred to a 37°C incubator at day 15, and a microaerophilic environment was created using a desiccator candle jar. The medium was exchanged daily from day 1 to day 17, and SMFAs were performed on days 15 to 18 postculture initiation.
Approximately 50 female A. gambiae mosquitoes were distributed into pint-sized cups and starved of sugar and water for ∼12 h prior to feeding. P. falciparum (NF54) day 15 to 18 gametocyte cultures were pelleted and diluted to 0.25 or 0.1% gametocytemia with human blood at 50% hematocrit. Blood was washed with RPMI medium and brought to 50% hematocrit with normal AB serum. Gametocytemic blood was kept at 37°C until feeding. Parthenin was diluted to appropriate concentrations in a 20-μl total volume plus 2 μl of 10× phosphate-buffered saline (PBS). Each parthenin dilution (22-μl total volume) was added to 200 μl of gametocytemic blood. Controls consisted of 22 μl of the water-acetone mixture into which the parthenin was dissolved. The gametocytemic blood was placed inside water-jacketed membrane feeders at 37°C, and mosquitoes were allowed to feed for 60 min. After blood feeding, non-blood-fed mosquitoes were removed from each treatment, and the fed mosquitoes were kept at 26°C and 70% humidity with a 10% sucrose solution to allow for oocyst development and maturation.
Control and treated midguts were dissected 8 days postfeeding and stained with 0.2% mercurochrome in water for 20 min. Midguts were placed on a slide with a drop of PBS, overlaid with a coverslip, and examined for oocysts using bright-field microscopy at a total magnification of ×200. Due to the expected nonnormal distribution of oocyst counts, a zero-inflated generalized linear mixed model (GLMM) was used to evaluate differences in oocyst intensities between control and treatment groups.
Ex vivo ookinete immunofluorescence assay (IFA).
Mosquito midguts were dissected at 22 h postinfectious blood feed. The blood meal was separated from the midgut by nicking the bottom of the midgut and using the surface tension of the PBS to collect the blood meal contents. The midgut was discarded, and the individual blood meals were homogenized and fixed in 100 μl of 4% paraformaldehyde. Samples were centrifuged at 3,000 × g, washed with PBS, and blocked with 3% bovine serum albumin in PBS for at least 30 min. Samples were probed with anti-Pfs25 antibody (1:1,000) for 1 h at room temperature and protected from light. Blood meal contents were then washed three times in PBS and probed with an Alexa Fluor 488-conjugated anti-mouse secondary antibody (Invitrogen, Carlsbad, CA). Samples were washed three times in PBS, and then 10 μl was spotted onto a glass microscope slide. After samples were allowed to dry, a drop of Prolong gold antifade reagent with 4′,6′-diamidino-2-phenylindole (DAPI; Invitrogen) was added before a glass coverslip was applied. Slides were imaged at a total magnification of ×1,000 using a Nikon 90i digital microscope and Volocity imaging software (PerkinElmer).
Plasmodium berghei ookinete in vitro culture.
Donor mice were infected with a frozen stock of a strain of P. berghei expressing green fluorescent protein (GFP)-tagged circumsporozoite protein/thrombospondin-related adhesive protein (CTRP) (42), according to approved Animal Care and Use Committee (ACUC) protocols (JHU Animal Welfare Assurance number A3272-01). After 7 to 10 days another set of mice was injected with 200 μl of 0.63% phenylhydrazine in PBS to induce reticulocytosis. After 3 days, phenylhydrazine-treated mice were infected with blood from a donor mouse at ≥15% parasitemia. At 3 days postinfection, mice were given 15 mg/liter sulfadiazine in their drinking water to reduce the number of asexual parasites. After 24 h the number of exflagellation centers was measured for each mouse, and those with parasitized blood presenting with ≥15 exflagellation centers/×20 magnification field were sacrificed, and blood was collected by heart puncture using a heparinized needle. Blood was placed directly into complete ookinete culture medium (RPMI medium with HEPES and l-glutamine [Sigma-Aldrich], 20% heat-inactivated fetal bovine serum [FBS], 0.2% weight per volume NaHCO3, 0.05% weight per volume hypoxanthine [Sigma-Aldrich], 100 μM xanthurenic acid [XA; Sigma-Aldrich], 50 units/ml penicillin, 50 μg/ml streptomycin [100× penicillin and streptomycin; Invitrogen], pH 8.0), and rocked gently for 48 h at 19°C.
The culture was allowed to progress for 48 h to ensure that all parasites that had committed to asynchronous ookinete development would have ample time to complete the process. The zygotes, retorts, and mature ookinetes were enriched using a MidiMACs QuadroMACS magnet (Miltenyi Biotec) fitted with LS columns equilibrated with 5 ml of incomplete ookinete medium. The blood from the 48-h ookinete culture was run through the LS column twice. The column was washed three times with 5 ml of complete ookinete medium. The LS column was then removed from the magnet, and the ookinetes were eluted with 3 ml of complete ookinete medium. Parasites were spun down at 3,500 × g for 3 min, and extra medium was removed to concentrate the samples as needed for imaging flow cytometry.
Quantification of ookinetes by imaging flow cytometry.
P. berghei CTRP-GFP ookinetes were prepared in 24-well plates as described above. Each individual well contained 460 µl of total volume consisting of 400 µl of complete ookinete medium, 20 µl of gametocytemic blood from a single mouse, and 40 µl of parthenin dissolved in acetone to give final concentrations of 100, 50, 25, 12.5, and 6.25 µg/ml of parthenin. A total of 40 µl of the 9:1 water-acetone mixture (used to dissolve the parthenin) was also added to a row of wells in place of the parthenin as a control for the effects of the acetone on the cells. Samples were spun down at 3,500 × g for 3 min to concentrate them to a 50-μl total volume in complete ookinete medium. After enrichment, approximately 1 to 8% of the cells were GFP-expressing parasites.
Samples were analyzed on an Amnis ImageStreamX Mark II flow cytometer (EMD Millipore) with high-resolution imaging capabilities. Individual images of each event were recorded. The events were gated based on bright-field area and GFP intensity. Images were taken at a magnification of ×60 and run at high speed with a 7-μm core size. To increase the speed of the analysis and overcome the throughput limitation of the Imagestream, only an equivalent portion of each sample was analyzed, and the total number of ookinetes per sample was estimated based on the proportion analyzed.
