Abstract
Arylimidamides (AIAs) have been shown to have considerable biological activity against intracellular pathogens, including Trypanosoma cruzi, which causes Chagas disease. In the present study, the activities of 12 novel bis-AIAs and 2 mono-AIAs against different strains of T. cruzi in vitro and in vivo were analyzed. The most active was m-terphenyl bis-AIA (35DAP073), which had a 50% effective concentration (EC50) of 0.5 μM for trypomastigotes (Y strain), which made it 26-fold more effective than benznidazole (Bz; 13 μM). It was also active against the Colombiana strain (EC50 = 3.8 μM). Analysis of the activity against intracellular forms of the Tulahuen strain showed that this bis-AIA (EC50 = 0.04 μM) was about 100-fold more active than Bz (2 μM). The trypanocidal effect was dissociated from the ability to trigger intracellular lipid bodies within host cells, detected by oil red labeling. Both an active compound (35DAP073) and an inactive compound (26SMB060) displayed similar activation profiles. Due to their high selectivity indexes, two AIAs (35DAP073 and 35DAP081) were moved to in vivo studies, but because of the results of acute toxicity assays, 35DAP081 was excluded from the subsequent tests. The findings obtained with 35DAP073 treatment of infections caused by the Y strain revealed that 2 days of therapy induced a dose-dependent action, leading to 96 to 46% reductions in the level of parasitemia. However, the administration of 10 daily doses in animals infected with the Colombiana strain resulted in toxicity, preventing longer periods of treatment. The activity of the combination of 0.5 mg/kg of body weight/day 35DAP073 with 100 mg/kg/day Bz for 10 consecutive days was then assayed. Treatment with the combination resulted in the suppression of parasitemia, the elimination of neurological toxic effects, and survival of 100% of the animals. Quantitative PCR showed a considerable reduction in the parasite load (60%) compared to that achieved with Bz or the amidine alone. Our results support further investigations of this class with the aim of developing novel alternatives for the treatment of Chagas disease.
INTRODUCTION
Chagas disease (CD) is caused by the obligate intracellular protozoan Trypanosoma cruzi. More than a hundred years after its discovery (1), CD is still an important public health problem, and about 6 million to 7 million people, mostly in Latin America, are estimated to be infected worldwide (2). Of these infected individuals, 30 to 40% develop cardiomyopathy and/or digestive syndromes (3). For over 4 decades, nifurtimox [3-methyl-4-(5′-nitrofurfurylideneamine tetrahydro-4H-1,4-tiazine-1,1-dioxide)] and benznidazole (Bz; N-benzyl-2-nitroimidazole acetamide), which have been used as empirical therapy for CD, have remained the sole treatment options (4). Both have several limitations, including a variety of adverse effects and limited efficacy in the later chronic phase of infection (5, 6), characteristics which strengthen the need for new trypanocidal compounds that overcome these limitations.
Classic aromatic diamidines (ADs) are DNA minor groove binders with recognized broad-spectrum antimicrobial and antitumor activities (7). ADs, such as pentamidine, diminazene, and propamidine, have been used as therapies in human and veterinary medicine for decades, but despite their excellent antiparasitic effects, they have relevant drawbacks that include low bioavailability and side effects. To overcome these limitations, new analogues have been synthesized and tested in vivo and in vitro (7). Several ADs and related compounds have been screened for their activities against T. cruzi and have shown promising results, at least in part due to their capacity to alter the kinetoplast DNA molecule (8, 9). Arylimidamides (AIAs; formerly called “reversed” amidines, because of the reversed position of the nitrogen and carbon atoms compared to their orientation in classic aromatic amidines) are the most effective amidine analogues tested against T. cruzi in vitro and in vivo (9–12).
Lipid bodies (LBs) consist of a nucleus of cholesteryl esters and triglycerides surrounded by a single monolayer of phospholipids. LBs are considered not only lipid storage compartments but also intracellular sites for many biological events, like cellular signaling and activation, regulation of lipid metabolism, membrane trafficking, and regulation of inflammatory mediators (13, 14). Recent studies proposed a dynamic role of LBs in the control of pathogen infections due to their localization inside the parasitophorous vacuoles in intracellular parasites like T. cruzi (15).
Our present study focused on an analysis of the activities of 12 novel bis-AIAs and 2 mono-AIAs against bloodstream trypomastigotes (BTs) and intracellular forms of T. cruzi (the Tulahuen, Y, and Colombiana strains) in vitro. The most potent and most selective compound tested, 35DAP073, underwent further screening in experimental mouse models of acute parasite infection (with strains Y and Colombiana) alone or combined with the reference drug (Bz) with the aim of contributing to the search for novel alternative protocols for the treatment of CD.
MATERIALS AND METHODS
Synthesis of the arylimidamides.
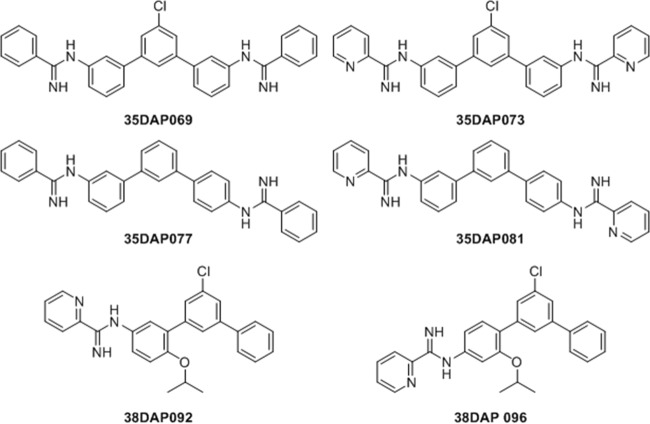
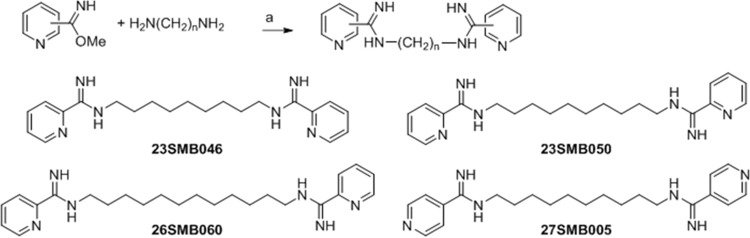
The synthesis of the four m-terphenyl bisarylimidamides 35DAP069, 35DAP073, 35DAP077, and 35DAP081 and the two monoarylimidamides 35DAP092 and 38DAP096 (Fig. 1) has been described previously (16), wherein the final step was the reaction of the appropriate terphenyl amine or diamine with benzonitrile or 2-cyanopyridine using sodium bis(trimethylsilyl)amide in tetrahydrofuran. Similar reactions involving 1,4-diphenylenediamine and 2,5- or 2,6-diaminopyridine were employed for the synthesis of 19SAB003, 19SAB005, 19SAB007, and 28SMB008 (17) (Fig. 2). Compounds 23SMB046, 23SMB050, 26SMB060, and 27SMB005 (Fig. 3) were prepared by a previously described methodology from the methyl imidate derivatives of 2- or 4-cyanopyridine and the appropriate α,ω-diaminoalkane (18). All 14 compounds were isolated as their hydrochloride salts. Experimental details and physical data for the compounds shown in Fig. 2 and 3 are given in Text S1 in the supplemental material.
FIG 1.
