Abstract
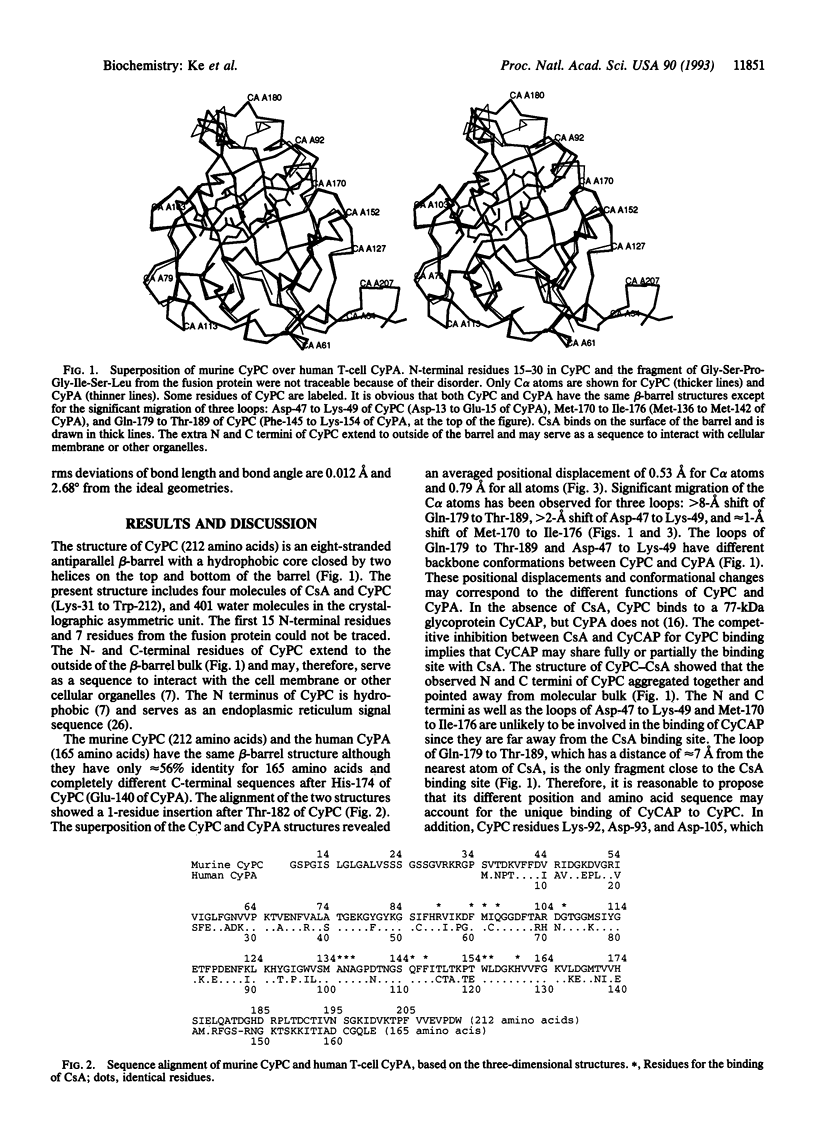
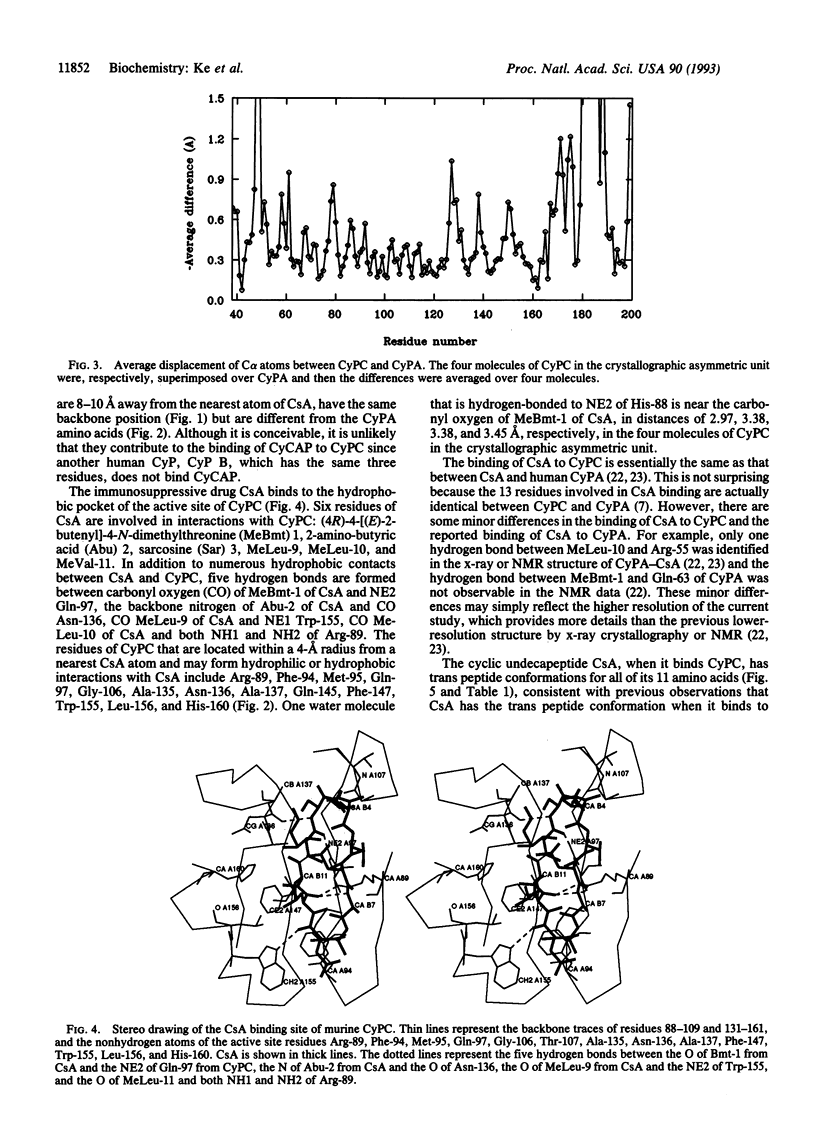
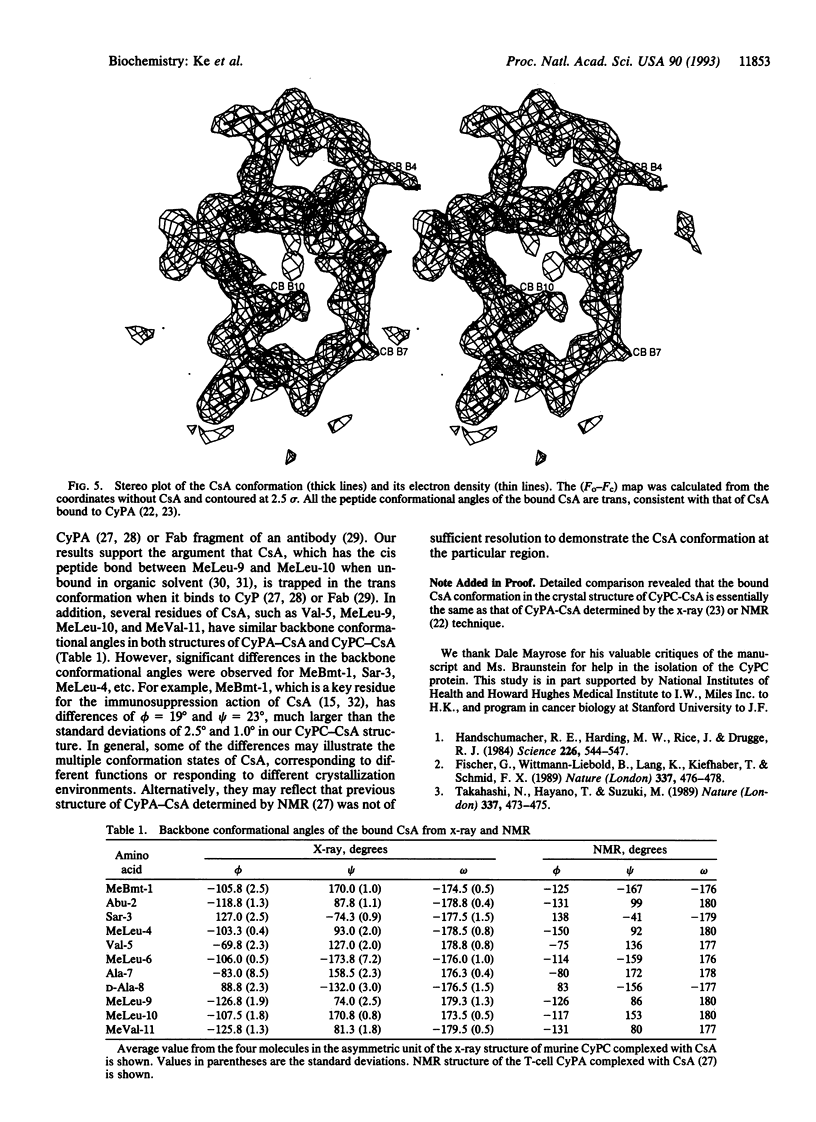
Cyclophilin is a cellular receptor for the immunosuppressive drug cyclosporin A (CsA). Cyclophilin C (CyPC) is highly expressed in murine kidney, making it a potential mediator of the nephrotoxic effects of CsA. The structure of murine CyPC complexed with CsA has been solved and refined to an R factor of 0.197 at a 1.64-A resolution. Superposition of the CyPC-CsA structure with the unligated cyclophilin A (CyPA) revealed significant migration of three loops: Gln-179 to Thr-189, Asp-47 to Lys-49, and Met-170 to Ile-176. The proximity of the loop Gln-179 to Thr-189 to the CsA binding site may account for the unique binding of a 77-kDa glycoprotein, CyPC binding protein (CyCAP), to CyPC. The binding of CsA to CyPC is similar to that of CsA to human T-cell cyclophilin A (CyPA). However, the conformation of CsA when bound to CyPC is significantly different from that when bound to CyPA. These differences may reflect conformational variation of CsA when bound to different proteins. Alternatively, the previous CyPA-CsA structure at low resolution may not provide sufficient details for a comparison with the CyPC-CsA structure.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschuh D., Vix O., Rees B., Thierry J. C. A conformation of cyclosporin A in aqueous environment revealed by the X-ray structure of a cyclosporin-Fab complex. Science. 1992 Apr 3;256(5053):92–94. doi: 10.1126/science.1566062. [DOI] [PubMed] [Google Scholar]
- Bergsma D. J., Eder C., Gross M., Kersten H., Sylvester D., Appelbaum E., Cusimano D., Livi G. P., McLaughlin M. M., Kasyan K. The cyclophilin multigene family of peptidyl-prolyl isomerases. Characterization of three separate human isoforms. J Biol Chem. 1991 Dec 5;266(34):23204–23214. [PubMed] [Google Scholar]
