Abstract
We conducted a retrospective study of 2,149 clinical Salmonella strains to help document the historical emergence of antimicrobial resistance. There were significant increases in resistance to older drugs, including ampicillin, chloramphenicol, streptomycin, sulfamethoxazole, and tetracycline, which were most common in Salmonella enterica serotype Typhimurium. An increase in multidrug resistance was observed for each decade since the 1950s. These data help show how Salmonella evolved over the past 6 decades, after the introduction of new antimicrobial agents.
TEXT
Approximately 1.2 million people in the United States contract salmonellosis annually, resulting in 23,128 hospitalizations and >452 deaths (1). Infections caused by antimicrobial-resistant Salmonella have been associated with increased hospitalization rates, morbidity, mortality, and economic costs (2–4). Fluoroquinolones or expanded-spectrum cephalosporins are frontline treatments in cases of life-threatening salmonellosis (5). In the United States, systematic monitoring of resistance in foodborne pathogens began in 1996 with implementation of the National Antimicrobial Resistance Monitoring System (NARMS) (6). There are very few data on resistance trends among foodborne pathogens before then. To help document the evolution of resistance in salmonellae prior to NARMS, we tested banked historical isolates, collected over the past 6 decades, for susceptibility to the antimicrobial agents used in NARMS and compared these data with recent surveillance data.
A total of 2,149 Salmonella isolates, representing >145 serotypes, obtained from human clinical cases between 1948 and 1995 were included in this study (Table 1). These isolates were mainly from culture collections at the Centers for Disease Control and Prevention. We focused on five serotypes commonly associated with human foodborne infections, namely, Salmonella enterica serotypes Enteritidis, Typhimurium, Newport, Heidelberg, and Saintpaul. Most of the isolates were recovered from stool, and a few were recovered from blood and urine. Historical isolates were maintained on Trypticase soy agar stabs sealed with paraffin and stored at room temperature. NARMS isolates were frozen in Trypticase soy broth containing 30% glycerol at −70°C for prolonged storage.
TABLE 1.
Distribution of Salmonella isolates, overall and five common serotypes, by decade
| S. enterica serotype | No. of isolates in: |
||||||
|---|---|---|---|---|---|---|---|
| 1940s (n = 63) | 1950s (n = 614) | 1960s (n = 177) | 1970s (n = 458) | 1980s (n = 380) | 1990–1995 (n = 457) | Total (n = 2,149) | |
| Enteritidis | 2 | 23 | 12 | 169 | 46 | 32 | 284 |
| Typhimuriuma | 3 | 2 | 57 | 35 | 112 | 54 | 263 |
| Newport | 6 | 1 | 43 | 75 | 20 | 33 | 178 |
| Heidelberg | 0 | 12 | 12 | 45 | 49 | 31 | 149 |
| Saintpaul | 0 | 6 | 9 | 54 | 20 | 16 | 105 |
| Other serotypes | 51 | 549 | 42 | 75 | 66 | 286 | 1,069 |
| Not serotyped | 1 | 21 | 2 | 5 | 67 | 5 | 101 |
Includes S. Typhimurium variant Copenhagen.
Antimicrobial MICs were determined with the Sensititre system (Thermo Fisher Scientific, Trek Diagnostics, Cleveland, OH). Results were interpreted according to the Clinical and Laboratory Standards Institute guidelines, where CLSI breakpoints are available (7). The tested antimicrobial agents are listed in Table 2. Multidrug resistance (MDR) was defined as resistance to three or more antimicrobial classes. The Mann-Kendall test, a nonparametric test, was used to detect the resistance trend over time. The magnitude of the change was estimated by using Sen's nonparametric method and calculated with the Excel template MAKESENS (8). A P value of <0.05 was considered significant.
TABLE 2.
