Abstract
A total of 55 cefotaxime-resistant Escherichia coli isolates were obtained from retail meat products purchased in Shenzhen, China, during the period November 2012 to May 2013. Thirty-seven of these 55 isolates were found to harbor a blaCTX-M gene, with the blaCTX-M-1 group being the most common type. blaCMY-2 was detected in 16 isolates, alone or in combination with other extended-spectrum β-lactamase (ESBL) determinants. Importantly, the fosA3 gene, which encodes fosfomycin resistance, was detected in 12 isolates, with several being found to reside in the conjugative plasmid that harbored the blaCTX-M gene. The insertion sequence IS26 was observed upstream of some of the blaCTX-M-55 and fosA3 genes. Conjugation experiments showed that blaCTX-M genes from 15 isolates were transferrable, with Inc I1 and Inc FII being the most prevalent replicons. High clonal diversity was observed among the blaCTX-M producers, suggesting that horizontal transfer of the blaCTX-M genes among E. coli strains in retail meats is a common event and that such strains may constitute an important reservoir of blaCTX-M genes, which may be readily disseminated to other potential human pathogens.
INTRODUCTION
Escherichia coli causes a wide range of opportunistic infections and may be regarded as a key vector in the dissemination of resistance elements among Gram-negative human pathogens. Among the range of antibiotic-resistant Gram-negative bacterial pathogens known to date, organisms producing CTX-M-type extended-spectrum β-lactamases (ESBLs) pose a particularly serious public health threat worldwide (1). In the past few years, rapid dissemination of E. coli strains that produce ESBLs has been reported in various parts of the world, with the CTX-M-type enzymes being the most common ESBLs detected among clinical isolates (2, 3). The CTX-M family can be divided into five major groups (CTX-M-1, CTX-M-2, CTX-M-8, CTX-M-9, and CTX-M-25), with less than 90% identity between different groups and more than 95% identity among members of a group (4).
Fosfomycin is a naturally occurring antibacterial agent with a broad spectrum of antimicrobial activity against both Gram-positive and Gram-negative bacteria, except Acinetobacter baumannii (5). The agent has been used to treat urinary tract infections, but development of bacterial resistance during therapy frequently occurred, rendering it unsuitable for sustained therapy of severe infections (6, 7). Recently, renewed attention has been paid to fosfomycin for the treatment of both urinary tract and systemic infections due to rapid dissemination of multidrug-resistant Gram-negative bacteria, especially strains of Enterobacteriaceae species that are resistant to traditionally used agents (8). Compared to other agents, fosfomycin seems to have retained antimicrobial activity against a substantial percentage of clinical isolates, in particular E. coli. Recently, a fosfomycin resistance gene, fosA3, has been reported in E. coli and Klebsiella pneumoniae isolates (9–13). The gene is normally plasmid mediated and flanked by the IS26 transposase genes and is often detected in blaCTX-M-producing and multidrug-resistant E. coli strains recovered from animals, as well as patients, in China, Japan, and Korea (9–13). It has been suggested that the increasing prevalence of the fosA3 gene is due to dissemination of the Inc I, Inc N, and Inc FII plasmids among E. coli isolates rather than to clonal expansion of specific strains (11, 14).
The origins of ESBL and fosA3 genes in clinical strains, as well as the factors facilitating the dissemination of such elements, remain ill defined. Recent reports of recovery of ESBL-producing bacterial isolates in animals have raised concerns about the possibility that such isolates are becoming causative agents of human clinical infections (15). In particular, we postulate that meat products constitute a potential source of blaCTX-M- and fosA3-bearing organisms that mediate drug-resistant infections. In the present study, we tested this hypothesis by examining the prevalence of E. coli strains that harbor such elements in retail meat, followed by characterization of their phenotypic and genotypic features.
MATERIALS AND METHODS
Bacterial isolation.
E. coli isolates were collected from fresh pork and chicken samples purchased from wet markets (markets that sell fresh and unprocessed meat products) in Shenzhen, Guangdong Province, China, during the period November 2012 to May 2013. E. coli was isolated on MacConkey agar plates and identified by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) using a Bruker MicroFlex LT mass spectrometer (Bruker Daltonics). E. coli isolates were further confirmed with an API20E test strip (bioMérieux, Inc.). Only one E. coli isolate from each sample was used for further analysis.
Antimicrobial susceptibility testing.
Antimicrobial susceptibility was determined using the agar dilution method and interpreted according to the Clinical and Laboratory Standards Institute (CLSI) recommendations (16). E. coli strain ATCC 25922 was used as a quality control strain in antimicrobial susceptibility testing. Isolates that exhibited resistance to at least three different classes of agents were classified as multidrug resistant.
Detection of β-lactamase genes.
β-Lactamase genes in the E. coli isolates were determined by PCR as previously described (17). The PCR amplicons were then sequenced and subjected to BLAST analysis against the NR database available at GenBank to investigate the degree of genetic similarity between the detected sequence and known ESBL genes.
