Abstract
There are limited pharmacokinetic data for use of the first-line antituberculosis drugs during infancy (<12 months of age), when drug disposition may differ. Intensive pharmacokinetic sampling was performed in infants routinely receiving antituberculosis treatment, including rifampin, isoniazid, pyrazinamide, and ethambutol, using World Health Organization-recommended doses. Regulatory-approved single-drug formulations, including two rifampin suspensions, were used on the sampling day. Assays were conducted using liquid chromatography-mass spectrometry; pharmacokinetic parameters were generated using noncompartmental analysis. Thirty-nine infants were studied; 14 (36%) had culture-confirmed tuberculosis. Fifteen (38%) were premature (<37 weeks gestation); 5 (13%) were HIV infected. The mean corrected age and weight were 6.6 months and 6.45 kg, respectively. The mean maximum plasma concentrations (Cmax) for rifampin, isoniazid, pyrazinamide, and ethambutol were 2.9, 7.9, 41.9, and 1.3 μg/ml, respectively (current recommended adult target concentrations: 8 to 24, 3 to 6, 20 to 50, and 2 to 6 μg/ml, respectively), and the mean areas under the concentration-time curves from 0 to 8 h (AUC0–8) were 12.1, 24.7, 239.4, and 5.1 μg · h/ml, respectively. After adjusting for age and weight, rifampin exposures for the two formulations used differed in Cmax (geometric mean ratio [GMR], 2.55; 95% confidence interval [CI], 1.47 to 4.41; P = 0.001) and AUC0–8 (GMR, 2.52; 95% CI, 1.34 to 4.73; P = 0.005). HIV status was associated with lower pyrazinamide Cmax (GMR, 0.85; 95% CI, 0.75 to 0.96; P = 0.013) and AUC0–8 (GMR, 0.79; 95% CI, 0.69 to 0.90; P < 0.001) values. No other important differences were observed due to age, weight, prematurity, ethnicity, or gender. In summary, isoniazid and pyrazinamide concentrations in infants compared well with proposed adult target concentrations; ethambutol concentrations were lower but similar to previously reported pediatric studies. The low rifampin exposures require further investigation. (This study has been registered at ClinicalTrials.gov under registration no. NCT01637558.)
INTRODUCTION
Infants (age <12 months) in settings with high-burden tuberculosis (TB) and HIV are at high risk of Mycobacterium tuberculosis exposure, infection, disease, and mortality, emphasizing the need for rigorous evidence from pharmacokinetic studies to guide optimal antituberculosis treatment in this vulnerable population. Up to 50% of infants exposed to and infected with M. tuberculosis will develop TB disease in the absence of preventive therapy, with up to 30% of these progressing to severe pulmonary or disseminated disease (1). A 4-fold increase in mortality has been reported among infants with maternal HIV-associated TB in India (2), while 24% mortality was observed in South African infants <3 months of age with culture-confirmed TB (3).
In 2010, the World Health Organization (WHO) revised pediatric TB dosing guidelines by recommending considerably higher doses of first-line antituberculosis drugs in children (rifampin [RMP], 10 to 20 versus 8 to 12 mg/kg of body weight/day; isoniazid [INH], 10 to 15 versus 4 to 6 mg/kg/day; pyrazinamide [PZA], 30 to 40 versus 20 to 30 mg/kg/day; and ethambutol [EMB], 15 to 25 versus 15 to 20 mg/kg/day) (4, 5). These guidelines were based on evidence from pharmacokinetic studies involving mainly older children, which showed that higher mg/kg body weight dosing was necessary to achieve equivalent adult target concentrations (6–10). The pharmacokinetic and safety profiles of antituberculosis drugs in infants at these higher doses remain largely unknown. Infants undergo developmental and physiological changes that may influence drug disposition and susceptibility to toxicity (11, 12). Enzyme maturation, efflux transporter activity, increased drug clearance for body weight, and formulations and their preparations are all important factors determining drug exposure in young children (12). Few pharmacokinetic studies have been completed in children <2 years of age with TB (13, 14), and none were specifically in infants. A study in Indian children found significantly lower maximum plasma concentrations (Cmax) and areas under the concentration-time curves (AUC) for RMP, INH, and PZA in children <3 years of age compared to older children (ages 3.1 to 12.0 years) when the drugs were given thrice weekly, highlighting the need for age-dependent dosing considerations (14). The aim of our study was to determine key pharmacokinetic parameters for RMP, INH, PZA, and EMB at the revised WHO-recommended doses in infants routinely treated for TB.
MATERIALS AND METHODS
Study design and setting.
An observational cohort study with intensive pharmacokinetic sampling (single day) was conducted among HIV-infected and -uninfected infants routinely initiating first-line antituberculosis treatment in Cape Town, South Africa, from March 2014 through March 2015. Participants were recruited from Tygerberg Children's Hospital, Khayelitsha District Hospital, and Brooklyn Chest Hospital.
Study population and antituberculosis drugs.
HIV-infected and -uninfected infants were eligible after at least 2 weeks of intensive phase first-line antituberculosis treatment, allowing for RMP autoinduction (15). Infants were consecutively recruited after parental informed consent was obtained. HIV testing was routinely performed. TB treatment consisted of a standard 2-month intensive phase of RMP, INH, and PZA, with or without EMB, given once daily, 7 days a week, followed by a 4-month continuation phase of INH and RMP at the same doses (16). Weight-banded doses were consistent with the WHO interim guidelines for treatment of TB in young children using the available dispersible fixed-dose combinations for children. EMB was added at clinician discretion (typically in the case of more severe TB disease), as per national guidelines (16). Routine South African national TB program antituberculosis drugs, consisting of a fixed-dose combination (FDC) tablet of RMP and INH (60:60 mg) (Sandoz, South Africa) and a 500-mg PZA tablet (Sandoz, South Africa) with or without a 400-mg EMB tablet (Sandoz, South Africa), were given for the duration of TB treatment. Nevirapine (NVP) as part of prevention of mother-to-child transmission and combination antiretroviral therapy (cART) consisting of abacavir, lamivudine, and lopinavir/ritonavir were given to HIV-exposed and -infected infants, respectively (17).
Pharmacokinetic sampling was deferred in critically ill infants, those with a weight of <1.8 kg, and those with a hemoglobin level of <8 g/dl. On the pharmacokinetic sampling day only, routine antituberculosis drugs were replaced by single-drug formulations. This allowed for more accurate dosing in the very young study population, more closely reflecting the revised WHO-recommended dosing guidelines, since the routinely used FDC tablets (1:1 relation of RMP and INH) were not suitable for accurate dosing and the new FDC tablets (1.5:1 relation of RMP and INH) supporting the revised WHO dosing recommendations were not yet available at the time of the study. INH, PZA, and EMB were available in tablets of 50, 150, and 100 mg, respectively, and RMP was a granulate for suspension (100 mg/5 ml; Eremfat, referred to here as RMP formulation 1). The RMP, INH, and EMB formulations were manufactured by Riemser Arzneimittel, Germany, were registered with a stringent regulatory authority, and appeared on the global fund list of approved medicines. Pyrazinamide (Svizera Laboratories, Pty Ltd., the Netherlands) was manufactured in a WHO-certified facility complying with good manufacturing practices. In June 2014, Riemser temporarily halted the manufacturing of Eremfat, necessitating its substitution with a second RMP suspension, R-cin (100 mg/5 ml; manufactured by Aspen Pharmacare, South Africa, and registered by the Medicines Control Council of South Africa; referred to here as RMP formulation 2).
Sample size calculations.
