Abstract
BACKGROUND
In patients undergoing percutaneous coronary intervention (PCI), drug-eluting stents (DES) reduce repeat revascularizations compared with bare metal stents (BMS), but their effects on death and myocardial infarction (MI) are mixed. Few studies have focused on patients with end-stage renal disease (ESRD).
OBJECTIVES
We compared mortality and cardiovascular morbidity during PCI with DES and with BMS in dialysis patients.
METHODS
We identified 36,117 dialysis patients from the U.S. Renal Data System who had coronary stenting in the U.S. between 4/23/03 and 12/31/10, and examined the association of DES versus BMS with 1-year outcomes: death; death or MI; and death, MI or repeat revascularization. We conducted a temporal analysis by dividing the study period into 3 DES eras: Transitional (4/23/03 – 6/30/04); Liberal (7/1/04 – 12/31/06); and Selective (1/1/07 – 12/31/10).
RESULTS
One-year event rates were high, with 38 deaths, 55 death or MI events and 71 death, MI or repeat revascularization events per 100 person-years. DES was associated with a significant 18% lower risk of death, 16% lower risk of death or MI, and 13% lower risk of death, MI or repeat revascularization, compared with BMS. DES use varied, from 56% in the Transitional era to 85% in the Liberal era and 62% in the Selective era. DES outcomes in the Liberal era were significantly better than in the Transitional Era, but not significantly better than in the Selective Era.
CONCLUSIONS
DES for PCI appears safe in U.S. dialysis patients, and is associated with lower rates of death, MI and repeat revascularization.
Keywords: Cardiovascular disease, end-stage renal disease, hemodialysis, ischemic heart disease, renal insufficiency, epidemiology
INTRODUCTION
Coronary heart disease affects 30 to 60% of patients with end-stage renal disease (ESRD) on dialysis (1–3), and the number of such patients undergoing percutaneous coronary intervention (PCI) has increased by nearly 50% over the past decade (4). Numerous clinical trials in patients with preserved kidney function demonstrate that drug-eluting stents (DES) reduce repeat revascularizations compared with bare metal stents (BMS), but effects on the risk of myocardial infarction (MI) and mortality are less consistent (5,6). A meta-analysis of 76 randomized clinical trials comparing DES and BMS showed that short-term risk of MI was reduced in DES relative to BMS, but with no benefit on short-term mortality (5). Pooled analysis of data from 3 randomized trials showed similar results consistently across different levels of kidney function, although patients with ESRD were excluded (6).
Current guidelines recommend using DES rather than BMS in patients with ESRD (7), based on extrapolation of data from involving patients with normal or near-normal kidney function. However, uremia, inflammation, or dialysis itself may lead patients with ESRD to have more diffuse coronary disease and more vascular calcification (1), making them more prone to post-procedural complications than patients without ESRD (8). There has been only 1 randomized clinical trial directly comparing DES with BMS that included patients with ESRD on dialysis, and it showed lower rates of target vessel revascularization at 12 months with DES (9). Only 22 participants in that trial had ESRD, and 1-year mortality was much lower than the national annual death rate. Those results may therefore not be generalizable to the overall ESRD population. Observational studies have yielded mixed results; some show a benefit of DES over BMS (8,10), while others do not (11–17). Many of the studies involved small cohorts or in largely Asian populations, or did not adequately adjust for potential confounders–perhaps accounting for some of the heterogeneity in outcomes.
Despite the paucity of evidence, use of DES rose rapidly in ESRD patients after approval of the first DES in the United States in 2003 (10,18). When reports suggested higher rates of MI, cardiovascular mortality and stent thrombosis with DES compared to BMS in 2006 (19–24), use of DES in patients with ESRD declined markedly until 2007, after which the use of DES stabilized following publication of studies showing longer term benefit and safety of DES (25).
We studied patients with ESRD on maintenance dialysis undergoing PCI with stenting, hypothesizing that DES would be associated with lower risks of death, MI and repeat revascularization compared with BMS. Due to concerns about residual confounding by indication in this study, we leveraged changing patterns of DES use in a temporal analysis that compared outcomes among 3 DES eras (26): Transitional (4/23/03–6/30/04); Liberal (7/1/04–12/31/06); and Selective (1/1/07–12/31/10). We hypothesized that patients undergoing PCI during the Liberal DES era would have better outcomes than patients in the 2 eras with more restrictive DES use.
METHODS
STUDY POPULATION
Data on all fee-for-service claims from Medicare Parts A and B were linked to the U.S. Renal Data System (USRDS), the national registry of patients with ESRD (27). As mandated in the Social Security Amendments of 1972 (28), almost all patients with ESRD qualify for federal health benefits through Medicare, irrespective of age or disability status.
Study patients were ≥18 years of age, had ESRD and underwent PCI with stenting (ascertained from International Classification of Diseases, Ninth Edition [ICD-9] procedure codes 36.00, 36.01, 36.02, 36.05, 36.06, 36.07, 36.09, or 00.66 reported in the institutional detail datasets) after approval of the first DES by the U.S. Food and Drug Administration on April 22, 2003 (Figure 1) and through December 31, 2010.
Figure 1. Cohort assembly flow diagram.
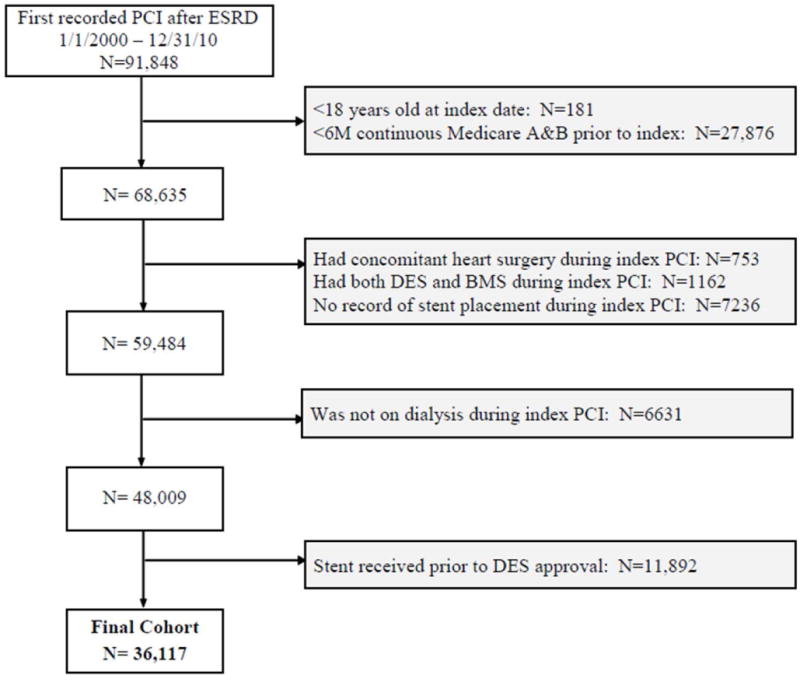
Assembly of cohort of patients with ESRD on dialysis who underwent percutaneous coronary intervention with stenting after drug-eluting stents became available in the United States.
