Abstract
The Clinical and Laboratory Standards Institute (CLSI) revised cefepime (CFP) breakpoints for Enterobacteriaceae in 2014, and MICs of 4 and 8 μg/ml were reclassified as susceptible-dose dependent (SDD). Pediatric dosing to provide therapeutic concentrations against SDD organisms has not been defined. CFP pharmacokinetics (PK) data from published pediatric studies were analyzed. Population PK parameters were determined using NONMEM, and Monte Carlo simulation was performed to determine an appropriate CFP dosage regimen for SDD organisms in children. A total of 664 CFP plasma concentrations from 91 neonates, infants, and children were included in this analysis. The median patient age was 1.0 month (interquartile range [IQR], 0.2 to 11.2 months). Serum creatinine (SCR) and postmenstrual age (PMA) were covariates in the final PK model. Simulations indicated that CFP dosing at 50 mg/kg every 8 h (q8h) (as 0.5-h intravenous [i.v.] infusions) will maintain free-CFP concentrations in serum of >4 and 8 μg/ml for >60% of the dose interval in 87.1% and 68.6% of pediatric patients (age, ≥30 days), respectively, and extending the i.v. infusion duration to 3 h results in 92.3% of patients with free-CFP levels above 8 μg/ml for >60% of the dose interval. CFP clearance (CL) is significantly correlated with PMA and SCR. A dose of 50 mg/kg of CFP every 8 to 12 h does not achieve adequate serum exposure for older children with serious infections caused by Gram-negative bacilli with a MIC of 8 μg/ml. Prolonged i.v. infusions may be useful for this population.
INTRODUCTION
Health care-associated infections (HAI), such as ventilator-associated pneumonia, catheter-associated urinary tract infection, surgical site infection, and catheter-related bloodstream infection, may lead to morbidity and mortality in children (1–3). Infections caused by Gram-negative bacilli (GNB) are a major cause of HAI, including infections caused by Pseudomonas aeruginosa and the Enterobacteriaceae, such as Klebsiella pneumoniae and Escherichia coli (4, 5). The incidence of multidrug-resistant GNB, based on the presence of extended-spectrum β-lactamases (ESBLs) and AmpC β-lactamases, is increasing globally (6–9). Infections caused by these organisms are associated with a poor prognosis (10, 11). Further complicating this clinical challenge is that development of new antibiotics effective against these important pathogens has been slow (12). It is important to use existing antibiotics effectively whenever possible, using pharmacokinetics (PK) and pharmacodynamics (PD) analyses. Cefepime (CFP), a fourth-generation cephalosporin, is widely used to treat infections caused by GNB in both adult and pediatric patients (13, 14). Several studies have shown the effectiveness and safety of CFP in treating urinary tract and lower respiratory infections in children (15, 16). Further, CFP is also an important choice to treat multidrug-resistant GNB, such as AmpC β-lactamase-producing strains and several strains of ESBL-producing organisms with MICs that correspond to clinically achievable plasma concentrations of CFP (17, 18). The Clinical and Laboratory Standards Institute (CLSI) had initially defined susceptible CFP MICs for Enterobacteriaceae as 8 μg/ml or less (19). This interpretation was based on providing a standard dose of CFP. However, clinical failures were noted for infections caused by isolates with CFP MICs of 8 μg/ml with the usual CFP doses originally approved by regulatory agencies (20). Thus, CLSI revised CFP breakpoints for Enterobacteriaceae in 2014 (21). The MICs of 4 and 8 μg/ml have been reclassified in the susceptible-dose-dependent (SDD) category (21). SDD interpretation is a new interpretive category for antibacterial susceptibility testing. This category implies that susceptibility of an isolate is dependent on the dosing regimen and the MIC. Higher doses or more frequent dosing that leads to higher systemic drug exposure is needed to treat the patients infected with an organism with higher MICs, classified as the SDD category (21). Pediatric CFP dosing to provide therapeutic antibiotic exposure against SDD organisms has not been defined. Thus, we performed a population PK study of CFP to assess the appropriate CFP dosing regimen for treating infections caused by SDD organisms in neonates, infants, and children.
(Part of this research was presented in part at the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 5 to 9 September 2014.)
MATERIALS AND METHODS
CFP PK from the data set of two published studies in pediatric patients with a suspected or proven infection and who received CFP intravenously (i.v.) were assembled for analysis (22, 23). In both studies, CFP concentrations were measured by a validated high-performance liquid chromatography method. Patients who received CFP solely by the intramuscular route were excluded. The data for samples that were below the quantifiable limit (BQL) were also excluded. The following information was collected: postnatal age (PNA), gestational age (GA), postmenstrual age (PMA), body weight, gender, serum creatinine (SCR), and CFP dose, dosing interval, and serum concentrations.
Pharmacokinetic analysis.