Antimicrobial resistance phenotypes of Salmonella, overall and top five serotypes in strain set
| Antimicrobial class | Antimicrobial agent | Resistance breakpoint (μg/ml) | % resistance in: |
Timeline for clinical use of antimicrobials | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Enteritidis | Typhimurium | Newport | Heidelberg | Saintpaul | Other serotypes | Not serotyped | ||||
| β-Lactamase inhibitor combinations | Amoxicillin-clavulanic acid | ≥32/≥16 | 0.6 | 0 | 2.3 | 0 | 1.3 | 1.9 | 0.2 | 1 | 1984 |
| Aminopenicillins | Ampicillin | ≥32 | 7.2 | 5.3 | 22.9 | 11.2 | 8.1 | 12.4 | 2.4 | 13.9 | 1961 |
| Cephems | Cefoxitin | ≥32 | 0.7 | 0.4 | 0.8 | 0.6 | 0 | 1 | 1 | 0 | 1977 |
| Ceftiofur | ≥8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1988 | |
| Ceftriaxone | ≥4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1984 | |
| Cephalothin | ≥32 | 3.6 | 2.1 | 8.4 | 7.9 | 3.4 | 7.6 | 2.2 | 1 | 1964 | |
| Phenicols | Chloramphenicol | ≥32 | 2.9 | 1.1 | 11.8 | 2.2 | 2 | 1.9 | 0.8 | 9.9 | 1947 |
| Aminoglycosides | Gentamicin | ≥16 | 1.1 | 0 | 2.3 | 0 | 8.1 | 1 | 0.5 | 0 | 1963 |
| Kanamycin | ≥64 | 3.1 | 1.1 | 10.6 | 2.3 | 9.4 | 6.7 | 0.3 | 5.9 | 1957 | |
| Streptomycin | ≥64 | 12.4 | 4.2 | 24.7 | 13.5 | 34.2 | 18.1 | 7.6 | 14.9 | 1943 | |
| Quinolones | Nalidixic acid | ≥32 | 0.5 | 0 | 1.9 | 0.6 | 0 | 0 | 0.5 | 0 | 1962 |
| Ciprofloxacin | ≥4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1987 | |
| Tetracyclines | Tetracycline | ≥16 | 9.9 | 2.5 | 23.6 | 12.9 | 20.1 | 13.3 | 5.5 | 17.8 | 1948 |
| Folate pathway inhibitors | Sulfamethoxazole | ≥512 | 10 | 2.5 | 24 | 15.2 | 24.2 | 16.2 | 4.7 | 14.9 | 1936 |
| Trimethoprim-sulfamethoxazole | ≥4/≥76 | 0.6 | 0 | 1.9 | 0.6 | 0 | 1 | 0.1 | 5 | 1968 | |
Overall, 429 of 2,149 (20%) isolates were resistant to at least one antimicrobial, and 165 (7.7%) isolates were MDR, with 62 (2.9%) isolates showing resistance to five or more classes of drugs. Resistance was higher for older drugs, such as streptomycin (12.4%), sulfamethoxazole (10%), tetracycline (9.9%), and ampicillin (7.2%) (Table 2). Resistance to newer agents, such as ceftiofur, ceftriaxone, and ciprofloxacin, was not detected in any isolate. The most common serotype was S. Enteritidis (13.2%), followed by S. Typhimurium (12.2%), S. Newport (8.3%), S. Heidelberg (6.9%), and S. Saintpaul (4.9%). The frequency of resistance varied by serotype; S. Typhimurium often exhibited resistance to more agents than other serotypes, and S. Enteritidis exhibited resistance to the fewest agents.
Resistance increased steadily with each decade for streptomycin, sulfamethoxazole, tetracycline, and ampicillin (Table 2), which was consistent with historical trends in Escherichia coli (9). Resistance to chloramphenicol, streptomycin, sulfamethoxazole, and tetracycline has long been recognized in Salmonella spp. (10), including those causing infections in animals (11). Comparison of these historical trends with recent NARMS data showed that resistance to the older compounds increased steadily until the late 1990s, often linked together in MDR strains, and then began a steady decline (12).
For sulfamethoxazole, resistance increased from 1.6% in the 1940s to 19.7% in the 1980s, followed by a slight drop to 13.8% in the 1990s. A similar decreasing trend in sulfonamide resistance was reported among human nontyphoidal Salmonella isolates between 1996 and 2009 (12). Sulfonamides were introduced into human medicine in the 1930s and have been in continuous use for >70 years, usually as a single agent before the 1970s and in combination with trimethoprim since the 1970s. Sulfonamides are still used in the veterinary and agricultural fields (13).
