Abstract
Pharmacokinetic modeling has often been applied to evaluate vancomycin pharmacokinetics in neonates. However, clinical application of the model-based personalized vancomycin therapy is still limited. The objective of the present study was to evaluate the clinical utility and safety of a model-based patient-tailored dose of vancomycin in neonates. A model-based vancomycin dosing calculator, developed from a population pharmacokinetic study, has been integrated into the routine clinical care in 3 neonatal intensive care units (Robert Debré, Cochin Port Royal, and Clocheville hospitals) between 2012 and 2014. The target attainment rate, defined as the percentage of patients with a first therapeutic drug monitoring serum vancomycin concentration achieving the target window of 15 to 25 mg/liter, was selected as an endpoint for evaluating the clinical utility. The safety evaluation was focused on nephrotoxicity. The clinical application of the model-based patient-tailored dose of vancomycin has been demonstrated in 190 neonates. The mean (standard deviation) gestational and postnatal ages of the study population were 31.1 (4.9) weeks and 16.7 (21.7) days, respectively. The target attainment rate increased from 41% to 72% without any case of vancomycin-related nephrotoxicity. This proof-of-concept study provides evidence for integrating model-based antimicrobial therapy in neonatal routine care.
INTRODUCTION
Neonatal bacterial sepsis, exacerbated by neonatal immunodeficiency (1), remains a major cause of mortality and morbidity in newborns (2). Vancomycin is widely used for the treatment of late-onset sepsis caused by methicillin-resistant Staphylococcus aureus and coagulase-negative staphylococci in neonatal intensive care units (NICUs) (3); however, the clinical use of vancomycin is still hampered by its narrow therapeutic index and high pharmacokinetic variability (4). Indeed, de Hoog et al. reported that vancomycin clearance and half-life varied between 0.63 and 1.4 ml/kg/min and between 3.5 and 10 h in neonates, respectively (4). The common adverse effects of vancomycin are nephrotoxicity and ototoxicity; however, it has been shown that neonates tolerated vancomycin better than adults. Safety data for a high dosing regimen and long-term follow-up are still lacking.
The pharmacokinetic modeling approach is often applied to evaluate and optimize antimicrobial therapy in neonates (5). To date, vancomycin is one of the best-studied antimicrobials, and numerous studies have been conducted to characterize its pharmacokinetic parameters and to identify individual factors influencing variability (6). However, the clinical application of model-based personalized vancomycin therapy is still limited. The objective of the present study was to evaluate the clinical utility and safety of a patient-tailored dose of vancomycin in neonates.
MATERIALS AND METHODS
Neonates receiving vancomycin as a continuous infusion in one of three NICUs (Cochin Port Royal, Robert Debré, and Clocheville hospitals) were enrolled between June 2012 and November 2014. This study was designed in accordance with legal requirements and the Declaration of Helsinki, registered at the Commission Nationale Informatique et Liberté (CNIL), and approved by the local research ethics committee (Comité d' Evaluation de l'Ethique des Projets de Recherche Biomédicale [CEERB]).
Model-based patient-tailored dose of vancomycin.
Special training was conducted in each NICU. We first informed the staff of the clinical pharmacology of vancomycin, its pharmacokinetic variability, and a large variation of dosage schedules currently used. We then explained the principles of individual dosage adaptation and how we developed the Excel dosing calculator using the results from our published population pharmacokinetic model (6).
In order to calculate the patient-tailored dosing of vancomycin for each neonate, neonatologists had to enter four patient covariates in the calculator, including birth weight (in grams), current weight (in grams), postnatal age (PNA; in days), and serum creatinine concentration (in micromoles per liter) measured within 48 h of starting vancomycin treatment. The developed calculator was locked, and no other information was required. The patient-tailored dose is calculated automatically by using the following pharmacokinetic equations.
| (1) |
| (2) |
where the loading dose is in milligrams, the maintenance dose is in milligrams per 24 h, the target concentration is in milligrams per liter, V (volume of distribution) is in liters, and CL (clearance) is in liters per hour; V and CL are calculated based on the model (6)
| (3) |
| (4) |
where current weight and birth weight are in grams, PNA is in days, and serum creatinine is in micromoles per liter.