For patients with multiple PCIs, the first recorded PCI was the index PCI. We required that patients have continuous Medicare Part A and B coverage as primary payer for at least 6 months prior to the index hospitalization admission date to allow determination of baseline comorbid conditions. Patients undergoing any type of heart surgery during the index hospitalization were excluded, as were patients who received both BMS and DES or who had no record of stent placement during the index PCI. All patients were required to be on maintenance dialysis during the index PCI, per USRDS-defined modality file (rxhist60).
FOLLOW-UP AND OUTCOMES
We examined 3 outcomes within 1-year after index PCI: 1) death from any cause, 2) death or MI; and 3) death, MI or repeat revascularization (Online Table 1). Death was determined from the USRDS. MI occurring during the index hospitalization was not considered an outcome, since it may have occurred prior to the revascularization. Because determination of MI and repeat revascularization required claims information, follow-up for composite outcomes was censored at the time of loss of Medicare Part A and B coverage. We conducted additional sensitivity analysis censoring patients at the time of loss of Medicare Part A and B coverage or kidney transplantation.
COVARIATES
Data included age, sex, race, Hispanic ethnicity, dialysis modality (hemodialysis or peritoneal), and presumed cause of ESRD from the USRDS patient and treatment history files at the index date. We included time since first treatment for ESRD (i.e. dialysis “vintage”) as a covariate, given its association with mortality and other outcomes in this patient population (29). We also included kidney transplant wait list status (obtained from the wait list file), because patients with an upcoming kidney transplant may be more likely to receive a BMS, which requires shorter duration of dual antiplatelet therapy. Wait list status is also an aggregate indicator of the overall health of the patient and associated with outcomes (30).
We defined comorbid conditions using ICD-9 diagnosis and procedure codes requiring at least 1 inpatient or 2 outpatient encounters separated by ≥ 1 day using all available historical data prior to (but not including) the index date (Online Table 1) (18), an approach that yields less bias than fixed observation windows (31). We used all inpatient and outpatient physician billing claims included in the USRDS institutional claims detail and physician supplier datasets. To adjust for differences in health care utilization (18), we identified the number of non-nephrology outpatient visits, number of hospitalized days, and nursing home stays for 6 months prior to the index date. We also categorized patients into 1 of 9 U.S. census regions based on the zip code in which they first received ESRD treatment.
STATISTICAL ANALYSIS
Baseline characteristics among patients who received BMS versus DES were compared using standardized differences (32), which are not influenced by sample size (32,33). A standardized difference of >10% represents meaningful imbalance between treatment groups.
We estimated exposure propensity scores (34) for each patient from a multivariable logistic regression model with receipt of BMS as the dependent variable that included as predictors all baseline variables listed in Table 1, plus census region. We then used the propensity scores in 2 ways. First, we applied a greedy matching algorithm (35) to tightly match 1 patient who received a BMS to 1 patient who received a DES (maximum difference in propensity scores between matched pairs = 0.1). We required that all matched pairs match exactly by index year (and thus did not include index year in the logistic regression model). Second, propensity scores were used to conduct inverse probability of treatment weighted (IPTW) estimation with stabilized weights (36,37). A strength of IPTW is that the entire cohort remains available for analysis, and results are generalizable to the entire population from which the observed sample was derived, in contrast to propensity score-matching which necessarily excludes the unmatched portion of the cohort (32).
Table 1.
Baseline characteristics of patients with ESRD on dialysis undergoing PCI with stenting in the overall cohort, and after propensity score-matching and applying the inverse probability of treatment weighting.
| Full Cohort | Propensity Score-Matched | Inverse Probability of Treatment Weighted | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| BMS | DES | Std Diff | BMS | DES | Std Diff | BMS | DES | Std Diff | |
|
| |||||||||
| N | 11202 | 24915 | 10751 | 10751 | 11202 | 24915 | |||
|
| |||||||||
| Demographics | |||||||||
| Age, mean (SD), years | 64.6 (12.1) | 64.0 (11.8) | 5.0 | 64.6 (12.1) | 64.5 (11.7) | 0.8 | 64.2 (11.9) | 64.2 (11.8) | 0.0 |
| Male | 58.3 | 55.3 | 6.1 | 58.1 | 58.0 | 0.3 | 56.5 | 56.2 | 0.6 |
| Race | |||||||||
| White | 64.2 | 64.2 | 0.02 | 64.3 | 64.8 | 1.1 | 65.1 | 64.4 | 1.5 |
| Black | 30.5 | 29.4 | 2.5 | 30.4 | 30.0 | 1.0 | 28.9 | 29.6 | 1.5 |
| Asian | 3.2 | 4.1 | 5.0 | 3.2 | 3.3 | 0.5 | 3.8 | 3.8 | 0.2 |
| Other/Unknown | 2.1 | 2.3 | 1.5 | 2.1 | 1.9 | 1.1 | 2.2 | 2.2 | 0.1 |
|
| |||||||||
| Peritoneal Dialysis | 7.2 | 7.2 | 0.2 | 7.1 | 7.1 | 0.0 | 7.0 | 7.2 | 0.6 |
|
| |||||||||
| Vintage, median (IQR), years | 3.1 (1.6–5.3) | 2.7 (1.4–4.8) | 10.0 | 3.0 (1.5–5.3) | 2.9 (1.5–5.2) | 0.0 | 2.9 (1.5–5.0) | 2.8 (1.4–5.0) | 0.0 |
|
| |||||||||
| Cause of end-stage renal disease | |||||||||
| Diabetes | 52.4 | 59.8 | 15.0 | 53.1 | 54.0 | 1.8 | 57.7 | 57.5 | 0.5 |
| Hypertension | 27.8 | 23.9 | 9.0 | 27.5 | 27.3 | 0.5 | 25.1 | 25.1 | 0.1 |
| Glomerulonephritis | 8.4 | 6.5 | 7.3 | 8.1 | 7.9 | 0.6 | 7.0 | 7.1 | 0.4 |