Data analysis was performed using the IDEAS, version 6.0, software. A total of 1 × 105 to 1 × 106 fluorescence units was selected as the range of GFP intensities that are indicative of parasite stages postfertilization, based on a visual confirmation of the corresponding images. The parasites were also gated based on bright-field area to exclude events too small or large to be parasites. For experimental counts, each event within the gate was individually verified as a parasite or another cell type.
Quantification of exflagellation centers.
P. falciparum gametocytemic cultures treated immediately with the compound or the acetone-water control were dropped (4 μl) on a glass slide, overlaid with a coverslip, and incubated at room temperature for 16 min. After 16 min, exflagellation centers were counted across 10 random fields at a total magnification of ×200 on a compound microscope using phase-contrast microscopy. Counts for each sample were performed in triplicate for controls and at 6.25 to 100 μg/ml parthenin. Statistical significance at each concentration was determined using repeated-measures analysis of variance (ANOVA) with a Dunnett's post hoc test. Stepwise linear regression (SPSS) was also used to compare the effect of the compound on exflagellation.
Assessment of in vivo functional activity of stage V gametocytes.
Gametocytes were cultured as described previously except that on days 15 and 16, stages IV and V gametocytes were exposed to 1,000 ng/ml, 100 ng/ml, 10 ng/ml, and 1 ng/ml parthenin for 24 h. The control gametocytes were exposed to the water-acetone mixture over the 24-h time period. After 24 h (days 16 and 17), the treated and control media were washed out and replaced with fresh media and returned to the incubator for another 24 h of exposure to the fresh media. The gametocytes (days 18 and 19) were harvested and used in an SMFA. Midguts were dissected at day 8 post-blood feeding, and oocyst numbers were determined, as described above, as a proxy measurement of gametocyte viability and infectivity to mosquitoes.
Giemsa stain.
Gametocyte cultures at 2.5% gametocytemia were smeared onto glass slides, dried, and fixed in methanol for 15 s before being stained for 10 min in 15% Giemsa stain (Sigma) diluted 1:40 in Sorensen buffer. Slides were then washed with water, dried, and imaged using oil immersion at a total magnification of ×1,000.
Male microgamete immunofluorescence assay.
The cultured day 17 and 18 male gametocytes that were present in the infectious blood meal that was used for the SMFA were also assessed for functional activity. The blood meal (4 μl) was deposited on a slide and incubated at room temperature for 16 min. Once exflagellation was observed, the samples were fixed in 4% paraformaldehyde. After 1 h the samples were centrifuged at 3,000 × g and washed with PBS three times and then blocked with 3% bovine serum albumin for at least 30 min. Samples were probed with an anti-α-tubulin antibody(12G10; 1:1,000 in blocking buffer) for 1 h at room temperature. The samples were washed three times in PBS before being probed with an Alexa Fluor 594-conjugated anti-mouse secondary antibody (Invitrogen, Carlsbad, CA). Samples were washed three times in PBS, and then 10 μl was spotted onto a glass microscope slide. After samples were dried, a drop of Prolong gold antifade reagent with DAPI (Invitrogen) was added before slides were covered with a glass coverslip. Slides were imaged at a total magnification of ×630 using a Nikon 90i digital microscope and Volocity imaging software.
RESULTS
Exposing P. falciparum gametocytes to parthenin in the blood meal decreases midgut oocyst intensity.
The addition of parthenin to gametocytemic blood at 100, 50, 25, 12.5, and 6.25 μg/ml (382 μM to 24 μM) prior to ingestion by the mosquitoes was shown to have a significant impact (P value of <0.05) on oocyst intensity at 100, 50, 25, and 12.5 μg/ml across all three replicates (Fig. 1A; see also Fig. S3 in the supplemental material) (P values and statistics for all GLMM analyses can be found in Table S1 in the supplemental material). At the lowest concentration of 6.25 μg/ml, the median oocyst counts decreased by 40 to 80% although the infection prevalence remained high at 88 to 91% (Fig. 1B). The water-acetone mixture used in the control feeds was previously observed to have a negligible impact on the number of oocysts (data not shown). Inclusion of parthenin in the blood meal effectively confirmed the transmission-blocking activity of parthenin. However, since oocyst numbers reflect a proxy measure for successful midgut invasion by ookinetes, it is possible that parthenin may have acted on the parasite at any of the developmental steps preceding oocyst formation.
FIG 1.
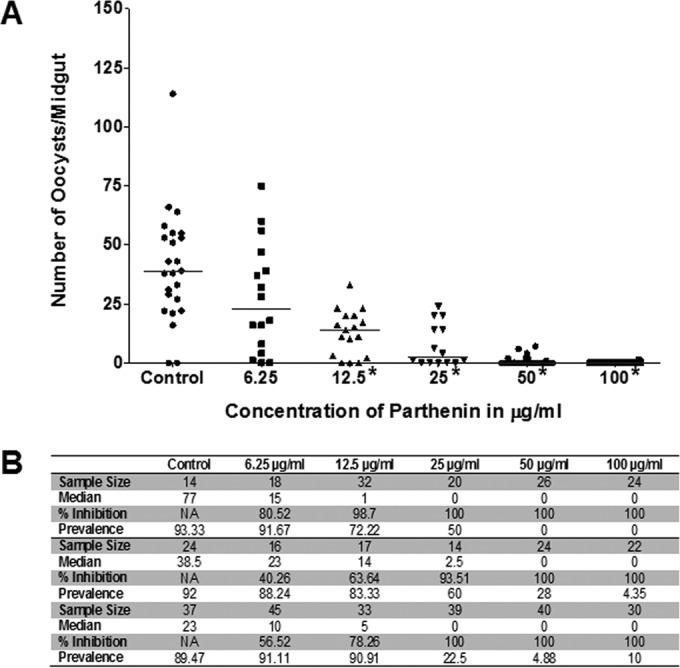
Parthenin treatment of gametocytemic blood concurrent to blood feeding results in lower oocyst counts. (A) Replicate 2 showing statistically significant decreases in oocyst counts (denoted by asterisks and determined using zero-inflated GLMM analysis and a P value of <0.05). P values were <0.0001 for 100, 50, 25, and 12.5 μg/ml and above the 0.05 cutoff for 6.25 μg/ml. (B) Table summarizing the sample size, median oocyst number, percent inhibition in median oocyst numbers, and prevalence of infection for all three replicates (replicates 1 and 3 are shown in Fig. S3 in the supplemental material).
Parthenin decreases the numbers of zygotes and ookinetes in P. falciparum in vivo and P. berghei in vitro.