The loading dose is infused over 60 min and followed by the maintenance dose administered as a continuous infusion over 24 h. Only patients with complete information were included in the final analysis.
Clinical utility and safety.
The target attainment rate was selected as the endpoint for evaluating the clinical utility of the patient-tailored dose. It was defined as the percentage of patients with a vancomycin serum concentration within the target window of 15 to 25 mg/liter, calculated using the first therapeutic drug monitoring (TDM) sample taken 6 to 24 h after starting vancomycin treatment. This TDM practice has become a part of the routine clinical care in the 3 NICUs. One TDM sample per patient (0.5 ml) was required. The relative error to the concentration of 20 mg/liter {calculated using the equation (observed concentration [in milligrams per liter] − 20)/20} was also used to evaluate the impact of covariates on the performance of the patient tailored-dose.
The safety evaluation was focused on nephrotoxicity, which was evaluated based on changes in serum creatinine concentrations from baseline obtained within 48 h of starting vancomycin administration. Nephrotoxicity was defined as either a 2-fold increase or an increase by at least 0.6 mg/dl from the start and any time until the end of vancomycin therapy (7). The causality of nephrotoxicity was analyzed individually to assess the potential relation to vancomycin.
Analysis of vancomycin.
The serum vancomycin trough concentrations were determined by fluorescence polarization immunoassay using a Cobas Integra system (Roche Diagnostics, Meylan, France). The lower limit of quantification and coefficients of variation were 0.74 mg/liter and <3.3%, respectively. The TDM samples were analyzed locally in the pharmacology laboratory of each hospital according to clinical practice. All three laboratories used the same analytical method and followed the same external quality control program and good clinical practice.
Statistical analysis.
Results are presented as means, standard deviations (SD), and ranges. The simple linear regression was used to evaluate the potential impact of patient characteristics (birth weight, current weight, postnatal age, and baseline serum concentration) on the relative error to target concentration. The permutational two-sample t test was used to compare the characteristics of patients who had vancomycin concentration within versus out of the targeted range. Statistical analyses were conducted using R software (V.3.0). A P value of <0.05 was considered statistically significant.
RESULTS
Study population.
The pharmacokinetic and safety data from 190 neonates were available. The mean (SD) current weight and postnatal age were 1,755.0 (872.5) g (range, 540 to 4,750) and 16.7 (21.7) days (range, 0 to 196) days, respectively. A summary of the population characteristics is presented in Table 1.
TABLE 1.
Baseline characteristics of the 190 neonates
| Characteristic | Mean (SD) | Median (range) |
|---|---|---|
| Gestational age (wks) | 31.1 (4.9) | 30 (24–42) |
| Birth wt (g) | 1,563.0 (844.0) | 1,290 (512–4,180) |
| Current wt (at time of dosing) (g) | 1,755.0 (872.5) | 1,525 (540–4,750) |
| Postnatal age (days) | 16.7 (21.7) | 10 (0–196) |
| Baseline serum creatinine concna (μmol/liter) | 48.6 (21.8) | 46 (14.0–125.0) |
| Vancomycin loading dose (mg/kg/day) | 11.1 (0.6) | 11.1 (9.9–12.6) |
| Vancomycin maintenance dose (mg/kg/day) | 28.3 (9.9) | 25.4 (13.0–61.0) |
Within 48 h before inclusion.
Eight patients were excluded because of incomplete information (creatinine concentration was not measured). Patients were treated for suspected or proven late-onset sepsis mainly caused by coagulase-negative staphylococci. The durations of treatment were 7 to 10 days for proven infections and 2 to 5 days for suspected infections.
Clinical utility of patient-tailored dose.