| Other/Unknown | 11.4 | 9.8 | 5.2 | 11.3 | 10.8 | 1.6 | 10.2 | 10.3 | 0.2 |
|
| |||||||||
| Non-nephrology outpatient visits, 6M prior, median (IQR) | 20 (12–31) | 21 (13–31) | 2.8 | 20 (12–31) | 20 (12–31) | 1.1 | 20 (12–31) | 20 (12–31) | 0.0 |
|
| |||||||||
| Hospital days in 6m prior, median (IQR) | 3 (1–10) | 3 (1–9) | 4.0 | 3 (1–10) | 3 (1–10) | 0.0 | 3 (1–10) | 3 (1–10) | 0.0 |
|
| |||||||||
| Any nursing home stay | 7.7 | 7.3 | 1.4 | 7.8 | 7.7 | 0.5 | 7.1 | 7.4 | 1.0 |
|
| |||||||||
| On kidney transplant waiting list | 15.3 | 15.1 | 0.5 | 15.2 | 14.9 | 0.9 | 15.2 | 15.2 | 0.1 |
|
| |||||||||
| Multivessel intervention | 13.2 | 17.4 | 11.6 | 13.6 | 13.7 | 0.2 | 15.8 | 16.1 | 0.6 |
|
| |||||||||
| Index Presentation | |||||||||
|
| |||||||||
| Stable coronary artery disease | 58.7 | 64.7 | 12.3 | 59.4 | 59.6 | 0.4 | 62.8 | 62.9 | 0.2 |
| ST-elevation MI | 10.7 | 5.9 | 17.2 | 9.7 | 8.7 | 3.2 | 7.4 | 7.4 | 0.2 |
| Non-ST elevation MI | 30.2 | 28.9 | 2.9 | 30.5 | 31.3 | 1.7 | 29.3 | 29.3 | 0.1 |
| Unstable angina | 0.4 | 0.5 | 1.0 | 0.4 | 0.4 | 0.4 | 0.5 | 0.5 | 0.1 |
|
| |||||||||
| Cardiovascular Comorbidities | |||||||||
|
| |||||||||
| CABG prior to index | 26.2 | 24.0 | 5.1 | 26.1 | 26.2 | 0.3 | 25.2 | 24.7 | 1.0 |
|
| |||||||||
| PCI prior to index | 13.2 | 15.8 | 7.2 | 13.4 | 13.9 | 1.3 | 15.5 | 15.0 | 1.3 |
|
| |||||||||
| Myocardial infarction | 53.6 | 48.4 | 10.3 | 52.9 | 53.3 | 0.7 | 50.1 | 50.0 | 0.3 |
|
| |||||||||
| Angina | 56.1 | 59.8 | 7.5 | 56.3 | 56.6 | 0.5 | 58.9 | 58.6 | 0.6 |
|
| |||||||||
| Heart failure | 78.5 | 78.5 | 0.1 | 78.6 | 79.0 | 1.0 | 78.9 | 78.5 | 0.8 |
|
| |||||||||
| Hypertension | 99.9 | 99.8 | 2.5 | 99.9 | 99.8 | 1.2 | 99.8 | 99.8 | 0.6 |
|
| |||||||||
| Atrial fibrillation | 30.2 | 27.1 | 7.0 | 30.1 | 29.8 | 0.7 | 27.9 | 28.0 | 0.1 |
|
| |||||||||
| Other arrhythmia | 34.1 | 30.8 | 7.0 | 34.0 | 33.8 | 0.3 | 31.7 | 31.8 | 0.2 |
|
| |||||||||
| Stroke/Transient Ischemic Attack | 25.3 | 24.5 | 1.9 | 25.4 | 26.4 | 2.1 | 25.0 | 24.7 | 0.7 |
|
| |||||||||
| Valvular disease | 45.8 | 42.4 | 6.8 | 45.8 | 45.4 | 0.7 | 43.9 | 43.5 | 0.9 |
|
| |||||||||
| Peripheral arterial disease | 56.4 | 55.9 | 0.9 | 56.6 | 56.8 | 0.4 | 56.2 | 56.1 | 0.3 |
|
| |||||||||
| Cerebrovascular disease | 25.9 | 25.7 | 0.4 | 26.1 | 26.6 | 1.1 | 25.8 | 25.8 | 0.1 |
|
| |||||||||
| Other comorbid conditions | |||||||||
|
| |||||||||
| Diabetes mellitus | 77.2 | 81.9 | 11.6 | 77.9 | 79.2 | 3.2 | 80.6 | 80.4 | 0.4 |
|
| |||||||||
| Hyperlipidemia | 72.6 | 74.2 | 3.7 | 73.2 | 74.3 | 2.4 | 73.9 | 73.7 | 0.4 |
|
| |||||||||
| Gastrointestinal bleeding | 31.1 | 27.7 | 7.6 | 30.8 | 30.5 | 0.6 | 28.9 | 28.7 | 0.3 |
|
| |||||||||
| Peptic ulcer disease | 8.8 | 7.6 | 4.3 | 8.6 | 8.7 | 0.2 | 7.9 | 7.9 | 0.1 |
|
| |||||||||
| Intracranial hemorrhage | 2.8 | 2.0 | 4.9 | 2.7 | 2.8 | 0.7 | 2.3 | 2.3 | 0.1 |
|
| |||||||||
| Liver disease | 17.3 | 15.6 | 4.5 | 17.2 | 17.2 | 0.0 | 16.2 | 16.1 | 0.1 |
|
| |||||||||
| Chronic lung disease | 47.3 | 45.1 | 4.5 | 47.4 | 48.0 | 1.3 | 46.3 | 45.8 | 1.0 |
|
| |||||||||
| Smoking history | 17.9 | 14.8 | 8.2 | 17.7 | 17.5 | 0.3 | 15.9 | 15.8 | 0.3 |
|
| |||||||||
| Dementia | 7.5 | 7.0 | 1.9 | 7.6 | 7.7 | 0.6 | 6.9 | 7.1 | 0.8 |
|
| |||||||||
| Depression | 23.3 | 22.7 | 1.5 | 23.3 | 24.4 | 2.4 | 22.7 | 22.9 | 0.3 |
|
| |||||||||
| Cancer | 15.1 | 12.9 | 6.3 | 14.9 | 14.6 | 0.9 | 13.6 | 13.6 | 0.01 |
|
| |||||||||
| Hypothyroid | 19.1 | 19.1 | 0.1 | 19.1 | 19.4 | 0.8 | 18.9 | 19.1 | 0.5 |
|
| |||||||||
| Obesity | 17.2 | 17.8 | 1.7 | 17.4 | 18.1 | 1.8 | 17.8 | 17.6 | 0.3 |
All values are % unless otherwise noted. Abbreviations: BMS = bare metal stent; DES = drug eluting stent; Std Diff – standardized differences; SD = standard deviation; IQR = interquartile range; MI = myocardial infarction; PCI = percutaneous coronary intervention; CABG = coronary artery bypass grafting;
We estimated hazard ratios (HR) and corresponding 95% confidence intervals (CI) for outcomes of interest using proportional hazards regression models. Because all baseline variables were well balanced (i.e. standardized differences <10%) in the IPTW and propensity-score-matched cohorts (Table 1), we made no further adjustments to the models. In the IPTW analyses, we used robust standard errors. We tested the proportionality assumption using Schoenfeld residual plots.
Given the possibility of residual confounding by indication, we conducted an analysis leveraging the temporal trends in DES use during the study period. Rather than directly compare DES with BMS use, we divided the cohort into 3 distinct DES eras based on previous studies (26) and based empirically on the proportion of DES use observed in our cohort: Transitional (4/23/03–6/30/04); Liberal (7/1/04–12/31/06); and Selective (1/1/07–12/31/10). We compared baseline characteristics in the Transitional and Selective eras with the Liberal era using standardized differences as above. Event rates among Transitional, Liberal and Selective DES eras were estimated using the Kaplan-Meier method and compared using the log-rank estimate. We used proportional hazards regression to compare outcomes in the Transitional and Selective eras relative to the Liberal DES era, adjusting for the variables listed in Table 2. Although previous studies used enrollment year or rates of DES use as an instrumental variable (38,39), we felt that DES era did not satisfy 2 key assumptions of a formal instrumental variable analysis: 1) that DES era was associated with outcomes only through its association with receipt of DES or BMS; and 2) that the instrumental variable randomizes patients such that patients are similar with respect to measured and unmeasured variables across levels of the instrument.
Table 2.
