Abstract
Clostridium difficile-associated diarrhea has been associated with disruption of the normal intestinal microbiota, particularly the Bacteroides fragilis group and Prevotella species. Surotomycin is a bactericidal cyclic lipopeptide in development for treatment of Clostridium difficile-associated diarrhea that has selective and potent activity against C. difficile and other Gram-positive bacteria and a minimal impact on intestinal Gram-negative organisms. The impacts of ascending doses of surotomycin on major organism groups in the gut microbiota of healthy volunteers were evaluated during a randomized, double-blind, placebo-controlled, multiple-dose phase 1 study. Thirty volunteers were randomized into 3 cohorts, using a 4:1 ratio, to receive 250 mg, 500 mg, or 1,000 mg of surotomycin, or placebo, twice daily for 14 days. Stool samples collected at baseline (days 0 and 1) and at the end of treatment (days 13 to 15) were cultured quantitatively. The B. fragilis group, the Bacteroides/Prevotella group, and Enterobacteriaceae were also quantified by quantitative real-time PCR. Baseline and end-of-treatment stool samples showed 1- to 2-log10 CFU/g reductions in total bacterial counts for most volunteers. Various decreases in clostridial, Lactobacillus-Bifidobacterium group, and enterococcus-streptococcus group counts occurred while patients were receiving surotomycin, whereas the enterobacteria and the B. fragilis group persisted at the end of treatment. There was no change in enterococcus MICs of surotomycin, nor was vancomycin-resistant Enterococcus detected after exposure. Surotomycin at doses of up to 1,000 mg twice daily had only modest disruptive effects on the gut microbiota. The potential sparing of the gut microbiota by surotomycin may decrease the risk of disease recurrence.
INTRODUCTION
The human intestinal tract is colonized with a variety of commensal beneficial microorganisms that reside in a delicate balance, providing protection against potential pathogens (1). Alteration of the microbial diversity renders the environment supportive to Clostridium difficile or other intestinal pathogens (2, 3).
C. difficile infection, also known as C. difficile-associated diarrhea (CDAD), is the leading cause of health care-associated diarrhea in the world (4). Over the past decade, the incidence and severity of C. difficile infection have increased throughout the United States, Canada, and Europe (5–9). The most commonly used therapies for CDAD are metronidazole and vancomycin. However, these treatments are suboptimal, as disease recurrence occurs in 15 to 35% of patients (10–12).
Surotomycin (CB-183,315) is an orally administered, minimally absorbed, selective, bactericidal cyclic lipopeptide in phase 3 development for the treatment of CDAD (13). A selective and potent activity of surotomycin against C. difficile and other Gram-positive bacteria, with minimal impact on intestinal Gram-negative organisms, has been demonstrated in vitro (14, 15). In the LCD-CDAD-DR-09-03 phase 2 trial, clinical cure rates were similar between both doses of surotomycin (125 mg and 250 mg) twice daily and vancomycin at 125 mg given four times daily; however, disease recurrence was significantly reduced with surotomycin at 250 mg compared with vancomycin (17.2% versus 35.6%, respectively; P = 0.035) (16). The objective of the current study was to determine the impact of surotomycin on major groups of organisms in the gut microbiota of healthy volunteers enrolled in a phase 1 clinical trial.
MATERIALS AND METHODS
Study population.
Eligible volunteers included males and females aged ≥18 years and ≤75 years who were considered to be in good health. Volunteers did not have any evidence of significant gastrointestinal inflammatory disease, such as inflammatory bowel disease. Additional exclusion criteria included known sensitivity to daptomycin, administration of antibiotics within the past 30 days, and use of prescribed or over-the-counter medication for volunteers between 18 and 49 years of age. For volunteers who were >49 years of age, use of medication had to be approved by both the medical monitor and the investigator.
Study design.
This study was a double-blind, randomized, placebo-controlled, multiple-dose phase 1 study of ascending oral surotomycin doses in healthy volunteers. Thirty eligible volunteers were recruited and sequentially enrolled into one of three dose cohorts, receiving 250 mg (cohort 1), 500 mg (cohort 2), or 1,000 mg (cohort 3) orally twice daily for 14 days. The 10 volunteers for each cohort were randomized at a 4:1 ratio to receive surotomycin (8 volunteers) or placebo (2 volunteers). Randomization was stratified by gender to achieve equal numbers of male and female volunteers in each cohort. One stool sample each was collected at baseline (days 0 and 1), midstudy (days 7 to 9), and the end of treatment (days 13 to 15) for all 4 arms. All stool samples were frozen at −70°C until analysis.