Population PK parameters were determined using NONMEM, version 7.2 (Icon Development Solutions, Ellicott City, MD), with the first-order conditional estimation with interaction (FOCE-I) subroutine. A two-compartment model (ADVAN 3 TRANS 3) was selected. The typical value of clearance (TVCL) was scaled allometrically by the subject weight (weight0.75), and the typical value of the steady-state volume of distribution (TVVss) was also scaled by subject weight (weight1.0) before evaluation of other covariates. The potential impact of clinical covariates on PK parameters was screened by a univariate, followed by a multivariate, analysis. Covariates that decreased the objective function by at least 3.84 (P < 0.05) were included in the multivariate analysis. The multivariate analysis utilized backward elimination, and covariates which decreased the objective function by more than 7.88 (P < 0.005) were retained. Empirical Bayesian estimates of individual patient PK parameters were generated from the final model using the POSTHOC subroutine. The appropriateness of the final model was confirmed by the bootstrap validation method (n = 1,000). Monte Carlo simulation (MCS) was performed using the final population PK model to determine appropriate CFP doses and intervals for SDD organisms. Joint distributions of age, weight, and serum creatinine from infants (including preterm) and children were used that included 59 different PNA/GA combinations. Term neonates (GA of 36 to 40 weeks) and preterm neonates (GA of 24 to 34 weeks) were included at 1, 7, 14, and 28 days of age. Older children, from 1 month to 18 years, were also included. Various doses and dosing intervals, such as 30 or 50 mg/kg and every 6 to 12 h (q6h to q12h) were tested. Standard and prolonged duration of infusion (30 min and 3 h, respectively) were also assessed. The primary pharmacodynamic target for CFP is percent time above MIC (TAM). We used 20% protein binding to calculate free cefepime in our MCS (24). For our analysis, we considered 60% free-drug TAM to represent a conservative target, reflecting nearly maximal bactericidal effects in an animal model, which we believe to be more appropriate for pediatric patient populations with some degree of immune compromise, such as neonates (25, 26). MCS was performed with 2,000 replications for each of the 59 different PNA/GA combinations (118,000 virtual subjects in total). The individual predicted steady-state concentrations at various time points during the dose interval from the Monte Carlo simulation were used to calculate the time above the MIC.
Statistical analysis.
Statistical analyses were performed by using the statistical software R, version 3.2.0. Correlations between PK parameters and covariates were evaluated by a Spearman test.
RESULTS
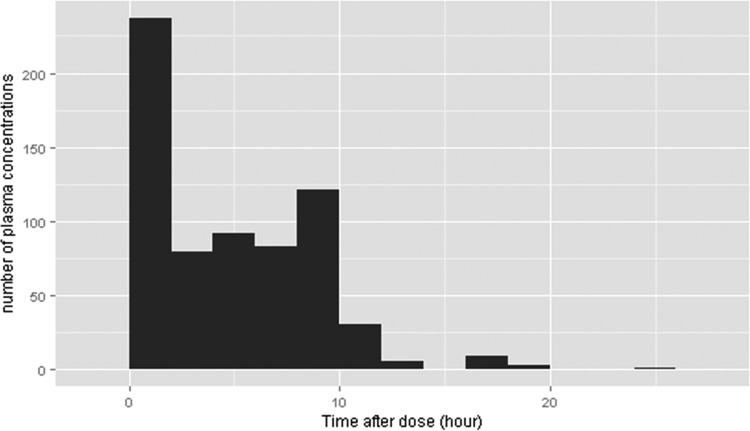
Patient characteristics are shown in Table 1. A total of 91 subjects had 725 plasma concentration measurements. Forty-eight (6.6%) plasma concentrations were collected after intramuscular CFP administration, and four (0.6%) that were BQL were excluded. Through the data-cleaning process, nine (1.2%) concentration values that were considered assay, sampling time, or transcription errors were excluded. Finally, 664 plasma concentrations were used in our analysis. The median patient age was 1 month, but the oldest patient was 16 years of age. The patient age distribution included a large portion of subjects under 1 year of age. Gestational age was available only for those patients under 2 months of age. In this patient population, many of patients were preterm, with a median GA of 29 weeks. The sampling time for measuring the CFP concentrations is shown in Fig. 1. The majority of samples were obtained within 10 h after CFP administration and included a high portion of samples obtained within 1 h to allow for characterization of the distribution phase.
TABLE 1.
Baseline characteristics
| Characteristic or statistic (n = 91) | Median value for the parameter | Range |
|---|---|---|
| Patient age (mo [IQR]) | 0.99 (0.23–11.19) | 0.03–197.30 |
| Age distribution (no. of patients [%]) | NAc | |
| 0–7 days | 25 (27.5) | |
| 8–29 days | 19 (20.9) | |
| 30 days–1 yr | 26 (28.6) | |
| ≥1 yr | 21 (23.1) | |
| No. (%) of preterm infantsa | 43 (78.2) | NA |
| No. (%) of term infantsb | 12 (21.8) | NA |
| Gestational age (weeks [IQR])b | 29.00 (26.05–33.00) | 22.10–42.29 |
| Gender female/male | NA | |
| No. (%) female patients | 31 (34.1) | |
| No. (%) male patients | 60 (65.9) | |
| Weight (kg [IQR]) | 3.10 (1.44–8.28) | 0.58–75.00 |
| Serum creatinine level (mg/dl [IQR]) | 0.60 (0.40–0.85) | 0.10–1.50 |
| Race (no. of patients [%]) | NA | |
| Caucasian | 37 (40.7) | |
| African American | 31 (34.1) | |
| Asian | 3 (3.3) | |
| Hispanic | 20 (22.0) |
Preterm, gestational age of <36 weeks.