Baseline characteristics of patients with end-stage renal disease on dialysis undergoing percutaneous coronary intervention with stenting, stratified by drug-eluting stent era.
| Transitional 4/23/03–6/30/04 | Liberal 7/1/04–12/31/06 | Selective 1/1/07–12/31/10 | Std Diff Transition al vs Liberal | Std Diff Selective vs Liberal | |
|---|---|---|---|---|---|
|
| |||||
| N | 5372 | 12349 | 18396 | – | – |
|
| |||||
| Demographics | |||||
| Age, mean (SD), years | 63.8 (12.0) | 64.2 (11.9) | 64.2 (11.8) | 3.7 | 0.5 |
| Male | 55.1 | 56.0 | 56.7 | 1.8 | 1.4 |
| Race | |||||
| White | 64.3 | 63.4 | 64.8 | 1.8 | 2.8 |
| Black | 30.1 | 30.5 | 29.1 | 0.9 | 3.1 |
| Asian | 3.6 | 3.6 | 4.0 | 0.2 | 2.2 |
| Other/Unknown | 2.0 | 2.5 | 2.1 | 2.8 | 2.3 |
|
| |||||
| Peritoneal Dialysis | 7.8 | 7.1 | 7.1 | 2.8 | 0.1 |
|
| |||||
| Vintage, median (IQR), years | 2.7 (1.4–4.9) | 2.7 (1.4–4.8) | 2.9 (1.5–5.1) | 1.5 | 6.3 |
|
| |||||
| Cause of end-stage renal disease | |||||
| Diabetes | 56.4 | 57.6 | 57.8 | 2.5 | 0.2 |
| Hypertension | 25.6 | 24.8 | 25.2 | 1.8 | 1.0 |
| Glomerulonephritis | 8.3 | 7.2 | 6.6 | 4.1 | 2.3 |
| Other/Unknown | 9.7 | 10.4 | 10.4 | 2.1 | 0.0 |
|
| |||||
| Non-nephrology outpatient visits, 6M prior, median (IQR) | 20 (12–29) | 20 (12–31) | 21 (13–32) | 6.4 | 8.0 |
|
| |||||
| Hospital days in 6m prior, median (IQR) | 3 (1–10) | 3 (1–10) | 3 (1–10) | 0.1 | 0.0 |
|
| |||||
| Any nursing home stay | 5.7 | 7.1 | 8.2 | 5.8 | 4.3 |
|
| |||||
| On kidney transplant waiting list | 14.3 | 15.0 | 15.5 | 1.9 | 1.5 |
|
| |||||
| Multivessel intervention | 9.2 | 16.0 | 18.2 | 20.4 | 5.9 |
|
| |||||
| Index Presentation | |||||
|
| |||||
| Stable coronary artery disease | 67.3 | 66.8 | 58.9 | 0.9 | 16.5 |
| ST-elevation myocardial infarction | 8.2 | 6.9 | 7.6 | 4.9 | 2.7 |
| Non-ST elevation myocardial infarction | 24.1 | 25.8 | 33.1 | 4.0 | 16.2 |
| Unstable angina | 0.5 | 0.5 | 0.4 | 0.2 | 1.7 |
|
| |||||
| Cardiovascular Comorbidities | |||||
|
| |||||
| Coronary artery bypass grafting prior to index | 24.6 | 24.9 | 24.6 | 0.7 | 0.6 |
|
| |||||
| Percutaneous coronary intervention prior to index | 12.9 | 13.9 | 16.3 | 3.0 | 6.5 |
|
| |||||
| Myocardial infarction | 46.6 | 46.4 | 53.4 | 0.5 | 14.2 |
|
| |||||
| Angina | 60.9 | 60.6 | 56.7 | 0.6 | 7.9 |
|
| |||||
| Heart failure | 76.0 | 77.9 | 79.6 | 4.4 | 4.2 |
|
| |||||
| Hypertension | 99.5 | 99.8 | 99.9 | 4.4 | 2.6 |
|
| |||||
| Atrial fibrillation | 26.8 | 27.7 | 28.6 | 2.1 | 2.0 |
|
| |||||
| Other arrhythmia | 29.1 | 30.8 | 33.3 | 3.7 | 5.5 |
|
| |||||
| Stroke/Transient ischemic attack | 22.5 | 23.1 | 26.5 | 1.3 | 7.9 |
|
| |||||
| Valvular disease | 40.7 | 43.5 | 44.2 | 5.8 | 1.3 |
|
| |||||
| Peripheral arterial disease | 53.7 | 55.4 | 57.2 | 3.3 | 3.8 |
|
| |||||
| Cerebrovascular disease | 23.7 | 24.3 | 27.4 | 1.5 | 7.1 |
|
| |||||
| Other comorbid conditions | |||||
|
| |||||
| Diabetes mellitus | 76.9 | 79.1 | 82.4 | 5.4 | 8.4 |
|
| |||||
| Hyperlipidemia | 61.8 | 69.5 | 80.0 | 16.2 | 24.5 |
|
| |||||
| Gastrointestinal bleeding | 27.6 | 28.7 | 29.1 | 2.3 | 1.0 |
|
| |||||
| Peptic ulcer disease | 8.3 | 8.1 | 7.7 | 0.6 | 1.6 |
|
| |||||
| Intracranial hemorrhage | 1.7 | 1.9 | 2.7 | 1.1 | 5.7 |
|
| |||||
| Liver disease | 14.4 | 15.9 | 16.8 | 4.0 | 2.7 |
|
| |||||
| Chronic lung disease | 40.8 | 44.6 | 48.0 | 7.7 | 6.7 |
|
| |||||
| Smoking history | 13.6 | 15.1 | 16.9 | 4.3 | 4.9 |
|
| |||||
| Dementia | 5.2 | 6.6 | 8.0 | 5.8 | 5.5 |
|
| |||||
| Depression | 19.5 | 21.5 | 24.7 | 4.9 | 7.7 |
|
| |||||
| Cancer | 12.1 | 13.1 | 14.4 | 3.0 | 3.8 |
|
| |||||
| Hypothyroid | 17.1 | 18.1 | 20.3 | 2.6 | 5.4 |
|
| |||||
| Obesity | 12.7 | 15.6 | 20.4 | 8.3 | 12.4 |
All values are % unless otherwise noted. Abbreviations: SD = standard deviation; IQR = interquartile range;
The Institutional Review Board of Stanford University approved the study. All analyses were conducted using SAS Enterprise Guide 6.1 (Cary, North Carolina).
RESULTS
Overall, 36,117 patients on maintenance dialysis underwent PCI with stenting between 2003 and 2010 and passed inclusion and exclusion criteria (Figure 1). Sixty-nine percent of the final cohort received a DES. Mean age was 64 years, and there was a high prevalence of diabetes, hypertension and hyperlipidemia. Patients who received DES had higher prevalence of diabetes mellitus, but patients who received BMS more often had a prior history of MI, and more often presented with a ST-elevation MI (Table 1).
DRUG-ELUTING STENTS VERSUS BARE METAL STENTS
In the logistic regression model on stent type (c = 0.6; Hosmer and Lemeshow Goodness-of-Fit Test, p = 0.86; Online Table 2), female sex, multivessel intervention, and presence of heart failure and diabetes mellitus were associated with higher odds of receiving a BMS versus DES. We matched 96% of BMS patients to a corresponding DES patient. All baseline variables were well balanced among patients receiving BMS and DES after propensity score matching and after applying the IPTW (Table 1).