- Bram R. J., Hung D. T., Martin P. K., Schreiber S. L., Crabtree G. R. Identification of the immunophilins capable of mediating inhibition of signal transduction by cyclosporin A and FK506: roles of calcineurin binding and cellular location. Mol Cell Biol. 1993 Aug;13(8):4760–4769. doi: 10.1128/mcb.13.8.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Francis C. W., Totterman S., Kessler C. M., Rao A. K., Rubin R., Kwaan H. C., Gabriel K. R., Marder V. J. Quantitation of venous clot lysis with the D-dimer immunoassay during fibrinolytic therapy requires correction for soluble fibrin degradation. Circulation. 1990 Jun;81(6):1818–1825. doi: 10.1161/01.cir.81.6.1818. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Fesik S. W., Gampe R. T., Jr, Eaton H. L., Gemmecker G., Olejniczak E. T., Neri P., Holzman T. F., Egan D. A., Edalji R., Simmer R. NMR studies of [U-13C]cyclosporin A bound to cyclophilin: bound conformation and portions of cyclosporin involved in binding. Biochemistry. 1991 Jul 2;30(26):6574–6583. doi: 10.1021/bi00240a030. [DOI] [PubMed] [Google Scholar]
- Fischer G., Schmid F. X. The mechanism of protein folding. Implications of in vitro refolding models for de novo protein folding and translocation in the cell. Biochemistry. 1990 Mar 6;29(9):2205–2212. doi: 10.1021/bi00461a001. [DOI] [PubMed] [Google Scholar]
- Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F. X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989 Feb 2;337(6206):476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- Friedman J., Trahey M., Weissman I. Cloning and characterization of cyclophilin C-associated protein: a candidate natural cellular ligand for cyclophilin C. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6815–6819. doi: 10.1073/pnas.90.14.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J., Weissman I. Two cytoplasmic candidates for immunophilin action are revealed by affinity for a new cyclophilin: one in the presence and one in the absence of CsA. Cell. 1991 Aug 23;66(4):799–806. doi: 10.1016/0092-8674(91)90123-g. [DOI] [PubMed] [Google Scholar]
- Handschumacher R. E., Harding M. W., Rice J., Drugge R. J., Speicher D. W. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984 Nov 2;226(4674):544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- Humes H. D., Jackson N. M., O'Connor R. P., Hunt D. A., White M. D. Pathogenetic mechanisms of nephrotoxicity: insights into cyclosporine nephrotoxicity. Transplant Proc. 1985 Aug;17(4 Suppl 1):51–62. [PubMed] [Google Scholar]
