Abstract
Objective: Studies have shown an increase in the use of antipsychotics to preschoolers for disruptive behavior and aggression. This study investigated the use of atypical antipsychotics in children ≤6 years of age in Kentucky who were on Medicaid.
Methods: Kentucky Medicaid prescription claims data between 2001 and 2010 were examined for all children ≤6 years of age who had received an atypical antipsychotic. Drug type, diagnosis codes, and geographic trends were analyzed using descriptive statistics.
Results: A total of 70,777 prescriptions were written to 6915 distinct children ≤6 years of age. The use of atypical antipsychotics in this age group increased over the years 2001–2010 with a peak ∼ 1.0% in 2004, and averaged 0.75% in 2010. Older male children were more likely to receive atypical antipsychotics, and risperidone accounted for two thirds of the prescriptions written. Mood disorders, primarily bipolar disorder, accounted for almost 75% of the diagnoses provided. Only 32% of the prescriptions were written by child psychiatrists. Geographic analysis showed significantly higher use in the Western part of the state (more than three times the state mean in some counties).
Conclusions: The use of atypical antipsychotics in children ≤6 years of age has declined from its peak, but remains substantial. The prescription rates for atypical antipsychotics by providers other than child psychiatrists, and the marked geographic variation in use across the state of Kentucky suggest that improved systems of mental healthcare for this population are needed.
Introduction
Trends of increased use of atypical antipsychotics to children ≤6 years of age have been identified. In a study of the new use of antipsychotics in patients on Medicaid 2–18 years of age, preschoolers had a 61% increase in use from 1996 to 2001 (Cooper et al. 2004). Also, an analysis of a seven state Medicaid program showed a doubling of usage in preschoolers for all psychotropic medications, including antipsychotics, from 1995 to 2001 (Zito et al. 2007). In addition, Olfson has reported an increase in the annualized rate of antipsychotic treatment to children 2–5 years of age with private insurance (Olfson et al. 2010).
The use of antipsychotics in children ≤6 years of age raises concerns. These medications have few United States Food and Drug Administration (FDA) indications for this age group, and little evidence exists to guide clinicians. The use of antipsychotics in this population can be associated with serious side effects, and may lead to ongoing medical concerns (Correll 2008). The pharmacological treatment of preschoolers is not recommended without ongoing psychosocial therapies (Gleason et al. 2007). Therefore, the decision to prescribe antipsychotics for preschoolers is a difficult one for providers, with important health ramifications.
We sought to evaluate trends of atypical antipsychotic use in preschoolers in Kentucky over the years 2001–2010, the diagnoses reported for the patients, and geographical variation. We also aimed to study the proportion of children who received prescriptions specifically from child psychiatrists.
Methods
This study was a limited data set review. The study's objectives, design, and protocol were approved by the institutional review boards (IRBs) from the University of Louisville and the Kentucky Cabinet for Health and Family Services. Data regarding filled prescriptions for atypical antipsychotics between 2001 and 2010 to children ≤6 years in Kentucky receiving Medicaid were obtained from Kentucky's Department for Medicaid Services. The data were analyzed using descriptive statistics in Microsoft Excel®. Each of the 70,777 entries (to 6915 children in total) included the date; medication; days prescribed; refills; amount paid by Medicaid; the recipient's age, race, gender, and ZIP code; diagnosis code; and prescriber identification number (ID). Providers reported the diagnostic codes on prior authorization forms starting in 2006. A random unique identifier was assigned to each Medicaid member to track duplicates.
A listing of each provider ID and specialty (if known) was included. Numbers of Medicaid recipients ≤6 years in each county from 2001 to 2010 were provided so that prescription rates could be calculated. ZIP codes were matched to their corresponding county to aid in geographic analysis. Data from the 16 county Passport Health Plan regions (which include the metropolitan area for the most populous city in the state) were not available for this study. Provider identification numbers were used to establish specialty type. Basic data summarization tools were used to examine the influence of selected variables and perform subgroup analysis.