The aim of the study was to enroll approximately 40 infants, based on prior knowledge of RMP variability, reported RMP exposures in adults and children, and the feasibility of enrolling this substantial number of infants with TB over a 12- to 18-month accrual period.
Pharmacokinetic investigation.
Infants were fasted for 2 h prior to TB drug dosing to facilitate improved absorption (18, 19). A nasogastric tube (NGT) was inserted to ensure accurate dosing, and the pH of gastric aspirates was measured using an instacheck 0-13 pHydrion pH test kit (Micro Essential Laboratory, USA). An indwelling peripheral venous catheter was inserted, and a predose (0-h) blood sample was obtained for drug concentration determination and for albumin and alanine aminotransferase (ALT) levels. The RMP suspension, followed by INH and PZA with or without EMB tablets crushed and mixed with water, was given via NGT, which was then flushed with 5 ml of water. Five more samples of 0.6 ml each at 1, 2, 4, 6, and 8 h postdose were collected in EDTA-coated tubes and centrifuged to separate the plasma before freezing at −80°C within 30 min of sampling. Drug concentrations were determined at the pharmacology laboratory at the University of Cape Town using validated liquid chromatography-mass spectrometry methods. The methods were validated over the concentration ranges of 0.0977 to 26.0 μg/ml for isoniazid, 0.117 to 30.0 μg/ml for rifampin, 0.200 to 80.0 μg/ml for pyrazinamide, and 0.0844 to 5.46 μg/ml for ethambutol (20). All samples that were below the level of quantification (BLQ) were set to half of the lower limit of quantification (LLOQ). ALT measures were repeated at the end of 6 months of TB treatment.
Pharmacokinetic parameters and statistical analysis.
Pharmacokinetic parameters were calculated using noncompartmental analysis (NCA). Stata 12.1 SE software (StataCorp 2011, College Station, TX, USA) was used for analyses. Cmax and time to Cmax (Tmax) were recorded directly from the concentration-time data. The AUC0–8 was calculated using the linear trapezoidal rule. The elimination half-life (t1/2) was denoted as ln2/Kel, where Kel (elimination rate constant) was the negative slope of the log-linear regression of three final data points of the concentration-time curve. The Cmax, AUC0–8, and t1/2 for RMP, INH, PZA, and EMB were compared by the clinical covariates of age at time of pharmacokinetic study evaluation (0 to 6 versus 7 to 12 months), nutritional status (Weight-for-age Z-score [WAZ] < −2 versus ≥ −2), prematurity (premature, <37 weeks, versus term), HIV status, ethnicity (black versus mixed race), and gender as well as, for RMP pharmacokinetic parameters only, by RMP formulation and RMP dose (10 to 15 versus 15 to 20 mg/kg). All comparisons were generated using t tests. For preterm infants, ages corrected for gestational age were calculated. Age was analyzed with and without corrected ages; no differences were found, so unadjusted age at the time of pharmacokinetic sampling was used. WAZs were calculated using the United Kingdom WHO preterm growth chart, in which preterm infant (<37 weeks) WAZs were adjusted for gestational age (21). Clearance was calculated for all first-line antituberculosis drugs using dose and AUC0–8 for age comparisons. Adverse events were classified using the division of AIDS (DAIDS) tables. TB treatment outcomes were defined using standard international criteria (22): cured or treatment completion was considered favorable, while treatment failure, death, and loss to follow-up, including treatment interruption of >2 months, were classified as unfavorable outcomes. TB treatment outcome was compared by the Cmax and AUC0–8 for the first-line antituberculosis drugs.
Multivariable regression models were generated to determine if any clinical covariates were associated with the INH, RMP, or PZA Cmax or AUC0–8. In total, six models were generated using the INH, RMP, and PZA log-transformed Cmax and AUC0–8 values as dependent variables, each analyzed separately. The geometric mean ratios (GMR) were reported along with 95% confidence intervals (CI) and P values. The clinical covariates considered for inclusion into the models were age at time of pharmacokinetic study (continuous), weight on pharmacokinetic day (continuous), HIV status, prematurity (premature versus term), ethnicity, nutritional status (WAZ < −2 versus WAZ ≥ −2), and gender. Covariates with a P value of <0.10 and factors known to affect drug disposition (age and weight) were included in each multivariable model. To determine if RMP dose was associated with Cmax or AUC0–8, a subanalysis was done using multivariable linear regression stratified by RMP formulation, where dose was analyzed, categorically controlling for continuous age and weight. The resulting regression coefficients (βs) for dose, along with the corresponding 95% CI and P values, are reported.
Regulatory approval.
The study (NCT01637558) was approved by Medicines Control Council of South Africa, by the research ethics committees of Stellenbosch University (N13/03/031), and by the University of Cape Town (180/2011).
RESULTS
Patient characteristics at pharmacokinetic assessment.
Table 1 displays the characteristics of the 39 infants, of whom 15 (39%) were documented as premature. At pharmacokinetic sampling, the mean age, corrected for gestational age, and weight were 6.6 months and 6.5 kg, respectively. M. tuberculosis infection was confirmed in 14 (36%) infants. Twenty-two (56%) infants were born to HIV-infected mothers; 5 infants seroconverted shortly after birth. All 5 HIV-infected infants were established on cART at the time of pharmacokinetic assessment. All infants received RMP, PZA, and INH; 16 (41%) also received EMB. Ethionamide was given in 7 (18%) infants (n = 2 with TB meningitis, n = 1 with miliary TB, and n = 4 due to clinician preference). The dosing ranges and summary statistics for the pharmacokinetic parameters for RMP (i.e., formulation 1 and formulation 2), INH, PZA, and EMB are displayed in Table 2. No difference was noted for clearance of the first-line antituberculosis drugs by age (data not shown).
TABLE 1.
Characteristics of infants with tuberculosis at the time of pharmacokinetic sampling (n = 39a)
| Characteristic | Value |
|---|---|
| No. (%) male | 26 (67) |
| No. (%) black ethnicity | 29 (74) |
| Mean (SD) corrected age, mo | 6.6 (3.3) |
| Mean (SD) weight, kg | 6.45 (1.67) |
| Mean (SD) WAZb | −1.62 (1.53) |
| Mean (SD) WLZc | −0.41 (1.26) |
| No. (%) with premature birth (<37 wk gestational age) | 15 (38) |
| Median (IQRd) birth weight, kg | 2.70 (2.33–3.08) |
| Mean (SD) hemoglobin, g/dl | 10.9 (1.6) |
| Mean (SD) albumin, g/liter | 42 (3) |
| No. (%) with gastric pH ≤3 (n = 11) | 11 (100) |
| Maternal HIV status | |
| No. (%) with HIV-infected mother | 22 (56) |
| Median maternal log viral load (n = 15) | 2.8 |
| No. (%) with maternal cART/PMTCTe (n = 21) | 15 (71) |
| Infant HIV status | |
| No. (%) HIV infected (n = 39) | 5 (13)f |
| Median (IQR) infant CD4 percentage | 35.4 (26.9–38.5) |
| Median (IQR) infant log viral load | 6.33 (6.14–6.7) |
| Median (IQR) cART in weeks before PK study | 8 (5–8) |
| Infant TB characteristics | |
| No. (%) with culture and/or Xpert MTBg/rifampin confirmed | 14 (36)h |
| Median (IQR) duration of TB treatment in weeks at PK | 7 (5–9) |
| No. (%) with BCGi vaccination history/scar | 32 (82) |
| No. (%) with positive tuberculin skin test (n = 16) | 7 (44) |
| No. (%) with documented TB source case (n = 28) | 28 (72) |
| No. (%) with mother as source case | 15 (54) |
| No. (%) with other household TB source case | 8 (29) |
| No. (%) with nonhousehold TB source case | 5 (17) |
| No. (%) with smear/culture and/or Xpert positive source case | 18 (64) |
| No. (%) with type of TBj | |
| PTB | 36 (92) |
| EPTB (n = 1 TBM) | 1 (3) |
| PTB and EPTB (n = 1 miliary TB; n = 1 TBM) | 2 (5) |
| Mean (range) routine antituberculosis drug dose, mg/kg | |
| Rifampin (n = 38) | 13.8 (9.0–19.7) |
| Isoniazid (n = 38) | 13.8 (9.0–19.7) |
| Pyrazinamide (n = 39) | 31.5 (18.7–44.6) |
| Ethambutol (n = 16) | 19.9 (13.3–29.0) |
| No. (%) with concomitant medicationsk | |
| ABC, 3TC, LPV/rl | 5 (12) |
| Prednisone | 1 (3) |
| Diuretics and spironolactone | 1 (3) |
Total number of infants evaluated was 39, unless otherwise noted in table.