- Kallen J., Spitzfaden C., Zurini M. G., Wider G., Widmer H., Wüthrich K., Walkinshaw M. D. Structure of human cyclophilin and its binding site for cyclosporin A determined by X-ray crystallography and NMR spectroscopy. Nature. 1991 Sep 19;353(6341):276–279. doi: 10.1038/353276a0. [DOI] [PubMed] [Google Scholar]
- Kallen J., Walkinshaw M. D. The X-ray structure of a tetrapeptide bound to the active site of human cyclophilin A. FEBS Lett. 1992 Apr 6;300(3):286–290. doi: 10.1016/0014-5793(92)80865-e. [DOI] [PubMed] [Google Scholar]
- Ke H. M., Zydowsky L. D., Liu J., Walsh C. T. Crystal structure of recombinant human T-cell cyclophilin A at 2.5 A resolution. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9483–9487. doi: 10.1073/pnas.88.21.9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke H., Mayrose D., Cao W. Crystal structure of cyclophilin A complexed with substrate Ala-Pro suggests a solvent-assisted mechanism of cis-trans isomerization. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3324–3328. doi: 10.1073/pnas.90.8.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke H. Similarities and differences between human cyclophilin A and other beta-barrel structures. Structural refinement at 1.63 A resolution. J Mol Biol. 1992 Nov 20;228(2):539–550. doi: 10.1016/0022-2836(92)90841-7. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Draetta G. F., Hubbard M. J. Calcineurin. Adv Enzymol Relat Areas Mol Biol. 1988;61:149–200. doi: 10.1002/9780470123072.ch4. [DOI] [PubMed] [Google Scholar]
- Koletsky A. J., Harding M. W., Handschumacher R. E. Cyclophilin: distribution and variant properties in normal and neoplastic tissues. J Immunol. 1986 Aug 1;137(3):1054–1059. [PubMed] [Google Scholar]
- Liu J., Albers M. W., Wandless T. J., Luan S., Alberg D. G., Belshaw P. J., Cohen P., MacKintosh C., Klee C. B., Schreiber S. L. Inhibition of T cell signaling by immunophilin-ligand complexes correlates with loss of calcineurin phosphatase activity. Biochemistry. 1992 Apr 28;31(16):3896–3901. doi: 10.1021/bi00131a002. [DOI] [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Marks W. H., Harding M. W., Handschumacher R., Marks C., Lorber M. I. The immunochemical distribution of cyclophilin in normal mammalian tissues. Transplantation. 1991 Aug;52(2):340–345. doi: 10.1097/00007890-199108000-00030. [DOI] [PubMed] [Google Scholar]