The study protocol was approved by institutional review boards at the participating institutions, and all participants provided written informed consent.
Microbiological evaluation.
Fecal specimens were thawed and inoculated onto selective and nonselective media for recovery of aerobic and anaerobic bacteria (Table 1). Because of previous reports of vancomycin-resistant Enterococcus (VRE) appearing in conjunction with vancomycin and metronidazole therapy (17), enterococci were quantitated using Enterococcosel agar with 16 μg/ml or 4 μg/ml vancomycin or without drug. Quantitative plating was achieved by weighing approximately 1 g of stool and adding sufficient saline to make a 1:10 dilution. Further 10-fold dilutions were prepared, and 0.1 ml was plated on Enterococcosel agar. Semiquantitative cultures of other organisms were prepared by plating 0.01 ml of the 1:10 dilution onto the selective and nonselective media listed in Table 1 and streaking plates for isolation. Total counts were determined from quantitative 10-fold dilutions. Anaerobic culture plates were incubated in an anaerobic chamber (Bactron IV; Sheldon Manufacturing Inc., Cornelius, OR), and enterococcal and other aerobic culture plates were incubated in the ambient atmosphere at 36°C for 2 to 5 days. Isolates were identified to the genus or group level by using growth characteristics on selective media, Gram staining, and catalase production assay. The lower level of detection was 1 × 102 CFU/g.
TABLE 1.
Plating media for anaerobic and aerobic bacterial isolates
| Bacterial group | Medium | Supplier |
|---|---|---|
| Anaerobes | ||
| Bacteroides | Bacteroides bile esculin agar (BBE) | Anaerobe Systems, Morgan Hill, CA |
| Prevotella and Bacteroides | Laked blood with kanamycin and vancomycin (LKV) agar | Anaerobe Systems |
| Lactobacilli and bifidobacteria | Lactobacillus MRS agar | Hardy Diagnostics, Santa Maria, CA |
| Gram-positive organisms | Phenylethyl alcohol blood agar (PEA) | Anaerobe Systems |
| Clostridia | Egg yolk agar (EYA) after ethanol treatment | Anaerobe Systems |
| Total anaerobes | Brucella blood agar | Anaerobe Systems |
| Aerobes | ||
| Enteric Gram-negative rods | MacConkey agar | Hardy Diagnostics |
| Enterococci | Enterococcosel agar, with and without surotomycin (4 and 16 μg/ml) | BBL, Sparks, MD |
| Gram-positive bacteria | Rose agar | Hardy Diagnostics |
| Total aerobes | Blood agar | Hardy Diagnostics |
PCR analysis.
Total nucleic acid was prepared for SYBR green quantitative PCR analyses by extracting 0.1 g of stool in stool transport and recovery buffer (Roche Molecular, Pleasanton, CA), using a NucliSENS easyMAG extraction system (bioMérieux, Durham, NC) running the Specific A program. Before extraction, stool samples were spiked with 10 μl of the Simplexa extraction and amplification control set (SEAC; Focus Diagnostics, Cypress, CA) to monitor PCR inhibition. The Uni331F/Bac708R primer set was used to amplify species within the Bacteroides fragilis group, whereas the CFB286F/CFB719R primer set more broadly amplified the members of the Bacteroides/Prevotella group. Purified nucleic acid (1 μl) was added to each quantitative PCR mix, which contained 0.3 μM (each) primers (Biosearch Technologies, Petaluma, CA) and 2× QuantiTect SYBR green master mix (Qiagen, Valencia, CA). Quantitative PCR was performed in 10-μl reaction mixtures by use of an integrated cycler machine (3M, St. Paul, MN). Cycling parameters included an initial 10 min at 95°C for denaturation followed by 45 cycles of 95°C for 30 s, 50°C (for the B. fragilis group and the Bacteroides/Prevotella group) or 55°C (for Enterobacteriaceae) for 30 s, and 72°C for 30 s. Standard curves were constructed by extracting preparations of cultured isolates (with known numbers of CFU per milliliter) of Escherichia coli (ATCC 25922), B. fragilis (ATCC 25285), and Prevotella melaninogenica (ATCC 25845). The primer sequences used to quantify shifts in major components of the gut microbiota are shown in Table 2 (18–21).