Structures of mono- and bisarylimidamide derivatives of m-terphenyl.
FIG 2.
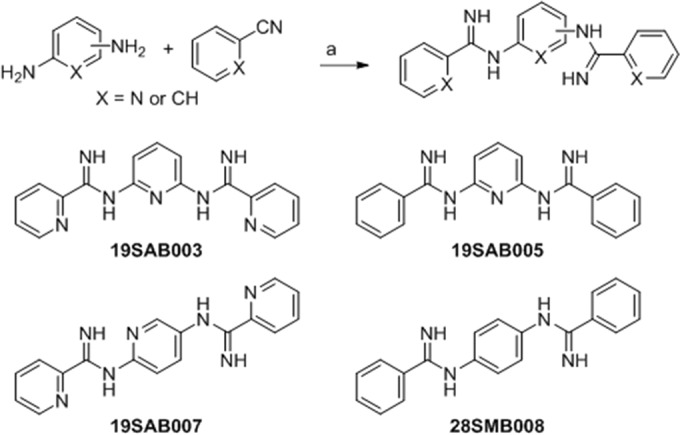
Synthesis of bisarylimidamide derivatives of benzene and pyridine. Reagents and conditions were as follows: a, sodium bis(trimethylsilyl)amide; tetrahydrofuran; and 25°C.
FIG 3.
Synthesis of bisarylamidamide derivatives of n-alkanes. Reagents and conditions were as follows: a, 4 M HCl in dioxane; methanol; and 25°C.
Stock solutions of the tested compounds.
The studied molecules were prepared in dimethyl sulfoxide (DMSO; Sigma-Aldrich), with the final concentration of the solvent (obtained by dilution using RPMI 1640 medium) never exceeding 0.6% and 10% for the in vitro and in vivo analyses, respectively. The solvent did not exert toxicity on parasites or mammalian host cells (data not shown). Bz was purchased from the Laboratório Farmacêutico do Estado de Pernambuco (LAFEPE), Brazil. Bz was dissolved in distilled and sterile water supplemented with 3% Tween 80 and does not have any detectable effect on mice (19).
Mammalian cell cultures.
Primary cultures of embryonic cardiac cells (CCs) were obtained from Swiss Webster mice as previously reported (20). After purification, the CCs were seeded into 24- and 96-well microplates containing gelatin-coated coverslips at densities of 0.2 × 106 and 0.05 × 106 cells/well, respectively, as reported previously (18). The cardiac cell cultures were then sustained at 37°C in Dulbecco's modified Eagle medium (DMEM; without phenol red; Sigma-Aldrich) supplemented with 10% horse serum, 5% fetal bovine serum, 2.5 mM CaCl2, 1 mM l-glutamine, and 2% chicken embryo extract. Additionally, mouse L929 fibroblasts were cultivated (4 × 103 cells/well in 96-well microplates) at 37°C in RPMI 1640 medium (pH 7.2 to 7.4) without phenol red (Gibco BRL) supplemented with 10% fetal bovine serum and 2 mM glutamine (RPMI), as reported previously (21).
In vitro cytotoxicity tests.
CCs and L929 cell cultures were incubated at 37°C for different periods of time (24 to 96 h) with increasing concentrations of each compound (up to 96 μM) diluted in DMEM (without phenol red). Next, mammalian cell morphology and spontaneous contractibility (of CCs) were evaluated by light microscopy, and cell viability was determined by a colorimetric assay using 10 μl alamarBlue (Invitrogen), which was added to each well. After incubation for 24 h, the absorbance at 570 and 600 nm was determined and the results were determined following the manufacturer's instructions. Then, the concentration that reduced cell viability by 50% (LC50) was calculated as reported previously (21).
Parasites.
Bloodstream trypomastigote (BT) forms of the Y and Colombiana strains of T. cruzi were obtained from the blood of infected male Swiss Webster mice at the peak of parasitemia (12). Immediately after the purification step, the parasites were resuspended in RPMI 1640 medium (pH 7.2 to 7.4) without phenol red (Gibco BRL) supplemented with 10% fetal bovine serum (FBS) and 2 mM glutamine, as reported previously (18). The effect against the intracellular forms was investigated through the use of L929 cell lineages infected with tissue culture-derived trypomastigotes (the Tulahuen strain expressing the Escherichia coli β-galactosidase gene), using a 10:1 parasite/host cell ratio. The incubation with the tested compounds was performed for 96 h, and previously established protocols were followed (21). Alternatively, CCs were infected (ratio, 10:1) with bloodstream trypomastigotes (Y strain). After 24 h of interaction, the infected cultures were rinsed and then exposed for 48 h to nontoxic concentrations of the amidines, which had previously been screened on mammalian host cells.
Trypanocidal analysis.
The BT forms of the Y and Colombiana strains (5 × 106 per ml) were incubated for up to 24 h at 37°C in RPMI in the presence of serial dilutions of the compounds (0 to 32 μM), and parasites incubated with culture medium alone were used as controls. After incubation with the compound, the parasite death rates were determined by light microscopy through the direct quantification of the number of live parasites using a Neubauer chamber, and the compound concentration that reduced the number of parasites by 50% (the 50% effective concentration [EC50]) was calculated. Also, the compound concentration that reduced the number of parasites by 90% (EC90) was further calculated in intracellular assays using the Y strain (18). For the assay with intracellular forms, cardiac cell cultures and cells of the L929 cell line were used as hosts for infection with the Y and Tulahuen strains, respectively. Briefly, Tulahuen-infected L929 cell cultures were exposed to 10 μM (corresponding to the EC90 value of Bz, as reported elsewhere [18]) each compound diluted in RPMI, and the compounds that resulted in a ≥50% reduction of the parasite infection index were further screened at increased concentrations to determine the EC50s (18). After 96 h of incubation with the compound at 37°C, chlorophenol red glycoside (500 μM; Sigma-Aldrich) in 0.5% Nonidet P-40 was added to each well, and the plate was incubated for 18 h at 37°C. Next, the absorbance at 570 nm was measured. Uninfected and T. cruzi-infected cultures exposed to the vehicle and Bz were run in parallel. The results are expressed as the percentage of T. cruzi growth inhibition in compound-tested host cells compared to that in the infected but untreated cells (21). Triplicate samples were run in the same plate, and at least two assays were performed in each analysis. For the analysis of the effects of the test compounds against intracellular amastigotes of the Y strain, after 24 h of parasite-host cell interaction, the infected CCs were washed to remove free parasites and then incubated for another 48 h with increasing concentrations of the test compounds. CCs were maintained at 37°C in an atmosphere of 5% CO2 and air, and the medium was replaced every 24 h. Then, the samples were fixed and stained with Giemsa solution (Sigma-Aldrich), and the number of infected host cells and the number of parasites per infected host cell were determined by light microscopy analysis (18). Only characteristic T. cruzi cells with nuclei and kinetoplasts were counted as surviving parasites, since irregular structures could indicate parasites undergoing death. Compound activity was estimated by calculating the percentage of inhibition upon the infection index (the percentage of infected host cells multiplied by the average number of intracellular amastigotes per infected host cell) (12). At least two assays were performed in duplicate in each analysis.
LB labeling.