WAZ, weight for age Z-score.
WLZ, weight for length Z-score.
IQR, interquartile range.
cART, combination antiretroviral therapy; PMTCT, prevention of mother-to-child transmission.
Three of these mothers received no PMTCT or cART before delivery.
Xpert: Gene Xpert for MTB/rifampin.
Culture and Xpert positive for 10 infants (n = 3, only culture; n = 1, only Gene Xpert).
BCG, bacillus Calmette-Guérin.
Types of TB: PTB, pulmonary TB; EPTB, extrapulmonary TB.
Nevirapine therapy was completed for HIV-exposed infants; steroid therapy, for severe airway compression; diuretics, for ventral septal defect.
ABC, abacavir; 3TC, lamivudine; LPV/r, lopinavir and ritonavir.
TABLE 2.
Summary statistics for infant pharmacokinetic parameters of rifampin, isoniazid, pyrazinamide, and ethambutol (n = 39a)
| First-line antituberculosis drug | Recommended dose (range) (mg/kg) | Mean (range) actual dose (mg/kg) | Mean (range) Cmax (μg) | Mean (IQRb) Tmax (h) | Mean (range) AUC0–8 (μg · h/ml) | Mean (range) t1/2 (h)c | Mean (range) CL/Fd (liter/h/kg) |
|---|---|---|---|---|---|---|---|
| Rifampin | 15 (10–20) | 15.37 (10.13–20.51) | 2.90 (0.59–7.96) | 2.0 (2.0–2.0) | 12.12 (1.78–33.01) | 2.05 (1.06–4.06) | 2.57 (0.37–16.68) |
| Formulation 1 (n = 14) | 12.91 (10.13–18.18) | 4.13 (0.65–7.96) | 2.0 (1.0–2.0) | 16.77 (1.59–33.01) | 2.07 (1.11–4.06) | 1.55 (0.37–7.07) | |
| Formulation 2 (n = 25) | 16.75 (10.13–20.51) | 2.22 (0.59–6.94) | 2.0 (2.0–2.0) | 9.52 (1.78–32.29) | 2.04 (1.06–3.93) | 3.14 (0.58–16.68) | |
| Isoniazid | 10 (10–15) | 12.80 (10.31–15.38) | 7.92 (3.97–11.30) | 1.0 (1.0–1.0) | 24.68 (11.56–50.18) | 2.00 (1.00–3.92) | 0.57 (0.26–1.00) |
| Pyrazinamide | 35 (30–40) | 33.32 (28.48–38.46) | 41.9 (26.3–68.4) | 1.0 (1.0–2.0) | 239.4 (147.1–450.0) | 8.01 (4.40–20.63) | 0.15 (0.08–0.20) |
| Ethambutol (n = 16) | 20 (15–25) | 20.18 (15.38–24.10) | 1.26 (0.24–2.01) | 2.0 (1.5–3.0) | 5.09 (1. 24–8.87) | 3.59 (1.61–6.94) | 4.69 (2.13–12.45) |
Total number of infants evaluated was 39 unless otherwise noted.
IQR, interquartile range.
t1/2, half-life of the drug. Due to late peaks, t1/2 was not calculated in the following infant samples: n = 4 rifampin (of n = 36); n = 1 isoniazid (of n = 38); n = 1 pyrazinamide (of n = 38); and n = 1 ethambutol (of n = 15).
CL, clearance; F, fraction absorbed.
Rifampin.
None of the 39 infants achieved the target adult peak RMP concentration of ≥8 μg/ml. Table 3 provides the RMP pharmacokinetic parameter comparisons for Cmax, AUC0–8, and t1/2 by clinical covariates; the comparison for RMP Cmax, AUC0–8, and t1/2 by RMP formulation and RMP dose are also displayed. On the pharmacokinetic sampling day, 14 of 39 (36%) infants received RMP formulation 1 (median dose, 12.1 mg/kg) and 25 of 39 (64%) infants received RMP formulation 2 (median dose, 18.6 mg/kg; difference between the median doses of RMP formulations was P = 0.025). Despite the lower dose range of RMP formulation 1, the RMP Cmax and AUC0–8 for RMP formulation 1 were higher than those of RMP formulation 2 (Cmax: 4.1 versus 2.2 μg/ml, P = 0.007; AUC0–8: 16.8 versus 9.5 μg · h/ml; P = 0.021). While this comparison was not adjusted for dose, it is worthwhile to note that the lower-dosed formulation resulted in higher Cmax and AUC0–8. When the analysis of formulation was adjusted for dose, the differences in Cmax and AUC0–8 remained, and the formulation effect was increased in the same direction (results not shown). In multivariable analysis stratified on formulation, RMP dose (10 to 15 versus 15 to 20 mg/kg) was not associated with either Cmax or AUC0–8 after adjusting for age and weight (Cmax for subset of RMP formulation 1: βdose = 1.33, 95% CI, −0.43 to 3.09, P = 0.132; Cmax for subset of RMP formulation 2: βdose = 2.02, 95% CI, −1.30 to 5.34, P = 0.206; AUC0–8 for subset of RMP formulation 1: βdose = 5.93, 95% CI, −2.17 to 14.03, P = 0.143; AUC0–8 for subset of RMP formulation 2: βdose = 9.81, 95% CI, −3.68 to 23.31, P = 0.136). Figure 1 illustrates RMP concentrations over time by formulation, including suggested adult target values (23, 24). In multivariable regression adjusting for the effects of age and weight, RMP formulation was associated with RMP peak concentrations and exposure. For Cmax, the GMR for the RMP formulation 1 to the RMP formulation 2 was 2.55 (95% CI, 1.47 to 4.41; P = 0.001); for AUC0–8, the GMR for the same comparison was 2.52 (95% CI, 1.34 to 4.73; P = 0.005).
TABLE 3.