For patients of postnatal age of over 63 days, information about gestational age was not available.
NA, not applicable.
FIG 1.
Distribution of sampling time.
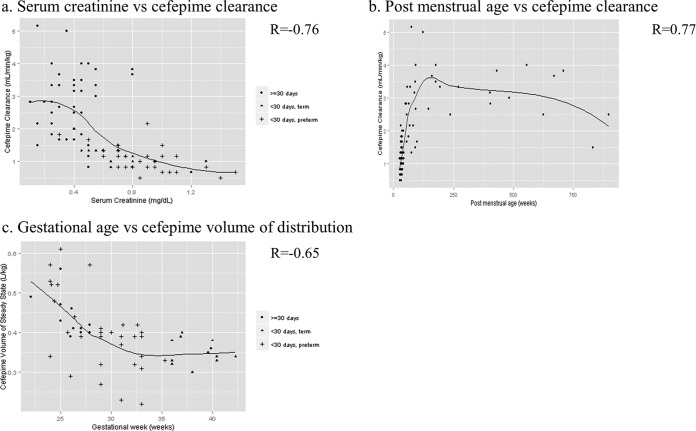
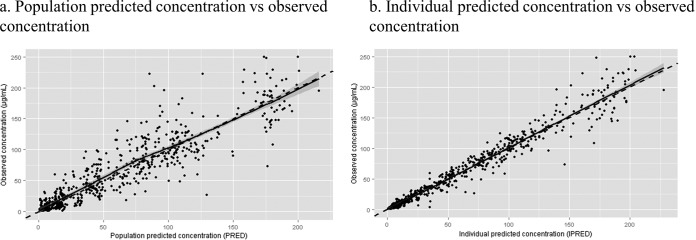
Table 2 shows the effect of each covariate on the base model, with each covariate added one by one into the base model. All covariates except gender significantly improved the model when linked to clearance (CL). Among them, PMA using a nonlinear effect on CL resulted in a better model than PMA using a linear effect; thus, nonlinear PMA was selected. For steady-state volume of distribution (Vss), only GA added a significant improvement to the model. Finally, the full model for multivariate analysis was constructed using these covariates: nonlinear PMA and SCR for CL and GA for Vss. Deleting each covariate one by one demonstrated that all of the covariates were significant. Therefore, this full model was determined as the final model. The relationship between CFP PK parameters from the final model with the noted covariates is shown in Fig. 2. The CL/kg correlated well with the patient's serum creatinine levels (R = −0.76) and PMA (R = 0.77). The age-dependent increase in CL reached a plateau around 2 to 3 years of age. A negative relation (R = −0.65) was found between GA and Vss/kg. The final population PK model is shown in Table 3. The CFP CL/kg and Vss/kg for typical age groups were calculated by using this model. Typical CL for the age groups were 1.12, 3.33, and 2.42 ml/min/kg for preterm infants (PMA, 32 weeks [GA, 30 weeks; PNA, 14 days]), toddlers (age 2 years), and teens (age 14 years), respectively. We also summarized the PK profile of CFP in different age groups in Table 4. Last, the relationship between the observed concentration and the predicted concentration in the final model is shown in Fig. 3. These values are distributed equally, indicating that the model describes CFP PK data well and without bias. The bootstrap validation was performed for checking the stability and reproducibility of this model, and the results are summarized in Table 5. The successful rate of each run was over 85%, and the median of each model parameter was similar to the respective parameter from the final model. Also the 95% confidence intervals of these parameters from the bootstrap validation excluded 0, indicating accurate parameter estimation.
TABLE 2.
The impact of each covariate
| Analysis method and covariate groupa | Nameb | Δ in objective function |
|---|---|---|
| Univariate analysis (covariate added) | ||
| CL | SCR | −50.49 |
| GA | −69.07 | |
| PNA | −111.31 | |
| PMA | −93.51 | |
| PNA-NL | −122.87 | |
| PMA-NL | −146.78 | |
| Gender | −3.10 | |
| Vss | SCR | −2.62 |
| GA | −11.56 | |
| PNA | −0.14 | |
| PMA | −0.72 | |
| Gender | −0.00 | |
| Multivariate analysis (covariate removed) | ||
| CL | SCR | 17.06 |
| PMA-NL | 110.90 | |
| Vss | GA | 9.43 |
CL, clearance; Vss, volume of steady state.
SCR, serum creatinine; GA, gestational age; PNA, postnatal age; PMA, postmenstrual age; NL, nonlinear.
FIG 2.
Relationship between pharmacokinetic parameters and covariates. (a) Serum creatinine versus cefepime clearance. (b) Postmenstrual age versus cefepime clearance. (c) Gestational age versus cefepime volume of distribution. R is calculated by the Spearman test.
TABLE 3.