TABLE 2.
16S rRNA gene probes used for quantitative real-time PCR to quantify shifts in major components of the gut microbiota
| Target group | Primer | Primer sequencea | Standard | Reference |
|---|---|---|---|---|
| Bacteroides | Uni331F | F-TCCTACGGGAGGCAGCAGT | Bacteroides fragilis | 18 |
| Bac708R | R-CAATCGGAGTTCTTCGTG | 19 | ||
| Prevotella | CFB286F | F-GTAGGGGTTCTGAGAGGA | Prevotella melaninogenica | 20 |
| CFB719R | R-AGCTGCCTTCGCAATCGG | 20 | ||
| Enterobacteriaceae | Eco1457F | F-CATTGACGTTACCCGCAGAAGAAGC | Escherichia coli | 21 |
| Eco1652R | R-CTCTACGAGACTCAAGCTTGC | 21 |
F, forward; R, reverse.
MIC analysis.
Enterococci recovered on Enterococcosel agar were subcultured onto blood agar plates for identification and further testing. MICs of surotomycin, daptomycin, and vancomycin against enterococci were determined by broth microdilution according to methods described in Clinical and Laboratory Standards Institute document M7-A7 (22). Cation-adjusted Mueller-Hinton broth was prepared according to the manufacturer's specifications. Tests for daptomycin and surotomycin were adjusted to contain a final concentration of 50 mg/liter Ca2+ (23). The baseline MIC values were compared to the postbaseline values to determine if drug exposure selected for decreased susceptibility to surotomycin or emergence of vancomycin-resistant strains.
RESULTS
Volunteer population.
A total of 24 volunteers were randomized to receive 250 mg (n = 8), 500 mg (n = 8), or 1,000 mg (n = 8) of surotomycin twice daily; an additional 2 patients per cohort received a placebo (n = 6). A total of 29 volunteers completed the study.
Impact of surotomycin on gut microbiota as determined by bacterial culture.
Stool samples collected at baseline (days 0 and 1) and at the end of treatment (days 13 to 15) were cultured. Total bacterial counts and specific bacterium concentrations at these two time points were compared. In all three cohorts, total bacterial counts for the majority of volunteers were reduced. In the 250- and 1,000-mg twice-daily cohorts, 1- to 2-log10 CFU/g reductions in bacterial counts were observed in seven of the eight volunteers, whereas in the 500-mg twice-daily cohort, four of the seven volunteers had decreases of 1 to 2 log10 CFU/g.
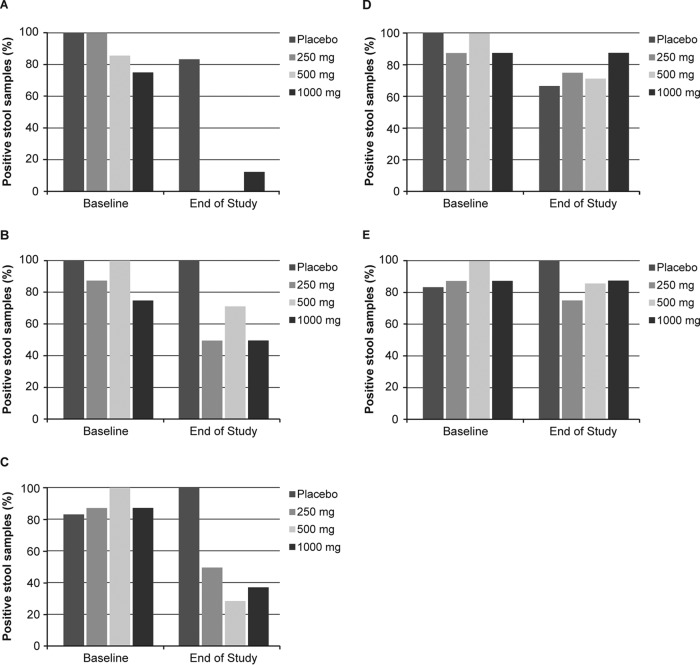
The impacts of surotomycin on the different organism groups found in the gut are summarized in Fig. 1. Enterococci and streptococci were recovered at baseline from 75 to 100% of volunteers. At the end of treatment, these bacteria persisted in only one patient from the 1,000-mg twice-daily cohort (Fig. 1A). When all three cohorts were combined, anaerobic Gram-positive rods resembling the Lactobacillus-Bifidobacterium group persisted at the end of treatment in 13 of 23 (57%) volunteers (Fig. 1B). Clostridium spp. were retained in four (50%) volunteers in the 250-mg twice-daily cohort, two (29%) volunteers in the 500-mg twice-daily cohort, and three (38%) volunteers in the 1,000-mg twice-daily cohort (Fig. 1C). Enterobacteria (Fig. 1D) and the B. fragilis-Prevotella-Porphyromonas group (Fig. 1E) were recovered at the end of treatment from 18 of 23 (78%) and 19 of 23 (83%) volunteers, respectively. Recovery of Staphylococcus aureus at baseline or the end of treatment was rare.