Conjugation experiments.
The horizontal-transfer efficiencies of the blaCTX-M genes were assessed by performing conjugation using the filter-mating method as previously described (18). Transconjugants were selected on MacConkey agar containing cefotaxime (2 μg/ml) and sodium azide (100 μg/ml) and tested for antimicrobial susceptibility and the presence of blaCTX-M genes.
Epidemiological typing.
The genetic relatedness between the test isolates and the corresponding transconjugants was determined by pulsed-field gel electrophoresis (PFGE) using the CHEF-MAP-PER System (Bio-Rad Laboratories, Hercules, CA, USA) as described previously (19). The results were interpreted according to the criteria of Tenover et al. (20). Plasmids harbored by the transconjugants were analyzed by S1 nuclease-PFGE and Southern blot hybridization, using probes that target specific resistance genes.
Plasmid replicon typing.
Plasmids extracted from transconjugants were characterized by plasmid-based replicon typing. Eighteen pairs of primers were designed to perform 5 multiplex and 3 simplex PCRs targeting the FIA, FIB, FIC, HI1, HI2, I1-Iγ, L/M, N, P, W, T, A/C, K, B/O, X, Y, F, and FIIA replicons, as previously described (21).
Multilocus sequence typing and phylogenetic grouping.
E. coli strains were subjected to multilocus sequence typing (MLST) and phylogenetic grouping. Internal fragments of seven housekeeping genes (adk, fumC, gyrB, icd, mdh, purA, and recA) were sequenced (22), followed by assignment of sequence types (STs) in accordance with the E. coli multilocus sequence typing scheme (http://mlst.warwick.ac.uk/mlst/dbs/Ecoli). Phylogenetic grouping was performed for all the isolates as described previously (23).
Analysis of the genetic environment of blaCTX-M genes.
The genetic environment of blaCTX-M genes was probed by PCR, using specific primer pairs (ISEcp1, ISCR1, IS26, ORF477, IS903, and those targeting group 1 and group 9 blaCTX-M genes, as well as the fosA3 gene). The PCR products were subjected to bidirectional DNA sequencing and BLAST analysis to identify the insertion sequence concerned.
RESULTS
A total of 70 pork and 50 chicken samples were purchased during the isolation period. Eighty E. coli isolates were obtained—45 E. coli isolates from pork (64% isolation rate) and 35 E. coli isolates from chicken (70% isolation rate). Among these 80 E. coli isolates, 55 were resistant to cefotaxime and were further characterized in this study. These cefotaxime-resistant E. coli isolates also showed resistance to other antibiotics, such as tetracycline (78%), nalidixic acid (71%), sulfamethoxazole-trimethoprim (65%), chloramphenicol (58%), ciprofloxacin (56%), kanamycin (47%), and fosfomycin (22%). However, all the isolates were susceptible to meropenem, and only two strains (4%) were resistant to amikacin. Thirty-seven out of the 55 isolates harbored the blaCTX-M gene, 16 harbored a blaCMY-2-like gene, and 4 possessed a blaACT-2-like gene (see Table S1 in the supplemental material). Three isolates harbored only blaTEM-1, suggesting that cephalosporin resistance mechanisms other than those we screened for may be present. Further investigation will be needed to discover the exact cefotaxime resistance mechanisms in these isolates. The combined presence of different β-lactamase genes was commonly observed in the isolates, as shown in Table S1 in the supplemental material. The fosA3 gene was also detected in 12 of the 55 E. coli isolates tested, representing 22% and 33% of the cefotaxime-resistant and blaCTX-M-positive E. coli isolates, respectively. It should be noted that fosA3 was detected only in blaCTX-M-positive isolates and not in strains containing the blaACT-2-or blaCMY-2-like gene.
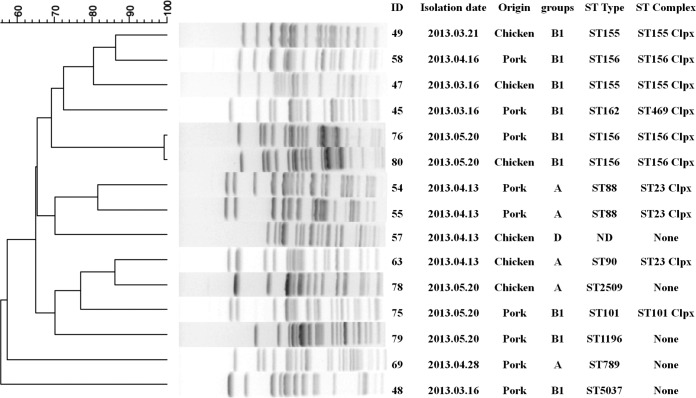
Among the 37 blaCTX-M-positive E. coli isolates tested, 15 were found to be able to successfully transfer their cefotaxime resistance phenotypes to the E. coli recipient strain J53 via conjugation. PFGE analysis of these 37 isolates revealed high genetic diversity of the isolates, with 29 arbitrary pulsotypes at a 90% threshold and 3 untypeable isolates (see Fig. S1 in the supplemental material). Among the 15 E. coli isolates with conjugative plasmids, 14 arbitrary pulsotypes at a 90% threshold were detected (Fig. 1). These data suggested that the blaCTX-M genes recovered in the test isolates were acquired as a result of horizontal transfer from existing resistant organisms, rather than through clonal expansion of specific resistant strains. The results of phylogenetic and MLST analyses also revealed a great diversity of MLST types. The most prevalent sequence type was ST156, followed by ST155 and ST88. Most of these strains belonged to the clonal complexes ST156cc, ST155cc, ST23cc, ST101cc, and ST469cc and to phylogenetic group B1, followed by A and D (Fig. 1).