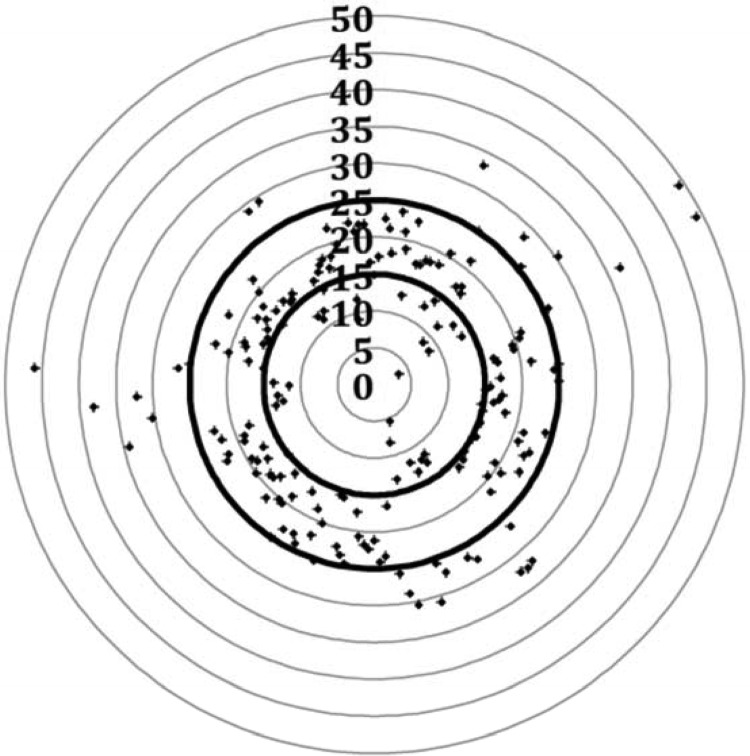
The mean loading dose and maintenance dose were 11.1 mg/kg and 28.3 mg/kg/day, respectively. After the subjects received these patient-tailored doses, the mean of the first vancomycin TDM concentrations was 20.0 mg/liter (10th to 90th percentiles: 13.1 to 27.6). The target attainment rate was 72% (n = 136) within the range of 15 to 25 mg/liter (91% within the range of 10 to 30 mg/liter). Only 6 patients (3.1%) had concentrations of <10 mg/liter, and 12 (6.3%) had concentrations of >30 mg/liter. TDM results are illustrated in Fig. 1.
FIG 1.
Vancomycin first TDM concentrations after receiving the model-based patient-tailored dose regimen in 190 neonates. The first therapeutic drug monitoring samples were taken 6 to 24 h after the start of vancomycin treatment (one sample per patient). The bold lines represent the boundaries of the target concentrations. The points represent observed concentrations (in milligrams per liter).
The relative error between observed and target TDM concentrations was not significantly linked to birth weight (P = 0.35), current weight (P = 0.26), postnatal age (P = 0.54), or baseline serum creatinine concentration (P = 0.90). Moreover, no significant differences were found between patients who had vancomycin concentrations within versus out of the target range in terms of birth weight (P = 0.67), current weight (P = 0.65), postnatal age (P = 0.69), or serum creatinine concentration (P = 0.80).
Safety of patient-tailored dose.
Among the 190 neonates receiving the patient-tailored dose of vancomycin, only 2 (1.1%) patients developed nephrotoxicity. The two neonates were 22 days old (birth weight, 760 g) and 10 days old (birth weight, 990 g). Both of them were treated on the basis of proven coagulase-negative staphylococcus infections, and the increases of serum creatinine levels (the first one from 0.35 to 1.62 mg/dl and the other one from 0.52 to 1.22 mg/dl) were concomitant with the onset of hemodynamic instability requiring vasoactive drugs (noradrenaline and dopamine). The altered renal function was resolved after restitution of the hemodynamic state, without any cessation of vancomycin therapy or reduction of dose. Therefore, the rise in serum creatinine level was not considered related to vancomycin for these patients.