Final population pharmacokinetic model
| Modela | Estimated levelb |
|---|---|
| CL (liters/h) | 0.395 × [−0.09 + 1.09 × {1 − exp(−0.00958 × PMA)}] × wt0.75 × (SCR/0.6)−0.392 |
| Vss (liters) | 0.406 × wt × (GA/30)−0.548 |
| Intersubject variability (%) | |
| CL | 31.8 |
| Vss | 22.2 |
| Residual variability (%) | 66.3 |
CL, clearance; Vss, volume distribution at steady state.
PMA, postmenstrual age (weeks); wt, weight (kg); GA, gestational age; SCR; serum creatinine.
TABLE 4.
Pharmacokinetic parameters by different age group
| Parametera | Mean value (±SD) by patient groupb |
|||
|---|---|---|---|---|
| All ages | Pediatric | Term | Preterm | |
| No. of patients | 91 | 47 | 12 | 32 |
| CL (ml/min/kg) | 1.86 ± 1.12 | 2.59 ± 1.10 | 1.24 ± 0.30 | 1.03 ± 0.40 |
| Vss (liters/kg) | 0.38 ± 0.08 | 0.37 ± 0.07 | 0.35 ± 0.03 | 0.40 ± 0.10 |
| t1/2(β) (h) | 3.53 ± 2.06 | 2.45 ± 1.77 | 3.59 ± 0.61 | 5.09 ± 1.80 |
| Css min (μg/ml)b | 16.07 ± 18.77 | 6.38 ± 13.40 | 15.42 ± 6.72 | 30.56 ± 19.52 |
| Css max (μg/ml)b | 181.04 ± 27.50 | 171.89 ± 23.47 | 192.92 ± 19.64 | 190.02 ± 31.25 |
CL, clearance; Vss, volume of distribution at steady state; Css min, minimum concentration at steady state; Css max, maximum concentration at steady state; GA, gestational age; t1/2(β), elimination phase half-life.
Pediatric, age of ≥30 days; term, age of <30 days and GA of ≥36 weeks; preterm, age of <30 days and GA of <36 weeks. Actual milligram doses that patients received were used for estimation. Dosing intervals were set as q12h for estimation. Actual milligram doses that patients received were as follows: all patients, 48.8 ± 4.7 mg/kg/dose; pediatric patients, 48.2 ± 5.9 mg/kg/dose; term patients, 49.9 ± 0.2 mg/kg/dose; preterm patients, 49.2 ± 3.3 mg/kg/dose.
FIG 3.
Final population model's goodness of fit. (a) Population predicted concentration versus observed concentration. (b) Individual predicted concentration versus observed concentration. In each panel, the solid line represents the smoothed regression of the data, and the dashed line represents the line of identity.
TABLE 5.
Bootstrap validation of the population pharmacokinetic parameters
| Model parameterb | Final estimate ± SE | Bootstrap validation (n = 1,000)a |
|---|---|---|
| θ1 (liters/h/kg0.75) | 0.395 ± 0.049 | 0.396 (0.315 to 0.550) |
| θ2 (liters/kg) | 0.406 ± 0.023 | 0.404 (0.361 to 0.451) |
| θ3 | 0.460 ± 0.020 | 0.452 (0.002 to 0.487) |
| θ4 (liters/h/kg0.75) | 0.575 ± 0.049 | 0.580 (0.488 to 2.570) |
| θ5 | 0.705 ± 0.039 | 0.705 (0.598 to 0.785) |
| θ6 | 1.09 ± 0.087 | 1.09 (0.945 to 1.320) |
| θ7 | 0.010 ± 0.003 | 0.010 (0.004 to 0.016) |
| θ8 | −0.392 ± 0.101 | −0.396 (−0.620 to −0.191) |
| θ9 | −0.548 ± 0.221 | −0.555 (−1.01 to −0.106) |
| %ωCL | 31.80 ± 12.90 | 30.6 (25.1 to 35.8) |
| %ωVss | 22.20 ± 12.60 | 21.1 (14.1 to 28.7) |
| %σ | 66.30 ± 37.30 | 66.1 (48.6 to 98.8) |
Values are medians (2.5th to 97.5th percentile). The success rate was 86.9%.
θ1, TVCL after maturation; θ2, TVVss; θ3, ratio of TVV/TVVss; θ4, typical value of intercompartmental clearance; θ5, fraction of additive and proportional error; θ6, fraction of mature CL at birth; θ7, rate constant for CL gain; θ8, exponent for serum creatinine for CL; θ9, exponent for gestational age for Vss. CL, clearance; Vss, steady-state volume of distribution.