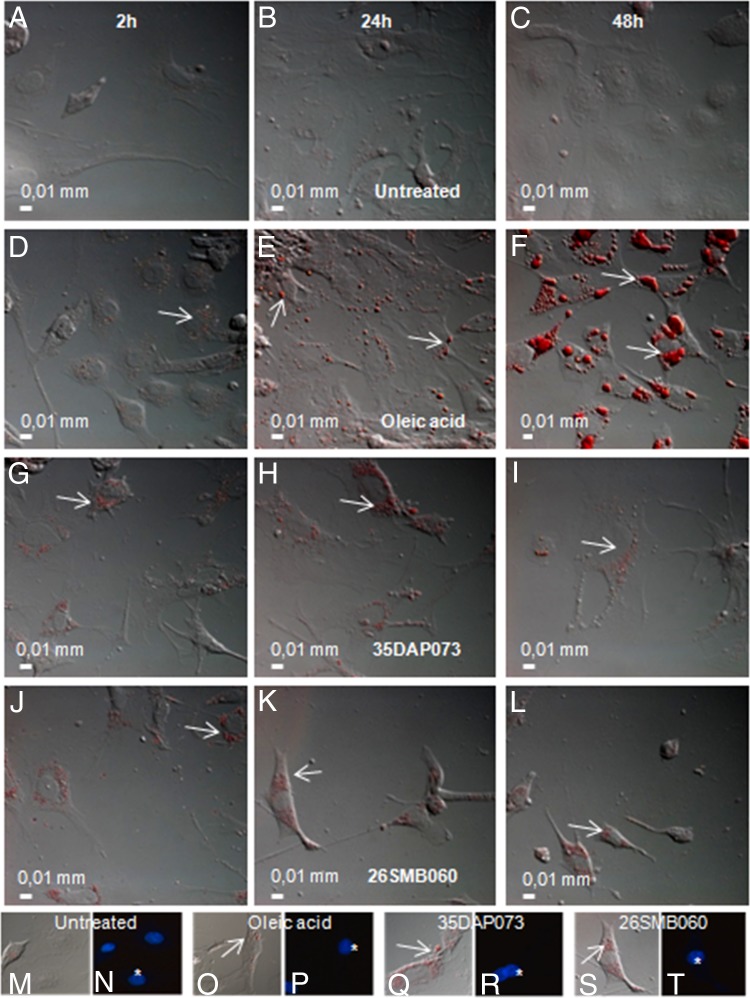
To determine the biogenesis of lipid bodies (LBs) in cardiac cell cultures exposed to amidines, untreated CCs were incubated for 2 to 48 h with the studied compounds at the corresponding EC50s (against intracellular forms of the Y strain determined earlier) and then fixed for 10 min with 3.7% formaldehyde in Ca2+- and Mg2+-free Hanks balanced salt solution (pH 7.4). Next, the samples were rinsed twice using distilled water, incubated for 5 min in absolute propylene glycol, and stained for 10 min in 0.5% oil red (Sigma-Aldrich). The cultures were incubated for 3 min with 85% propylene glycol and, finally, rinsed twice using distilled water. The samples were incubated or not with 1 μg/ml 4,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) for DNA staining (host cells nuclei), rinsed with saline buffer, dried, and mounted using aqueous mounting medium [2.5% 1.4-diazabicyclo-(2.2.2)octane (DABCO)], and the fluorescence was analyzed with a ×63 oil objective in a Zeiss photomicroscope (AxioCam) equipped with epifluorescence (Zeiss Inc., Thornwood, NY) and by using a filter set for UV-excited probes. Images were captured using the software AnalySIS OPTI. As positive control, 2 μM oleic acid (Sigma-Aldrich) was used to trigger the biogenesis of the lipid bodies (22).
In vivo acute toxicity.
In order to determine the no-observed-adverse-effect level (NOAEL), increasing doses of the tested compounds (up to 200 mg/kg of body weight) were injected by the intraperitoneal (i.p.) route individually in female Swiss Webster mice (weight, 20 to 23 g; n = 2 mice per assay for two assays). Treated animals were inspected for toxic and subtoxic symptoms according to the Organization for Economic Cooperation and Development (OECD) guidelines. At 48 h after compound injection, the NOAEL values were determined as reported previously (23).
Biochemical analysis.
At 48 h after compound administration, mouse blood was collected and immediately submitted to biochemical analysis for determination of the levels of plasma markers, including urea (blood urea nitrogen [BUN]), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and creatine kinase (CK), which was performed at the animal facilities (CECAL/Fiocruz platform) of the Oswaldo Cruz Foundation (Rio de Janeiro, Brazil) using an Ortho Clinical Vitros 250 chemistry system (Johnson & Johnson), as reported previously (23).
In vivo infection.
Male Swiss Webster mice (weight, 18 to 20 g) obtained from the animal facilities of CECAL were housed at a maximum of 6 per cage, kept in a specific-pathogen-free (SPF) room at 20 to 24°C under a 12- light and 12-h dark cycle, and provided sterilized water and chow ad libitum. The animals were allowed to acclimate for 7 days before starting the experiments. Infection was performed by i.p. injection of 104 and 5 × 103 bloodstream trypomastigotes (the Y and Colombiana strains, respectively). Age-matched noninfected mice were maintained under identical conditions (12).
Treatment schemes.
AIAs were first dissolved in DMSO and then freshly diluted with sterile distilled water. The stock solution of benznidazole (N-benzyl-2-nitroimidazole acetamide) was prepared in sterile distilled water with 3% Tween 80 (Sigma-Aldrich). The animals were divided into the following groups (5 animals per group): uninfected (noninfected and nontreated), untreated (infected but treated only with vehicle), and treated (infected and treated with the compounds) groups. The therapy (once a day) was performed by the use of different schemes (see Text S2 in the supplemental material). In the first set of assays, T. cruzi (Y strain)-infected mice were treated for only 2 days (at 5 days postinfection [dpi] and at 8 dpi, which correspond to the time of parasitemia onset and the time of peak parasitemia in this experimental model, respectively), using 5 to 20 mg/kg/day AIA (administered i.p.) and 100 mg/kg/day Bz (administered orally). In the second set of experiments, mice were infected with the Colombiana strain and the therapy started at 10 dpi (the time of parasitemia onset in this animal model) were treated for 10 consecutive days with 35DAP073 alone (5 mg/kg/day) or in combination with Bz (0.5 mg/kg/day AIA plus 100 mg/kg/day of Bz). In all assays, only mice with positive parasitemia were used in the infected groups.
Parasitemia and mortality rates.
Parasitemia was individually checked by direct microscopic counting of the number of parasites in 5 μl of blood, and the mice were checked for mortality daily until 30 days posttreatment. Mortality is expressed as the percent cumulative mortality (CM) as described before (18).
Determination of blood parasite load by qPCR.
For quantitative real-time PCR (qPCR), 500 μl blood was diluted in a 1:2 volume of guanidine solution (6 M guanidine-HCl, 0.2 M EDTA; Sigma-Aldrich) and heated for 90 s in boiling water. Blood samples in guanidine-EDTA were processed using a QIAamp DNA minikit (Qiagen) (24). Quantitative real-time multiplex PCR assays using TaqMan probes targeting T. cruzi satellite nuclear DNA and the internal amplification control (IAC; plasmid pZErO-2 containing an insert from the Arabidopsis thaliana aquaporin gene) were performed as described previously (25). The standard curves for absolute quantification were constructed with serial dilutions of total DNA ranging from 105 parasite equivalents to 0.5 parasite equivalents per ml of blood obtained with a negative blood sample in guanidine-EDTA spiked with 105 parasite equivalents per milliliter of blood.