One-year event rates were high, with 38 deaths, 55 death or MI events and 71 death, MI or repeat revascularization events per 100 person-years. Unadjusted rates of death, death or MI and death, MI, or repeat revascularization were lower in DES patients compared to BMS patients (Table 3). In the propensity score-matched cohort, DES was associated with an 18% (CI, 14% to 22%) lower risk of death, 16% (CI, 13% to 19%) lower risk of death or MI, and 13% (CI, 9% to 16%) lower risk of death, MI or repeat revascularization compared with BMS (Central Illustration). These results were similar using the IPTW approach (Central Illustration), and were not materially changed in sensitivity analyses that censored patients at the time of kidney transplantation (data not shown).
Table 3.
Unadjusted 1-year event rates for specified outcomes in the full cohort and by drug-eluting stent era.
| Death | Death or Myocardial Infarction | Death, Myocardial Infarction or Repeat Revascularization | ||||
|---|---|---|---|---|---|---|
| N events | Events per 100 p-y | N events | Events per 100 p-y | N events | Events per 100 p-y | |
| Full cohort | 11086 | 37.8 | 14690 | 55.1 | 17493 | 71.2 |
| Drug-eluting stent | 7134 | 34.5 | 9600 | 51.0 | 11618 | 67.0 |
| Bare metal stent | 3952 | 45.5 | 5090 | 65.2 | 5875 | 81.3 |
| Propensity-Matched cohort | 7014 | 40.9 | 9179 | 59.1 | 10773 | 75.1 |
| Drug-eluting stent | 3233 | 36.7 | 4307 | 53.8 | 5151 | 69.7 |
| Bare metal stent | 3781 | 45.3 | 4872 | 64.8 | 5622 | 80.8 |
| Transitional Era | 1654 | 38.0 | 2187 | 55.2 | 2679 | 74.3 |
| Liberal Era | 3666 | 36.3 | 4759 | 51.1 | 5752 | 67.3 |
| Selective Era | 5766 | 38.7 | 7744 | 57.9 | 9062 | 73.1 |
TEMPORAL ANALYSIS: TRANSITIONAL, LIBERAL AND SELECTIVE DES ERAS
Average use of DES during PCI changed significantly over the study period, from 56% in the Transitional era, to 85% in the Liberal era and 62% in the Selective era (Figure 2). Patients who underwent PCI in the Transitional era were less likely to have a non-ST elevation MI on index presentation or require multivessel coronary intervention, and had fewer nursing home stays and lower prevalence of most comorbid conditions compared with patients undergoing PCI in the other 2 eras (Table 2).
Figure 2. Changing patterns of drug-eluting stent use in patients on dialysis from April 2003 – Dec 2010.
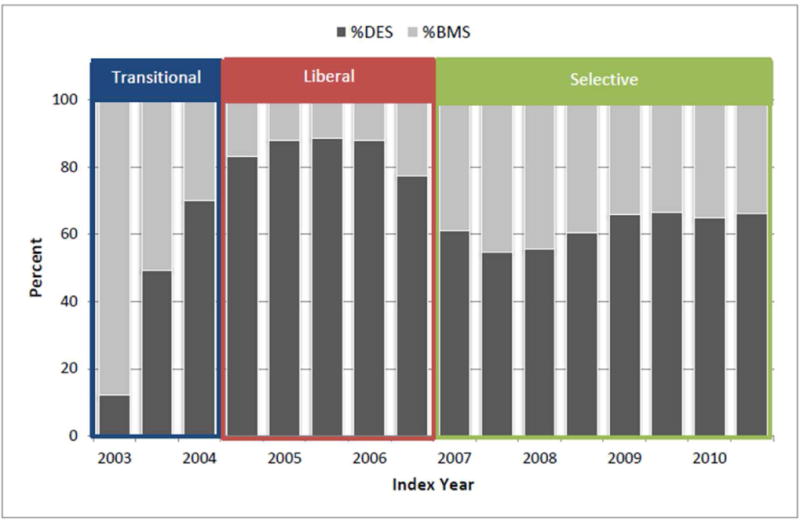
Proportion of patients with end-stage renal disease on dialysis receiving drug-eluting stents (DES) or bare metal stents (BMS) during percutaneous coronary intervention in 3 different drug-eluting stent eras: Transitional: 4/23/03–6/30/04; Liberal: 7/1/04–12/31/06; and Selective: 1/1/07–12/31/10.
In unadjusted analyses, patients who underwent PCI in the Liberal era tended to have lower rates of death, death or MI and death, MI or repeat revascularization than patients who underwent PCI in the Transitional or Selective eras (Table 3, Figure 3). After adjusting for baseline characteristics, outcomes for patients undergoing PCI in the Transitional era were consistently worse than for patients in the Liberal era, while the outcomes of PCI during the Liberal versus Selective eras were similar, with only the composite of death and MI being significantly worse in the Selective era (HR = 1.05; CI, 1.01–1.09; Figure 3). Results were not changed in sensitivity analyses that censored patients at the time of kidney transplantation (data not shown).
Figure 3. Temporal analysis comparing outcomes by drug-eluting stent era in patients on dialysis.
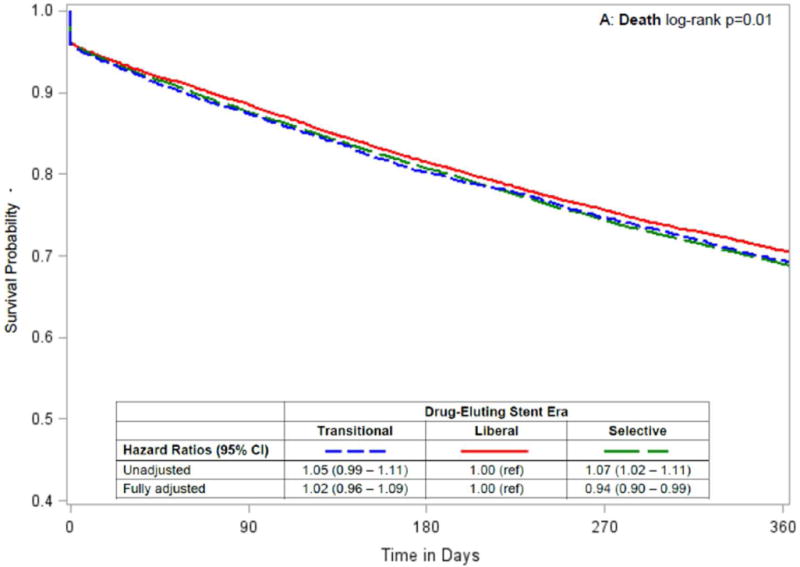
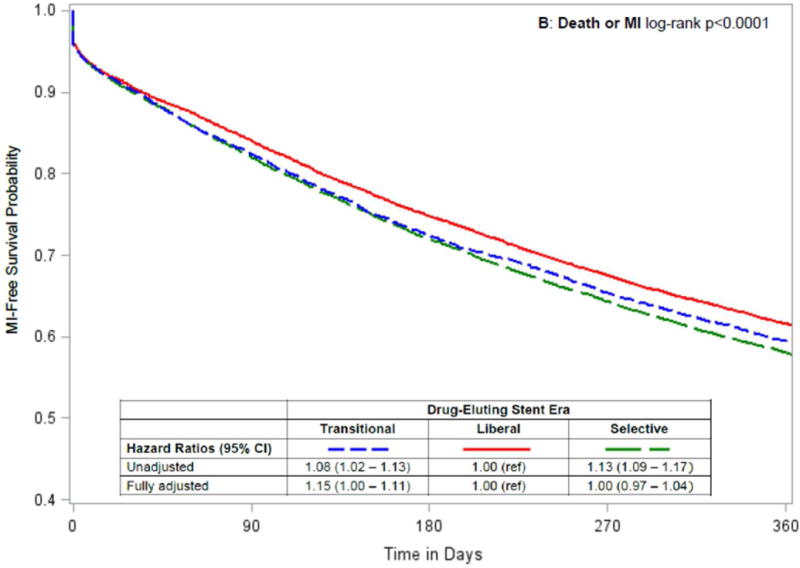
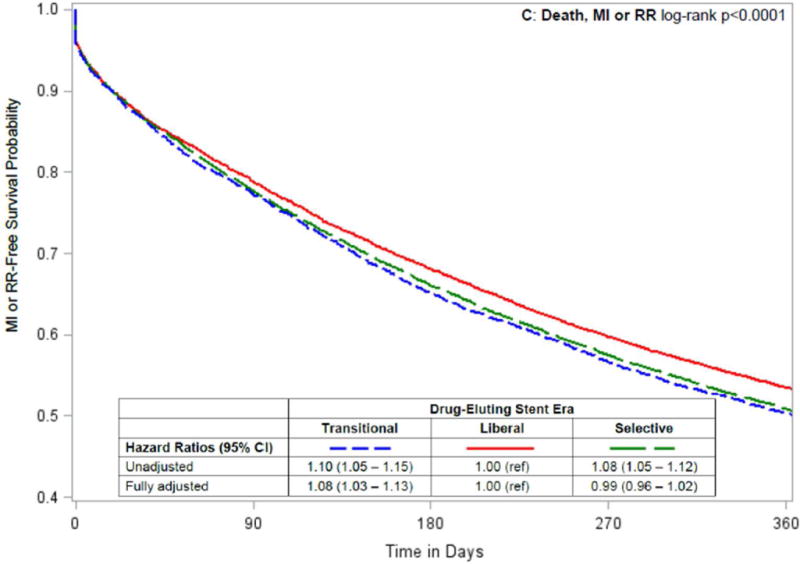
Kaplan-Meier curves by drug-eluting stent era, with unadjusted and adjusted hazard ratios (95% confidence intervals) comparing Transitional and Selective versus Liberal drug-eluting stent eras for the following 1-year outcomes after percutaneous coronary intervention: (A) death; (B) death or myocardial infarction [MI]; and (C) death, myocardial infarction or repeat revascularization [RR].
DISCUSSION
In this large, representative cohort of patients with ESRD on dialysis, use of DES rather than BMS during PCI was associated with 18% lower risk of death, 6% lower risk of death or MI and 13% lower risk of death, MI or repeat revascularization (Central Illustration). DES-associated reduction in need for repeat revascularization among patients with reduced kidney function was recently demonstrated in the RENAL-DES (Randomized Comparison of Xience V and Multi-Link Vision Coronary Stents in the Same Multivessel Patient with Chronic Kidney Disease) trial, which enrolled 215 patients with estimated creatinine clearance < 60 mL/min and multivessel coronary disease to receive DES or BMS (9). Mean creatinine clearance was 47 mL/min, with 10% of the cohort (N = 22) on dialysis. Results from RENAL-DES showed that the incidence of ischemia-driven target vessel revascularization at 12 months was 8.7% lower in the DES group (p <0.001). Differences were even larger for patients with a creatinine clearance < 30 mL/min or who were on dialysis (BMS = 24.2% vs. DES = 3.1%; absolute risk reduction = 21.1%; p = 0.005). However, the patient cohort in this trial was highly selected, reflected in its 1-year death rate of only 3.7%, which is much lower than the annual death rates for patients with chronic kidney disease and coronary heart disease overall (40). Our study extends the results of RENAL-DES to a population better reflecting patients treated in actual clinical practice.