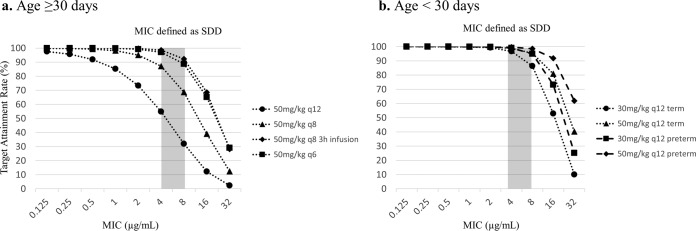
The Monte Carlo simulation of various CFP dosages was performed to evaluate the appropriate dosing strategy for SDD organisms. The target attainment rates of 60% TAM for various MIC values are shown in Fig. 4 and Table S1 in the supplemental material. The free-CFP concentrations were calculated using the value of 20% protein binding (24). For preterm (GA of <36 weeks) neonates less than 30 days of age, CFP doses of 30 and 50 mg/kg q12h easily achieved >60% TAM for a MIC of 8 μg/ml, with a target attainment rate exceeding 94% at both doses. For term (GA of ≥36 weeks) neonates less than 30 days of age, CFP doses of 30 and 50 mg/kg q12h achieved >60% TAM for a MIC of 8 μg/ml, with target attainment rates of 86.4 and 95.3%, respectively. However, for patients older than 30 days of age, the target attainment rate for 60% TAM of CFP at doses of 50 mg/kg q12h and 50 mg/kg q8h for pathogens with a CFP MIC of 8 μg/ml were only 32.0% and 68.6%, respectively. In this age group, a dose of 50 mg/kg q8h as a prolonged infusion of 3 h provides sufficient antimicrobial exposure, achieving a greater than 90% target attainment rate for 60% TAM at the MIC of 8 μg/ml. We also summarize the target attainment rates for different percent TAM targets for a MIC of 8 μg/ml in Fig. S1 in the supplemental material. The dosing regimen of 50 mg/kg q12h for term neonates was still sufficient to achieve an attainment rate of approximately 90% when the need for 70% TAM was assessed, while premature infants achieved an attainment rate of 96% using this dosing regimen.
FIG 4.
Monte Carlo simulation evaluation of 60% time above MIC for various dosages, intervals, and infusion rates. (a) Age of ≥30 days. (b) Age of <30 days. SDD, susceptible-dose dependent.
DISCUSSION
CFP has an important role in the treatment of bacterial infection caused by GNB in neonates, infants, and children. Recently, CLSI revised the CFP breakpoint based on treatment failure for organisms with MICs of 4 to 8 μg/ml. CLSI has classified MICs of 4 and 8 μg/ml as SDD (21). The CLSI recommends using the highest doses of CFP originally approved by the FDA for empirical therapy of febrile neutropenia to treat organisms with a MIC of 8 μg/ml (2 g i.v. q8h) (20, 21). However, PK/pharmacodynamics (PD) analysis in children was required to define the appropriate dosing regimen for GNB with a MIC of 4 or 8 μg/ml, realizing that children will often have greater renal clearance of β-lactams than adults. Cephalosporin antibiotics show time-dependent bacterial killing, and assessment of percent TAM is used as an indicator of clinical efficacy. However, specific targets of percent TAM for each cephalosporin and each patient group are still not well defined and require assessments of factors that may impact host response to the infection, such as age and immune status, and factors that impact the treating clinician's willingness to take a risk of treatment failure (e.g., the severity of infection). To achieve the maximum bactericidal effect in animal models, 60 to 70% TAM is required, particularly for cephalosporins (27, 28), and several recent articles used 60% TAM of β-lactams as their target (25, 29, 30). Therefore, we also used this value in our current analysis. However, these targets have not been prospectively evaluated in clinical trials in children, and a further prospective study is needed to determine the appropriate target percent TAM for each of the various pediatric populations.
CFP is usually prescribed as 50 mg/kg q8h to q12h for infants and children and 30 to 50 mg/kg q8h to q12h for neonates (31). For young infants older than 30 days with greater CFP CL values, Monte Carlo simulation shows that the FDA-approved usual maximum dosage regimen of CFP, 50 mg/kg q8h infused over 30 min, did not provide a sufficient attainment rate of 60% TAM for organisms with a MIC of ≥8 μg/ml. Based on several reports indicating a poor prognosis of infections caused by organisms with a higher CFP MIC (20, 32), there is clearly a need to revise the treatment options for infections caused by these organisms. On the other hand, sufficiently high target attainment rates were observed for neonates with a relatively low CFP CL with infections caused by organisms with a MIC of 8 μg/ml. Currently existing dosage regimens of 50 mg/kg q12h achieved a target attainment rate of approximately 90% when the need for 70% TAM was assessed. This dosing regimen might be appropriate to treat organisms with a MIC of 4 to 8 μg/ml in neonates.
CFP is usually administered intravenously using an infusion of 30 min. However, if dosing achieves an insufficient TAM, it is important to consider the use of prolonged or continuous i.v. CFP infusion without altering the daily mg/kg dose to provide an increase in the TAM to one associated with clinical and microbiologic cure. By using a prolonged 3-h i.v. infusion of 50 mg/kg q8h, we showed high rates of target attainment in assessing the 60% TAM for children over 30 days of age who had an infection caused by an organism with a CFP MIC of 8 μg/ml. Although the clinical utility of prolonged β-lactam antibiotic infusions in pediatric patients is still unclear (33), we believe that prolonged infusion of CFP for pediatric patients might be useful for infections caused by organisms with MICs of 8 μg/ml.