Ethics.
All procedures were carried out in accordance with the guidelines established by the FIOCRUZ Committee of Ethics for the Use of Animals (CEUA LW16/14).
Statistical analysis.
The data represent the means ± standard deviations (SDs) from 2 experiments run in duplicate, and statistical analysis was performed by analysis of variance (ANOVA), with significance being set at a P value of ≤0.05 (23).
RESULTS
The phenotypic analysis of BT forms (Y strain, discrete typing unit [DTU] II) showed that all studied compounds were active against the parasite. The response to compounds 35DAP073, 35DAP081, 35DAP077, and 35DAP092 was time dependent, with the compounds exhibiting EC50s ranging from 0.7 to 28 μM and 0.5 to 10 μM after 2 and 24 h of incubation, respectively, while the EC50 of Bz was 13 μM after 24 h (Table 1). Among these four compounds, the most active were 35DAP073 and 35DAP081, and both also displayed the highest selectivity indexes (SIs; 64 and 53, respectively). Statistical analysis revealed that 35DAP073, 35DAP081, and 35DAP092 presented efficacy superior to that of Bz (P < 0.05). When BT forms of the Colombiana strain (DTU I) were assayed, both amidines showed a trypanocidal effect, with the EC50s of 35DAP073 and 35DAP081 being 3.8 ± 2.8 and 1.9 ± 0.4 μM, respectively. 35DAP073 and 35DAP081 were very active against intracellular forms of both the Tulahuen (Table 2) and Y (Table 3) strains of T. cruzi, which are representatives of DTUs VI and II, respectively, and were more potent than Bz (P ≤ 0.05). The m-terphenyl bis-AIA 35DAP073 showed higher activity, indicated by the lower EC90 values (87 nM) after 48 h of incubation, and was about 126-fold more effective than Bz, and it also exhibited the highest SI (SI, 2,000) (Table 3).
TABLE 1.
In vitro activities (EC50s) and SIs of the tested compounds against BT forms of Trypanosoma cruzi Y
| Compound | EC50 (μM) |
SI at 24 h | |
|---|---|---|---|
| 2 h | 24 h | ||
| 35DAP096 | >32 | >32 | >1 |
| 35DAP073 | 0.7 ± 0.14 | 0.5 ± 0.2a | 64 |
| 35DAP077 | 28 ± 0.3 | 10 ± 5.5 | 2 |
| 35DAP081 | 2 ± 0.8 | 0.6 ± 0.2a | 53 |
| 38DAP092 | 16 ± 11 | 7 ± 1.2a | 5 |
| 38DAP096 | >32 | 24 ± 1 | 1.3 |
| 19SAB003 | >32 | >32 | >1 |
| 19SAB005 | >32 | >32 | >1 |
| 19SAB007 | >32 | >32 | >1 |
| 28SMB008 | >32 | >32 | >1 |
| 23SMB046 | >32 | >32 | >1 |
| 23SMB050 | >32 | >32 | >1 |
| 26SMB060 | >32 | >32 | >1 |
| 27SMB005 | >32 | >32 | >1 |
| Benznidazole | >100 | 13 ± 2 | 77b |
P < 0.05 by ANOVA.
The datum is from Timm et al. (18).
TABLE 2.
In vitro activities (EC50s) and SIs of the tested compounds against intracellular forms of Trypanosoma cruzia
The Trypanosoma cruzi Tulahuen strain expressing the Escherichia coli β-galactosidase gene was used to infect L929 cells, which were treated for 96 h at 37°C.
P < 0.05 by ANOVA.
TABLE 3.
In vitro activities (EC50s) and SIs of the tested compounds against intracellular forms of Trypanosoma cruzi Y in CCs at 48 h after incubation at 37°C
| Compound | EC50 (μM) | EC90 (μM) | SIa |
|---|---|---|---|
| 35DAP073 | 0.016 ± 0.007b | 0.087 ± 0.009b | 2,000 |
| 35DAP081 | 0.23 ± 0.06b | 0.9 ± 0.05b | 139 |
| Benznidazole | 3.6 ± 1.7 | 11 ± 2.7 | 277 |
The SI data are related to the EC50 values.
P < 0.05 by ANOVA.
As 35DAP073 was more effective against intracellular forms than BT forms (Y strain; Tables 1 and 3) and the biogenesis of LBs may be induced by different stimuli (26), the induction of LB biogenesis during incubation of uninfected CCs with this amidine was compared with that during incubation of uninfected CCs with another molecule not active against T. cruzi in vitro (26SMB060). After incubation for different lengths of time (2 to 48 h), our data revealed that both amidines were able to induce LB biogenesis at similar levels very quickly, since strong LB formation compared to that in the untreated group was seen as soon as only 2 h posttreatment, and the profile was similar to that obtained with the positive control (oleic acid) (Fig. 4). A large number of LBs was also observed after 24 h of amidine exposure, and the levels were maintained up to 48 h, although oleic acid caused the progressive and continuous formation of LB (increases in the number and volume) (Fig. 4).
FIG 4.
Differential interference contrast (DIC) microscopy analysis of oil red labeling of cardiac cells untreated (A to C) and exposed to oleic acid (D to F), 35DAP073 (G to I), and 26SMB060 (J to L) for 2 h (A, D, G, J), 24 h (B, E, H, K), and 48 h (C, F, I, L) at 37°C. (M to T) Detailed oil red labeling of cardiac cells by differential interference contrast (M, O, Q, and S) and fluorescence microscopy using DAPI staining of mammalian cell nuclei (N, P, R, and T) after 24 h of incubation with 35DAP073 (Q and R) and 35DAP060 (S and T), showing the accumulation of lipid bodies randomly distributed throughout the cytoplasm.
Next, due to the high activity and selectivity of 35DAP073 and 35DAP081, Swiss Webster mice were used to determine the NOAEL values of the two compounds. 35DAP073 proved to be less toxic at all doses tested, presenting an NOAEL of 25 mg/kg, while 35DAP081 showed an NOAEL of <12.5 mg/kg (Table 4). Plasma biochemical analysis (P ≤ 0.1) after 48 h of compound administration did not show any statistically significant differences in the levels of ALT, AST, CK, and BUN among all tested groups (Table 5). Due to the findings of these preliminary acute toxicity studies, only 35DAP073 was evaluated in in vivo models of acute T. cruzi infection using nontoxic doses (up to 25 mg/kg/day). In parallel, a control group was orally treated with Bz (100 mg/kg/day) (Fig. 5).
TABLE 4.
Results of acute toxicity analysisa
| Compound | Result after treatment at the following dose (mg/kg): |
NOAEL (mg/kg) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 12.5 | 25 | 50 | 100 | 200 | ||
| 35DAP073 | NDE | NDE | NDE | Loss of mouse body wt | Tremors, ataxia | Death | 25 |
| 35DAP081 | NDE | Ataxia, tremors, excitation and vocalizationb | Ataxia, tremors, excitation and vocalizationb | Ataxia, tremors, excitation and vocalizationb | Ataxia, tremors, excitation and vocalizationb | Ataxia, tremors, excitation and vocalizationb | <12. 5 |
Escalating doses starting at 12.5 mg/kg and increasing to 200 mg/kg were administered i.p. in a 0.1-ml final volume to female Swiss Webster mice, using a single mouse per dose. NOAEL, no-observed-adverse-effect level (for noninvasive parameters); NDE, no detectable effect.