To determine the precise developmental stage affected by parthenin, we examined multiple developmental steps preceding oocyst formation. We initially explored the effect of parthenin on ookinete maturation by feeding mosquitoes with P. falciparum NF54 gametocytes with a high concentration of parthenin (500 μg/ml, a concentration at which oocysts were never observed) and scoring for the presence or absence of ookinetes in the midgut blood meal bolus by IFA. At this concentration, ookinete development in the midgut of treated mosquitoes was completely abrogated (see Fig. S4A to D in the supplemental material). We considered that this initial observation might simply be a result of the intrinsic cytotoxicity of parthenin at this very high concentration. However, we observed that a 10-fold lower dose (50 μg/ml, 191 μM) resulted in the complete abrogation of oocyst development, suggesting compound activity beyond any general cytotoxic effect (see Fig. S4E). Due to the inherent subjectivity associated with obtaining accurate counts of total ookinetes ex vivo, we transitioned to an ookinete in vitro culture model using a P. berghei line that expresses GFP under the control of the CTRP promoter in fertilized female macrogametes (42). This approach permitted the precise titration of parthenin, under controlled culture conditions, and quantification of ookinetes by imaging flow cytometry.
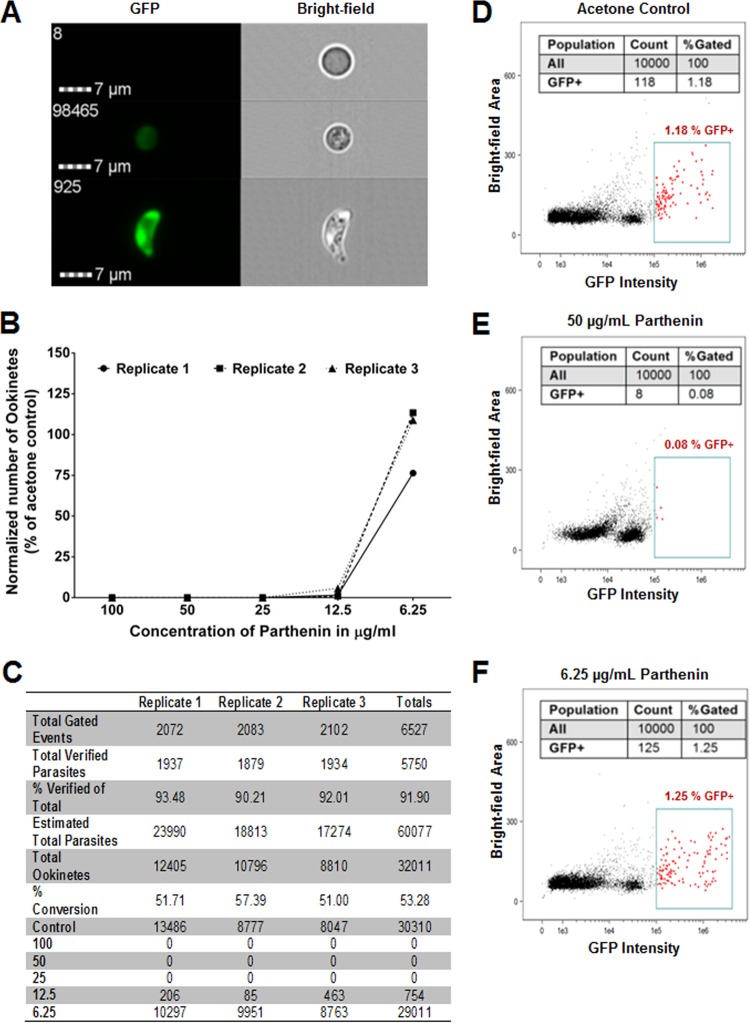
The Amnis ImageStreamX Mark II system quantifies and captures an image of each GFP-positive event (parasite), which represents fertilized female macrogametes and zygotes (round forms), elongated retorts, and banana-shaped mature ookinetes. Despite the reduction in throughput compared to that of more traditional flow cytometry platforms, the capability of the Amnis system to match an image with each event (at a magnification of ×600) was significant as this allowed for visual confirmation of the quantified, intact parasites, as opposed to cellular debris or aggregates of lysed cells. A red blood cell could be easily distinguished from a zygote or an ookinete (Fig. 2A), and even parasites obscured by other cellular debris were also verified (see Fig. S5 in the supplemental material).
FIG 2.
Parthenin treatment inhibits ookinete maturation. (A) Example images from the Amnis ImageStreamX Mark II depicting a red blood cell (top), zygote (middle), and ookinete (bottom); images like these were used for the manual verification of each GFP-positive event. (B) Graph depicting total numbers of P. berghei zygotes and ookinetes at different concentrations that were identified by flow cytometry and individually verified. The parthenin was added at 1 h postaddition of infectious blood to the ookinete medium to ensure that the compound was affecting only ookinete maturation and not fertilization. (C) Table summarizing total gated events, verified parasites from those events, percentage of gated events that were verified to be parasites, estimated total parasites based on volume, total ookinetes, and percent conversion, based on the total number of ookinetes divided by the total number of verified parasites. Also listed are total parasite numbers for each concentration and all three replicates. (D to F) Representative flow cytometry data from replicate 3 shown in Fig. S6 in the supplemental material depicting results from the control, 50-μg/ml parthenin sample that contained no verified parasites, and 6.25-μg/ml parthenin sample that contained a large amount of parasites.
The manually verified counts of total zygotes and ookinetes demonstrated that parthenin in the blood meal decreases the total number of parasites prior to midgut cell invasion and oocyst formation (Fig. 2D to F; see also Fig. S6 in the supplemental material), corroborating our initial observations (Fig. 1). It could not be ascertained definitively whether the observed ookinetes were viable or functional as the parasites were not sorted, nor was viability directly measured; however, in the context of quantifying round, intermediate, and mature ookinete forms, we assume this to be the case, given the normal ookinete morphology (Fig. 2A) and the observed development of oocysts present in vivo with matched concentrations of parthenin (Fig. 1).
To increase the likelihood that parthenin targeted only ookinete maturation and not gametogenesis or fertilization, which occurs within the first 15 to 20 min, we repeated the experiments described above with one important alteration. Instead of having the compound present in the culture medium, parthenin was added 1 h after the addition of gametocytemic blood, well after gametogenesis activation. This allowed the parasites to undergo fertilization in the absence of the compound. Despite the change in the timing of exposure, the results remained unchanged, and ookinete development was inhibited in a dose-dependent manner (Fig. 2B and C). We then explored the possibility of additional effects on parasite development directly preceding zygote formation and development.