- Myers B. D., Ross J., Newton L., Luetscher J., Perlroth M. Cyclosporine-associated chronic nephropathy. N Engl J Med. 1984 Sep 13;311(11):699–705. doi: 10.1056/NEJM198409133111103. [DOI] [PubMed] [Google Scholar]
- Pflügl G., Kallen J., Schirmer T., Jansonius J. N., Zurini M. G., Walkinshaw M. D. X-ray structure of a decameric cyclophilin-cyclosporin crystal complex. Nature. 1993 Jan 7;361(6407):91–94. doi: 10.1038/361091a0. [DOI] [PubMed] [Google Scholar]
- Price E. R., Zydowsky L. D., Jin M. J., Baker C. H., McKeon F. D., Walsh C. T. Human cyclophilin B: a second cyclophilin gene encodes a peptidyl-prolyl isomerase with a signal sequence. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1903–1907. doi: 10.1073/pnas.88.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal N. H., Dumont F., Durette P., Siekierka J. J., Peterson L., Rich D. H., Dunlap B. E., Staruch M. J., Melino M. R., Koprak S. L. Is cyclophilin involved in the immunosuppressive and nephrotoxic mechanism of action of cyclosporin A? J Exp Med. 1991 Mar 1;173(3):619–628. doi: 10.1084/jem.173.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Hayano T., Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature. 1989 Feb 2;337(6206):473–475. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- Thériault Y., Logan T. M., Meadows R., Yu L., Olejniczak E. T., Holzman T. F., Simmer R. L., Fesik S. W. Solution structure of the cyclosporin A/cyclophilin complex by NMR. Nature. 1993 Jan 7;361(6407):88–91. doi: 10.1038/361088a0. [DOI] [PubMed] [Google Scholar]
- Weber C., Wider G., von Freyberg B., Traber R., Braun W., Widmer H., Wüthrich K. The NMR structure of cyclosporin A bound to cyclophilin in aqueous solution. Biochemistry. 1991 Jul 2;30(26):6563–6574. doi: 10.1021/bi00240a029. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M. Issues in the pathophysiology of nephrotoxic renal tubular cell injury pertinent to understanding cyclosporine nephrotoxicity. Transplant Proc. 1985 Aug;17(4 Suppl 1):81–90. [PubMed] [Google Scholar]