FIG 1.
Origins, molecular characteristics, and genetic relatedness of 15 ESBL-producing E. coli strains that harbored conjugative plasmids.
Sequencing of the full-length β-lactamase genes revealed that blaCTX-M-55 was the most prevalent (8/15) in these 15 E. coli isolates, followed by blaCTX-M-15 (4/15), blaCTX-M-14 (2/15), and blaCTX-M-123 (1/15). For transconjugants, the most commonly detected blaCTX-M gene was blaCTX-M-55, followed by blaCTX-M-15, blaCTX-M-14, and blaCTX-M-123. It should be noted that all except one gene in the blaCTX-M-1 group were transferrable to the recipient, whereas conjugation was successful in only two out of four strains of the blaCTX-M-9 group, suggesting that the blaCTX-M-1-type genes were located more commonly on conjugative plasmids (Table 1). A notable case was isolate 63, the parental strain of which produced both blaCTX-M-14 and blaCTX-M-15, but no β-lactamase gene could be detected in the recipient strain by PCR, suggesting the existence of a novel β-lactamase gene in the conjugative plasmid.
TABLE 1.
Antibiotic susceptibility and β-lactamase gene profiles of plasmid-mediated ESBL-producing E. coli strains and corresponding transconjugants
| Strain or transconjuganta | Susceptibility [MIC (μg/ml)]b |
β-Lactamase gene(s) detected in plasmid(s) | Estimated plasmid size(s) (kb)c | Plasmid typed | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMK | CTX | CIP | KAN | STR | CRO | TET | CHL | NAL | SXT | ||||
| 45 | 2 | >16 | 0.03 | 2 | 4 | >16 | 2 | 4 | 4 | 16 | TEM-1, CTX-M-55 | 244, 138, 78 | |
| TC45 | 1 | >16 | 0.015 | 4 | 16 | >16 | 2 | 4 | 4 | 0.5 | CTX-M-55 | 78 | Inc I1 |
| 47 | >128 | >16 | >16 | >128 | >128 | >16 | 32 | >64 | >64 | >16 | TEM-1, CTX-M55, fosA3 | 130, 104, 78 | |
| TC47 | 0.5 | >16 | 0.03 | 1 | 1 | >16 | 0.5 | 1 | 4 | 2 | CTX-M-55 | 130 | Inc FIV |
| 48 | 2 | >16 | 0.5 | >128 | 64 | >16 | 16 | 32 | 8 | >16 | CTX-M-55 | 78 | |
| TC48 | 1 | >16 | 0.06 | 128 | 32 | >16 | 16 | 64 | 16 | 1 | CTX-M-55 | 138 | ND |
| 49 | 1 | >16 | 0.03 | >128 | 64 | >16 | 16 | 32 | 2 | >16 | CTX-M-123, fosA3 | 216,130 | |
| TC49 | 0.5 | >16 | 0.03 | >128 | 1 | >16 | 8 | 1 | 4 | 2 | CTX-M-123, fosA3 | 130 | Inc I1 |
| 54 | 1 | >16 | 1 | 16 | 128 | >16 | 16 | 32 | 8 | >16 | CTX-M15, CMY-2, CTXM14 | 167,138,78 | |
| TC54 | 0.5 | >16 | 0.03 | 1 | 1 | >16 | 1 | 1 | 4 | ≤0.25 | CTX-M-15 | 78 | Inc I1 |
| 55 | 1 | >16 | >16 | >128 | 128 | >16 | 32 | 32 | >64 | >16 | TEM-1, CTX-M55 | 200, 78 | |
| TC55 | 0.5 | >16 | 0.015 | 1 | 2 | >16 | 1 | 2 | 4 | ≤0.25 | CTX-M-55 | 78 | Inc I1 |
| 57 | 1 | >16 | >16 | >128 | 32 | >16 | 16 | >64 | >64 | 4 | CTX-M-55, fosA3 | 138, 130, 104, 78 | |
| TC57 | 1 | >16 | 0.06 | 128 | 32 | >16 | 16 | 64 | 16 | 1 | CTX-M-55, fosA3 | 130 | Inc FII |
| 58 | 2 | >16 | >16 | >128 | >128 | >16 | >32 | >64 | >64 | >16 | TEM-1, CTX-15 | 130 | |
| TC58 | 1 | >16 | 0.06 | 128 | 32 | >16 | 16 | 64 | 16 | 1 | CTX-M-15 | 138 | ND |