Results from observational studies in ESRD examining outcomes of death and MI have been less consistent. Some smaller studies (sample size ranging from 74 to 505) in dialysis patients, conducted mostly in Asian cohorts, showed no differences in death with DES versus BMS (11–17), while 2 larger, U.S.-based observational studies in patients with ESRD (8,10) showed benefit to DES consistent with our results. One was a descriptive analysis using USRDS data that showed lower crude 1-year survival rates for patients on dialysis receiving a BMS (63%) than for patients receiving DES (71%) (10). The other included only older patients undergoing PCI, and showed a 15% (CI, 6% to 14%) lower risk of death with DES versus BMS in the subgroup of dialysis patients (8). Our results expand upon those findings by adjusting for potential confounders using propensity score-matching and IPTW analyses, and by studying a more diverse patient cohort.
Patients with ESRD on dialysis are at higher risk of serious bleeding events due to chronic heparin exposure, uremia-induced platelet dysfunction, and concomitant use of anticoagulants (41–43). Such patients are also more likely to discontinue clopidogrel or other antiplatelet agents prematurely (44), which can lead to in-stent thrombosis and subsequent MI (45). Concerns about adverse bleeding effects or medication non-adherence may lead physicians to choose BMS for sicker, less adherent patients, because DES requires a longer duration of dual antiplatelet therapy. We used different statistical approaches to account for these factors (i.e. propensity score-matching and IPTW), but as with any observational study comparing 2 treatment options, residual confounding remained a concern. Rather than directly compare outcomes associated with DES versus BMS, we leveraged changing provider preferences for DES versus BMS during the study period and conducted a temporal analysis by DES era. We found that patients undergoing PCI during the Transitional era when DES use was at its lowest (56% overall), had a 9% to 12% higher adjusted risk of death and cardiovascular events compared with PCI with stenting during the Liberal era, when DES use was at its highest (85% overall; Central Illustration). These results persisted despite the fact that patients in the Transitional era had a lower prevalence of most measured comorbid conditions and may have been expected to have better outcomes. In contrast, undergoing PCI in the Selective era (DES use 62% overall) compared with the Liberal era was not consistently associated with higher risks of death and cardiovascular outcomes, although patients in the Selective era had a higher prevalence of many comorbid conditions traditionally associated with poorer outcomes (e.g. diabetes mellitus, heart failure, peripheral arterial disease). In 2007 (the start of our Selective era), the recommended duration of dual antiplatelet therapy was extended for DES to at least 1 year and for BMS for up to 1 year (46), which may partly account for the lack of differences we observed in comparisons of the Selective and Liberal eras. Although not definitive, our results suggest no increased risk of death or MI with DES, an important finding, given the potential safety concerns in this population.
STUDY LIMITATIONS
Despite our use of complementary analytical strategies to test the robustness of our results, in the absence of randomization to DES or BMS, the potential for residual confounding remains. In addition, our analysis relied on administrative claims data and we did not have access to laboratory information (e.g., cardiac troponins), clinical variables (e.g., left ventricular ejection fraction), or coronary angiography data (e.g., lesion length and complexity). We cannot exclude the possibility that there were differences in the indication for any PCI (using BMS or DES) across the 3 eras and how such marginal patients may have influenced our results. While we were able to examine any repeat revascularization procedure, we were unable to distinguish among target vessel revascularization, target lesion revascularization, or a de novo revascularization. Further, we did not have information on the specific type of DES used (e.g., sirolimus-eluting, paclitaxel-eluting or a newer generation agent), which can differ in efficacy (5). Finally, we did not have information on the concomitant use of medical therapies, including antihypertensive agents and antiplatelet agents.