DISCUSSION
The present work provides evidence-based data to demonstrate the clinical utility and renal safety of a model-based patient-tailored dose of vancomycin in neonates. The findings from a population pharmacokinetic study were successfully integrated into neonatal clinical practice to individualize vancomycin therapy, showing that the percentage of patients achieving the target concentrations rose from 41% (using the standard dosing regimen) in our previous study (6) to 72% in the present study. The patient characteristics are similar between the two studies (mean postmenstrual age of 33.8 weeks and weight of 1,700 g in our previous study). Most of the patients were preterm neonates and had low birth weights.
Due to the wide interindividual pharmacokinetic variability of many drugs evidenced in neonates (8), dosage individualization is a key issue faced by neonatologists and pediatric pharmacologists to optimize neonatal drug treatment (9). Traditionally, the antimicrobial pharmacokinetic study in neonates was focused on average drug exposure to achieve adult levels, and the neonatal recommended dose is usually administered on a milligram-per-kilogram basis (10). This approach obviously simplifies the analysis of developmental changes in drug disposition and the effects of clinical conditions on pharmacokinetic parameters. It assumes an “average newborn” with an “average weight” and therefore a simple linear maturation relationship between weight and drug clearance. This simplification of the complex developmental pharmacokinetics resulted in only 41% of patients achieving the vancomycin target concentrations of 15 to 25 mg/liter using the standard milligram-per-kilogram-based vancomycin dose (6).
According to regulatory guidelines, antibacterial agents are a good example of drugs for which the exposure-response relationship can be assumed to be similar in adults and neonates. An AUC0–24/MIC ratio (area under the concentration-time curve over 24 h at steady state divided by the MIC) over 400 h has been shown to best predict the clinical and bacteriological responses for invasive methicillin-resistant Staphylococcus aureus infections in adults (11). Therefore, the target AUC0–24 of 400 mg · h/liter (assuming a standard MIC of 1 mg/liter) was often used as a surrogate of efficacy to optimize dose in neonates. A positive correlation between vancomycin trough concentration and risk of nephrotoxicity was demonstrated in adults (12). A vancomycin (continuous infusion) target concentration of 15 to 25 mg/liter allowed achieving this target AUC and demonstrated a good safety profile in the present study.
An appropriate neonatal dosage regimen needs to integrate the rapid developmental changes during the neonatal period, as reflected by covariates influencing drug disposition (13). As vancomycin is almost entirely eliminated by the kidneys, covariates reflecting both renal maturation and renal function should be taken into account to personalize the vancomycin dosage. The pharmacokinetic modeling approach has been used for many years to evaluate pharmacokinetics of vancomycin (14, 15) and identify the major covariates in neonates, including weight, age, and creatinine concentration. The next step is now to implement such a mathematic tool in clinical practice. Using our developed population pharmacokinetic model, the patient-tailored dose regimen was established and tested prospectively using the identified covariates. In the present study, we have shown a great improvement in the target attainment rate using the patient-tailored dose. The clinical benefits of such personalized therapy are clear: the target attainment rate is reached early and will increase efficacy while reducing the risks of bacterial resistance and toxicity. In addition, the earlier target achievement will reduce the numbers of TDM samples and dosage adjustments. Obviously, the reduced blood loss in newborns has a considerable clinical benefit. Of note, there is still a considerable number of neonates (28%) for whom the first TDM concentration was out of target range, making TDM still recommended for vancomycin therapy in neonates.
Only 2 of the 190 (1.1%) neonates showed nephrotoxicity as quantified by serum creatinine concentration during vancomycin treatment, and this was most probably related to the patients' clinical condition and hemodynamic instability. In a previous study, Bhatt-Metha et al. reported 8.7% (6 of 69) newborn infants with nephrotoxicity (using the same definition as in our study) while receiving a standard vancomycin dose administered as an intermittent infusion (7). Although it is not possible to directly compare these safety results because of the different method of administration, our study did not evidence any deleterious effect on renal function with the patient-tailored dose of vancomycin administered as a continuous infusion.