Rifampin pharmacokinetic parameters by age, nutritional status, prematurity, HIV status, ethnicity, gender, formulation, and dose (n = 39)
| Variable | Parameter data for: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Cmax (μg/ml) |
AUC0–8 (μg · h/ml) |
t1/2 (h)a |
|||||||
| No. evaluated | Mean (SD) value | P | No. evaluated | Mean (SD) value | P | No. evaluated | Mean (SD) value | P | |
| Age at PK sampling, mo | |||||||||
| 0–6 | 20 | 2.89 (2.16) | 20 | 12.78 (9.72) | 17 | 2.17 (0.70) | |||
| 7–12 | 19 | 2.92 (2.24) | 0.975 | 19 | 11.43 (9.63) | 0.664 | 19 | 1.95 (0.88) | 0.425 |
| Nutritional status on day of PK | |||||||||
| ≥−2.0 | 21 | 2.65 (1.94) | 21 | 10.60 (8.20) | 20 | 2.02 (0.78) | |||
| <−2.0 | 18 | 3.21 (2.44) | 0.429 | 18 | 13.90 (10.93) | 0.289 | 16 | 2.08 (0.84) | 0.830 |
| Prematurity | |||||||||
| Term (≥38 wk) | 24 | 2.73 (1.90) | 24 | 11.46 (8.65) | 23 | 1.99 (0.84) | |||
| Premature (<37 wk) | 15 | 3.18 (2.60) | 0.538 | 15 | 13.18 (11.12) | 0.593 | 13 | 2.17 (0.73) | 0.520 |
| HIV status | |||||||||
| HIV infected | 5 | 3.67 (3.31) | 5 | 16.46 (15.00) | 5 | 2.27 (1.14) | |||
| HIV uninfected | 34 | 2.79 (2.00) | 0.404 | 34 | 11.48 (8.66) | 0.283 | 31 | 2.02 (0.75) | 0.522 |
| Ethnicity | |||||||||
| African | 29 | 3.00 (2.37) | 29 | 12.04 (10.20) | 28 | 1.88 (0.64) | |||
| Mixed race | 10 | 2.63 (1.52) | 0.644 | 10 | 12.37 (7.94) | 0.926 | 8 | 2.67 (1.04) | 0.011 |
| Gender | |||||||||
| Female | 13 | 3.27 (2.39) | 13 | 13.00 (10.01) | 13 | 1.75 (0.57) | |||
| Male | 26 | 2.72 (2.08) | 0.468 | 26 | 11.68 (9.52) | 0.692 | 23 | 2.22 (0.87) | 0.088 |
| Rifampin formulation | |||||||||
| Formulation 1 | 14 | 4.13 (2.35) | 14 | 16.77 (10.50) | 14 | 2.07 (0.84) | |||
| Formulation 2 | 25 | 2.22 (1.77) | 0.007 | 25 | 9.52 (8.11) | 0.021 | 22 | 2.04 (0.79) | 0.918 |
| Rifampin dose, mg/kg | |||||||||
| 10–15 | 17 | 2.74 (2.34) | 17 | 10.86 (10.01) | 17 | 1.91 (0.71) | |||
| 15–20 | 22 | 3.03 (2.09) | 0.691 | 22 | 13.10 (9.34) | 0.447 | 19 | 2.17 (0.87) | 0.340 |
t1/2, half-life of the drug.
FIG 1.
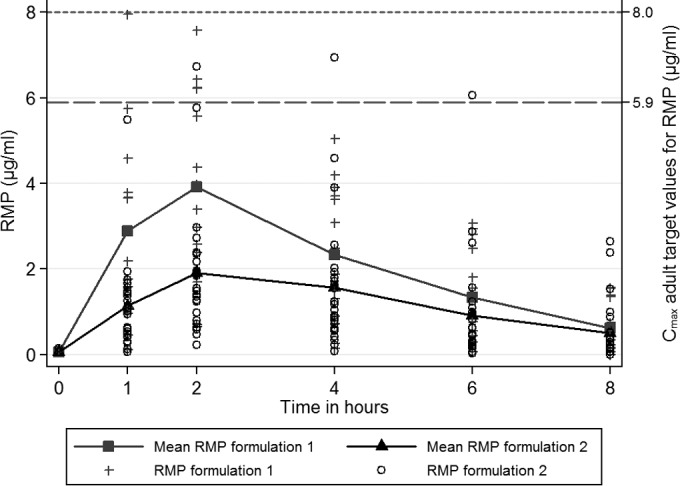
Mean plasma rifampin (RMP) concentrations (μg/ml) after the intake of a mean dose of 12.9 mg/kg for RMP formulation 1 (n = 14) and of a mean dose of 16.7 mg/kg for RMP formulation 2 (n = 25). Cmax adult target values, 8 to 24 μg/ml when RMP is administered at 600 mg daily in American adults (23); Cmax, 5.9 μg/ml when RMP is administered at 10.9 mg/kg in South African adults (24).
Isoniazid.
All 39 infants achieved the target adult peak INH concentration of ≥3 μg/ml. Table S1 in the supplemental material provides the INH pharmacokinetic parameter comparisons for Cmax, AUC0–8, and t1/2. Infants with a lower WAZ (≤ −2.0) had a shorter t1/2 (1.77 versus 2.2 h; P = 0.046). Figure 2 shows the INH concentration over time for infant participants, including reported adult target values (23, 24). No significant associations were found in log-transformed multivariable regression.
FIG 2.
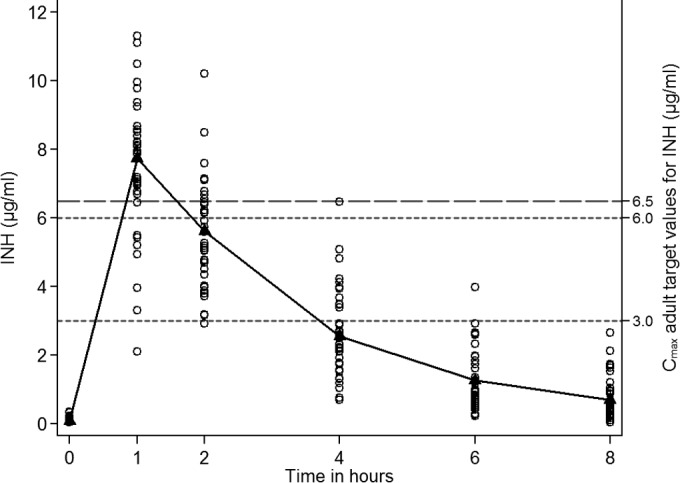
Mean plasma isoniazid (INH) concentrations (μg/ml) after the intake of a mean dose of 12.8 mg/kg of INH (n = 39). Adult target Cmax values, 3 to 6 μg/ml when INH is administered at 300 mg daily in American adults (23); Cmax, 6.5 μg/ml when INH is administered at 6.5 mg/kg in South African adults (24).
Pyrazinamide.
All 39 infants achieved adult peak PZA concentrations of ≥20 μg/ml, and 31 of 39 (80%) achieved a Cmax of ≥35 μg/ml. Table S2 in the supplemental material provides the PZA pharmacokinetic parameters comparisons for Cmax, AUC0–8, and t1/2. Younger infants had a longer t1/2 (8.92 versus 7.01 h; P = 0.047), while malnourished infants, versus those with normal nutrition, had a higher AUC0–8 (268.09 versus 214.72 μg · h/ml; P = 0.018). Figure 3 shows the PZA concentration over time, including reported adult target values (23, 24). Multivariable analyses from a log-transformed regression model for PZA showed that HIV-infected infants had a lower Cmax (GMR, 0.85; 95% CI, 0.75 to 0.96; P = 0.013) and a lower AUC0–8 (GMR, 0.79; 95% CI, 0.69 to 0.90; P = 0.001), controlling for age and weight. No other significant associations were observed.
FIG 3.