Parthenin inhibits male microgamete exflagellation.
Microgametogenesis and exflagellation occur within 15 min of entering the mosquito midgut and involve three rounds of DNA replication (1n to 8n) and partitioning of nuclear material to flagella. This phenomenon suggests that exflagellation may be vulnerable to the hypothesized effects of parthenin because parthenin has been shown to damage DNA in quickly replicating cancerous cell lines (18, 22–24), and artemisinin has been shown to have similar effects (43). To control for total numbers of male gametocytes present in each sample, we tested the various concentrations of parthenin against two different gametocyte densities: medium (0.5%) and high (1%). As suspected, after the induction of gametogenesis and the addition of parthenin, we observed a decrease in the number of exflagellation centers as the concentration of parthenin increased from 6.25 μg/ml to 100 μg/ml (Fig. 3). A P value of <0.0001 and R of 0.695 were obtained using a stepwise method for linear regression analyses.
FIG 3.
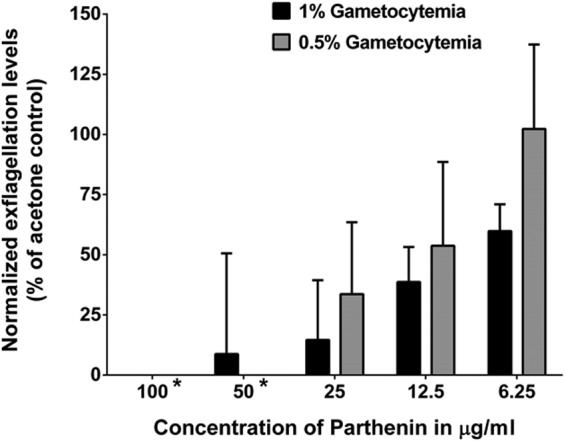
Parthenin decreases the number of exflagellation center events. Each bar represents the percent change in mean exflagellation levels compared to that of the acetone control (normalized to 100% exflagellation). The mean numbers of exflagellation centers were counted across 30 fields at a total magnification of ×200 at 16 to 18 min postinduction; error bars represent the normalized standard errors of the means. We found that 100- to 12.5-μg/ml doses were statistically significant at both parasite concentrations (P < 0.05), using a repeated-measures ANOVA with a Dunnett's posttest. At 1% gametocytemia, all six concentrations of parthenin were statistically significant, and for 0.5% gametocytemia, concentrations higher than 12.5 μg/ml were statistically significant. For all concentrations combined, a P value of <0.0001 and R value of 0.695 were obtained using a stepwise method for linear regression analyses.
Parthenin inactivates stage V gametocytes.
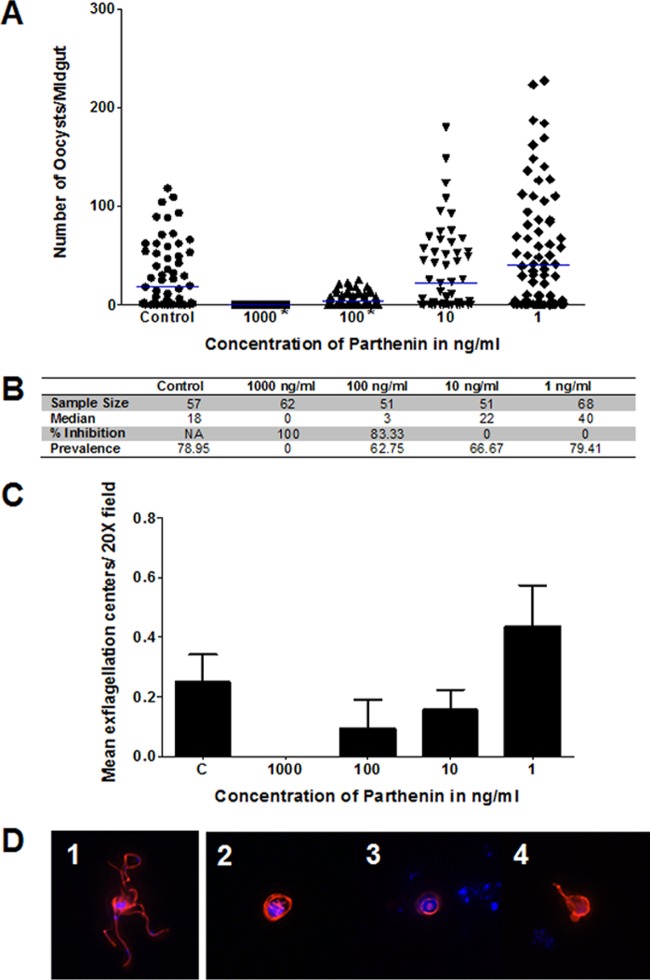
Mature P. falciparum stage V gametocytes that circulate in the peripheral blood have proven difficult to eliminate by standard antimalarial drugs that are effective at killing asexual stages (44, 45). Artemisinin is active against early gametocyte developmental stages but not mature forms (44, 46, 47). Stage V gametocytes were incubated with parthenin from 1,000 ng/ml to 1 ng/ml (3.82 μM to 3.82 nM) in 10-fold dilutions for 24 h, after which the parthenin was washed out, and the parasites were resuspended in untreated culture medium for 24 h prior to inclusion in an SMFA. There was a complete absence of oocysts at 1,000 ng/ml (P < 0.0001), an 83% reduction in median oocyst number at 100 ng/ml (P < 0.0001), and a return to control levels at ≤10 ng/ml (Fig. 4A and B).
FIG 4.
Coincubation of parthenin with stage V gametocytes results in lack of exflagellation or oocysts. (A) Oocyst intensity was reduced by 100% at 1,000 ng/ml (P < 0.0001) and by 83% at 100 ng/ml (P < 0.0001) but returned to control levels with 10 ng/ml. (B) Table showing the sample size, median oocyst number, and percent inhibition for oocyst counts from the graph shown in panel A. (C) Normalized changes in mean exflagellation counts with the standard errors of the means corresponding to the same gametocytemic blood used for the feed in panel A. (D) DAPI and tetramethyl rhodamine isothiocyanate overlay of microgametes at 16 min postactivation. Frame 1, control microgamete not exposed to parthenin undergoing exflagellation; frames 2 to 4, microgametes that were previously exposed to 1,000 ng/ml parthenin are unable to properly complete exflagellation.