| 63 | 4 | >16 | >16 | >128 | 64 | >16 | 32 | >64 | >64 | 1 | CTX-M-15, CTX-M-14 | 138, 104 | |
| TC63 | 1 | 4 | 0.015 | 1 | 2 | 16 | 1 | 2 | 4 | 0.25 | / | 104 | Inc I1 |
| 69 | 4 | 16 | 2 | 2 | 16 | 8 | 16 | 2 | >64 | 0.5 | TEM-1, OXA-1, CTX-M-55 | 104, 78 | |
| TC69 | 1 | >16 | 0.015 | 4 | 16 | >16 | 2 | 4 | 4 | 0.5 | CTX-M-55 | 78 | Inc I1 |
| 75 | 4 | 8 | 2 | >128 | 128 | >16 | >32 | >64 | 64 | >32 | TEM-1, CTX-M-14, fosA3 | 244, 104 | |
| TC75 | 0.25 | 4 | 0.03 | >128 | 32 | 4 | 16 | 32 | 4 | >16 | TEM-1, CTX-M-14, fosA3 | 244 | ND |
| 76 | 4 | >16 | >16 | 16 | 128 | >16 | >32 | 64 | >64 | >32 | TEM-1, CTX-M-15, fosA3 | 138 | |
| TC76 | 0.5 | >16 | 0.06 | 128 | 64 | >16 | 16 | 64 | 16 | 2 | CTX-M-15, fosA3 | 138 | ND |
| 78 | 4 | >16 | >16 | >128 | >128 | >16 | 32 | >64 | >64 | >32 | TEM-1, CTX-M-55 | 100 | |
| TC78 | 0.5 | >16 | 0.015 | >128 | 64 | >16 | 16 | 32 | 4 | ≤0.25 | TEM-1, CTX-M-55 | 104 | Inc FII |
| 79 | 4 | >16 | >16 | 64 | 8 | >16 | >32 | >64 | >64 | >32 | CTX-M-14 | 104, 78 | |
| TC79 | 2 | 8 | 0.03 | 32 | 2 | 16 | 1 | 4 | 4 | ≤0.25 | CTX-M14 | 104 | Inc FII |
| 80 | 4 | >16 | 8 | >128 | >128 | >16 | >32 | >64 | >64 | >32 | TEM-1, CTX-M-55, fosA3 | 142, 138, 104 | |
| TC80 | 0.5 | >16 | 0.06 | 128 | 32 | >16 | 8 | 64 | 32 | 2 | CTX-M-55, fosA3 | 138 | ND |
TC, transconjugant.
AMK, amikacin; CTX, cefotaxime; CIP, ciprofloxacin; KAN, kanamycin; STR, streptomycin; CRO, ceftriaxone; TET, tetracycline; CHL, chloramphenicol; NAL, nalidixic acid; SXT, sulfamethoxazole-trimethoprim.
Number in bold denotes plasmid transferable to recipient strain.
ND, not determined.
S1-PFGE analysis showed that the parental strains carried multiple plasmids with sizes ranging from 78 to 244 kb (Table 1). In comparison, conjugative plasmids of four major sizes, (78, 104, 130, and 138 kb) were recovered (Table 1; see Fig. S2 in the supplemental material). Plasmid typing indicated that all five 78-kb conjugative plasmids were Inc I1 plasmids that harbored β-lactamase genes of the blaCTX-M-1 group, including blaCTX-M-55 and blaCTX-M-15; four 138-kb plasmids, which were untypeable, were found to harbor all blaCTX-M-1 group genes, including blaCTX-M-15 and blaCTX-M-55, with two of them also harboring the fosA3 gene; three 130-kb conjugative plasmids were found to belong to the Inc I1, FII, and FIV types and to harbor blaCTX-M-1 group β-lactamase genes, including blaCTX-M-15, blaCTX-M-55, and blaCTX-M123; two 104-kb plasmids were of the FII type, which harbored the blaCTX-M-55 element. One strain, TC75, was found to carry two conjugative plasmids with sizes of 244 kb and 50 kb, with the 244-kb plasmid comprising a blaCTX-M-9 group β-lactamase gene, blaCTX-M-14, together with a fosA gene (Table 1; see Fig. S2 in the supplemental material). The Inc FIV plasmid has rarely been described and has not been detected with any blaCTX-M ESBL gene before.