These effects were observed after 2 h of compound administration.
TABLE 5.
Plasma biochemical analysis of female mice after 48 h of compound administration
| Biochemical marker | Compound | Result after treatment with the following dose (mg/kg)a: |
|||||
|---|---|---|---|---|---|---|---|
| 0b | 12.5 | 25 | 50 | 100 | 200 | ||
| ALT | 35DAP073 | 57 ± 8 | 58 ± 0 | 51 ± 0 | 88 ± 51 | 77 ± 41 | ND |
| 35DAP081 | 56 ± 0.7 | 67 ± 13 | 82 ± 8 | 102 ± 23 | 116 ± 11 | ||
| CK | 35DAP073 | 935 ± 226 | 635 ± 80 | 368 ± 0 | 701 ± 260 | 870 ± 646 | ND |
| 35DAP081 | 503 ± 145 | 509 ± 145 | 737 ± 0 | 1,681 ± 310 | 1,162 ± 452 | ||
| AST | 35DAP073 | 183 ± 16 | 109 ± 0 | 113 ± 0 | 152 ± 37 | 242 ± 105 | ND |
| 35DAP081 | 105 ± 16 | 143 ± 21 | 285 ± 55 | 422 ± 132 | 376 ± 229 | ||
| BUN | 35DAP073 | 46 ± 11 | 51 ± 16 | 34 ± 0 | 43 ± 2 | 55 ± 12 | ND |
| 35DAP081 | 37 ± 7 | 29 ± 7 | 31 ± 10 | 29 ± 0 | 60 ± 29 | ||
Data are in milligrams per deciliter for blood urea nitrogen and units per liter for all other biochemical markers and are means ± SDs from two independent assays. Reference values (CECAL/Fiocruz) are as follows: blood urea nitrogen (BUN), up to 29 mg/dl; alanine aminotransferase (ALT), up to 132 U/liter; aspartate transaminase (AST), up to 247 U/liter; creatine kinase (CK), up to 1,070 U/liter. P was <0.05 by ANOVA for all samples. ND, not determined due to animal death at the higher dose.
Results are from one representative assay.
FIG 5.
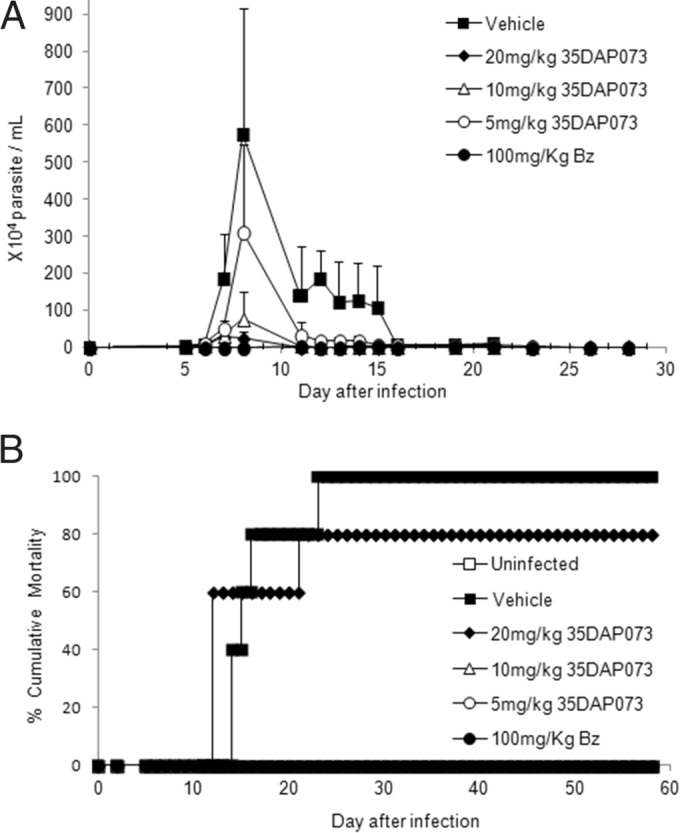
Effect of 35DAP073 (20 to 5 mg/kg/day) and of benznidazole (100 mg/kg/day) administration at 5 and 8 dpi in a mouse model of experimental T. cruzi infection (Y strain). (A) Parasitemia levels; (B) percent cumulative mortality.
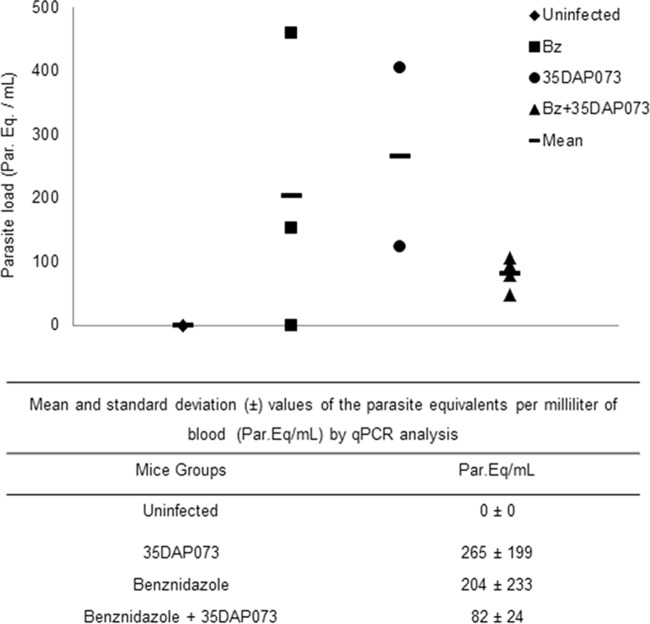
Our data demonstrated a dose-dependent effect of treatment with 35DAP073 at concentrations of up to 20 mg/kg/day, which led to 96 to 46% reductions in the levels of parasitemia (Fig. 5). The animal survival rates achieved 100% when the mice were treated with 5 and 10 mg/kg/day of 35DAP073, which was similar to the results obtained with 100 mg/kg/day Bz (Fig. 5). However, as the tested compound did not result in the complete suppression of the blood parasite load, a longer period of therapy (10 days using 5 mg/kg/day) was next investigated with mice infected with 5 × 103 bloodstream trypomastigote forms of the Colombiana strain. Also, 35DAP073 combined with Bz (treatment for 10 days using 0.5 mg/kg/day 35DAP073 and 100 mg/kg/day Bz) was assayed in parallel using the same experimental model described above, with the therapy being started at the time of parasitemia onset. Our findings showed that 5 mg/kg/day of 35DAP073 completely suppressed the parasitemia and gave 100% animal survival, as did Bz (Fig. 6). However, at the end of the therapy period (the last day of 35DAP073 administration), the mice presented some undesirable neurological toxic disorders (tremors, shaking, ataxia) that were no longer observed after 48 h posttreatment (data not shown). The combination of 0.5 mg/kg/day of this amidine with Bz resulted in parasitemia suppression, 100% animal survival, and no toxic events (Fig. 6). Analysis by qPCR showed that although no statistically significant difference in blood parasite loads was found among the tested groups (P > 0.05), monotherapy with Bz and 35DAP073 resulted in mean values of 204 and 265 parasite equivalents (Par. Eq.)/ml, respectively, while the association of Bz with the AIA induced a higher reduction (60%) of the blood parasite load (82 Par. Eq./ml) (Fig. 7).