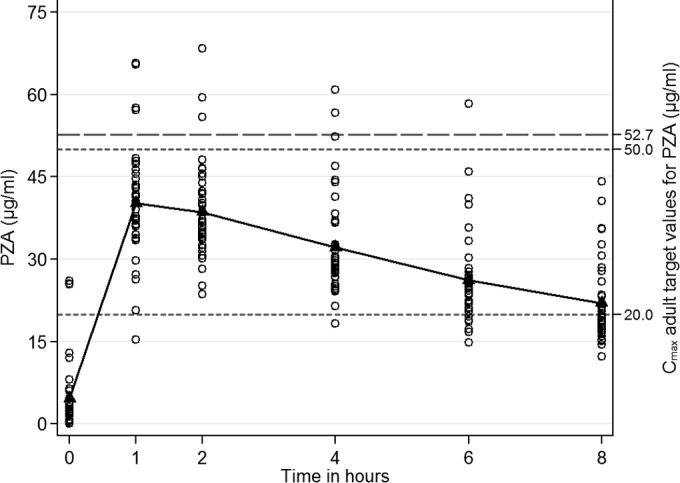
Mean plasma pyrazinamide (PZA) concentrations (μg/ml) after the intake of a mean dose of 33.3 mg/kg of PZA (n = 39). Adult target Cmax values, 20 to 50 μg/ml when PZA is administered at 25 mg/kg/day in American adults (23); Cmax, 52.7 μg/ml when INH is administered at 35.7 mg/kg in South African adults (24).
Ethambutol.
Only one of 16 infants (6%) had a Cmax >2 μg/ml, which is the recommended adult target value (Fig. 4). Table S3 in the supplemental material displays the EMB pharmacokinetic parameter comparisons for Cmax, AUC0–8, and t1/2. HIV-infected infants had a lower Cmax (0.4 versus 1.4 μg/ml; P = 0.004) and a lower AUC0–8 (2.0 versus 5.5 μg · h/ml; P = 0.008) than HIV-uninfected infants.
FIG 4.
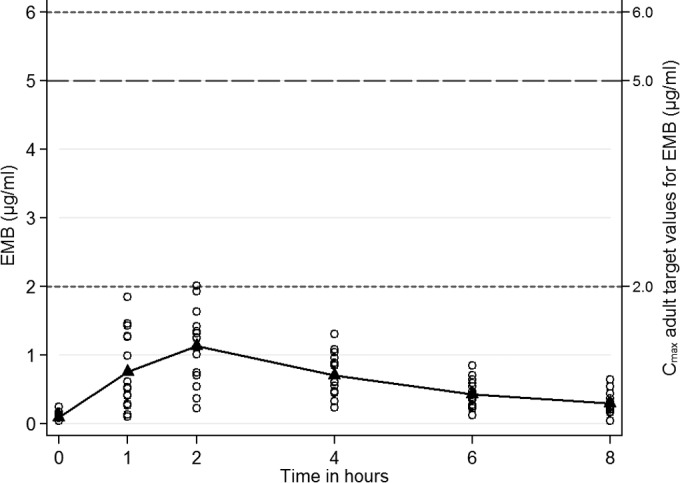
Mean plasma ethambutol (EMB) concentrations (μg/ml) after the intake of a mean dose of 20.2 mg/kg of EMB (n = 16). Adult target Cmax values, 2 to 6 μg/ml administered at 25 mg/kg/day in American adults (23); Cmax, 5 μg/ml when EMB is administered at 24.5 mg/kg in South African adults (24).
Safety.
The median ALT at the time of sampling was 21 U/liter (interquartile range [IQR], 15 to 35 U/liter; n = 37) and at treatment completion was 22 U/liter (IQR, 18 to 30 U/liter; n = 36). In total, 5 of 73 (7%) ALT values in 5 separate infants were elevated. Two grade 1 adverse events (elevated 2-fold) and one grade 3 event (elevated 7-fold) occurred on the pharmacokinetic visit; one grade 1 event and one grade 2 event (elevated 3-fold) occurred following 6 months of treatment. All resolved spontaneously at a follow-up measurement. These safety data are relevant only to the routine antituberculosis drugs, as study drugs were administered only on the PK sampling day.
TB treatment outcome.
Thirty-three of the infants (85%) had favorable TB treatment outcomes; cure occurred in 4, and treatment completion was documented in 29. Of six infants, all with poor social circumstances, initially classified as having unfavorable treatment outcomes, 4 infants (n = 1 HIV-infected and n = 3 HIV-exposed) initially classified as lost to follow-up were successfully reinitiated on TB treatment (n = 1 each at month 3, 4, 5, and 6) and completed 6 months of TB treatment. In the other 2 infants, the treatment outcome was unknown (both relocated to a different province after 3 months of treatment). Cmax and AUC0–8 for RMP, INH, PZA, and EMB were not associated with TB treatment outcome.
DISCUSSION
This is the first study reporting pharmacokinetic parameters for the four first-line antituberculosis drugs, using the 2010 revised WHO dosing guidelines, specifically in infants. Encouragingly, infants demonstrated INH and PZA concentrations similar to those reported in adult studies; EMB concentrations were low but comparable to other pediatric pharmacokinetic studies using the same dose. However, very low RMP exposures were observed for both RMP formulations studied. RMP formulation 2, which was dosed at a higher dosing range, had lower RMP Cmax and AUC0–8 than RMP formulation 1. The RMP dose was not associated with RMP Cmax and AUC0–8 when stratified by formulation; therefore, the formulation probably contributed more than the dose toward the observed difference in RMP pharmacokinetic parameters. PZA and EMB exposures were lower in the few HIV-infected infants studied. No other important associations were found for the key pharmacokinetic parameters of first-line antituberculosis drugs by age, weight, weight-for-age Z-score, prematurity, HIV status, ethnicity, gender, or TB treatment outcome. No infants developed symptomatic hepatotoxicity, and at least 85% of infants had a favorable TB treatment outcome.
A markedly low RMP mean Cmax of 2.9 μg/ml was observed in infants, even when dosed at 12 to 20 mg/kg/day. Desirable RMP 2-hour postdose concentrations of between 8 and 24 μg/ml have been suggested in adult healthy volunteers and in TB patients receiving a dose of 9 to 12 mg/kg (23); however, few adult and pediatric patients reach the often-recommended minimum RMP 2-hour concentration of 8 μg/ml (24, 25). In our study, RMP exposures were low using both RMP suspensions, and no infant achieved a 2-hour value of >8 μg/ml. Infants on RMP formulation 1 achieved significantly higher exposures than those on RMP formulation 2, despite the lower-mg/kg administered dose (Fig. 1). RMP dose itself was not associated with RMP Cmax, or AUC0–8; however, these findings and conclusions must be interpreted with caution. Further analysis showed that the formulation mainly contributed toward observed differences in pharmacokinetic parameters, emphasizing the importance of bioavailability of drug formulations and the use of high-quality formulations. High-performance liquid chromatography (HPLC) testing revealed similar RMP concentrations for both formulations, and, in a laboratory-based study, aliquots of the RMP formulation 1 used in the study that were left to stand for 30 min at room temperature in 3 nasogastric tubes had RMP concentrations similar to a control aliquot of the suspension (26). McIlleron et al. (26) also reported no impact of NGT drug delivery on the effect of RMP formulation on the AUC in the large DATiC (dosing antiretroviral- and tuberculosis drugs in children) study, where children were given an RMP suspension either by NGT or per os. The peak RMP concentrations of 4.0 and 2.2 μg/ml for the two different RMP formulations, respectively, were similar to those reported in other pediatric studies, documenting low peak concentrations of 2.8, 2.9, 3.5, 3.8, and 3.9 μg/ml following an oral administration of 10 to 20 mg/kg (27–31). Higher median RMP peak concentrations of 10 and 12 μg/ml were achieved when dosed at 10 and 15 mg/kg (P = 0.008), respectively, in Indian children (32). RMP mean peak concentrations of 6.4 and 11.7 μg/ml, when dosed at 10 and 15 mg/kg (P = 0.005), respectively, were observed in 11 South African children younger than 2 years of age using a different RMP formulation. The authors noted that children with high drug concentrations at the lower RMP dose did not necessarily have comparatively high drug concentrations following the higher dose (13). RMP formulations are known for considerable intra- and interindividual variability, which may partly be explained by differences in RMP bioavailability. In a study with 20 children, only 50% ± 22% of a freshly prepared oral suspension was absorbed compared to an intravenous dose (33). Although the cause for this variable RMP bioavailability remains unclear, the following factors have been proposed: raw material characteristics, changes in the crystalline habit of the RMP, excipients, manufacturing and/or process variables, degradation in the gastrointestinal tract, and inherent variability in absorption and metabolism (34). RMP is the only hydrophobic drug of the first-line antituberculosis drugs, is characterized by low solubility, is easily adsorbed by common pharmaceutical excipients, and also shows a pH-dependent solubility affecting its absorption (35). All of these factors highlight the need for stringent quality assurance of antituberculosis drugs in clinical care and research. A limitation of this study was that all of the study drugs were not purchased prior to the start of the study. The effect of gastric pH on RMP bioavailability remains unclear. A low gastric pH, as found in 11 study infants tested, was associated with low RMP exposures; Khalil et al. described reduced oral absorption of RMP when combined with antacids (36).There are limited data available on the ontogeny of the expression of p-glycoprotein, known to influence RMP absorption, with developmental differences in efflux transporter activity markedly altering the bioavailability of drugs (37). The specific relevance of p-glycoprotein in the dosing of young children with RMP requires further investigation in young children.