FIG 1.
Impacts of surotomycin on fecal bacterium groups. The graphs show percentages of stool samples showing the presence of enterococci and streptococci (A), lactobacilli and bifidobacteria (B), Clostridium spp. (C), E. coli-Enterobacter-Klebsiella (D), and the B. fragilis group-Prevotella-Porphyromonas (E) at baseline and at the end of treatment. The lower level of detection was 1 × 102 CFU/g.
Impact of surotomycin on gut microbiota as determined by quantitative PCR.
Stool samples collected at baseline and at the end of treatment were analyzed, and specific bacterium concentrations at these two time points were compared. Quantitative SYBR green PCR was used to quantify Enterobacteriaceae, Bacteroides, and Prevotella organisms. At baseline, the average log CFU/ml was 6.79, 8.33, and 7.40 for Enterobacteriaceae, the B. fragilis group, and the Bacteroides/Prevotella group, respectively. By the end of treatment, the Enterobacteriaceae counts increased by 1.77, 1.36, and 1.74 log CFU/ml in the 250-, 500-, and 1,000-mg twice-daily cohorts, respectively, compared with a 0.05-log CFU/ml increase in the placebo group (Fig. 2). The Bacteroides/Prevotella group counts decreased by 0.19 and 0.20 log CFU/ml in the 250- and 500-mg twice-daily cohorts, respectively, compared with a 0.04-log CFU/ml increase in the placebo group (Fig. 2). The counts increased by 0.25 log CFU/ml in the 1,000-mg twice-daily cohort (Fig. 2). The B. fragilis group count decreased by 0.19 and 0.49 log CFU/ml in the 250- and 500-mg twice-daily cohorts, respectively, compared with a 0.38-log CFU/ml increase in the placebo group (Fig. 2). Alternatively, the B. fragilis group count increased by 0.34 log CFU/ml in the 1,000-mg twice-daily cohort (Fig. 2). The relative precision of the quantitative PCR was limited, as the coefficient of variation for the PCR was approximately 8% using quantitative standards.
FIG 2.
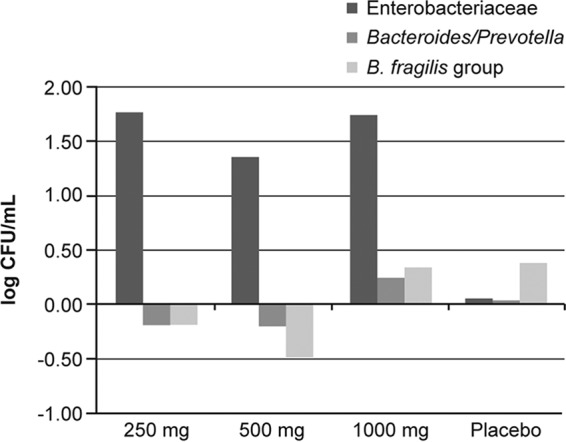
Changes in the number of CFU per milliliter for Enterobacteriaceae, the Bacteroides/Prevotella group, and the B. fragilis group for each treatment cohort as determined by quantitative real-time PCR.
Susceptibility testing of enterococci.
Susceptibility testing with surotomycin, daptomycin, and vancomycin was performed on all isolates. Surotomycin MIC values for all 114 isolates recovered from all cohorts ranged from ≤0.03 to 2 μg/ml, with an MIC90 of 1 μg/ml (Table 3) and an MIC50 of 0.5 μg/ml. Two volunteers who received surotomycin in each of the three cohorts did not have enterococci in their stools at baseline. In the 250-mg and 500-mg twice-daily cohorts, no enterococci were isolated from any volunteers midstudy or at the end of treatment. In the 1,000-mg twice-daily cohort, one volunteer had enterococci present midstudy, whereas another volunteer had enterococci present at the end of treatment (surotomycin MIC = 0.25 μg/ml). Among volunteers who received surotomycin, changes in MIC values for surotomycin, daptomycin, or vancomycin (±1 dilution from baseline) were not detected for any postbaseline isolates. Additionally, VRE were not recovered from any volunteer.