Southern hybridization was performed and confirmed that blaCTX-M and fosA3 genes were located in plasmids of various sizes. blaCTX-M-bearing plasmids of the same sizes as those in the parental strains could also be detected in transconjugants, with the exception of two strains, namely, 48 and 58. In these two cases, the blaCTX-M-55 gene was found to be located in plasmids with sizes of ∼78 kb and ∼104 kb, respectively, in the parental strains but in plasmids with a size of ∼138 kb in the transconjugants TC48 and TC58 (Table 1; see Fig. S3 in the supplemental material). Southern hybridization also confirmed that both blaCTX-M and fosA genes recovered from five strains (47, 57, 75, 76, and 80) were all located on the same plasmids, which could all be transferred to the transconjugants (see Fig. S4 in the supplemental material).
In an attempt to probe the genetic environment of the resistance genes, an IS26 element was detected upstream of five blaCTX-M-55 genes, with ORF477 and a 47-bp spacer detected downstream of the blaCTX-M-55 genes. However, ISEcp1, ISCR1, and IS903 were not detected in the rest of the 10 transconjugants. IS26 was detected upstream of the fosA3 gene in two transconjugants, C76 and C80, but not in the other three transconjugants in which IS26 was detectable (see Fig. S5 in the supplemental material).
DISCUSSION
Food animals colonized with ESBL-producing E. coli bacteria have been considered potential sources of resistant strains that commonly cause infections in the community (24). ESBL producers were rarely detected in animal isolates in China before 2005 (25–27) but have emerged at a dramatically increasing rate in recent years (27–29), presumably due to overuse of third-generation cephalosporins in raising food animals (30). A recent report that E. coli isolated from Dutch patients shared identical ESBL genes and plasmids with strains recovered from retail chicken meat and poultry suggests that transmission of ESBL-producing organisms from poultry to humans is a common event (31). In a similar study, Shiraki et al. (32) suggested that genetic determinants encoding CTX-M enzymes may have originated from animals and were transmitted to humans via the food chain. Therefore, tracking the transmission routes of food-borne E. coli strains in community settings is urgently required to prevent widespread dissemination of ESBL-producing organisms in China.
Resistance conferred by ESBLs is often associated with resistance to other classes of antibiotics, such as trimethoprim-sulfamethoxazole, aminoglycosides, and fluoroquinolones (33). In China, plasmid-mediated fosfomycin resistance has been frequently detected among CTX-M-producing isolates in animals (10, 34). It was unexpected, since the use of fosfomycin in food-producing animals is not approved in China. Nevertheless, we also observed a high rate of fosfomycin resistance among the blaCTX-M-producing isolates. Our finding is also consistent with those of previous studies, in which blaCTX-M genes were found to be located predominantly in Inc FII- or Inc I1-type plasmids in E. coli isolates recovered from food animals (35). blaCTX-M genes were often found in plasmids of different sizes (from 78 kb to 138 kb), implying that a diversity of blaCTX-M genetic environments exist. The ISEcp1 element is known to play an important role in blaCTX-M expression and gene transfer and is frequently located upstream of blaCTX-M genes (18, 36, 37). Surprisingly, such an element was not detected upstream of the blaCTX-M gene in these conjugative plasmids. No blaCTX-M gene was found to be associated with ISCR1 in the conjugative plasmids, which is consistent with the previous observation in that ISCR1 was much less commonly associated with blaCTX-M genes. In previous studies, ORF477 and IS903 were frequently detected downstream of the blaCTX-M-1 and blaCTX-M-9 group genes, respectively. ORF477 was also frequently detected in this study, whereas IS903 was not, presumably because only two transconjugants that belonged to the blaCTX-M-9 group were analyzed. Surprisingly, the blaCTX-M gene in a strain that contained the fosA3 element, with the genetic structure IS26–blaCTX-M-55–ORF477, located in a plasmid of 138 kb, was different from those reported in all previous studies in that the blaCTX-M gene in the same genetic environment was often located in plasmids 60 kb to 100 kb in size. In addition, blaCTX-M-1 group genes were found to be more transferable than the blaCTX-M-9 group genes in this study, presumably due to the difference in the genetic environments.
In this study, most of the transferable isolates belonged to the phylogenetic group B1, followed by A, and none belonged to phylogenetic group B2, ST131, which is the most prevalent type worldwide at present. Since virulent organisms causing infections are mainly known to belong to group B2, and to a lesser extent to group D, whereas most commensal strains belong to groups B1 and A (38), our data indicate that most of the transferable isolates tested in this study belonged to commensal strains. This finding is therefore consistent with previous reports in that commensal E. coli strains in food animals were likely to be a reservoir of ESBL genes (24) and to play a role in dissemination of such resistance elements. ST156 was the most prevalent ST detected and the only type isolated from humans (39, 40). ST131 (26%) and ST156 (11.1%) were the most prevalent STs identified in carbapenemase-producing E. coli strains in 83 hospitals in Spain (41). Various other reports also showed that ST156 was detected in water and animals (42–44). The strong linkage of blaCTX-M-15 ST156 isolates from humans, animals, and the environment warrants further attention to and research on this type of E. coli bacteria.