FIG 6.
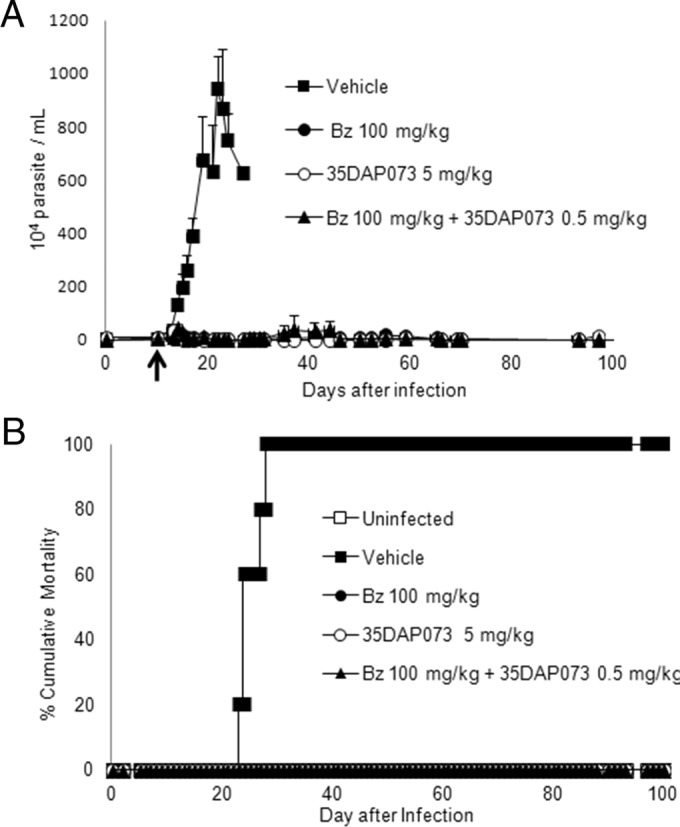
Effect of daily administration of 35DAP073 (5 mg/kg/day), benznidazole (100 mg/kg/day), and 35DAP073 plus Bz (0.5 mg/kg/day and 100 mg/kg/day, respectively) for 10 days in mouse models of experimental T. cruzi infection (Colombiana strain) when compounds were administered starting at 10 dpi. (A) Parasitemia levels; (B) percent cumulative mortality.
FIG 7.
qPCR analysis of the parasite load (in numbers of parasite equivalents per milliliter [Par. Eq./ml]) in the blood of uninfected mice and mice infected with the Colombiana strain submitted to each different treatment regimen: monotherapies (benznidazole at 100 mg/kg/day and 35DAP073 at 5 mg/kg/day) and combined therapy (benznidazole at 100 mg/kg/day plus 35DAP073 at 0.5 mg/kg/day). Each symbol represents an individual value, and the bars represent the respective mean values.
DISCUSSION
The current therapies for Chagas disease remain unsatisfactory due to their various side effects and limited efficacies (27). Amidines have been used as antiparasitic agents for decades in human and veterinary medicine but show important limitations, such as the need for parenteral administration and undesirable side effects (7). In order to overcome these limitations, novel amidines have been synthesized and phenotypic analysis of novel amidines has been conducted in vivo and in vitro (12, 28). Among these, arylimidamides (AIAs) showed considerable biological activity against several intracellular pathogens, including T. cruzi (29), with the bis-AIAs being the most effective of the group (19).
A previous study reported the synthesis of dicationic m-terphenyl derivatives and their biological effect against Trypanosoma brucei rhodesiense, Plasmodium falciparum, Leishmania amazonensis, and T. cruzi (16). Among these, bispyridylimidamides were the most potent against the intracellular amastigote form of the T. cruzi (Tulahuen strain, DTU VI). In the present study, we further explored the phenotypic activity of 14 bis- and monoarylimidamides against BTs and intracellular forms of T. cruzi in vitro and in vivo and extended the analysis to other parasite strains (the Y and Colombiana strains, which belong to DTUs II and I, respectively).
Structural variations of the test compounds used in this study included those in AIA moieties as well as the central cores of the molecules. Maximum potencies were observed with 35DAP073 and 35DAP081, each of which has two 2-pyridylimidamide groups attached to an m-terphenyl nucleus and in which at least one of the two AIA groups is meta to the central ring. These two compounds were more potent than the corresponding bisphenylimidamides, 35DAP069 and 35DAP077. The difference in potencies between 35DAP073 and 35DAP069 was greater than 60-fold but was less pronounced than that between 35DAP081 and 35DAP077. The bispyridylimidamides 35DAP073 and 35DAP081 were also more potent than the monopyridylimidamides 35DAP092 and 38DAP096, thus demonstrating the importance of two AIA moieties. Compound 38DAP092, in which the AIA group is meta to the central ring, was more potent than its regioisomer, 38DAP096, which bears a para-AIA moiety. None of the bis-AIAs with lone aromatic rings or aliphatic chains between the two AIA functionalities (Fig. 2 and 3) showed detectable activity.
The most active molecule, the m-terphenyl bis-AIA 35DAP073, showed an EC50 of 0.5 μM against trypomastigotes (Y strain) after 24 h and was more effective than the reference drug, Bz (13 μM). This amidine was also active against a highly naturally resistant strain (Colombiana), exhibiting an EC50 of 3.8 μM. Analysis of its activity against intracellular forms (Tulahuen strain) showed that this bis-AIA (EC50 = 0.04 μM) was about 50-fold more active than Bz (2 μM). The greater effect against intracellular forms than BT forms was dissociated from the ability of the amidines to induce LB accumulation within mammalian host cells. The biogenesis of lipid bodies is a central event in several processes of cellular homeostasis, as well as during intracellular pathogen infection (30, 31). LBs are recognized to be dynamic organelles composed of a triglyceride and cholesteryl ester core with a surrounding monolayer of phospholipid, cholesterol, and a variety of associated proteins with diverse functions in cell metabolism, signaling, and inflammation (14, 32). Under inflammatory and infectious conditions, prostaglandins and other lipid mediators are mainly produced by LBs (33). Also, recent findings showed that antimicrobial compounds may induce an accumulation of lipid droplets in the cytoplasm of Candida spp. (34) and protozoans (35). In Mycobacterium leprae-infected cells, LBs showed a strong ability to fuse, forming giant lipid droplets (36). Since amidines can modulate the functional activities of mammalian host cells linked to the control of parasite proliferation and/or survival (37, 38) and LBs act on cellular metabolism, inflammation, and infectious conditions (14, 32), in the present study we investigated whether the studied AIAs could interfere with LB biogenesis and parasitism control in vitro. Our data also demonstrated that 35DAP073 and 28SMB060 were able to induce LB biogenesis in cardiac cells at all times tested (2 to 48 h), with cells treated with the two compounds exhibiting similar morphological profiles. No correlation between the trypanocidal effect of amidines and LB accumulation in CCs was found, but further studies are desirable to better understand the consequences of the higher LB levels induced by the amidines in the parasite as well the consequences on the mammalian cell physiology, since this modulation may influence the growth of intracellular parasites (36).