CONCLUSIONS
The use of DES in patients with ESRD on dialysis mirrored trends in the general population: rapidly increasing after its introduction to the U.S. market in mid-2003, decreasing in 2006 over concerns regarding higher risks of stent thrombosis, and stabilizing from 2007 onwards. Several analytical techniques suggest that DES was associated with better outcomes in terms of death and cardiovascular events compared with BMS in patients with ESRD on dialysis. Until a randomized clinical trial is conducted, our study provides additional evidence to support current guidelines recommending preferential use of DES over BMS in this high-risk patient population.
Supplementary Material
Central Illustration: Stent Type and Outcomes in Dialysis: Direct comparison of drug-eluting stents and bare metal stents in patients on dialysis.
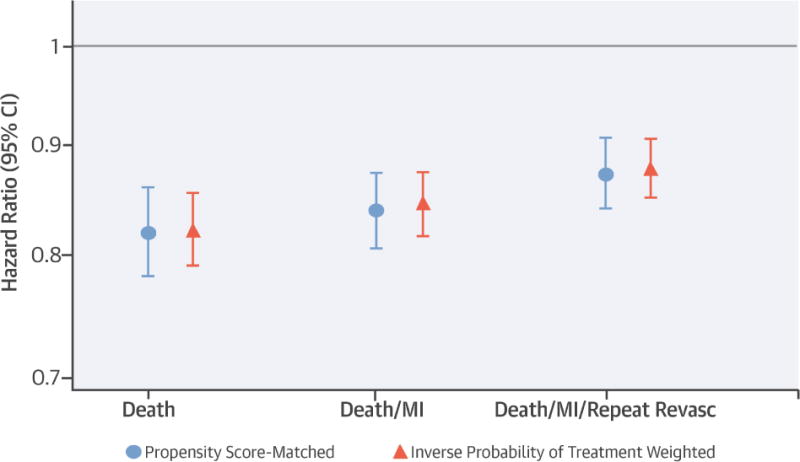
Hazard ratios comparing drug-eluting stents with bare-metal stents after percutaneous coronary intervention in patients with end-stage renal disease on dialysis for the specified outcomes using 2 different analytical approaches. Error bars represent 95% confidence intervals [CI]. Abbreviations: MI = myocardial infarction; Revasc = revascularization. Abbreviations: ESRD = end-stage renal disease on dialysis; PCI = percutaneous coronary intervention; DES = drug-eluting stent; BMS = bare metal stent.
PERSPECTIVES.
Competency in Medical Knowledge
Coronary artery disease is common in patients with end-stage renal disease (ESRD) on dialysis and rates of percutaneous coronary intervention with stenting have risen over the past decade.
Competency in Patient Care
Current guidelines recommend use of drug-eluting stents over bare metal stents in patients with ESRD, based largely on extrapolation of evidence from trials in patients without ESRD.
Translational Outlook
Future interventional studies should consider inclusion of patients across a broad range of kidney function, including patients with ESRD on dialysis.
Acknowledgments
This work was conducted under a data use agreement between Dr. Winkelmayer and the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK). An NIDDK officer reviewed this manuscript for research compliance and approved of its submission for publication. Data reported herein were supplied by the USRDS. Interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government.
Dr. Chang is supported by a grant from the NIDDK (5K23DK095914).
Funding: There are no relevant relationships with industry to report.
Abbreviations
- BMS
bare metal stent(s)
- CI
confidence interval
- DES
drug-eluting stent(s)
- ESRD
end-stage renal disease
- HR
hazard ratios
- ICD-9
International Classification of Diseases, Ninth Edition
- IPTW
inverse probability of treatment weighted
- MI
myocardial infarction
- PCI
percutaneous coronary intervention
- USRDS
United States Renal Data System
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Charytan D, Kuntz RE, Mauri L, DeFilippi C. Distribution of coronary artery disease and relation to mortality in asymptomatic hemodialysis patients. Am J Kidney Dis. 2007;49:409–16. doi: 10.1053/j.ajkd.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 2.Kumar N, Baker CS, Chan K, et al. Cardiac survival after pre-emptive coronary angiography in transplant patients and those awaiting transplantation. Clin J Am Soc Nephrol. 2011;6:1912–9. doi: 10.2215/CJN.08680910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joki N, Hase H, Takahashi Y, et al. Angiographical severity of coronary atherosclerosis predicts death in the first year of hemodialysis. Int Urol Nephrol. 2003;35:289–97. doi: 10.1023/b:urol.0000020356.82724.37. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Renal Data System. USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011. [Google Scholar]
- 5.Bangalore S, Kumar S, Fusaro M, et al. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation. 2012;125:2873–91. doi: 10.1161/CIRCULATIONAHA.112.097014. [DOI] [PubMed] [Google Scholar]
- 6.Garg P, Charytan DM, Novack L, et al. Impact of moderate renal insufficiency on restenosis and adverse clinical events after sirolimus-eluting and bare metal stent implantation (from the SIRIUS trials) Am J Cardiol. 2010;106:1436–42. doi: 10.1016/j.amjcard.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 7.National Kidney Foundation. KDOQI Clinical Practice Guidelines for Cardiovascular Disease in Dialysis Patients. Am J Kidney Dis. 2005;45(4 suppl 3):S1–S153. [PubMed] [Google Scholar]
- 8.Tsai TT, Messenger JC, Brennan JM, et al. Safety and efficacy of drug-eluting stents in older patients with chronic kidney disease: a report from the linked CathPCI Registry-CMS claims database. J Am Coll Cardiol. 2011;58:1859–69. doi: 10.1016/j.jacc.2011.06.056. [DOI] [PubMed] [Google Scholar]
- 9.Tomai F, Ribichini F, De Luca L, et al. Randomized Comparison of Xience V and Multi-Link Vision Coronary Stents in the Same Multivessel Patient With Chronic Kidney Disease (RENAL-DES) Study. Circulation. 2014;129:1104–12. doi: 10.1161/CIRCULATIONAHA.113.005186. [DOI] [PubMed] [Google Scholar]
- 10.Shroff GR, Solid CA, Herzog CA. Long-term survival and repeat coronary revascularization in dialysis patients after surgical and percutaneous coronary revascularization with drug-eluting and bare metal stents in the United States. Circulation. 2013;127:1861–9. doi: 10.1161/CIRCULATIONAHA.112.001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aoyama T, Ishii H, Toriyama T, et al. Sirolimus-eluting stents vs bare metal stents for coronary intervention in Japanese patients with renal failure on hemodialysis. Circ J. 2008;72:56–60. doi: 10.1253/circj.72.56. [DOI] [PubMed] [Google Scholar]
- 12.Das P, Moliterno DJ, Charnigo R, et al. Impact of drug-eluting stents on outcomes of patients with end-stage renal disease undergoing percutaneous coronary revascularization. J Invasive Cardiol. 2006;18:405–8. [PubMed] [Google Scholar]
- 13.Ichimoto E, Kobayashi Y, Iijima Y, Kuroda N, Kohno Y, Komuro I. Long-term clinical outcomes after sirolimus-eluting stent implantation in dialysis patients. Int Heart J. 2010;51:92–7. doi: 10.1536/ihj.51.92. [DOI] [PubMed] [Google Scholar]
- 14.Ishii H, Toriyama T, Aoyama T, et al. Percutaneous coronary intervention with bare metal stent vs. drug-eluting stent in hemodialysis patients. Circ J. 2012;76:1609–15. doi: 10.1253/circj.cj-12-0078. [DOI] [PubMed] [Google Scholar]