TABLE 3.
Surotomycin MIC ranges (μg/ml) for enterococcia
| Visit | Surotomycin group data |
Placebo group data |
||||||
|---|---|---|---|---|---|---|---|---|
| Cohort 1 (250 mg b.i.d.) |
Cohort 2 (500 mg b.i.d.) |
Cohort 3 (1,000 mg b.i.d.) |
All cohorts |
|||||
| n | MIC range | n | MIC range | n | MIC range | n | MIC range | |
| Baseline (days 0 and 1) | 8 | 0.06–1 | 8 | 0.06–1 | 8 | 0.03–1 | 6 | 0.06–2 |
| Midstudy (days 7 to 9) | 8 | — | 7 | — | 8 | 0.25 | 5 | 0.06–1 |
| End of study (days 13 to 15) | 8 | — | 7 | — | 8 | 0.25 | 6 | 0.06–0.5 |
n, number of volunteers with stool samples provided and evaluated; —, none of the evaluable volunteers had any enterococcal isolates in the stool samples. MIC ranges are given for volunteers with enterococci isolated.
DISCUSSION
Despite increased awareness and knowledge of CDAD, the incidence and severity of this infection have increased over the past decade (5–9). Rates of morbidity and mortality are high, as CDAD can quickly progress from watery diarrhea to fulminant colitis to toxic megacolon and bowel perforation (24). Both vancomycin and metronidazole, the primary antibiotics used to treat CDAD, disrupt the protective intestinal microbiota, have high rates of disease recurrence, and can lead to VRE colonization in the intestinal tract (1, 17, 25).
Fidaxomicin, a macrocyclic antibiotic recently approved by the U.S. Food and Drug Administration for treatment of CDAD, preserves the intestinal microbiota and is associated with a greater sustained clinical response than that observed with vancomycin (25). Still, new and improved antibiotics that target C. difficile without disrupting the normal intestinal microbiota are needed.
Surotomycin is a novel agent under investigation for treatment of CDAD. It is a cyclic lipopeptide with activity against C. difficile and limited activity against Gram-negative pathogens. Given surotomycin's improved selectivity for C. difficile versus Gram-negative bacteria (14, 15), the current analysis, conducted as part of a randomized, double-blind, placebo-controlled, multiple-dose phase 1 clinical trial, evaluated the impacts of surotomycin on major organism groups in the gut microbiota of healthy volunteers.
In the current study, oral surotomycin doses of 250, 500, and 1,000 mg twice daily for 14 days had a minimal disruptive effect on the normal gut microbiota. Whereas postexposure counts for clostridia and the enterococcus and streptococcus groups were lowered, enterobacteria and the B. fragilis group persisted in the majority of volunteers at the end of the study (days 13 to 15). A slight increase in Enterobacteriaceae counts was observed by PCR. This finding is in agreement with previously published data for mice (26) and supports findings from a recent phase 2 study that evaluated the effects of surotomycin and vancomycin on the intestinal microbiota (27). That study demonstrated that surotomycin was associated with little discernible change in the counts of Gram-negative anaerobes, particularly the B. fragilis group and Prevotella, whereas vancomycin exposure greatly suppressed these microorganisms during and after treatment (27).
The B. fragilis group and Prevotella have an important role in colonization resistance and maintaining a healthy environment within the intestinal tract by preventing overgrowth of potential pathogens, such as C. difficile (1, 25). Preserving the normal balance of these protective bacteria could minimize CDAD recurrence following treatment. In a recent phase 2 clinical trial, surotomycin at doses of 125 and 250 mg twice daily was safe and well tolerated in patients with CDAD (16). Clinical cure rates were similar between surotomycin at 125 and 250 mg and vancomycin (92.4%, 86.6%, and 89.4%, respectively) (16, 28). However, 4 weeks after treatment, surotomycin at 250 mg was associated with a lower recurrence rate than that observed with vancomycin (17.2% versus 35.6%, respectively; P = 0.035) (16). More than 80% of the recurrences were considered relapses, as the recurrent isolate was genetically identical to the baseline pathogen (27). Investigators theorized that fewer CDAD relapses and recurrences following surotomycin therapy could be a result of this agent's minimal effect on the normal bowel biota.