These results suggest that parthenin is taken up by gametocytes, preventing exflagellation at 24 h postexposure, resulting in the prevention of mosquito infection (oocyst development) 8 days later (Fig. 4A to C). The treated and control male and female gametocytes appeared to be morphologically similar after Giemsa staining (see Fig. S7 in the supplemental material), and we did not observe cell lysis during this short time period. However, our day 17 and 18 gametocyte cultures routinely result in a male/female ratio of 1:4; therefore, the Giemsa analysis is limited to assaying for normal morphologies for mostly female stage V gametocytes. To investigate the functional viability of the treated male gametocytes, gametocytes were induced and fixed after 16 min and stained with anti-α-tubulin antibody to visualize microgamete flagella during exflagellation. We observed that some of the male gametes treated at 1,000 ng/ml undergo some of the steps prior to exflagellation but failed to properly release the flagellated DNA packets (Fig. 4D; see Fig. S8 in the supplemental material). The normal flagellar structural changes preceding and during exflagellation were based on the published microscopy data from Straschil et al. (48) and were readily observed in the control samples. Taken together, the data suggest that the gametocytes are not completely inactivated after treatment with parthenin and that while initiation of microgametogenesis is evident, there is an apparent failure to complete the exflagellation process.
Parthenolide, similar to parthenin, exhibits inhibitory activities.
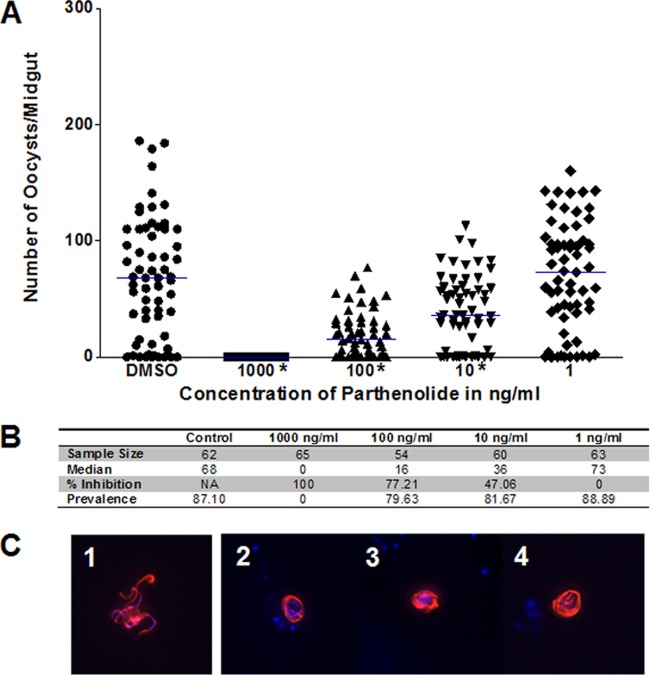
Parthenolide also proved effective against stage V gametocytes and may be more effective at the same dosing levels as parthenin (Fig. 5). We observed the absence of oocyst formation at 1,000 ng/ml (4 μM), and even at 10 ng/ml (40 nM) we noted a 47% reduction in median oocyst numbers (Fig. 5A and B). The decrease in oocyst numbers was deemed statistically significant at 1,000 ng/ml (P < 0.0001), 100 ng/ml (P < 0.0001), and 10 ng/ml (P = 0.0021). Parthenolide also showed a similar effect on microgamete exflagellation when microgametes were stained with anti-α-tubulin antibody (Fig. 5C; see also Fig. S9 in the supplemental material). Although we did not explore the effects of parthenolide to the same depth as parthenin on these other stages, the outcomes parallel those of parthenin at similar concentrations (see Fig. S10).
FIG 5.
Coincubation of parthenolide with stage V gametocytes results in lack of exflagellation or oocysts. (A) Dimethyl sulfoxide (DMSO) controls contained the same amount of dimethyl sulfoxide (0.03%) as the treatments. Oocyst intensity was again reduced by 100% at 1,000 ng/ml, and the decrease was deemed statistically significant at 1,000 ng/ml (P < 0.0001), 100 ng/ml (P < 0.0001), and 10 ng/ml (P = 0.0021). (B) Table summarizing study characteristics, including the sample size, median oocyst number, and percent inhibition for oocyst counts from the graph shown in panel A. (C) DAPI and tetramethyl rhodamine isothiocyanate overlay of microgametes at 16 min postactivation. Frame 1, control microgamete not exposed to parthenin undergoing exflagellation; frames 2 to 4, microgametes that were previously exposed to 1,000 ng/ml parthenin are unable to properly complete exflagellation.
DISCUSSION
The demonstrated ability of both parthenin and parthenolide to inhibit the development of Plasmodium, especially the inactivation of stage V gametocytes, provides a strong argument for further investigation of these compounds and their derivatives as potential transmission-blocking drugs. The parthenolide analog dimethylamino-parthenolide (DMAPT; LC-1) has shown acceptable pharmacological characteristics for a potential cancer drug that targets leukemia stem cells (34, 39). This, along with the relative safety of artemisinin derivatives, suggests that it may be possible to make parthenin or parthenolide derivatives that maintain robust activity against Plasmodium with a better safety profile. Since parthenolide is also an anti-inflammatory agent and a fever reducer, this compound could serve a multifold purpose beyond the reduction of the transmission potential of gametocytes. It is possible that when partnered with other antimalarials, parthenolide can reduce the number of blood stage parasites as well as the concomitant fever. This study also suggests that it would be a worthwhile endeavor to mine more broadly all related SQLs for transmission-blocking activity. The potential of SQLs as antimalarial drugs is also supported by the discovery of other SQLs with activity against blood stage parasites (49–51). There appears to be great potential within the SQLs as a whole for future effective therapies for treating the symptoms of malaria while also interrupting the transmission of parasites from human to mosquito.
The results from this study offer preliminary insights into affected pathways. The reduction in exflagellation illustrates the speed at which parthenin acts on the parasite as exflagellation centers were measured after a 16-min exposure to the compound. Damage to the DNA or induction of reactive oxygen species during this time of intense DNA replication and organelle partitioning, among a number of other possibilities, may be responsible for the observed effects. It is also interesting that the parasites fail to properly execute exflagellation even if the compound has been washed out. If parthenin was internalized by the parasite, the washing step could have failed to remove the compound; or parthenin could have already compromised the parasite internally so that it appeared morphologically similar to control parasites but was not fully functional when ingested by the mosquito. One possible mechanism of parthenin's action that loses support in this assay is that parthenin inhibits oxidative phosphorylation (25). Male gametes appear to lack mitochondria and have been shown to rely on glycolysis quite heavily for exflagellation (52, 53). Though this does not completely exclude this mechanism of action, it seems less important for the compound's effect at this stage.