The blaCTX-M producers observed in this study pose a serious challenge for treatment of human infections, since the test strains were resistant not only to cephalosporins, but also to other classes of antibiotics. Continued investigations and surveillance for better understanding of the transmission dynamics and evolutionary characteristics of such strains and the resistance elements that they harbor are necessary.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Chinese National Key Basic Research and Development (973) Program (2013CB127201) and the Shenzhen Key Laboratory of Food Biological Safety Control (ZDSYS20140509142430241).
We have no conflicts of interest to declare.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.03101-15.
REFERENCES
- 1.Ho PL, Lo WU, Yeung MK, Li Z, Chan J, Chow KH, Yam WC, Tong AH, Bao JY, Lin CH, Lok S, Chiu SS. 2012. Dissemination of pHK01-like incompatibility group IncFII plasmids encoding CTX-M-14 in Escherichia coli from human and animal sources. Vet Microbiol 158:172–179. doi: 10.1016/j.vetmic.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet R. 2004. Growing group of extended-spectrum beta-lactamases: the CTX-M enzymes. Antimicrob Agents Chemother 48:1–14. doi: 10.1128/AAC.48.1.1-14.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canton R, Coque TM. 2006. The CTX-M beta-lactamase pandemic. Curr Opin Microbiol 9:466–475. doi: 10.1016/j.mib.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 4.D'Andrea MM, Arena F, Pallecchi L, Rossolini GM. 2013. CTX-M-type beta-lactamases: a successful story of antibiotic resistance. Int J Med Microbiol 303:305–317. doi: 10.1016/j.ijmm.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Grassi GG, Ferrara A, Navone A, Peona V, Sala P. 1977. Antibacterial activity of fosfomycin. II. Determination of minimal bactericidal concentration in association with other antibiotics. G Ital Chemioter 23:87–94. (In Italian.). [PubMed] [Google Scholar]
- 6.Patel SS, Balfour JA, Bryson HM. 1997. Fosfomycin tromethamine. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy as a single-dose oral treatment for acute uncomplicated lower urinary tract infections. Drugs 53:637–656. [DOI] [PubMed] [Google Scholar]
- 7.Reeves DS. 1994. Fosfomycin trometamol. J Antimicrob Chemother 34:853–858. doi: 10.1093/jac/34.6.853. [DOI] [PubMed] [Google Scholar]
- 8.Falagas ME, Kastoris AC, Kapaskelis AM, Karageorgopoulos DE. 2010. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum beta-lactamase producing, Enterobacteriaceae infections: a systematic review. Lancet Infect Dis 10:43–50. doi: 10.1016/S1473-3099(09)70325-1. [DOI] [PubMed] [Google Scholar]
- 9.Ho PL, Chan J, Lo WU, Lai EL, Cheung YY, Lau TC, Chow KH. 2013. Prevalence and molecular epidemiology of plasmid-mediated fosfomycin resistance genes among blood and urinary Escherichia coli isolates. J Med Microbiol 62:1707–1713. doi: 10.1099/jmm.0.062653-0. [DOI] [PubMed] [Google Scholar]
- 10.Ho PL, Chan J, Lo WU, Law PY, Li Z, Lai EL, Chow KH. 2013. Dissemination of plasmid-mediated fosfomycin resistance fosA3 among multidrug-resistant Escherichia coli from livestock and other animals. J Appl Microbiol 114:695–702. doi: 10.1111/jam.12099. [DOI] [PubMed] [Google Scholar]
- 11.Lee SY, Park YJ, Yu JK, Jung S, Kim Y, Jeong SH, Arakawa Y. 2012. Prevalence of acquired fosfomycin resistance among extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae clinical isolates in Korea and IS26-composite transposon surrounding fosA3. J Antimicrob Chemother 67:2843–2847. doi: 10.1093/jac/dks319. [DOI] [PubMed] [Google Scholar]
- 12.Sato N, Kawamura K, Nakane K, Wachino J, Arakawa Y. 2013. First detection of fosfomycin resistance gene fosA3 in CTX-M-producing Escherichia coli isolates from healthy individuals in Japan. Microb Drug Resist 19:477–482. doi: 10.1089/mdr.2013.0061. [DOI] [PubMed] [Google Scholar]
- 13.Wachino J, Yamane K, Suzuki S, Kimura K, Arakawa Y. 2010. Prevalence of fosfomycin resistance among CTX-M-producing Escherichia coli clinical isolates in Japan and identification of novel plasmid-mediated fosfomycin-modifying enzymes. Antimicrob Agents Chemother 54:3061–3064. doi: 10.1128/AAC.01834-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou J, Yang X, Zeng Z, Lv L, Yang T, Lin D, Liu JH. 2013. Detection of the plasmid-encoded fosfomycin resistance gene fosA3 in Escherichia coli of food-animal origin. J Antimicrob Chemother 68:766–770. doi: 10.1093/jac/dks465. [DOI] [PubMed] [Google Scholar]