A limitation of our study is that all the patients were monitored until the end of hospitalization and long-term follow-up data were not available. Obviously, the developmental toxicity, e.g., ototoxicity, could not be evaluated in our study, and a long-term safety study of vancomycin in neonates is required (16). Despite the limitation of the use of creatinine concentration as a surrogate of nephrotoxicity in neonates, it allows us to compare the incidences of nephrotoxicity with those in previously published studies (4, 7). Numerous novel biomarkers of nephrotoxicity (N-acetylglucosaminidase, neutrophil gelatinase-associated lipocalin, cystatin C, etc.) are being studied; however, the clinical value of these biomarkers needs to be validated for neonates (17). The training regarding the correct use of dosing calculator is extremely important. The impact of entering imprecise covariate information into the calculator may introduce medication error. As highlighted by the excluded patients, the serum creatinine concentration changes rapidly at the beginning of the life. The individual dose, calculated using creatinine measured always from the day of starting treatment, may have a 2-fold difference. Obviously, the error of dose calculation will increase the risks of toxicity or treatment failure.
In summary, a model-based patient-tailored dose of vancomycin administered as a continuous infusion has been successfully implemented in routine care in 3 NICUs and demonstrated positive results in terms of pharmacokinetics and safety. This study provides a proof-of-concept for the clinical utility and safety of model-based patient-tailored dosing regimen of vancomycin in neonates. The next step will be to confirm our results with a prospective controlled trial. This innovative personalized dosing approach, certainly applicable to other antimicrobial therapies, is a promising way to optimize drug therapy in neonates.
ACKNOWLEDGMENTS
We thank all the children and their families for participating in this study.
We declare no conflict of interest related to this work.
The VANCO IVC (continuous intravenous administration of vancomycin) Study Group includes the following: from Hôpital Robert Debré, Stéphanie Leroux, Valérie Biran, Olivier Baud, Evelyne Jacqz-Aigrain, Wei Zhao, Daolun Zhang, Elsa Maksoud, and Thomas Storme; from Hôpital Clocheville, Emmanuel Lopez, Camille Wallon, Elie Saliba, Antoine Bouissou, Nadine Fakhri, Armelle Bridier, and Camille Dubois; and from Hôpital Cochin-Port Royal, Doriane Madeleneau, Elodie Zana-Taïeb, Pierre-Henri Jarreau, Juliana Patkaï, Marie-Stéphanie Aubelle, Mayass El Ayoubi, Maher Ben Laïba, Clémence Lopez, Charlotte Pierron, Marianne Reveret, Amélie Maillard, Jean-Françoise Boccara, Souad Kout, Marie-Lucie Brunet, and Camille Imbert.
Funding Statement
This work was supported by the Fundamental Research Funds and Young Scholars Program of Shandong University, the GRIP (Global Research in Pediatrics, European Commission FP7 project, grant agreement number 261060), and NeoVanc (European Commission FP7 project, grant agreement number 602041).