A limitation of this study is that some of the assays could evaluate only certain stages or sexual subsets of the parasite. For example, in measuring the levels of exflagellation, we disregarded the compound's effect on female macrogametes. It has been shown that macrogametes are generally less susceptible to drugs than their microgamete counterparts, and measuring their responses to drugs is done with greater difficulty (43). Due to the fact that a reduction in ookinete and oocyst count represents a proxy measure of activity of parthenin against fertilized macrogametes, we elected to focus our analysis on the microgamete response to the compound. It remains possible that parthenin can affect both male and female gametogenesis, and we are poised to examine this process further. We also did not investigate the effect of the compound on earlier stages of gametocytogenesis (stages I to IV). These stages fell outside the scope of the present study on transmissible stages as stage I to IV gametocytes are often sequestered in the bone marrow and are often unaffected by compounds present in the circulating blood (54). Another common criticism is that lab-based infections of mosquitoes and subsequent oocyst burdens are much higher than those normally observed in nature and therefore are not representative of what would happen under field conditions. It is true that oocyst counts are generally much higher in laboratory infections than those observed in the field, but allowing for abnormally high infections allows a better assessment of the transmission-blocking potential and more stringent evaluation of the range of prospective compounds. Arguably, a compound's ability to completely prevent the development of 300 oocysts will likely translate to comparable efficacy against smaller parasite numbers. In fact, a previous study showed that as gametocytemia decreased toward published field levels (i.e., 1 to 20 oocysts/midgut), the effect of a compound on the prevalence increased while the effect on the reduction in oocyst intensity remained high (55). These data suggest that the use of high infection rates in this study is not unreasonable and that these results could translate into similar results in the field.
In conclusion, both parthenin and parthenolide have demonstrated activity against stage V gametocytes and multiple mosquito-specific stages of the Plasmodium parasite. Further studies on the mode of action of parthenin could lead to the discovery of vulnerable cellular pathways in Plasmodium that are independent of artemisinin's proposed mechanism of action. Parthenolide derivatives currently undergoing clinical tests may have the potential to be developed as antimalarials, and the mining of other SQLs could reveal additional compounds with potent antimalarial activity. This greater breadth of knowledge about the molecular pathways affected and the general safety to nontarget cells made parthenolide a reasonable next step to further explore the potential anti-Plasmodium properties of SQLs. Subsequent studies on the mode of action of parthenin/parthenolide on mature gametocytes must consider the duration of inactivation following short exposure to the compound. Gametocytes can persist in peripheral blood, presumably sequestered in capillary beds, beyond the average half-life of existing antimalarials. Clearly, gametocyte uptake of parthenin/parthenolide and the observed delayed activity in a subsequent stage offer significant advantages for a next-generation antimalarial. Such valuable characteristics may potentially meet the target product profile of transmission-blocking drugs in the age of malaria elimination and eradication.
Supplementary Material
ACKNOWLEDGMENTS
We thank Hilary Hurd and Paul Eggleston for the Anopheles gambiae KEELE strain, Abhai Tripathi for gametocyte cultures, and Hanhvy Bui for assistance with the Amnis instrumentation.
Funding Statement
The study was funded in part by the Johns Hopkins Malaria Research Institute.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02002-15.
REFERENCES
- 1.World Health Organization. 2014. World Malaria Report 2014. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.White NJ. 1997. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob Agents Chemother 41:1413–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okebe J, Bojang K, D'Alessandro U. 2014. Use of artemisinin and its derivatives for the treatment of malaria in children. Pediatr Infect Dis J 33:522–524. doi: 10.1097/INF.0000000000000306. [DOI] [PubMed] [Google Scholar]
- 4.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel S. 2011. Harmful and beneficial aspects of Parthenium hysterophorus: an update. 3 Biotech 1:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lakshmi C, Srinivas CR. 2007. Parthenium: a wide angle view. Indian J Dermatol Venereol Leprol 73:296–306. doi: 10.4103/0378-6323.35732. [DOI] [PubMed] [Google Scholar]
- 7.Chippendale J, Panetta F. 1994. The cost of Parthenium weed to the Queensland cattle industry. Plant Prot Q 9:73–76. [Google Scholar]
- 8.Narasimhan T, Murthy BK, Harindranath N, Rao PS. 1984. Characterization of a toxin from Parthenium hysterophorus and its mode of excretion in animals. J Biosci 6:729–738. doi: 10.1007/BF02702716. [DOI] [Google Scholar]
- 9.Narsimhan T, Ananth M, Narayana Swamy M, Rajendra Babu M, Subba Rao P. 1977. Toxicity of Parthenium hysterophorus. Curr Sci 46:15–16. [Google Scholar]
- 10.Belz RG, Reinhardt CF, Foxcroft LC, Hurle K. 2007. Residue allelopathy in Parthenium hysterophorus L.—Does parthenin play a leading role? Crop Prot 26:237–245. doi: 10.1016/j.cropro.2005.06.009. [DOI] [Google Scholar]
- 11.Swaminathan C, Rai RV, Suresh K. 1990. Allelopathic effects of Parthenium hysterophorus on germination and seedling growth of a few multi-purpose trees and arable crops. Int Tree Crops J 6:143–150. doi: 10.1080/01435698.1990.9752880. [DOI] [Google Scholar]
- 12.Batish DR, Singh H, Kohli R, Saxena D, Kaur S. 2002. Allelopathic effects of parthenin against two weedy species, Avena fatua and Bidens pilosa. Environ Exp Bot 47:149–155. doi: 10.1016/S0098-8472(01)00122-8. [DOI] [Google Scholar]