Ampicillin, along with trimethoprim-sulfamethoxazole, was commonly used to treat salmonellosis in the 1980s (14). While we detected ampicillin resistance in isolates from 1949, resistance became a problem in the early 1980s (15), leading to a shift in clinical practice toward the use of quinolones and extended-spectrum cephalosporins. We documented a rise in ampicillin resistance in the 1980s that was followed by a drop in the 1990s and an uptick in the 2000s.
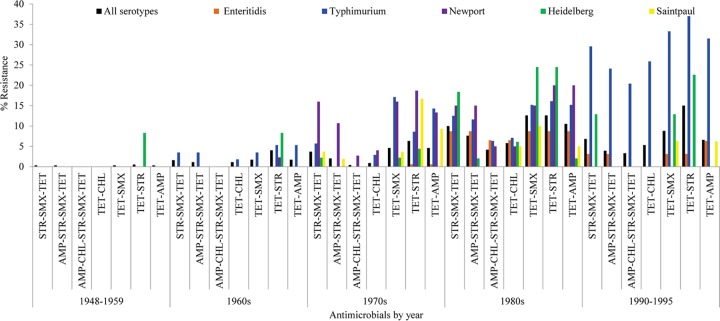
Current statistics on drug use show that tetracycline is the most commonly used antibiotic in food animal production (16), where it has been used historically for production and therapeutic purposes. Out data show that coresistance to tetracycline was frequent. Concurrent resistance to tetracycline-streptomycin was the most common coresistance phenotype (7.3%), followed by resistance to tetracycline-sulfamethoxazole (5.3%), ampicillin-streptomycin (4.9%), tetracycline-ampicillin (4.5%), tetracycline-sulfamethoxazole-streptomycin (4.2%), and tetracycline-ampicillin-streptomycin-sulfamethoxazole (2.8%) (Fig. 1). A total of 52 of 62 (83.9%) chloramphenicol-resistant Salmonella isolates were coresistant to tetracycline. Similarly, the use of sulfamethoxazole (and other agents) might help drive coselection of resistance determinants for other antibiotics. In our data, 63.7% and 53% of sulfamethoxazole-resistant Salmonella were coresistant to streptomycin and tetracycline, respectively. The presence of the sul1 gene as a constituent of class 1 integrons can mediate the spread of sulfonamide resistance in the absence of selective pressure (17).
FIG 1.
Changes in MDR phenotypes among the top 5 Salmonella serotypes in our strain set. STR, streptomycin; SMX, sulfamethoxazole; TET, tetracycline; AMP, ampicillin; CHL, chloramphenicol.
The frequency of resistance varied by serotype. S. Typhimurium often exhibited resistance to more agents than other serotypes, and S. Enteritidis exhibited resistance to the fewest agents (Table 2). S. Typhimurium has long been known to be more resistant than other common serotypes to antimicrobials (18, 19). Resistance of S. Typhimurium to tetracycline, chloramphenicol, and ampicillin increased with each decade. Tetracyclines were approved for use in the 1950s and have been used extensively in the prophylaxis and therapy of human and animal infections. Tetracyclines are by far the most common antibacterial agents currently used in food animal production (16). While chloramphenicol has been available since the 1940s, it is rarely used in the United States today due to toxicity issues. The related compound, florfenicol, is approved for use in swine, cattle, salmonids, and catfish. Chloramphenicol resistance persists in Salmonella and other enteric species (9, 12).