All infants had 2-hour plasma INH concentrations of >3 μg/ml following a mean dose of 12.8 mg/kg, which is above the suggested adult lower limit target range of 3 to 6 μg/ml (23). Infant INH concentrations also compared well with other pediatric pharmacokinetic studies with similar 2-hour concentrations reported of 4.5, 5.6, and 3.9 to 8.6, following 10 to 15 mg/kg (7, 27, 38). The effect of acetylator status on INH exposures is not reported here (future work).
PZA is probably the best absorbed among the first-line antituberculosis drugs, and infants achieved a high mean Cmax of 41.9 μg/ml at 1 h, following a dose of 33.3 mg/kg. A proposed PZA adult target range of 20 to 60 μg/ml has been suggested (23), but a study from Botswana showed that a Cmax of less than 35 μg/ml was associated with a poor outcome in adult pulmonary TB patients (39). Our results were similar to other pediatric studies reporting peak concentrations of 34.6 to 47.8 μg/ml when dosed at 30 to 40 mg/kg (8, 13, 28, 32).
The mean EMB Cmax of 1.3 μg/ml at 2 h following a mean dose of 20.2 mg/kg was low, with a proposed adult therapeutic 2-hour peak concentration of 2 μg/ml (23). Other pediatric studies reported similarly low concentrations of between 0.78 and 2.1 μg/ml when dosed at 10 to 20 mg/kg (27, 28, 32, 40, 41). Increasing EMB dosage in children has been reported to result in higher peak concentrations (42, 43); however, dose-dependent ocular toxicity is a limiting factor in dosing increases (44) especially in young children, in whom clinical evaluation is more challenging. In a recent study, 3 of 11 children <5 years of age developed reversible visual impairment secondary to 23, 25, and 27 mg/kg/day of EMB (45). Routine eye examinations are not common in resource-constrained settings; therefore, caution should be advised with increased EMB dosage. Ethionamide is sometimes preferentially given to infants due to clinical concerns of optic neuritis. EMB is the least efficacious of the first-line antituberculosis drugs and is used mainly to protect companion drugs against resistance. The clinical relevance of these findings in infants, who typically have paucibacillary TB and in whom the theoretical risk of acquision of drug resistance is low, needs further evaluation.
Age-dependent elimination of first-line antituberculosis drugs has been shown, with lower exposures documented in younger children for RMP (10, 14, 40), INH (14, 40, 46), PZA (8, 14), and EMB (42). These findings have mainly been attributed to the more rapid clearance of drugs and to a relatively larger liver-to-body size ratio. In our study, however, where all children were <1 year of age, age did not influence clearance. To our knowledge, this is the first study specifically performed in infants on antituberculosis drugs that also incorporated postconceptual age, and we found no remaining influence of prematurity. There is no clear evidence regarding the potential association between low TB drug exposures and concomitant cART (8, 13, 27, 47). In our study, we observed lower PZA and EMB exposures in HIV-infected infants; however, we studied only 5 HIV-coinfected infants. The higher PZA AUC0–8 observed in malnourished infants, compared to those with normal nutrition, was confounded by the fact that malnourished infants were dosed at the higher end of the dosing range.
Hepatotoxicity is the main adverse event associated with RMP, INH, and PZA. All infants were asymptomatic for symptoms and signs of hepatotoxicity, but ALT values were raised in 5 (7%) infants while on routine antituberculosis drugs. However, ALT values returned to normal within 3 months without intervention. Few pediatric studies have reported on hepatotoxicity at the revised WHO dosing recommendations. Hiruy et al. documented an ALT range of 9 to 71 U/liter in 31 children younger than 10 years at the revised higher doses (27). Two (3.1%) of 64 children developed hepatitis on the previously recommended lower doses, and 3 (4.8%) of 63 children did so on the revised higher doses in a study conducted in Indian children younger than 15 years of age (32).
The majority of infants had favorable TB treatment outcomes, despite the observed low RMP exposures for both formulations and the extensive disease in most. Higher RMP dosing strategies are currently being investigated in early bactericidal activity studies in adults; the short-term safety results have been reassuring. In adult RMP dose-escalation studies of up to 35 mg/kg/day for 2 weeks, higher doses showed a disproportionate plasma exposure increase with dose and a greater fall in bacterial load (48). Multiple factors influence the relationship between drug concentrations and TB treatment outcome, including M. tuberculosis genotype, gene polymorphisms, extent of TB disease, bacillary burden, and immune status, including HIV infection status (46). A limitation of our study is that the study drug was administered only on the pharmacokinetic day, while routine drugs were used for the 6-month treatment; this limits the interpretation of drug concentrations on TB treatment outcome.
NCA was used due to its timeliness and also for ease of interpretation to a broad clinical audience. Multivariable analysis was used to determine if pharmacokinetic parameters were associated with clinical covariates applicable to a clinical audience. In the absence of pediatric pharmacodynamic data, the optimal required target concentrations remain unclear and are typically extrapolated from adult studies. Furthermore, direct comparison of pharmacokinetic studies is complicated by variations in dose administered, type of formulation, single drugs versus fixed-dose combinations, daily versus intermittent therapy, and different sampling schedules, all of which influence plasma drug concentrations. In our study, INH, PZA, and EMB concentrations were comparable to other pediatric studies when dosed at the revised higher WHO recommendations, with INH and PZA reaching recommended adult target concentrations. However, RMP exposures were low regardless of the formulation used when administered at a dosing range of 10 to 20 mg/kg, highlighting the importance of using high-quality formulations and of bioavailability studies for RMP formulations in children and potentially arguing in favor of using dispersible tablets instead of liquid formulations in young children. In addition, the low RMP exposures argue for RMP dose-optimization studies in children. Reported TB treatment outcomes are generally good in children with drug-susceptible TB, independent of drug concentrations achieved, but this may be different for infants with immature immunity, for HIV-infected children, and in those with extensive and disseminated TB disease. Therefore, the low RMP exposures may become increasingly relevant in children who receive treatment-shortening regimens and in children with severe forms of TB; both populations are currently under evaluation.
Supplementary Material
ACKNOWLEDGMENTS
We thank all families for participation in the study, and we thank the Desmond Tutu TB Center and Brooklyn Chest Hospital pharmacokinetic unit personnel for their assistance.