Our analysis demonstrated the age-dependent change of CFP CL. CFP is primarily eliminated in the urine, and renal function is critical in best evaluating the CFP PK profile. Glomerular filtration rate (GFR) rapidly develops after birth and reaches a similar level as that of adults around the age of 2 years of life. Indeed, weight-adjusted CFP CL (ml/min/kg) reached a maximal value around 2 to 3 years of age in our evaluation. The same phenomenon has been observed for other antibiotics. Madigan et al. reported that vancomycin trough levels were lower in patients 2 to 5 years of age than in patients over 6 years of age with the same dosing regimen (34). Therefore, by 2 years of age, children might require higher doses, more frequent dosing, or more prolonged infusion regimens to maintain sufficient drug exposure, particularly for pathogens with higher MICs.
We summarize the CFP PK profile in different pediatric age groups based on the published literature (Table 6). Lima-Rogel et al. reported PK of cefepime from neonates and young infants (35). Their CL, Vss, and half-life values were similar to those in neonates in our study. Pediatric patients in this study showed a higher CL value than the adult population (36).
TABLE 6.
Comparison of pharmacokinetic date between current and reported studies
| Parametera | Mean value (±SD) by method and patient age and gender |
||||||
|---|---|---|---|---|---|---|---|
| Population PK |
Noncompartmental method (Barbhaiya et al. [36]) |
||||||
| Current study |
Lima-Rogel et al. (35) | Young adult (n = 24)f |
Elderly adult (n = 24)g |
||||
| ≥30 days (n = 47) | <30 days (n = 44) | 6–58 days (n = 31) | Male | Female | Male | Female | |
| CL (ml/min/kg) | 2.59 ± 1.10 | 1.08 ± 0.38 | 1.20 ± 0.49 | 1.54 ± 0.22 | 1.56 ± 0.22 | 1.11 ± 0.12 | 1.22 ± 0.19 |
| V or Vss (liters/kg) | 0.37 ± 0.07 | 0.39 ± 0.09 | 0.41 ± 0.12 | 0.21 ± 0.02 | 0.21 ± 0.02 | 0.23 ± 0.03 | 0.24 ± 0.06 |
| t1/2 (h) | 2.45 ± 1.77c | 4.68 ± 1.69c | 4.32 ± 1.8 | 2.26 ± 0.51 | 2.15 ± 0.33 | 3.05 ± 0.50 | 2.92 ± 0.38 |
| Css min (μg/ml)b | 6.38 ± 13.40 | 26.43 ± 18.24 | 18.39 ± 13.3e | NAd | |||
| Css max (μg/ml)b | 171.89 ± 23.47 | 190.81 ± 28.36 | 120.9 ± 38.5e | ||||
CL, clearance; V, volume of distribution; Vss; steady-state volume of distribution; Css min, minimum concentration at steady state; Css max, maximum concentration at steady state; t1/2, half-life.
Actual milligram doses that patients received were used for estimation. Dosing intervals were set as q12h for estimation. Actual milligram doses that patients received were 48.2 ± 5.9 mg/kg/dose (pediatric; age ≥30 days) and 49.4 ± 2.8 mg/kg/dose (neonates; age <30 days).
Elimination half-life value.
NA, not applicable.
Dose of 50 mg/kg q12h.
Mean age, 31.5 years.
Mean age, 68 years.
Our analysis has several limitations. First, our analysis was skewed to patients aged less than 12 months. To build a good prediction model about age, the patient's age distribution should be equally balanced. Second, external validation has not been performed in this study. Third, we performed PK analysis with only a Monte Carlo simulation and did not include the clinical outcomes in this analysis. Thus, we could not evaluate if a difference in clinical outcome depends on the various dosage regimens. Further prospective studies are needed to verify the utility of prolonged infusion for the infection of Enterobacteriaceae with MICs of ≥8 μg/ml.
In conclusion, CFP CL is strongly influenced by PMA and SCR. In our analysis, the standard dosage (50 mg/kg q8h to q12h) of CFP may not be sufficient for children older than 30 days who have severe infections caused by GNB with MICs of ≥8 μg/ml.
Supplementary Material
ACKNOWLEDGMENTS
Author contributions were as follows: K.S. analyzed data and wrote the manuscript; J.S.B, M.D.R, J.N.V.D.A., and C.D were involved in study design, data generation, and revision of the manuscript. E.V.C initiated, conceptualized, and supervised the study and wrote the manuscript together with K.S. All authors approved the final manuscript as submitted.
This study was supported by the National Center for Child Health and Development (NCCHD; grant NCCHD27-6 to K.S.) and the National Institute of Child Health and Human Development (NICHD; grant U54HD071601 to J.N.V.D.A. and U54HD071600 to J.S.B. and E.V.C.).
The funders (NCCHD and NICHD) had no role in study design, data collection and interpretation, or the decision to submit the work for publication
E.V.C has served as a consultant to Gilead and on a Drug and Safety Monitoring Board (DSMB) for The Medicines Company. He also has served as a consultant to Alexion and on a DSMB for Cempra. None of the other authors has any conflicts of interest to report.
Funding Statement
The funders (NCCHD and NICHD) had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02592-15.