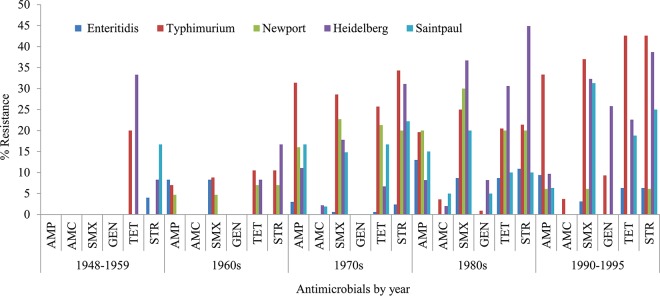
Resistance changes were most notable for S. Typhimurium. Comparing data from pre-1960 with those from post-1989, resistance rose from 0% to 33.3% for ampicillin, 0% to 25.9% for chloramphenicol, 0% to 42.6% for streptomycin, 20% to 42.6% for tetracycline, and 0% to 37% for sulfamethoxazole (Fig. 2). Comparing data from pre-1960 with those from post-1989, the proportion of pan-susceptible isolates declined from 87.3% to 62.7% for the five serotypes examined here. Simultaneously, MDR increased from 0% (0/55) to 16.9% (28/166). MDR was most common in S. Typhimurium, where 61 of 263 (23.2%) isolates were resistant to three or more antimicrobial classes, and 30 (11.4%) isolates were resistant to five or more classes. The other common serotypes with MDR phenotypes were S. Newport (22/178), S. Heidelberg (22/149), S. Saintpaul (12/105), and S. Enteritidis (9/284). MDR increased in S. Typhimurium (from 0.0% to 37.0%) and S. Heidelberg (from 0.0% to 16.1%) between pre-1960 and post-1989 (Table 3). MDR nontyphoidal Salmonella with the ampicillin-chloramphenicol-streptomycin-sulfamethoxazole-tetracycline pattern is of particular concern in the United States (20–22). S. Typhimurium DT104 emerged in the United States in the 1990s and has been one of the leading causes of animal and human salmonellosis (23–25). The typical S. Typhimurium DT104 exhibits the ampicillin-chloramphenicol-streptomycin-sulfamethoxazole-tetracycline resistance pattern. Medalla et al. (26) reported a significant decline in MDR among NARMS human Salmonella isolates between 1996 and 2009, driven mainly by decreased MDR among S. Typhimurium. Our study is limited by its reliance on preexisting culture collections, which resulted in a nonrandom sample and an uneven temporal distribution of isolates. In addition, we had no information regarding prior treatment, travel history, or the rationale for preserving the strains. In Salmonella, the spread of resistance is mainly via horizontal gene transfer (27). Because most of the historical strains were maintained at room temperature, it is possible that loss of plasmids (28) and any associated resistance traits may have affected our results. Regardless of these unavoidable limitations, the use of the same testing platform with historical strains, coupled with secular surveillance data, provides a broad picture of resistance development over time and helps characterize the evolution of drug resistance in Salmonella spp. since the beginning of the antibiotic age.
FIG 2.
Changes in select antimicrobial resistance among the top 5 Salmonella serotypes in our strain set. AMP, ampicillin; AMC, amoxicillin-clavulanic acid; SMX, sulfamethoxazole; GEN, gentamicin; TET, tetracycline; STR, streptomycin; SXT, trimethoprim-sulfamethoxazole.
TABLE 3.
Number of isolates with multidrug resistance, by serotypea
| S. enterica serotype | Total | Pan-susceptible (%) | MDR (%) | MDR, ≥5 drugs (%) | STR-SMX-TET (%) | AMP-STR-SMX-TET (%) | AMP-CHL-STR-SMX-TET (%) |
|---|---|---|---|---|---|---|---|
| All Salmonella | 2,149 | 1,720 (80.0) | 165 (7.7) | 62 (2.9) | 91 (4.2) | 60 (2.8) | 33 (1.5) |
| Enteritidis | 284 | 263 (92.6) | 9 (3.2) | 4 (1.4) | 5 (1.8) | 5 (1.8) | 3 (1.1) |
| Typhimurium | 263 | 164 (62.4) | 61 (23.2) | 30 (11.4) | 34 (12.9) | 28 (10.6) | 18 (6.8) |
| Newport | 178 | 136 (76.4) | 22 (12.4) | 10 (5.6) | 15 (8.4) | 11 (6.2) | 3 (1.7) |
| Heidelberg | 149 | 84 (56.4) | 22 (14.8) | 2 (1.3) | 14 (9.4) | 1 (0.7) | 0 (0) |
| Saintpaul | 105 | 76 (72.4) | 12 (11.4) | 4 (3.8) | 2 (1.9) | 1 (1) | 0 (0) |
| Other serotypes | 1,069 | 920 (86.1) | 23 (2.2) | 6 (0.6) | 13 (1.2) | 6 (0.6) | 4 (0.4) |
| Not serotyped | 101 | 77 (76.2) | 16 (15.8) | 6 (5.9) | 8 (7.9) | 8 (7.9) | 5 (5) |
MDR, multidrug resistant; STR, streptomycin; SMX, sulfamethoxazole; TET, tetracycline; AMP, ampicillin; CHL, chloramphenicol.