Next, due to the excellent selective indexes of 35DAP073 and 35DAP081, both were tested for acute toxicity. The results showed NOAEL values of 25 and 12.5 mg/kg for 35DAP073 and 35DAP081, respectively. Then, in vivo efficacy studies were conducted with 35DAP073 at concentrations of <25 mg/kg. Our data demonstrated that 35DAP073 treatment of mice infected with the Y and Colombiana strains resulted in a dose-dependent action of 35DAP073, leading to 96 to 46% reductions of parasitemia and 100% animal survival, with Bz showing a similar effect. Unfortunately, as the 10 daily doses (at 5 mg/kg i.p.) were toxic for the animals, combined therapy with 35DAP073 and Bz was tested with the aim of reducing the toxic effects of the amidine. Monotherapy with 35DAP073 at only 0.5 mg/kg/day was not attempted, as previous data with related amidines demonstrated that doses of ≤5 mg/kg/day had only a mild effect on parasitemia control (23). As was also found by our group (28) using the combination of Bz with the amidine DB289, the association of 0.5 mg/kg 35DAP073 with Bz in the present study improved the control of parasite proliferation, providing a 9-fold enhancement of activity compared to that of Bz alone. qPCR analysis showed that the combination therapy was the best regimen, exhibiting parasitemia suppression with no detectable toxicity to the animals. In this sense, our results support further experimental investigations of this compound class in association with licensed antiparasitic drugs with the aim of contributing to the identification of novel alternatives for the treatment of neglected parasitic diseases, such as Chagas disease.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Program for Technological Development in Tools for Health (PDTIS-Fiocruz) for the facilities with the real-time PCR RPT09A platform and RPT11G.
The present study was supported by grants from the Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional Desenvolvimento Científico e Tecnológico (CNPq), Fundação Oswaldo Cruz, PDTIS, PROEP/CNPq/Fiocruz, and CAPES. M.N.C.S. and C.B. are research fellows of CNPq. M.N.C.S. and C.B. are CNE researchers.
Funding Statement
The Bill and Melinda Gates Foundation through a subcontract with the Consortium for Parasitic Drug Development (CPDD) provided funding to R. R. Tidwell. FIOCRUZ provided funding to M. N. C. Soeiro. CAPES provided funding to F. H. Guedes-da-Silva as a fellowship.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01667-15.
REFERENCES
- 1.Chagas C. 1909. Nova tripanozomiase humana: estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Mem Inst Oswaldo Cruz 1:159–218. doi: 10.1590/S0074-02761909000200008. [DOI] [Google Scholar]
- 2.World Health Organization. 2015. Chagas disease. World Health Organization, Geneva, Switzerland: http://www.who.int/topics/chagas_disease/en/ Accessed 9 February 2015. [Google Scholar]
- 3.Rassi AJ, Rassi A, Marin-Neto A. 2010. Chagas disease. Lancet 375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 4.Rodriques Coura J, De Castro SL. 2002. A critical review on Chagas disease chemotherapy. Mem Inst Oswaldo Cruz 97:3–24. doi: 10.1590/S0074-02762002000100001. [DOI] [PubMed] [Google Scholar]
- 5.Soeiro MNC, de Castro SL. 2009. Trypanosoma cruzi targets for new chemotherapeutic approaches. Expert Opin Ther Targets 13:105–121. doi: 10.1517/14728220802623881. [DOI] [PubMed] [Google Scholar]
- 6.Machado FS, Tanowitz HB, Teixeira MM. 2010. New drugs for neglected infectious diseases: Chagas' disease. Br J Pharmacol 160:258–259. doi: 10.1111/j.1476-5381.2010.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soeiro MNC, Werbovetz K, Boykin DW, Wilson WD, Wang MZ, Hemphill A. 2013. Novel amidines and analogues as promising agents against intracellular parasites: a systematic review. Parasitology 140:929–951. doi: 10.1017/S0031182013000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daliry A, Pires MQ, Silva CF, Pacheco RS, Munde M, Stephens CE, Kumar A, Ismail MA, Liu Z, Farahat AA, Akay S, Som P, Hu Q, Boykin DW, Wilson WD, De Castro SL, Soeiro MN. 2011. The trypanocidal activity of amidine compounds does not correlate with their binding affinity to Trypanosoma cruzi kinetoplast DNA. Antimicrob Agents Chemother 55:4765–4773. doi: 10.1128/AAC.00229-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soeiro MNC, de Castro SL, de Souza EM, Batista DGJ, da Silva CF, Boykin DW. 2008. Diamidines activity upon trypanosomes: the state of the art. Curr Mol Pharmacol 1:151–161. doi: 10.2174/1874467210801020151. [DOI] [PubMed] [Google Scholar]
- 10.De Souza EM, Menna-Barreto R, Araújo-Jorge TC, Kumar A, Hu Q, Boykin DW, Soeiro MNC. 2006. Antiparasitic activity of aromatic diamidines is related to apoptosis-like death in Trypanosoma cruzi. Parasitology 133:75–79. doi: 10.1017/S0031182006000084. [DOI] [PubMed] [Google Scholar]
- 11.Da Silva CF, Batista MM, Mota RA, de Souza EM, Stephens CE, Som P, Boykin DW, Soeiro MNC. 2007. Activity of “reversed” diamidines against Trypanosoma cruzi in vitro. Biochem Pharmacol 73:1939–1946. doi: 10.1016/j.bcp.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Batista DDGJ, Batista MM, de Oliveira GM, do Amaral PB, Lannes-Vieira J, Britto CC, Junqueira A, Lima MM, Romanha AJ, Sales Júnior PA, Stephens CE, Boykin DW, Soeiro MDNC. 2010. Arylimidamide DB766, a potential chemotherapeutic candidate for Chagas' disease treatment. Antimicrob Agents Chemother 54:2940–2952. doi: 10.1128/AAC.01617-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tauchi-Sato K, Ozeki S, Houjou T, Taguchi R, Fujimoto T. 2002. The surface of lipid droplets is a phospholipid monolayer with a unique fatty acid composition. J Biol Chem 277:44507–44512. doi: 10.1074/jbc.M207712200. [DOI] [PubMed] [Google Scholar]
- 14.Bozza PT, Magellan KG, Weller PF. 2009. Leukocyte lipid bodies—biogenesis and functions in inflammation. Biochim Biophys Acta 1791:540–551. doi: 10.1016/j.bbalip.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Avila H, Freire-de-Lima CG, Roque NR, Teixeira L, Barja-Fidalgo C, Silva AR, Melo RC, Dosreis GA, Castro-Faria-Neto HC, Bozza PT. 2011. Host cell lipid bodies triggered by Trypanosoma cruzi infection and enhanced by the uptake of apoptotic cells are associated with prostaglandin E2 generation and increased parasite growth. J Infect Dis 204:951–961. doi: 10.1093/infdis/jir432. [DOI] [PubMed] [Google Scholar]
- 16.Patrick DA, Ismail MA, Arafa RK, Wenzler T, Zhu X, Pandharkar T, Jones SK, Werbovetz KA, Brun R, Boykin DW, Tidwell RR. 2013. Synthesis and antiprotozoal activity of dicationic m-terphenyl and 1,3-dipyridylbenzene derivatives. J Med Chem 56:5473–5494. doi: 10.1021/jm400508e. [DOI] [PubMed] [Google Scholar]