- 13.Singh HP, Batish DR, Kohli R, Saxena DB, Arora V. 2002. Effect of parthenin-A sesquiterpene lactone from Parthenium hysterophorus-on early growth and physiology of Ageratum conyzoides. J Chem Ecol 28:2169–2179. doi: 10.1023/A:1021089013754. [DOI] [PubMed] [Google Scholar]
- 14.Picman AK, Elliott R, Towers G. 1981. Cardiac-inhibiting properties of the sesquiterpene lactone, parthenin, in the migratory grasshopper, Melanoplus sanguinipes. Can J Zool 59:285–292. doi: 10.1139/z81-043. [DOI] [Google Scholar]
- 15.Datta S, Saxena DB. 2001. Pesticidal properties of parthenin (from Parthenium hysterophorus) and related compounds. Pest Manag Sci 57:95–101. doi:. [DOI] [PubMed] [Google Scholar]
- 16.Sharma G, Bhutani K. 1988. Plant based antiamoebic drugs. Part II. Amoebicidal activity of parthenin isolated from Parthenium hysterophorus. Planta Med 54:120–122. [DOI] [PubMed] [Google Scholar]
- 17.Hooper M, Kirby G, Kulkarni M, Kulkarni S, Nagasampagi B, O'Neill M, Phillipson J, Rojatkar S, Warhurst D. 1990. Antimalarial activity of parthenin and its derivatives. Eur J Med Chem 25:717–723. doi: 10.1016/0223-5234(90)90190-E. [DOI] [Google Scholar]
- 18.Ghantous A, Gali-Muhtasib H, Vuorela H, Saliba NA, Darwiche N. 2010. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov Today 15:668–678. doi: 10.1016/j.drudis.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Kumar A, Malik F, Bhushan S, Shah BA, Taneja SC, Pal HC, Wani ZA, Mondhe DM, Kaur J, Singh J. 2011. A novel parthenin analog exhibits anti-cancer activity: activation of apoptotic signaling events through robust NO formation in human leukemia HL-60 cells. Chem Biol Interact 193:204–215. doi: 10.1016/j.cbi.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Goswami A, Shah BA, Kumar A, Rizvi MA, Kumar S, Bhushan S, Malik FA, Batra N, Joshi A, Singh J. 2014. Antiproliferative potential of a novel parthenin analog P16 as evident by apoptosis accompanied by down-regulation of PI3K/AKT and ERK pathways in human acute lymphoblastic leukemia MOLT-4 cells. Chem Biol Interact 222C:60–67. doi: 10.1016/j.cbi.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Shah BA, Kaur R, Gupta P, Kumar A, Sethi VK, Andotra SS, Singh J, Saxena AK, Taneja SC. 2009. Structure–activity relationship (SAR) of parthenin analogues with pro-apoptotic activity: development of novel anti-cancer leads. Bioorg Med Chem Lett 19:4394–4398. doi: 10.1016/j.bmcl.2009.05.089. [DOI] [PubMed] [Google Scholar]
- 22.Amorim MHR, Gil da Costa RM, Lopes C, Bastos MM. 2013. Sesquiterpene lactones: adverse health effects and toxicity mechanisms. Crit Rev Toxicol 43:559–579. doi: 10.3109/10408444.2013.813905. [DOI] [PubMed] [Google Scholar]
- 23.Mehra R, Nargotra A, Shah BA, Taneja SC, Vishwakarma RA, Koul S. 2013. Pro-apoptotic properties of parthenin analogs: a quantitative structure-activity relationship study. Med Chem Res 22:2303–2311. doi: 10.1007/s00044-012-0225-5. [DOI] [Google Scholar]
- 24.Ramos A, Rivero R, Visozo A, Piloto J, Garcıìa A. 2002. Parthenin, a sesquiterpene lactone of Parthenium hysterophorus L. is a high toxicity clastogen. Mutat Res Genet Toxicol Environ Mutagen 514:19–27. doi: 10.1016/S1383-5718(01)00321-7. [DOI] [PubMed] [Google Scholar]
- 25.Narasimhan T, Kim H, Safe S. 1989. Effects of sesquiterpene lactones on mitochondrial oxidative phosphorylation. Gen Pharmacol 20:681–687. doi: 10.1016/0306-3623(89)90107-9. [DOI] [PubMed] [Google Scholar]
- 26.Das B, Reddy VS, Krishnaiah M, Sharma AVS, Ravi Kumar K, Rao JV, Sridhar V. 2007. Acetylated pseudoguaianolides from Parthenium hysterophorus and their cytotoxic activity. Phytochemistry 68:2029–2034. doi: 10.1016/j.phytochem.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Delves M, Plouffe D, Scheurer C, Meister S, Wittlin S, Winzeler EA, Sinden RE, Leroy D. 2012. The activities of current antimalarial drugs on the life cycle stages of Plasmodium: a comparative study with human and rodent parasites. PLoS Med 9:e1001169. doi: 10.1371/journal.pmed.1001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinden R. 1983. Sexual development of malarial parasites. Adv Parasitol 22:153–216. doi: 10.1016/S0065-308X(08)60462-5. [DOI] [PubMed] [Google Scholar]
- 29.Parish LA, Colquhoun DR, Mohien CU, Lyashkov AE, Graham DR, Dinglasan RR. 2011. Ookinete-interacting proteins on the microvillar surface are partitioned into detergent resistant membranes of Anopheles gambiae midguts. J Proteome Res 10:5150–5162. doi: 10.1021/pr2006268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manda H, Gouagna LC, Nyandat E, Kabiru E, Jackson R, Foster W, Githure J, Beier J, Hassanali A. 2007. Discriminative feeding behaviour of Anopheles gambiae ss on endemic plants in western Kenya. Med Vet Entomol 21:103–111. doi: 10.1111/j.1365-2915.2007.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manda H, Gouagna LC, Foster WA, Jackson RR, Beier JC, Githure JI, Hassanali A. 2007. Effect of discriminative plant-sugar feeding on the survival and fecundity of Anopheles gambiae. Malar J 6:113. doi: 10.1186/1475-2875-6-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nyasembe VO, Teal PE, Sawa P, Tumlinson JH, Borgemeister C, Torto B. 2014. Plasmodium falciparum infection increases Anopheles gambiae attraction to nectar sources and sugar uptake. Curr Biol 24:217–221. doi: 10.1016/j.cub.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manda H. 2007. Plant-feeding behaviour and its effects on the fitness and competence of the malaria vector Anopheles gambiae (Diptera: Culicidae). Ph.D. dissertation. Kenyatta University, Nairobi, Kenya. [Google Scholar]
- 34.Ghantous A, Sinjab A, Herceg Z, Darwiche N. 2013. Parthenolide: from plant shoots to cancer roots. Drug Discov Today 18:894–905. doi: 10.1016/j.drudis.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Hehner SP, Hofmann TG, Droge W, Schmitz ML. 1999. The antiinflammatory sesquiterpene lactone parthenolide inhibits NF-κB by targeting the IκB kinase complex. J Immunol 163:5617–5623. [PubMed] [Google Scholar]