The development and spread of VRE are a growing problem in hospitals. Unfortunately, >50% of CDAD cases have concurrent VRE colonization (29). Previous research has shown that treatment with both oral metronidazole and vancomycin for CDAD has produced VRE overgrowth in stool, leading to greater contamination of skin and environmental surfaces (17, 30). Additionally, VRE domination of the microbiota after antibiotic treatment has been shown to precede bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation (31). Our study showed no postbaseline changes in enterococcal MIC values for surotomycin, daptomycin, or vancomycin among volunteers who received surotomycin. This finding is consistent with a previous in vitro study which documented that the rate of spontaneous resistance to surotomycin was either low or below the limit of detection for C. difficile and enterococci, including VRE (32). VRE were not recovered from any volunteer at baseline, and exposure to surotomycin did not select for VRE in any study volunteer.
Due to the limitations of the procedures employed, the analysis of the impact of surotomycin exposure on the microbiota as determined by bacterial culture was not a formal quantitative study. Furthermore, fecal samples were not processed in an anaerobic environment, which could have negatively affected anaerobic bacterial growth and subsequently biased analyses of these samples. However, the molecular analysis confirmed the results generated by the semiquantitative methods. It should also be noted that analysis of bacterial groups within fecal samples for identification of microbial dysbiosis provides only an indirect measure of biological processes occurring at the mucosal surface of the gut (25).
Our research shows a potential benefit with the use of surotomycin for the treatment of CDAD. The microbiota-sparing trend observed with surotomycin in the current study supports the reduction in risk of recurrent disease observed in the LCD-CDAD-DR-09-03 phase 2 trial. Thus, these findings promote the continued clinical development of surotomycin for the treatment of CDAD.
ACKNOWLEDGMENTS
We acknowledge Eliza Leoncio for excellent technical assistance. Medical writing and editorial assistance was provided by Amy E. Ramsden and Cara L. Hunsberger of StemScientific, Lyndhurst, NJ, an Ashfield Company, part of UDG Healthcare PLC. This assistance was funded by Merck and Co., Inc., Kenilworth, NJ.
D.M.C. and K.L.T. work for the R. M. Alden Research Laboratory and were contracted by Merck and Co., Inc., for portions of this study. S.E.D. works for the ACM Global Central Laboratory and was contracted by Merck and Co., Inc., for portions of this study. L.C. is employed by Merck and Co., Inc., Kenilworth, NJ. E.J.C.G. works for the R. M. Alden Research Laboratory, was contracted by Merck and Co., Inc., for portions of this study, and has participated on advisory boards sponsored by Merck and Co., Inc., Cubist Pharmaceuticals Inc., Optimer Pharmaceuticals Inc., Bayer AG, Bio-K Plus International Inc., Summit Therapeutics PLC, Kindred Healthcare, Inc., Novartis Pharmaceuticals Corporation, Daichi Sankyo Company Ltd., and Rempex Pharmaceuticals, Inc. He has served as a speaker for Bayer AG, Merck and Co., Inc., Forest Laboratories, Inc., Optimer Pharmaceuticals Inc., and Cubist Pharmaceuticals Inc., and has received research grants from Merck and Co., Inc., Schering-Plough Pharmaceuticals LLC, Optimer Pharmaceuticals Inc., Theravance Biopharma, Cubist Pharmaceuticals Inc., Pfizer Inc., Astellas Pharma US, Inc., Cerexa, Inc., Forest Laboratories, Inc., Impax Laboratories, Inc., Novartis Pharmaceuticals Corporation, Clinical Microbiology Institute, Genzyme Corporation, a Sanofi company, Nanopacific Holdings, Inc., Romark Laboratories LC, ViroXis Corp., Warner Chilcott PLC, AvidBiotics Corp., GLSynthesis Inc., Immunome Inc., Salix Pharmaceuticals, Inc., Summit Therapeutics PLC, GlaxoSmithKline PLC, Rempex Pharmaceuticals, Inc., Symbiomix Therapeutics, Gynuity Health Projects, Durata Therapeutics, Inc., and Toltec Pharmaceuticals LLC.
This work was supported by Merck and Co., Inc., Kenilworth, NJ.