- 17.Brand RA, Bruma M, Kellman R, Marvel CS. 1978. Low-molecular-weight polybenzimidazoles from aromatic dinitriles and aromatic diamines. J Polym Sci Polym Chem Ed 16:2275–2284. doi: 10.1002/pol.1978.170160916. [DOI] [Google Scholar]
- 18.Timm BL, da Silva PB, Batista MM, da Silva FHG, da Silva CF, Tidwell RR, Patrick DA, Jones SK, Bakunov SA, Bakunova SM, Soeiro MDNC. 2014. In vitro and in vivo biological effects of novel arylimidamide derivatives against Trypanosoma cruzi. Antimicrob Agents Chemother 58:3720–3726. doi: 10.1128/AAC.02353-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Da Silva CF, Lima AJ, Romanha MM, Policarpo AJ, Stephens ASJ, Som PCE, Boykin DW, Soeiro MNC. 2011. In vitro trypanocidal activity of DB745B and other novel arylimidamides against Trypanosoma cruzi. J Antimicrob Chemother 66:1295–1297. doi: 10.1093/jac/dkr140. [DOI] [PubMed] [Google Scholar]
- 20.Meirelles MNL, Araujo-Jorge TC, Miranda CF, de Souza W, Barbosa HS. 1986. Interaction of Trypanosoma cruzi with heart muscle cells: ultrastructural and cytochemical analysis of endocytic vacuole formation and effect upon myogenesis in vitro. Eur J Cell Biol 41:198–206. [PubMed] [Google Scholar]
- 21.Romanha AJ, Castro SL, Soeiro MDN, Lannes-Vieira J, Ribeiro I, Talvani A, Bourdin B, Blum B, Olivieri B, Zani C, Spadafora C, Chiari E, Chatelain E, Chaves G, Calzada JE, Bustamante JM, Freitas-Junior LH, Romero LI, Bahia MT, Lotrowska M, Soares M, Andrade SG, Armstrong T, Degrave W, Andrade ZA. 2010. In vitro and in vivo experimental models for drug screening and development for Chagas disease. Mem Inst Oswaldo Cruz 105:233–238. doi: 10.1590/S0074-02762010000200022. [DOI] [PubMed] [Google Scholar]
- 22.Moreira LS, Piva B, Gentile LB, Mesquita-Santos FP, D'Avila H, Maya-Monteiro CM, Bozza PT, Bandeira-Melo C, Diaz BL. 2009. Cytosolic phospholipase A2-driven PGE2 synthesis within unsaturated fatty acids-induced lipid bodies of epithelial cells. Biochim Biophys Acta 1791:156–165. doi: 10.1016/j.bbalip.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Da Silva CF, Batista DGJ, Oliveira GM, de Souza EM, Hammer ER, da Silva PB, Daliry A, Araujo JS, Britto C, Rodrigues AC, Liu Z, Farahat AA, Kumar A, Boykin DW, Soeiro MNC. 2012. In vitro and in vivo investigation of the efficacy of arylimidamide DB1831 and its mesylated salt form—DB1965—against Trypanosoma cruzi infection. PLoS One 7:e30356. doi: 10.1371/journal.pone.0030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreira OC, Ramírez JD, Velázquez E, Melo MF, Lima-Ferreira C, Guhl F, Sosa-Estani S, Marin-Neto JA, Morillo CA, Britto C. 2013. Towards the establishment of a consensus real-time qPCR to monitor Trypanosoma cruzi parasitemia in patients with chronic Chagas disease cardiomyopathy: a substudy from the BENEFIT trial. Acta Trop 125:23–31. doi: 10.1016/j.actatropica.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Duffy T, Cura CI, Ramirez JC, Abate T, Cayo NM, Parrado R, Bello ZD, Velazquez E, Muñoz-Calderon A, Juiz NA, Basile J, Garcia L, Riarte A, Nasser JR, Ocampo SB, Yadon ZE, Torrico F, de Noya BA, Ribeiro I, Schijman AG. 2013. Analytical performance of a multiplex real-time PCR assay using TaqMan probes for quantification of Trypanosoma cruzi satellite DNA in blood samples. PLoS Negl Trop Dis 7:e2000. doi: 10.1371/journal.pntd.0002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melo RC, Dvorak AM. 2012. Lipid body-phagosome interaction in macrophages during infectious diseases: host defense or pathogen survival strategy? PLoS Pathog 8:e1002729. doi: 10.1371/journal.ppat.1002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatelain E. 2015. Chagas disease drug discovery: toward a new era. J Biomol Screen 20:22–35. doi: 10.1177/1087057114550585. [DOI] [PubMed] [Google Scholar]
- 28.Da Silva CF, Batista MM, Batista DG, De Souza EM, Da Silva PB, De Oliveira GM, Meuser AS, Shareef AR, Boykin DW, Soeiro MN. 2008. In vitro and in vivo studies of the trypanocidal activity of a diarylthiophene diamidine against Trypanosoma cruzi. Antimicrob Agents Chemother 52:3307–3314. doi: 10.1128/AAC.00038-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batista DGJ, Batista MM, Oliveira GM, Britto CC, Rodrigues ACM, Stephens CE, Boykin DW, Soeiro MNC. 2011. Combined treatment of heterocyclic analogues and benznidazole upon Trypanosoma cruzi in vivo. PLoS One 6:e22155. doi: 10.1371/journal.pone.0022155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bozza PT, Melo RC, Bandeira-Melo C. 2007. Leukocyte lipid bodies regulation and function: contribution to allergy and host defense. Pharmacol Ther 113:30–49. doi: 10.1016/j.pharmthera.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV Jr, Ory DS, Schaffer JE. 2003. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A 100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farese RV Jr, Walther TC. 2009. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell 139:855–860. doi: 10.1016/j.cell.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bozza PT, Payne JL, Morham SG, Langenbach R, Smithies O, Weller PF. 1996. Leukocyte lipid body formation and eicosanoid generation: cyclooxygenase-independent inhibition by aspirin. Proc Natl Acad Sci U S A 93:11091–11096. doi: 10.1073/pnas.93.20.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishida K, Fernandes R, Cammerer JCS, Urbina JA, Gilbert I, de Souza W, Rozental S. 2011. Synthetic arylquinuclidine derivatives exhibit antifungal activity against Candida albicans, Candida tropicalis and Candida parapsilopsis. Ann Clin Microbiol Antimicrob 10:3. doi: 10.1186/1476-0711-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Macedo-Silva ST, Urbina JA, de Souza W, Rodrigues JC. 2013. In vitro activity of the antifungal azoles itraconazole and posaconazole against Leishmania amazonensis. PLoS One 8:e83247. doi: 10.1371/journal.pone.0083247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elamin AA, Stehr M, Singh M. 2012. Lipid droplets and Mycobacterium leprae infection. J Pathog 2012:361374. doi: 10.1155/2012/361374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Souza EM, Lansiaux A, Bailly C, Wilson WD, Hu Q, Boykin DW, Batista MM, Araújo-Jorge TC, Soeiro MN. 2004. Phenyl substitution of furamidine markedly potentiates its anti-parasitic activity against T. cruzi and Leishmania amazonensis. Biochem Pharmacol 68:593–600. doi: 10.1016/j.bcp.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 38.Leepin A, Studli A, Brun R, Stephens EC, Boykin DW, Hemphill A. 2008. Host cells participate in the in vitro effects of novel analogues diamidine against tachyzoites of the intracellular apicomplexan parasites Neospora caninum and Toxoplasma gondii. Antimicrob Agents Chemother 52:1999–2008. doi: 10.1128/AAC.01236-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.