REFERENCES
- 1.Urrea M, Pons M, Serra M, Latorre C, Palomeque A. 2003. Prospective incidence study of nosocomial infections in a pediatric intensive care unit. Pediatr Infect Dis J 22:490–494. doi: 10.1097/01.inf.0000069758.00079.d3. [DOI] [PubMed] [Google Scholar]
- 2.Cavalcante SS, Mota E, Silva LR, Teixeira LF, Cavalcante LB. 2006. Risk factors for developing nosocomial infections among pediatric patients. Pediatr Infect Dis J 25:438–445. doi: 10.1097/01.inf.0000217377.54597.92. [DOI] [PubMed] [Google Scholar]
- 3.Urrea M, Iriondo M, Thio M, Krauel X, Serra M, LaTorre C, Jimenez R. 2003. A prospective incidence study of nosocomial infections in a neonatal care unit. Am J Infect Control 31:505–507. doi: 10.1016/S0196-6553(03)00077-4. [DOI] [PubMed] [Google Scholar]
- 4.Peleg AY, Hooper DC. 2010. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med 362:1804–1813. doi: 10.1056/NEJMra0904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richards MJ, Edwards JR, Culver DH, Gaynes RP. 1999. Nosocomial infections in pediatric intensive care units in the United States. National Nosocomial Infections Surveillance System. Pediatrics 103:e39. [DOI] [PubMed] [Google Scholar]
- 6.Denisuik AJ, Lagace-Wiens PR, Pitout JD, Mulvey MR, Simner PJ, Tailor F, Karlowsky JA, Hoban DJ, Adam HJ, Zhanel GG, Canadian Antimicrobial Resistance A . 2013. Molecular epidemiology of extended-spectrum beta-lactamase-, AmpC beta-lactamase- and carbapenemase-producing Escherichia coli and Klebsiella pneumoniae isolated from Canadian hospitals over a 5 year period: CANWARD 2007-11. J Antimicrob Chemother 68(Suppl 1):i57–i65. doi: 10.1093/jac/dkt027. [DOI] [PubMed] [Google Scholar]
- 7.Shu JC, Chia JH, Kuo AJ, Su LH, Wu TL. 2010. A 7-year surveillance for ESBL-producing Escherichia coli and Klebsiella pneumoniae at a university hospital in Taiwan: the increase of CTX-M-15 in the ICU. Epidemiol Infect 138:253–263. doi: 10.1017/S0950268809990409. [DOI] [PubMed] [Google Scholar]
- 8.Ding H, Yang Y, Lu Q, Wang Y, Chen Y, Deng L, Wang A, Deng Q, Zhang H, Wang C, Liu L, Xu X, Wang L, Shen X. 2008. The prevalence of plasmid-mediated AmpC beta-lactamases among clinical isolates of Escherichia coli and Klebsiella pneumoniae from five children's hospitals in China. Eur J Clin Microbiol Infect Dis 27:915–921. doi: 10.1007/s10096-008-0532-4. [DOI] [PubMed] [Google Scholar]
- 9.Park MJ, Kim TK, Song W, Kim JS, Kim HS, Lee J. 2013. An increase in the clinical isolation of acquired AmpC beta-lactamase-producing Klebsiella pneumoniae in Korea from 2007 to 2010. Ann Lab Med 33:353–355. doi: 10.3343/alm.2013.33.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melzer M, Petersen I. 2007. Mortality following bacteraemic infection caused by extended spectrum beta-lactamase (ESBL) producing E. coli compared to non-ESBL producing E. coli. J Infect 55:254–259. doi: 10.1016/j.jinf.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Folgori L, Livadiotti S, Carletti M, Bielicki J, Pontrelli G, Ciofi Degli Atti ML, Bertaina C, Lucignano B, Ranno S, Carretto E, Muraca M, Sharland M, Bernaschi P. 2014. Epidemiology and clinical outcomes of multidrug-resistant, gram-negative bloodstream infections in a European tertiary pediatric hospital during a 12-month period. Pediatr Infect Dis J 33:929–932. doi: 10.1097/INF.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 12.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 13.Adderson EE, Flynn PM, Hoffman JM. 2010. Efficacy and safety of cefepime in pediatric patients: a systematic review and meta-analysis. J Pediatr 157:490–495, 495 e491. doi: 10.1016/j.jpeds.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 14.Endimiani A, Perez F, Bonomo RA. 2008. Cefepime: a reappraisal in an era of increasing antimicrobial resistance. Expert Rev Anti Infect Ther 6:805–824. doi: 10.1586/14787210.6.6.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arrieta AC, Bradley JS. 2001. Empiric use of cefepime in the treatment of serious urinary tract infections in children. Pediatr Infect Dis J 20:350–355. doi: 10.1097/00006454-200103000-00034. [DOI] [PubMed] [Google Scholar]
- 16.Bradley JS, Arrieta A. 2001. Empiric use of cefepime in the treatment of lower respiratory tract infections in children. Pediatr Infect Dis J 20:343–349. doi: 10.1097/00006454-200103000-00033. [DOI] [PubMed] [Google Scholar]
- 17.Tamma PD, Girdwood SC, Gopaul R, Tekle T, Roberts AA, Harris AD, Cosgrove SE, Carroll KC. 2013. The use of cefepime for treating AmpC beta-lactamase-producing Enterobacteriaceae. Clin Infect Dis 57:781–788. doi: 10.1093/cid/cit395. [DOI] [PubMed] [Google Scholar]