- 15.Ishio N, Kobayashi Y, Takebayashi H, et al. Impact of drug-eluting stents on clinical and angiographic outcomes in dialysis patients. Circ J. 2007;71:1525–9. doi: 10.1253/circj.71.1525. [DOI] [PubMed] [Google Scholar]
- 16.Kim BK, Oh S, Jeon DW, et al. Long-term clinical outcomes and stent thrombosis of sirolimus-eluting versus bare metal stents in patients with end-stage renal disease: results of Korean multicenter angioplasty team (KOMATE) Registry. J Interv Cardiol. 2009;22:411–9. doi: 10.1111/j.1540-8183.2009.00495.x. [DOI] [PubMed] [Google Scholar]
- 17.Okada T, Hayashi Y, Toyofuku M, et al. One-year clinical outcomes of dialysis patients after implantation with sirolimus-eluting coronary stents. Circ J. 2008;72:1430–5. doi: 10.1253/circj.cj-08-0010. [DOI] [PubMed] [Google Scholar]
- 18.Chang TI, Shilane D, Kazi DS, et al. Multivessel coronary artery bypass grafting versus percutaneous coronary intervention in ESRD. J Am Soc Nephrol. 2012;23:2042–9. doi: 10.1681/ASN.2012060554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfisterer M, Brunner-La Rocca HP, Buser PT, et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol. 2006;48:2584–91. doi: 10.1016/j.jacc.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Curfman GD, Morrissey S, Jarcho JA, Drazen JM. Drug-eluting coronary stents — promise and uncertainty. N Engl J Med. 2007;356:1059–60. doi: 10.1056/NEJMe068306. [DOI] [PubMed] [Google Scholar]
- 21.Farb A, Boam AB. Stent thrombosis redux–the FDA perspective. N Engl J Med. 2007;356:984–7. doi: 10.1056/NEJMp068304. [DOI] [PubMed] [Google Scholar]
- 22.Lagerqvist B, James SK, Stenestrand U, et al. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med. 2007;356:1009–19. doi: 10.1056/NEJMoa067722. [DOI] [PubMed] [Google Scholar]
- 23.Maisel WH. Unanswered questions–drug-eluting stents and the risk of late thrombosis. N Engl J Med. 2007;356:981–4. doi: 10.1056/NEJMp068305. [DOI] [PubMed] [Google Scholar]
- 24.Mauri L, Hsieh WH, Massaro JM, et al. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356:1020–9. doi: 10.1056/NEJMoa067731. [DOI] [PubMed] [Google Scholar]
- 25.Krone RJ, Rao SV, Dai D, et al. Acceptance, panic, and partial recovery the pattern of usage of drug-eluting stents after introduction in the U.S. (a report from the American College of Cardiology/National Cardiovascular Data Registry) JACC Cardiovasc Interv. 2010;3:902–10. doi: 10.1016/j.jcin.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Venkitachalam L, Lei Y, Stolker JM, et al. Clinical and economic outcomes of liberal versus selective drug-eluting stent use: insights from temporal analysis of the multicenter Evaluation of Drug Eluting Stents and Ischemic Events (EVENT) registry. Circulation. 2011;124:1028–37. doi: 10.1161/CIRCULATIONAHA.110.978593. [DOI] [PubMed] [Google Scholar]
- 27.Saran R, Li Y, Robinson B, et al. US Renal Data System 2014 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2015;65(1 suppl 1):S1–305. doi: 10.1053/j.ajkd.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison L. Medicare from A to D: what every nephrologist needs to know. Clin J Am Soc Nephrol. 2008;3:899–904. doi: 10.2215/CJN.02430607. [DOI] [PubMed] [Google Scholar]
- 29.Chertow GM, Johansen KL, Lew N, et al. Vintage, nutritional status, and survival in hemodialysis patients. Kidney Int. 2000;57:1176–81. doi: 10.1046/j.1523-1755.2000.00945.x. [DOI] [PubMed] [Google Scholar]
- 30.Udayaraj UP, Steenkamp R, Caskey FJ, et al. Blood pressure and mortality risk on peritoneal dialysis. Am J Kidney Dis. 2009;53:70–8. doi: 10.1053/j.ajkd.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 31.Brunelli SM, Gagne JJ, Huybrechts KF, et al. Estimation using all available covariate information versus a fixed look-back window for dichotomous covariates. Pharmacoepidemiol Drug Saf. 2013;22:542–50. doi: 10.1002/pds.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Austin PC, Mamdani MM. A comparison of propensity score methods: a case-study estimating the effectiveness of post-AMI statin use. Stat Med. 2006;25:2084–106. doi: 10.1002/sim.2328. [DOI] [PubMed] [Google Scholar]
- 33.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 35.Kosanke J, Bergstralh E. In: GMATCH Macro: Match 1 or more controls to cases using the GREEDY algorithm. macro G, editor. Mayo Clinic College of Medicine; Rochester MN: 2004. [Google Scholar]
- 36.Brookhart MA, Wyss R, Layton JB, Sturmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes. 2013;6:604–11. doi: 10.1161/CIRCOUTCOMES.113.000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hernan MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health. 2006;60:578–86. doi: 10.1136/jech.2004.029496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Venkitachalam L, Lei Y, Magnuson EA, et al. Survival benefit with drug-eluting stents in observational studies: fact or artifact? Circ Cardiovasc Qual Outcomes. 2011;4:587–94. doi: 10.1161/CIRCOUTCOMES.111.960971. [DOI] [PubMed] [Google Scholar]
- 39.Yeh RW, Vasaiwala S, Forman DE, et al. Instrumental variable analysis to compare effectiveness of stents in the extremely elderly. Circ Cardiovasc Qual Outcomes. 2014;7:118–24. doi: 10.1161/CIRCOUTCOMES.113.000476. [DOI] [PubMed] [Google Scholar]
- 40.U.S. Renal Data System. Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2014. 2014. [Google Scholar]
- 41.Aggarwal A, Kabbani SS, Rimmer JM, et al. Biphasic effects of hemodialysis on platelet reactivity in patients with end-stage renal disease: a potential contributor to cardiovascular risk. Am J Kidney Dis. 2002;40:315–22. doi: 10.1053/ajkd.2002.34510. [DOI] [PubMed] [Google Scholar]
- 42.Kaw D, Malhotra D. Platelet dysfunction and end-stage renal disease. Semin Dial. 2006;19:317–22. doi: 10.1111/j.1525-139X.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 43.Holden RM, Harman GJ, Wang M, et al. Major bleeding in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:105–10. doi: 10.2215/CJN.01810407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang TI, Montez-Rath ME, Shen JI, et al. Thienopyridine use after coronary stenting in low income patients enrolled in medicare part D receiving maintenance dialysis. J Am Heart Assoc. 2014;3:e001356. doi: 10.1161/JAHA.114.001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mauri L, Kereiakes DJ, Yeh RW, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371:2155–66. doi: 10.1056/NEJMoa1409312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