REFERENCES
- 1.Chang JY, Antonopoulos DA, Kalra A, Tonelli A, Khalife WT, Schmidt TM, Young VB. 2008. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis 197:435–438. doi: 10.1086/525047. [DOI] [PubMed] [Google Scholar]
- 2.Buffie CG, Jarchum I, Equinda M, Lipuma L, Gobourne A, Viale A, Ubeda C, Xavier J, Pamer EG. 2012. Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to Clostridium difficile-induced colitis. Infect Immun 80:62–73. doi: 10.1128/IAI.05496-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buffie CG, Pamer EG. 2013. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol 13:790–801. doi: 10.1038/nri3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He M, Miyajima F, Roberts P, Ellison L, Pickard DJ, Martin MJ, Connor TR, Harris SR, Fairley D, Bamford KB, D'Arc S, Brazier J, Brown D, Coia JE, Douce G, Gerding D, Kim HJ, Koh TH, Kato H, Senoh M, Louie T, Michell S, Butt E, Peacock SJ, Brown NM, Riley T, Songer G, Wilcox M, Pirmohamed M, Kuijper E, Hawkey P, Wren BW, Dougan G, Parkhill J, Lawley TD. 2013. Emergence and global spread of epidemic healthcare-associated Clostridium difficile. Nat Genet 45:109–113. doi: 10.1038/ng.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC). 2005. Severe Clostridium difficile-associated disease in populations previously at low risk—four states, 2005. MMWR Morb Mortal Wkly Rep 54:1201–1205. [PubMed] [Google Scholar]
- 6.McDonald LC, Owings M, Jernigan DB. 2006. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996–2003. Emerg Infect Dis 12:409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redelings MD, Sorvillo F, Mascola L. 2007. Increase in Clostridium difficile-related mortality rates, United States,1999–2004. Emerg Infect Dis 13:1417–1419. doi: 10.3201/eid1309.061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burckhardt F, Friedrich A, Beier D, Eckmanns T. 2008. Clostridium difficile surveillance trends, Saxony, Germany. Emerg Infect Dis 14:691–692. doi: 10.3201/eid1404.071023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gravel D, Miller M, Simor A, Taylor G, Gardam M, McGeer A, Hutchinson J, Moore D, Kelly S, Boyd D, Mulvey M, Canadian Nosocomial Infection Surveillance Program. 2009. Health care-associated Clostridium difficile infection in adults admitted to acute care hospitals in Canada: a Canadian Nosocomial Infection Surveillance Program study. Clin Infect Dis 48:568–576. doi: 10.1086/596703. [DOI] [PubMed] [Google Scholar]
- 10.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. 2007. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 45:302–307. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 11.Pépin J, Valiquette L, Gagnon S, Routhier S, Brazeau I. 2007. Outcomes of Clostridium difficile-associated disease treated with metronidazole or vancomycin before and after the emergence of NAP1/027. Am J Gastroenterol 102:2781–2788. doi: 10.1111/j.1572-0241.2007.01539.x. [DOI] [PubMed] [Google Scholar]
- 12.Musher DM, Aslam S, Logan N, Nallacheru S, Bhaila I, Borchert F, Hamill RJ. 2005. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis 40:1586–1590. doi: 10.1086/430311. [DOI] [PubMed] [Google Scholar]
- 13.Mascio CTM, Mortin LI, Howland KT, Van Praagh AD, Zhang S, Arya A, Chuong CL, Kang C, Li T, Silverman JA. 2012. In vitro and in vivo characterization of CB-183,315, a novel lipopeptide antibiotic for the treatment of Clostridium difficile. Antimicrob Agents Chemother 56:5023–5030. doi: 10.1128/AAC.00057-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Citron DM, Tyrrell KL, Merriam CV, Goldstein EJ. 2012. In vitro activities of CB-183,315, vancomycin, and metronidazole against 556 strains of Clostridium difficile, 445 other intestinal anaerobes, and 56 Enterobacteriaceae species. Antimicrob Agents Chemother 56:1613–1615. doi: 10.1128/AAC.05655-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snydman DR, Jacobus NV, McDermott LA. 2012. Activity of a novel cyclic lipopeptide, CB-183,315, against resistant Clostridium difficile and other Gram-positive aerobic and anaerobic intestinal pathogens. Antimicrob Agents Chemother 56:3448–3452. doi: 10.1128/AAC.06257-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patino H, Stevens C, Louie T, Bernardo P, Friedland I. 2011. Efficacy and safety of the lipopeptide CB-183,315 for the treatment of Clostridium difficile infection, abstr K-205a. Abstr 51st Intersci Conf Antimicrob Agents Chemother. American Society for Microbiology, Washington, DC. [Google Scholar]