ACKNOWLEDGMENTS
The study isolates were collected by American Type Culture Collection (ATCC) through a contract agreement with the U.S. Food and Drug Administration, Center for Veterinary Medicine, Office of Research (contract 223-02-7010).
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy of the Department of Health and Human Services, the U.S. Food and Drug Administration, or the U.S. Government.
REFERENCES
- 1.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Travers K, Barza M. 2002. Morbidity of infections caused by antimicrobial-resistant bacteria. Clin Infect Dis 34(Suppl 3):S131–S134. [DOI] [PubMed] [Google Scholar]
- 3.Varma JK, Molbak K, Barrett TJ, Beebe JL, Jones TF, Rabatsky-Ehr T, Smith KE, Vugia DJ, Chang HG, Angulo FJ. 2005. Antimicrobial-resistant nontyphoidal Salmonella is associated with excess bloodstream infections and hospitalizations. J Infect Dis 191:554–561. doi: 10.1086/427263. [DOI] [PubMed] [Google Scholar]
- 4.Helms M, Vastrup P, Gerner-Smidt P, Molbak K. 2002. Excess mortality associated with antimicrobial drug-resistant Salmonella Typhimurium. Emerg Infect Dis 8:490–495. doi: 10.3201/eid0805.010267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hohmann EL. 2001. Nontyphoidal salmonellosis. Clin Infect Dis 32:263–269. doi: 10.1086/318457. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert JM, White DG, McDermott PF. 2007. The US national antimicrobial resistance monitoring system. Future Microbiol 2:493–500. doi: 10.2217/17460913.2.5.493. [DOI] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2010. Performance standards for antimicrobial susceptibility testing; 20th informational supplement. CLSI document M100-S20. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 8.Salmi T, Määttä A, Anttila P, Ruoho-Airola T, Amnell T. 2002. Detecting trends of annual values of atmospheric pollutants by the Mann-Kendall test and Sen's slope estimates: the excel template application MAKESENS. Finnish Meteorological Institute, Helsinki, Finland. [Google Scholar]
- 9.Tadesse DA, Zhao S, Tong E, Ayers S, Singh A, Bartholomew MJ, McDermott PF. 2012. Antimicrobial drug resistance in Escherichia coli from humans and food animals, United States, 1950-2002. Emerg Infect Dis 18:741–749. doi: 10.3201/eid1805.111153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bissett ML, Abbott SL, Wood RM. 1974. Antimicrobial resistance and R factors in Salmonella isolated in California (1971-1972). Antimicrob Agents Chemother 5:161–168. doi: 10.1128/AAC.5.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pocurull DW, Gaines SA, Mercer HD. 1971. Survey of infectious multiple drug resistance among salmonella isolated from animals in the United States. Appl Microbiol 21:358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.U.S. Food and Drug Administration. 2013. National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS): 2010 executive report. U.S. Food and Drug Administration, Rockville, MD. [Google Scholar]
- 13.Skold O. 2001. Resistance to trimethoprim and sulfonamides. Vet Res 32:261–273. doi: 10.1051/vetres:2001123. [DOI] [PubMed] [Google Scholar]
- 14.Cohen ML, Tauxe RV. 1986. Drug-resistant Salmonella in the United States: an epidemiologic perspective. Science 234:964–969. doi: 10.1126/science.3535069. [DOI] [PubMed] [Google Scholar]
- 15.Smith SM, Palumbo PE, Edelson PJ. 1984. Salmonella strains resistant to multiple antibiotics: therapeutic implications. Pediatr Infect Dis 3:455–460. doi: 10.1097/00006454-198409000-00017. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Food and Drug Administration. 2015. 2013 Summary report on antimicrobials sold or distributed for use in food-producing animals. U.S. Food and Drug Administration, Rockville, MD: Accessed 31 January 2016 http://www.fda.gov/downloads/ForIndustry/UserFees/AnimalDrugUserFeeActADUFA/UCM440584.pdf. [Google Scholar]