We declare no conflicts of interest.
A.B., H.S.S., H.M.M., and A.C.H. conceptualized the research. S.M. and P.R.D. assisted with the design and analysis of study. A.B. and L.V.D.L. implemented the study in the field, L.W. assisted with the pharmacokinetic assays, and H.R.D. completed statistical analysis of the data. All authors contributed to the writing of the manuscript and approved the final manuscript.
Funding Statement
Laboratory research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases (NIAID) (U01 AI068632), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) (AI068632) (University of Cape Town pharmacology laboratory). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02600-15.
REFERENCES
- 1.Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Obihara CC, Starke JJ, Enarson DA, Donald PR, Beyers N. 2004. The natural history of childhood intrathoracic tuberculosis: a critical review of literature from the prechemotherapy era. Int J Tuberc Lung Dis 8:392–402. [PubMed] [Google Scholar]
- 2.Gupta A, Nayak U, Ram M, Bhosale R, Patil S, Basavraj A, Kakrani A, Philip S, Deasi D, Sastry J, Bollinger RC, Byramjee Jeejeebhoy Medical College-John Hopkins University Study Group. 2007. Postpartum tuberculosis incidence and mortality among HIV-infected women and their infants in Pune, India, 2002-2005. Clin Infect Dis 45:241–9. doi: 10.1086/518974. [DOI] [PubMed] [Google Scholar]
- 3.Schaaf HS, Marais BJ, Whitelaw A, Hesseling AC, Eley B, Hussey GD, Donald PR. 2007. Culture-confirmed childhood tuberculosis in Cape Town, South Africa: a review of 596 cases. BMC Infect Dis 7:140. doi: 10.1186/1471-2334-7-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. 2010. Rapid advice: treatment of tuberculosis in children. WHO/HTM/TB/2010.13. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 5.World Health Organization. 2006. Guidance for national tuberculosis programs on the management of tuberculosis in children. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 6.McIlleron H, Willemse M, Werely CJ, Hussey GD, Schaaf HS, Smith PJ, Donald PR. 2009. Isoniazid plasma concentrations in a cohort of South African children with tuberculosis: implications for international pediatric dosing guidelines. Clin Infect Dis 48:1547–1553. doi: 10.1086/598192. [DOI] [PubMed] [Google Scholar]
- 7.Schaaf HS, Parkin DP, Seifart HI, Werely CJ, Hesseling PB, van Helden PD, Maritz JS, Donald PR. 2005. Isoniazid pharmacokinetics in children treated for respiratory tuberculosis. Arch Dis Child 90:614–618. doi: 10.1136/adc.2004.052175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham SM, Bell DJ, Nyirongo S, Hartkoorn R, Ward SA, Molyneux EM. 2006. Low levels of pyrazinamide and ethambutol in children with tuberculosis and impact of age, nutritional status, and human immunodeficiency virus infection. Antimicrob Agents Chemother 50:407–413. doi: 10.1128/AAC.50.2.407-413.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thee S, Detjen A, Wahn U, Magdorf K. 2008. Pyrazinamide serum levels in childhood tuberculosis. Int J Tuberc Lung Dis 12:1099–1101. [PubMed] [Google Scholar]
- 10.Thee S, Detjen A, Wahn U, Magdorf K. 2009. Rifampicin serum levels in childhood tuberculosis. Int J Tuberc Lung Dis 13:1106–1111. [PubMed] [Google Scholar]
- 11.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. 2003. Developmental pharmacology: drug disposition, action, and therapy in infants and children. N Engl J Med 349:1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 12.Bartelink IH, Rademaker CM, Schobben AF, van den Anker JN. 2006. Guidelines on pediatric dosing on the basis of developmental physiology and pharmacokinetic considerations. Clin Pharmacokinet 45:1077–1097. doi: 10.2165/00003088-200645110-00003. [DOI] [PubMed] [Google Scholar]
- 13.Thee S, Seddon JA, Donald PR, Seifart HI, Werely CJ, Hesseling AC, Rosenkranz B, Roll S, Magdorf K, Schaaf HS. 2011. Pharmacokinetics of isoniazid, rifampin, and pyrazinamide in children younger than two years of age with tuberculosis: evidence for implementation of revised World Health Organization recommendations. Antimicrob Agents Chemother 55:5560–5567. doi: 10.1128/AAC.05429-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramachandran G, Hemanth Kumar AK, Bhavani PK, Poorana Gangadevi N, Sekar L, Vijayasekaran D, Banu Rekha VV, Ramesh Kumar S, Ravichandran N, Mathevan G, Swaminathan S. 2013. Age, nutritional status, and INH acetylator status affect pharmacokinetics of anti-tuberculosis drugs in children. Int J Tuberc Lung Dis 17:800–806. doi: 10.5588/ijtld.12.0628. [DOI] [PubMed] [Google Scholar]
- 15.Acocella G, Pagani V, Marchetti M, Baroni GC, Nicolis FB. 1971. Kinetic studies on rifampicin. I. Serum concentration analysis in subjects treated with different oral doses over a period of two weeks. Chemotherapy 16:356–370. [DOI] [PubMed] [Google Scholar]
- 16.Department of Health, South Africa. 2013. Guidelines for the management of tuberculosis in children. http://www.sahivsoc.org/upload/documents/National-Childhood-TB-Guidelines-2013.pdf.
- 17.Department of Health, South Africa. 2010. Clinical guidelines: PMTCT (prevention of mother-to-child transmission). http://www.sahivsoc.org/upload/documents/NDOH_PMTCT.pdf.