- 17.Al-Nassir WN, Sethi AK, Li Y, Pultz MJ, Riggs MM, Donskey CJ. 2008. Both oral metronidazole and oral vancomycin promote persistent overgrowth of vancomycin-resistant enterococci during treatment of Clostridium difficile-associated disease. Antimicrob Agents Chemother 52:2403–2406. doi: 10.1128/AAC.00090-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadkarni MA, Martin FE, Jacques NA, Hunter N. 2002. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 148:257–266. doi: 10.1099/00221287-148-1-257. [DOI] [PubMed] [Google Scholar]
- 19.Bernhard AE, Field KG. 2000. Identification of nonpoint sources of fecal pollution in coastal waters by using host-specific 16S ribosomal DNA genetic markers from fecal anaerobes. Appl Environ Microbiol 66:1587–1594. doi: 10.1128/AEM.66.4.1587-1594.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loy A, Maixner F, Wagner M, Horn M. 2007. probeBase—an online resource for rRNA-targeted oligonucleotide probes: new features 2007. Nucleic Acids Res 35:D800–D804. doi: 10.1093/nar/gkl856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartosch S, Fite A, Macfarlane GT, McMurdo ME. 2004. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl Environ Microbiol 70:3575–3581. doi: 10.1128/AEM.70.6.3575-3581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 23.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing; twenty-fifth informational supplement M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 24.Hookman P, Barkin JS. 2009. Clostridium difficile associated infection, diarrhea and colitis. World J Gastroenterol 15:1554–1580. doi: 10.3748/wjg.15.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louie TJ, Cannon K, Byrne B, Emery J, Ward L, Eyben M, Krulicki W. 2012. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Clin Infect Dis 55(Suppl 2):S132–S142. doi: 10.1093/cid/cis338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hurless K, Cadnum JL, Chesnel L, Deshpande A, Donskey CJ. 2013. Effect of surotomycin, a novel cyclic lipopeptide antibiotic, on intestinal colonization with vancomycin-resistant enterococci and Klebsiella pneumoniae in mice, poster F628. Abstr 53rd Annu Intersci Conf Antimicrob Agents Chemother. American Society for Microbiology, Washington, DC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cannon K, Byrne B, Happe J, Louie T. 2012. Enteric microbiome profiles during a phase 2 clinical trial of CB-183,315 or vancomycin for treatment of Clostridium difficile infection. Clin Microbiol Infect 18(Suppl 3):659. [Google Scholar]
- 28.Chesnel L, Sambol S, Gerding D, Patino H, Therne G, Silverman J. 2012. Treatment of CDAD with oral CB-183 315: time to recurrence, relapse and re-infection rates compared with vancomycin. Clin Microbiol Infect 18(Suppl 3):380. [Google Scholar]
- 29.Fujitani S, George WL, Morgan MA, Nichols S, Murthy AR. 2011. Implications for vancomycin-resistant Enterococcus colonization associated with Clostridium difficile infections. Am J Infect Control 39:188–193. doi: 10.1016/j.ajic.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 30.Nerandzic MM, Mullane K, Miller MA, Babakhani F, Donskey CJ. 2012. Reduced acquisition and overgrowth of vancomycin-resistant enterococci and Candida species in patients treated with fidaxomicin versus vancomycin for Clostridium difficile infection. Clin Infect Dis 55(Suppl 2):S121–S126. doi: 10.1093/cid/cis440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ubeda C, Taur Y, Jeng RR, Equinda MJ, Son T, Samstein M, Viale A, Socci ND, van den Brink MR, Kamboj M, Pamer EG. 2010. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes blood stream invasion in humans. J Clin Invest 120:4332–4341. doi: 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mascio CT, Chesnel L, Thorne G, Silverman JA. 2014. Surotomycin demonstrates low in vitro frequency of resistance and rapid bactericidal activity in Clostridium difficile, Enterococcus faecalis and Enterococcus faecium. Antimicrob Agents Chemother 58:3976–3982. doi: 10.1128/AAC.00124-14. [DOI] [PMC free article] [PubMed] [Google Scholar]