- 17.Enne VI, Livermore DM, Stephens P, Hall LM. 2001. Persistence of sulphonamide resistance in Escherichia coli in the UK despite national prescribing restriction. Lancet 357:1325–1328. doi: 10.1016/S0140-6736(00)04519-0. [DOI] [PubMed] [Google Scholar]
- 18.Lee LA, Puhr ND, Maloney EK, Bean NH, Tauxe RV. 1994. Increase in antimicrobial-resistant Salmonella infections in the United States, 1989-1990. J Infect Dis 170:128–134. doi: 10.1093/infdis/170.1.128. [DOI] [PubMed] [Google Scholar]
- 19.MacDonald KL, Cohen ML, Hargrett-Bean NT, Wells JG, Puhr ND, Collin SF, Blake PA. 1987. Changes in antimicrobial resistance of Salmonella isolated from humans in the United States. JAMA 258:1496–1499. [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC). 2002. Outbreak of multidrug-resistant Salmonella Newport—United States, January-April 2002. Morbid Mortal Wkly Rep 51:545–548. [PubMed] [Google Scholar]
- 21.Gupta A, Fontana J, Crowe C, Bolstorff B, Stout A, Van Duyne S, Hoekstra MP, Whichard JM, Barrett TJ, Angulo FJ. 2003. Emergence of multidrug-resistant Salmonella enterica serotype Newport infections resistant to expanded-spectrum cephalosporins in the United States. J Infect Dis 188:1707–1716. doi: 10.1086/379668. [DOI] [PubMed] [Google Scholar]
- 22.Zhao S, Qaiyumi S, Friedman S, Singh R, Foley SL, White DG, McDermott PF, Donkar T, Bolin C, Munro S, Baron EJ, Walker RD. 2003. Characterization of Salmonella enterica serotype Newport isolated from humans and food animals. J Clin Microbiol 41:5366–5371. doi: 10.1128/JCM.41.12.5366-5371.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glynn MK, Bopp C, Dewitt W, Dabney P, Mokhtar M, Angulo FJ. 1998. Emergence of multidrug-resistant Salmonella enterica serotype Typhimurium DT104 infections in the United States. N Engl J Med 338:1333–1338. doi: 10.1056/NEJM199805073381901. [DOI] [PubMed] [Google Scholar]
- 24.Rabatsky-Ehr T, Whichard J, Rossiter S, Holland B, Stamey K, Headrick ML, Barrett TJ, Angulo FJ. 2004. Multidrug-resistant strains of Salmonella enterica Typhimurium, United States, 1997-1998. Emerg Infect Dis 10:795–801. doi: 10.3201/eid1005.030209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribot EM, Wierzba RK, Angulo FJ, Barrett TJ. 2002. Salmonella enterica serotype Typhimurium DT104 isolated from humans, United States, 1985, 1990, and 1995. Emerg Infect Dis 8:387–391. doi: 10.3201/eid0804.010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medalla F, Hoekstra RM, Whichard JM, Barzilay EJ, Chiller TM, Joyce K, Rickert R, Krueger A, Stuart A, Griffin PM. 2013. Increase in resistance to ceftriaxone and nonsusceptibility to ciprofloxacin and decrease in multidrug resistance among Salmonella strains, United States, 1996-2009. Foodborne Pathog Dis 10:302–309. doi: 10.1089/fpd.2012.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michael GB, Butaye P, Cloeckaert A, Schwarz S. 2006. Genes and mutations conferring antimicrobial resistance in Salmonella: an update. Microbes Infect 8:1898–1914. doi: 10.1016/j.micinf.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 28.Olsen JE, Brown DJ, Baggesen DL, Bisgaard M. 1994. Stability of plasmids in five strains of Salmonella maintained in stab culture at different temperatures. J Appl Bacteriol 77:155–159. doi: 10.1111/j.1365-2672.1994.tb03059.x. [DOI] [PubMed] [Google Scholar]