- 18.Goethaert K, Van Looveren M, Lammens C, Jansens H, Baraniak A, Gniadkowski M, Van Herck K, Jorens PG, Demey HE, Ieven M, Bossaert L, Goossens H. 2006. High-dose cefepime as an alternative treatment for infections caused by TEM-24 ESBL-producing Enterobacter aerogenes in severely ill patients. Clin Microbiol Infect 12:56–62. doi: 10.1111/j.1469-0691.2005.01290.x. [DOI] [PubMed] [Google Scholar]
- 19.CLSI. 2012. Performance standards for antimicrobial susceptibility testing; twenty-second informational supplement. Document M100-S22. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 20.Bhat SV, Peleg AY, Lodise TP Jr, Shutt KA, Capitano B, Potoski BA, Paterson DL. 2007. Failure of current cefepime breakpoints to predict clinical outcomes of bacteremia caused by Gram-negative organisms. Antimicrob Agents Chemother 51:4390–4395. doi: 10.1128/AAC.01487-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CLSI. 2014. Performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement. Document M100-S24 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 22.Capparelli E, Hochwald C, Rasmussen M, Parham A, Bradley J, Moya F. 2005. Population pharmacokinetics of cefepime in the neonate. Antimicrob Agents Chemother 49:2760–2766. doi: 10.1128/AAC.49.7.2760-2766.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reed MD, Yamashita TS, Knupp CK, Veazey JM Jr, Blumer JL. 1997. Pharmacokinetics of intravenously and intramuscularly administered cefepime in infants and children. Antimicrob Agents Chemother 41:1783–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bristol-Myers Squibb. 2009. Maxipime prescribing information. Bristol-Myers Squibb, Princeton, NJ: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/050679s032lbl.pdf. [Google Scholar]
- 25.Bradley JS, Sauberan JB, Ambrose PG, Bhavnani SM, Rasmussen MR, Capparelli EV. 2008. Meropenem pharmacokinetics, pharmacodynamics, and Monte Carlo simulation in the neonate. Pediatr Infect Dis J 27:794–799. doi: 10.1097/INF.0b013e318170f8d2. [DOI] [PubMed] [Google Scholar]
- 26.Lodise TP, Lomaestro BM, Drusano GL, Society of Infectious Diseases Pharmacists. 2006. Application of antimicrobial pharmacodynamic concepts into clinical practice: focus on beta-lactam antibiotics: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 26:1320–1332. doi: 10.1592/phco.26.9.1320. [DOI] [PubMed] [Google Scholar]
- 27.Drusano GL. 2004. Antimicrobial pharmacodynamics: critical interactions of “bug and drug.” Nat Rev Microbiol 2:289–300. [DOI] [PubMed] [Google Scholar]
- 28.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 29.Zasowski E, Bland CM, Tam VH, Lodise TP. 2015. Identification of optimal renal dosage adjustments for high-dose extended-infusion cefepime dosing regimens in hospitalized patients. J Antimicrob Chemother 70:877–881. doi: 10.1093/jac/dku435. [DOI] [PubMed] [Google Scholar]
- 30.Rich BS, Keel R, Ho VP, Turbendian H, Afaneh CI, Dakin GF, Pomp A, Nicolau DP, Barie PS. 2012. Cefepime dosing in the morbidly obese patient population. Obes Surg 22:465–471. doi: 10.1007/s11695-011-0586-8. [DOI] [PubMed] [Google Scholar]
- 31.Bradley JS, Nelson JD. 2015. Nelson's pediatric antimicrobial therapy, 21st ed American Academy of Pediatrics, Elk Grove Village, IL. [Google Scholar]
- 32.Lee NY, Lee CC, Huang WH, Tsui KC, Hsueh PR, Ko WC. 2013. Cefepime therapy for monomicrobial bacteremia caused by cefepime-susceptible extended-spectrum beta-lactamase-producing Enterobacteriaceae: MIC matters. Clin Infect Dis 56:488–495. doi: 10.1093/cid/cis916. [DOI] [PubMed] [Google Scholar]
- 33.Walker MC, Lam WM, Manasco KB. 2012. Continuous and extended infusions of beta-lactam antibiotics in the pediatric population. Ann Pharmacother 46:1537–1546. doi: 10.1345/aph.1R216. [DOI] [PubMed] [Google Scholar]
- 34.Madigan T, Sieve RM, Graner KK, Banerjee R. 2013. The effect of age and weight on vancomycin serum trough concentrations in pediatric patients. Pharmacotherapy 33:1264–1272. doi: 10.1002/phar.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lima-Rogel V, Medina-Rojas EL, Del Carmen Milan-Segovia R, Noyola DE, Nieto-Aguirre K, Lopez-Delarosa A, Romano-Moreno S. 2008. Population pharmacokinetics of cefepime in neonates with severe nosocomial infections. J Clin Pharm Ther 33:295–306. doi: 10.1111/j.1365-2710.2008.00913.x. [DOI] [PubMed] [Google Scholar]
- 36.Barbhaiya RH, Knupp CA, Pittman KA. 1992. Effects of age and gender on pharmacokinetics of cefepime. Antimicrob Agents Chemother 36:1181–1185. doi: 10.1128/AAC.36.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.