- 15.Carattoli A. 2008. Animal reservoirs for extended spectrum beta-lactamase producers. Clin Microbiol Infect 14(Suppl 1):S117–S123. doi: 10.1111/j.1469-0691.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- 16.CLSI. 2014. Performance standards for antimicrobial susceptibility testing; 24th informational supplement. CLSI document M100-S24. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 17.Dallenne C, Da Costa A, Decre D, Favier C, Arlet G. 2010. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J Antimicrob Chemother 65:490–495. doi: 10.1093/jac/dkp498. [DOI] [PubMed] [Google Scholar]
- 18.Eckert C, Gautier V, Arlet G. 2006. DNA sequence analysis of the genetic environment of various blaCTX-M genes. J Antimicrob Chemother 57:14–23. [DOI] [PubMed] [Google Scholar]
- 19.Gautom RK. 1997. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day. J Clin Microbiol 35:2977–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33:2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods 63:219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, Karch H, Reeves PR, Maiden MC, Ochman H, Achtman M. 2006. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol 60:1136–1151. doi: 10.1111/j.1365-2958.2006.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clermont O, Bonacorsi S, Bingen E. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol 66:4555–4558. doi: 10.1128/AEM.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smet A, Martel A, Persoons D, Dewulf J, Heyndrickx M, Herman L, Haesebrouck F, Butaye P. 2010. Broad-spectrum beta-lactamases among Enterobacteriaceae of animal origin: molecular aspects, mobility and impact on public health. FEMS Microbiol Rev 34:295–316. doi: 10.1111/j.1574-6976.2009.00198.x. [DOI] [PubMed] [Google Scholar]
- 25.Yang H, Chen S, White DG, Zhao S, McDermott P, Walker R, Meng J. 2004. Characterization of multiple-antimicrobial-resistant Escherichia coli isolates from diseased chickens and swine in China. J Clin Microbiol 42:3483–3489. doi: 10.1128/JCM.42.8.3483-3489.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu JH, Wei SY, Ma JY, Zeng ZL, Lu DH, Yang GX, Chen ZL. 2007. Detection and characterisation of CTX-M and CMY-2 beta-lactamases among Escherichia coli isolates from farm animals in Guangdong Province of China. Int J Antimicrob Agents 29:576–581. doi: 10.1016/j.ijantimicag.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Li L, Jiang ZG, Xia LN, Shen JZ, Dai L, Wang Y, Huang SY, Wu CM. 2010. Characterization of antimicrobial resistance and molecular determinants of beta-lactamase in Escherichia coli isolated from chickens in China during 1970-2007. Vet Microbiol 144:505–510. doi: 10.1016/j.vetmic.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Yuan L, Liu JH, Hu GZ, Pan YS, Liu ZM, Mo J, Wei YJ. 2009. Molecular characterization of extended-spectrum beta-lactamase-producing Escherichia coli isolates from chickens in Henan Province, China. J Med Microbiol 58:1449–1453. doi: 10.1099/jmm.0.012229-0. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Ma Y, Hu C, Jin S, Zhang Q, Ding H, Ran L, Cui S. 2010. Dissemination of cefotaxime-M-producing Escherichia coli isolates in poultry farms, but not swine farms, in China. Foodborne Pathog Dis 7:1387–1392. doi: 10.1089/fpd.2010.0581. [DOI] [PubMed] [Google Scholar]
- 30.Lei T, Tian W, He L, Huang XH, Sun YX, Deng YT, Sun Y, Lv DH, Wu CM, Huang LZ, Shen JZ, Liu JH. 2010. Antimicrobial resistance in Escherichia coli isolates from food animals, animal food products and companion animals in China. Vet Microbiol 146:85–89. doi: 10.1016/j.vetmic.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 31.Leverstein-van Hall MA, Dierikx CM, Cohen Stuart J, Voets GM, van den Munckhof MP, van Essen-Zandbergen A, Platteel T, Fluit AC, van de Sande-Bruinsma N, Scharinga J, Bonten MJ, Mevius DJ, National ESBL Surveillance Group . 2011. Dutch patients, retail chicken meat and poultry share the same ESBL genes, plasmids and strains. Clin Microbiol Infect 17:873–880. doi: 10.1111/j.1469-0691.2011.03497.x. [DOI] [PubMed] [Google Scholar]