REFERENCES
- 1.Schlapbach LJ, Mattmann M, Thiel S, Boillat C, Otth M, Nelle M, Wagner B, Jensenius JC, Aebi C. 2010. Differential role of the lectin pathway of complement activation in susceptibility to neonatal sepsis. Clin Infect Dis 51:153–162. doi: 10.1086/653531. [DOI] [PubMed] [Google Scholar]
- 2.Makhoul IR, Sujov P, Smolkin T, Lusky A, Reichman B, Israel Neonatal Network. 2005. Pathogen-specific early mortality in very low birth weight infants with late-onset sepsis: a national survey. Clin Infect Dis 40:218–224. doi: 10.1086/426444. [DOI] [PubMed] [Google Scholar]
- 3.Jacqz-Aigrain E, Zhao W, Sharland M, van den Anker JN. 2013. Use of antibacterial agents in the neonate: 50 years of experience with vancomycin administration. Semin Fetal Neonatal Med 18:28–34. doi: 10.1016/j.siny.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 4.de Hoog M, Mouton JW, van den Anker JN. 2004. Vancomycin: pharmacokinetics and administration regimens in neonates. Clin Pharmacokinet 43:417–440. doi: 10.2165/00003088-200443070-00001. [DOI] [PubMed] [Google Scholar]
- 5.Marsot A, Boulamery A, Bruguerolle B, Simon N. 2012. Population pharmacokinetic analysis during the first 2 years of life: an overview. Clin Pharmacokinet 51:787–798. doi: 10.1007/s40262-012-0015-8. [DOI] [PubMed] [Google Scholar]
- 6.Zhao W, Lopez E, Biran V, Durrmeyer X, Fakhoury M, Jacqz-Aigrain E. 2013. Vancomycin continuous infusion in neonates: dosing optimisation and therapeutic drug monitoring. Arch Dis Child 98:449–453. doi: 10.1136/archdischild-2012-302765. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt-Mehta V, Schumacher RE, Faix RG, Leady M, Brenner T. 1999. Lack of vancomycin-associated nephrotoxicity in newborn infants: a case-control study. Pediatrics 103:e48. doi: 10.1542/peds.103.4.e48. [DOI] [PubMed] [Google Scholar]
- 8.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. 2003. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med 349:1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 9.Zhao W, Leroux S, Jacqz-Aigrain E. 2014. Dosage individualization in children: integration of pharmacometrics in clinical practice. World J Pediatr 10:197–203. doi: 10.1007/s12519-014-0493-x. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Avant D, Green D, Seo S, Fisher J, Mulberg AE, McCune SK, Burckart GJ. 2015. A survey of neonatal pharmacokinetic and pharmacodynamic studies in pediatric drug development. Clin Pharmacol Ther 98:328–335. doi: 10.1002/cpt.149. [DOI] [PubMed] [Google Scholar]
- 11.Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. 2004. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet 43:925–942. doi: 10.2165/00003088-200443130-00005. [DOI] [PubMed] [Google Scholar]
- 12.Pritchard L, Baker C, Leggett J, Sehdev P, Brown A, Bayley KB. 2010. Increasing vancomycin serum trough concentrations and incidence of nephrotoxicity. Am J Med 123:1143–1149. doi: 10.1016/j.amjmed.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Smits A, Annaert P, Allegaert K. 2013. Drug disposition and clinical practice in neonates: cross talk between developmental physiology and pharmacology. Int J Pharm 452:8–13. doi: 10.1016/j.ijpharm.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 14.Lo YL, van Hasselt JG, Heng SC, Lim CT, Lee TC, Charles BG. 2010. Population pharmacokinetics of vancomycin in premature Malaysian neonates: identification of predictors for dosing determination. Antimicrob Agents Chemother 54:2626–2632. doi: 10.1128/AAC.01370-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Hoog M, Schoemaker RC, Mouton JW, van den Anker JN. 2000. Vancomycin population pharmacokinetics in neonates. Clin Pharmacol Ther 67:360–367. doi: 10.1067/mcp.2000.105353. [DOI] [PubMed] [Google Scholar]
- 16.Jacqz-Aigrain E, Leroux S, Zhao W, van den Anker JN, Sharland M. 2015. How to use vancomycin optimally in neonates: remaining questions. Expert Rev Clin Pharmacol 8:635–648. doi: 10.1586/17512433.2015.1060124. [DOI] [PubMed] [Google Scholar]
- 17.Elyasi S, Khalili H, Dashti-Khavidaki S, Mohammadpour A. 2012. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur J Clin Pharmacol 68:1243–1255. doi: 10.1007/s00228-012-1259-9. [DOI] [PubMed] [Google Scholar]