- 36.Juliana C, Fernandes-Alnemri T, Wu J, Datta P, Solorzano L, Yu JW, Meng R, Quong AA, Latz E, Scott CP, Alnemri ES. 2010. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J Biol Chem 285:9792–9802. doi: 10.1074/jbc.M109.082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathema VB, Koh Y, Thakuri BC, Sillanpää M. 2012. Parthenolide, a sesquiterpene lactone, expresses multiple anti-cancer and anti-inflammatory activities. Inflamm 35:560–565. doi: 10.1007/s10753-011-9346-0. [DOI] [PubMed] [Google Scholar]
- 38.Guzman ML, Rossi RM, Karnischky L, Li X, Peterson DR, Howard DS, Jordan CT. 2005. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood 105:4163–4169. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guzman ML, Rossi RM, Neelakantan S, Li X, Corbett CA, Hassane DC, Becker MW, Bennett JM, Sullivan E, Lachowicz JL, Vaughan A, Sweeney CJ, Matthews W, Carroll M, Liesveld JL, Crooks PA, Jordan CT. 2007. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood 110:4427–4435. doi: 10.1182/blood-2007-05-090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Posner GH, O'Neill PM. 2004. Knowledge of the proposed chemical mechanism of action and cytochrome P450 metabolism of antimalarial trioxanes like artemisinin allows rational design of new antimalarial peroxides. Acc Chem Res 37:397–404. doi: 10.1021/ar020227u. [DOI] [PubMed] [Google Scholar]
- 41.Nyasembe VO, Cheseto X, Kaplan F, Foster W, Teal PEA, Tumlinson JA, Borgemeister C, Torto B. 2015. The invasive American weed Parthenium hysterophorus can negatively impact malaria control in Africa. PLoS One 10:e0137836. doi: 10.1371/journal.pone.0137836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vlachou D, Zimmermann T, Cantera R, Janse CJ, Waters AP, Kafatos FC. 2004. Real-time, in vivo analysis of malaria ookinete locomotion and mosquito midgut invasion. Cell Microbiol 6:671–685. doi: 10.1111/j.1462-5822.2004.00394.x. [DOI] [PubMed] [Google Scholar]
- 43.Delves MJ, Ruecker A, Straschil U, Lelievre J, Marques S, Lopez-Barragan MJ, Herreros E, Sinden RE. 2013. Male and female Plasmodium falciparum mature gametocytes show different responses to antimalarial drugs. Antimicrob Agents Chemother 57:3268–3274. doi: 10.1128/AAC.00325-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bousema T, Okell L, Shekalaghe S, Griffin JT, Omar S, Sawa P, Sutherland C, Sauerwein R, Ghani AC, Drakeley C. 2010. Research revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar J 9:136. doi: 10.1186/1475-2875-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bousema JT, Schneider P, Gouagna LC, Drakeley CJ, Tostmann A, Houben R, Githure JI, Ord R, Sutherland CJ, Omar SA, Sauerwein RW. 2006. Moderate effect of artemisinin-based combination therapy on transmission of Plasmodium falciparum. J Infect Dis 193:1151–1159. doi: 10.1086/503051. [DOI] [PubMed] [Google Scholar]
- 46.Humphreys G, WWARN Gametocyte Carriage Study Group . 2014. The effect of artemisinin-combination treatment options on P. falciparum gametocyte carriage: a pooled analysis of individual patient data. Malar J 13(Suppl 1):P44. doi: 10.1186/1475-2875-13-S1-P44. [DOI] [Google Scholar]
- 47.Tangpukdee N, Krudsood S, Srivilairit S, Phophak N, Chonsawat P, Yanpanich W, Kano S, Wilairatana P. 2008. Gametocyte clearance in uncomplicated and severe Plasmodium falciparum malaria after artesunate-mefloquine treatment in Thailand. Korean J Parasitol 46:65–70. doi: 10.3347/kjp.2008.46.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Straschil U, Talman AM, Ferguson DJ, Bunting KA, Xu Z, Bailes E, Sinden RE, Holder AA, Smith EF, Coates JC, Rita T. 2010. The Armadillo repeat protein PF16 is essential for flagellar structure and function in Plasmodium male gametes. PLoS One 5:e12901. doi: 10.1371/journal.pone.0012901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bischoff TA, Kelley CJ, Karchesy Y, Laurantos M, Nguyen-Dinh P, Arefi AG. 2004. Antimalarial activity of lactucin and lactucopicrin: sesquiterpene lactones isolated from Cichorium intybus L. J Ethnopharmacol 95:455–457. doi: 10.1016/j.jep.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 50.Chea A, Hout S, Long C, Marcourt L, Faure R, Azas N, Elias R. 2006. Antimalarial activity of sesquiterpene lactones from Vernonia cinerea. Chem Pharm Bull 54:1437–1439. doi: 10.1248/cpb.54.1437. [DOI] [PubMed] [Google Scholar]
- 51.Pedersen MM, Chukwujekwu JC, Lategan CA, Van Staden J, Smith PJ, Staerk D. 2009. Antimalarial sesquiterpene lactones from Distephanus angulifolius. Phytochemistry 70:601–607. doi: 10.1016/j.phytochem.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 52.Talman AM, Prieto JH, Marques S, Ubaida-Mohien C, Lawniczak M, Wass MN, Xu T, Frank R, Ecker A, Stanway RS, Krishna S, Sternberg MJ, Christophides GK, Graham DR, Dinglasan RR, Yates JR, Sinden RE. 2014. Proteomic analysis of the Plasmodium male gamete reveals the key role for glycolysis in flagellar motility. Malar J 13:315. doi: 10.1186/1475-2875-13-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okamoto N, Spurck TP, Goodman CD, McFadden GI. 2009. Apicoplast and mitochondrion in gametocytogenesis of Plasmodium falciparum. Eukaryot Cell 8:128–132. doi: 10.1128/EC.00267-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aguilar R, Magallon-Tejada A, Achtman AH, Moraleda C, Joice R, Cistero P, Li Wai Suen CS, Nhabomba A, Macete E, Mueller I, Marti M, Alonso PL, Menendez C, Schofield L, Mayor A. 2014. Molecular evidence for the localization of Plasmodium falciparum immature gametocytes in bone marrow. Blood 123:959–966. doi: 10.1182/blood-2013-08-520767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mathias DK, Pastrana-Mena R, Ranucci E, Tao D, Ferruti P, Ortega C, Staples GO, Zaia J, Takashima E, Tsuboi T. 2013. A small molecule glycosaminoglycan mimetic blocks Plasmodium invasion of the mosquito midgut. PLoS Pathog 9:e1003757. doi: 10.1371/journal.ppat.1003757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.