- 32.Shiraki Y, Shibata N, Doi Y, Arakawa Y. 2004. Escherichia coli producing CTX-M-2 beta-lactamase in cattle, Japan. Emerg Infect Dis 10:69–75. doi: 10.3201/eid1001.030219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coque TM, Baquero F, Canton R. 2008. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Euro Surveill 13:19051 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19044. [PubMed] [Google Scholar]
- 34.Hou J, Huang X, Deng Y, He L, Yang T, Zeng Z, Chen Z, Liu JH. 2012. Dissemination of the fosfomycin resistance gene fosA3 with CTX-M beta-lactamase genes and rmtB carried on IncFII plasmids among Escherichia coli isolates from pets in China. Antimicrob Agents Chemother 56:2135–2138. doi: 10.1128/AAC.05104-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carattoli A. 2009. Resistance plasmid families in Enterobacteriaceae. Antimicrob Agents Chemother 53:2227–2238. doi: 10.1128/AAC.01707-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poirel L, Decousser JW, Nordmann P. 2003. Insertion sequence ISEcp1B is involved in expression and mobilization of a blaCTX-M beta-lactamase gene. Antimicrob Agents Chemother 47:2938–2945. doi: 10.1128/AAC.47.9.2938-2945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vinue L, Saenz Y, Martinez S, Somalo S, Moreno MA, Torres C, Zarazaga M. 2009. Prevalence and diversity of extended-spectrum beta-lactamases in faecal Escherichia coli isolates from healthy humans in Spain. Clin Microbiol Infect 15:954–957. doi: 10.1111/j.1469-0691.2009.02803.x. [DOI] [PubMed] [Google Scholar]
- 38.Johnson JR, Delavari P, Kuskowski M, Stell AL. 2001. Phylogenetic distribution of extraintestinal virulence-associated traits in Escherichia coli. J Infect Dis 183:78–88. doi: 10.1086/317656. [DOI] [PubMed] [Google Scholar]
- 39.Izdebski R, Baraniak A, Fiett J, Adler A, Kazma M, Salomon J, Lawrence C, Rossini A, Salvia A, Vidal Samso J, Fierro J, Paul M, Lerman Y, Malhotra-Kumar S, Lammens C, Goossens H, Hryniewicz W, Brun-Buisson C, Carmeli Y, Gniadkowski M, MOSAR WP2 and WP5 Study Groups . 2013. Clonal structure, extended-spectrum beta-lactamases, and acquired AmpC-type cephalosporinases of Escherichia coli populations colonizing patients in rehabilitation centers in four countries. Antimicrob Agents Chemother 57:309–316. doi: 10.1128/AAC.01656-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saez-Lopez E, Guiral E, Lopez Y, Montero I, Bosch J, Vila J, Soto SM. 2014. Characterization of CTX-M-14 and CTX-M-15 producing Escherichia coli strains causing neonatal sepsis. Microb Drug Resist 20:281–284. doi: 10.1089/mdr.2013.0190. [DOI] [PubMed] [Google Scholar]
- 41.Oteo J, Ortega A, Bartolome R, Bou G, Conejo C, Fernandez-Martinez M, Gonzalez-Lopez JJ, Martinez-Garcia L, Martinez-Martinez L, Merino M, Miro E, Mora M, Navarro F, Oliver A, Pascual A, Rodriguez-Bano J, Ruiz-Carrascoso G, Ruiz-Garbajosa P, Zamorano L, Bautista V, Perez-Vazquez M, Campos J, GEIH-GEMARA (SEIMC), REIPI. 2015. Prospective multicenter study of carbapenemase-producing Enterobacteriaceae from 83 hospitals in Spain reveals high in vitro susceptibility to colistin and meropenem. Antimicrob Agents Chemother 59:3406–3412. doi: 10.1128/AAC.00086-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Randall LP, Clouting C, Horton RA, Coldham NG, Wu G, Clifton-Hadley FA, Davies RH, Teale CJ. 2011. Prevalence of Escherichia coli carrying extended-spectrum beta-lactamases (CTX-M and TEM-52) from broiler chickens and turkeys in Great Britain between 2006 and 2009. J Antimicrob Chemother 66:86–95. doi: 10.1093/jac/dkq396. [DOI] [PubMed] [Google Scholar]
- 43.Pan YS, Liu JH, Hu H, Zhao JF, Yuan L, Wu H, Wang LF, Hu GZ. 2013. Novel arrangement of the blaCTX-M-55 gene in an Escherichia coli isolate coproducing 16S rRNA methylase. J Basic Microbiol 53:928–933. doi: 10.1002/jobm.201200318. [DOI] [PubMed] [Google Scholar]
- 44.Rashid M, Rakib MM, Hasan B. 2015. Antimicrobial-resistant and ESBL-producing Escherichia coli in different ecological niches in Bangladesh. Infect Ecol Epidemiol 5:26712. doi: 10.3402/iee.v5.26712. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.