- 18.Peloquin CA, Namdar R, Dodge AA, Nix DE. 1999. Pharmacokinetics of isoniazid under fasting conditions, with food, and with antacids. Int J Tuberc Lung Dis 3:703–710. [PubMed] [Google Scholar]
- 19.Peloquin CA, Namdar R, Singleton MD, Nix DE. 1999. Pharmacokinetics of rifampin under fasting conditions, with food, and with antacids. Chest 115:12–18. doi: 10.1378/chest.115.1.12. [DOI] [PubMed] [Google Scholar]
- 20.Kwara A, Enimil A, Gillani FS, Yang H, Sarfo AM, Dompreh A, Ortsin A, Osei-Tutu L, Owusu SK, Wiesner L, Norman J, Kurpewski J, Peloquin CA, Ansong D, Antwi S. 2015. Pharmacokinetics of first-line antituberculosis drugs using WHO revised dosage in children with tuberculosis with and without HIV coinfection. J Ped Infect Dis Soc pii:piv035. doi: 10.1093/jpids/piv035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole TJ, Wright CM, Williams AF, RCPCH Growth Chart Expert Group. 2012. Designing the new UK-WHO growth charts to enhance assessment of growth around birth. Arch Dis Child Fetal Neonatal Ed 97:F219–F222. doi: 10.1136/adc.2010.205864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. 2014. Global tuberculosis report 2014. WHO/HTM/TB/2014.08. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 23.Peloquin CA. 2002. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs 62:2169–2183. doi: 10.2165/00003495-200262150-00001. [DOI] [PubMed] [Google Scholar]
- 24.McIlleron H, Wash P, Burger A, Norman J, Folb PI, Smith P. 2006. Determinants of rifampin, isoniazid, pyrazinamide, and ethambutol pharmacokinetics in a cohort of tuberculosis patients. Antimicrob Agents Chemother 50:1170–1177. doi: 10.1128/AAC.50.4.1170-1177.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donald PR, Maritz JS, Diacon AH. 2011. The pharmacokinetics and pharmacodynamics of rifampicin in adults and children in relation to the dosage recommended for children. Tuberculosis 91:196–207. doi: 10.1016/j.tube.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 26.McIlleron H, Hundt H, Smythe W, Bekker A, Winckler J, Van der Laan L, Smith P, Zar H, Hesseling AC, Maartens G, Wiesner L, van Rie A. Bioavailability of two licensed paediatric rifampicin suspensions: implications for quality control programmes. Int J Tuberc Lung Dis, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiruy H, Rogers Z, Mbowane C, Adamson J, Ngotho L, Karim F, Gumbo T, Bishai W, Jeena P. 2015. Subtherapeutic concentrations of first-line anti-TB drugs in South African children treated according to current guidelines: the PHATISA study. J Antimicrob Chemother 70:1115–1123. doi: 10.1093/jac/dku478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mlotha R, Waterhouse D, Dzinjalamala F, Ardrey A, Molyneux E, Davies GR, Ward S. 2015. Pharmacokinetics of anti-TB drugs in Malawian children: reconsidering the role of ethambutol. J Antimicrob Chemother 70:1798–1803. doi: 10.1093/jac/dkv039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acocella G, Buniva G, Flauto U, Nicolis FB. 1969. Absorption and elimination of the antibiotic rifampicin in newborns and children, p 755–760. Proc 6th Int Congr Chemother, vol 2 University of Tokyo Press, Tokyo, Japan. [Google Scholar]
- 30.Mahajan M, Rohatgi D, Talwar V, Patni SK, Mahajan P, Agarwal DS. 1997. Serum and cerebrospinal fluid concentrations of rifampicin at two dose levels in children with tuberculous meningitis. J Commun Dis 29:269–274. [PubMed] [Google Scholar]
- 31.Seth V, Beotra A, Seth SD, Semwal OP, Kabra S, Jain Y, Mukhopadhya S. 1993. Serum concentrations of rifampicin and isoniazid in tuberculosis. Indian Pediatr 30:1091–1098. [PubMed] [Google Scholar]
- 32.Mukherjee A, Velpandian T, Singla M, Kanhiya K, Kabra SK, Lodha R. 2015. Pharmacokinetics of isoniazid, rifampicin, pyrazinamide and ethambutol in Indian children. BMC Infect Dis 15:126. doi: 10.1186/s12879-015-0862-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koup JR, Williams-Warren J, Viswanathan CT, Weber A, Smith AL. 1986. Pharmacokinetics of rifampin in children. II. Oral bioavailability. Ther Drug Monit 8:17–22. [DOI] [PubMed] [Google Scholar]
- 34.Laing R, Fourie B, Ellard G, Sesay M, Spinaci S, Blomberg B, Bryant D. 1999. Fixed-dose combination tablets for the treatment of tuberculosis: report of an informal meeting held in Geneva, Tuesday, 27 April 1999. WHO/CDS/CPC/TB/99.267. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 35.Panchagnula R, Agrawal S. 2004. Biopharmaceutic and pharmacokinetic aspects of variable bioavailability of rifampicin. Int J Pharm 271:1–4. doi: 10.1016/j.ijpharm.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 36.Khalil SAH, El-Khordagui LK, El-Gholmy ZA. 1984. Effect of antacids on oral adsorption of rifampicin. Int J Pharm 20:99–106. doi: 10.1016/0378-5173(84)90221-7. [DOI] [Google Scholar]
- 37.Hall SD, Thummel KE, Watkins PB, Lown KS, Benet LZ, Paine MF, Mayo RR, Turgeon DK, Bailey DG, Fontana RJ, Wrighton SA. 1999. Molecular and physical mechanisms of first-pass extraction. Drug Metab Dispos 27:161–166. [PubMed] [Google Scholar]
- 38.Bekker A, Schaaf HS, Seifart HI, Draper HR, Werely CJ, Cotton MF, Hesseling AC. 2014. Pharmacokinetics of isoniazid in low-birth-weight and premature infants. Antimicrob Agents Chemother 58:2229–2234. doi: 10.1128/AAC.01532-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chideya S, Winston CA, Peloquin CA, Bradford WZ, Hopewell PC, Wells CD, Reingold AL, Kenyon TA, Moeti TL, Tappero JW. 2009. Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana. Clin Infect Dis 48:1685–1694. doi: 10.1086/599040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verhagen LM, Lopez D, Hermans PW, Warris A, de Groot R, Garcia JF, de Waard JH, Aarnoutse RE. 2012. Pharmacokinetics of antituberculosis drugs in Venezuelan children younger than 16 years of age: supportive evidence for the implementation of revised WHO dosing recommendations. Trop Med Int Health 17:1449–1456. doi: 10.1111/tmi.12003. [DOI] [PubMed] [Google Scholar]
- 41.Zhu M, Burman WJ, Starke JR, Stambaugh JJ, Steiner P, Bulpitt AE, Ashkin D, Auclair B, Berning SE, Jelliffe RW, Jaresko GS, Peloquin CA. 2004. Pharmacokinetics of ethambutol in children and adults with tuberculosis. Int J Tuberc Lung Dis 8:1360–1367. [PubMed] [Google Scholar]
- 42.Thee S, Detjen A, Quarcoo D, Wahn U, Magdorf K. 2007. Ethambutol in pediatric tuberculosis: aspects of ethambutol serum concentration, efficacy and toxicity in children. Int J Tuberc Lung Dis 11:965–971. [PubMed] [Google Scholar]
- 43.Hussels H, Kroening U, Magdorf K. 1973. Ethambutol and rifampicin serum levels in children: second report on the combined administration of ethambutol and rifampicin. Pneumonologie 149:31–38. doi: 10.1007/BF02179950. [DOI] [PubMed] [Google Scholar]
- 44.Donald PR, Maher D, Maritz JS, Qazi S. 2006. Ethambutol dosage for the treatment of children: literature review and recommendations. Int J Tuberc Lung Dis 10:1318–1330. [PubMed] [Google Scholar]
- 45.Levy M, Rigaudiere F, de Lauzanne A, Koehl B, Melki I, Lorrot M, Faye A. 2015. Ethambutol-related impaired visual function in children less than 5 years of age treated for a mycobacterial infection: diagnosis and evolution. Pediatr Infect Dis J 34:346–350. doi: 10.1097/INF.0000000000000589. [DOI] [PubMed] [Google Scholar]
- 46.Ramachandran G, Kumar AK, Bhavani PK, Kannan T, Kumar SR, Gangadevi NP, Banurekha VV, Sekar L, Ravichandran N, Mathevan G, Sanjeeva GN, Dayal R, Swaminathan S. 2015. Pharmacokinetics of first-line antituberculosis drugs in HIV-infected children with tuberculosis treated with intermittent regimens in India. Antimicrob Agents Chemother 59:1162–1167. doi: 10.1128/AAC.04338-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schaaf HS, Willemse M, Cilliers K, Labadarios D, Maritz JS, Hussey GD, McIlleron H, Smith P, Donald PR. 2009. Rifampin pharmacokinetics in children, with and without human immunodeficiency virus infection, hospitalized for the management of severe forms of tuberculosis. BMC Med 7:19. doi: 10.1186/1741-7015-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boeree MJ, Diacon AH, Dawson R, Narunsky K, du Bois J, Venter A, Phillips PP, Gillespie SH, McHugh TD, Hoelscher M, Heinrich N, Rehal S, van Soolingen D, van Ingen J, Magis-Escurra C, Burger D, Plemper van Balen G, Aarnoutse RE, PanACEA Consortium . 2015. A dose-ranging trial to optimize the dose of rifampin in the treatment of tuberculosis. Am J Respir Crit Care Med 191:1058–1065. doi: 10.1164/rccm.201407-1264OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