Results
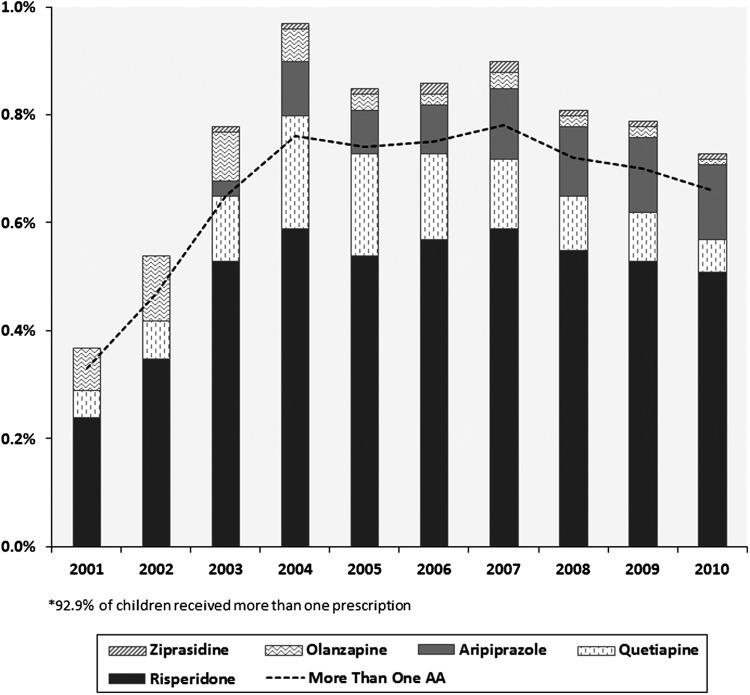
A total of 70,777 prescriptions were written to 6915 distinct children ≤6 years of age, for a mean of 10.2 prescriptions per child; 73% of prescriptions were to males. The proportion of children receiving Medicaid who were ≤6 years of age and prescribed an atypical antipsychotic peaked overall in 2004 at almost 1.0%, and has slowly trended downwards since, reaching nearly 0.75% by 2010 (Table 1).
Table 1.
Distribution of Children by Age
| Age in years | Number of children receiving a prescription (% of total AA scripts) | Percentage of children on Medicaid receiving an AA script |
|---|---|---|
| <1 | 17 (0.06) | 0.007 |
| 1 | 26 (0.07) | 0.009 |
| 2 | 175 (0.8) | 0.07 |
| 3 | 852 (5.6) | 0.37 |
| 4 | 2005 (16) | 0.90 |
| 5 | 3557 (30) | 1.66 |
| 6 | 5020 (47) | 2.43 |
Distribution of children by age and percentage of children on Medicaid in Kentucky who received an atypical antipsychotic (AA) over the period 2001–2010.
For the years 2001–2010, risperidone accounted for 66.3% of all prescriptions, quetiapine accounted for 18.6%, aripiprazole accounted for 9%, olanzapine accounted for 4.8%, and ziprasidone accounted for 1.2%. In 2007 or thereabouts, aripiprazole replaced quetiapine as the second most common agent used in the population, and the use of olanzapine diminished starting in 2004 (Fig. 1).
FIG. 1.
Percent of children ≤6 years of age who were prescribed an atypical antipsychotic.
The use of atypical antipsychotics increased with age and was greater in males than in females across all diagnostic categories. The median number of prescriptions of atypical antipsychotics increased with the age of the child, and almost one third of young children were prescribed risperidone for >12 months (Tables S1 and S2) (Supplementary Tables are available in the online article at http://www.liebertonline.com/cap). Medicaid did not require a prior authorization with a diagnostic code for atypical antipsychotics until 2006; therefore, 72% of prescriptions had no diagnosis; 118 unique diagnosis codes were provided for the remaining 19,630 prescriptions. Mood disorders (predominantly bipolar disorder) accounted for almost 75%, and psychotic disorders accounted for 12.5%. Autism (and pervasive developmental disorder [PDD]) accounted for 5.1% (Fig. S1) (Supplementary material is available in the online article at http://www.liebertonline.com/cap).
The prescriptions for atypical antipsychotics were written by 1997 unique providers, 90% of which could be categorized by specialty. Of these, 32.1% of prescriptions were written by psychiatrists, 28.9% by general practitioners, 10.5% by general pediatricians, and 9.7% by other specialists. The number of prescriptions written by nurse practitioners was 9.4% overall, but increased from minimal amounts to >15% by 2010 (Fig. S2) (Supplementary material is available in the online article at http://www.liebertonline.com/cap).
The proportion of children prescribed atypical antipsychotics varied by geographic region. In one Western Kentucky County (Union), a mean of 2.43% of all children in that age range had been prescribed an atypical antipsychotic, a rate three to four times the state mean. Counting only 5- and 6-year-olds, that number jumped to 7.2%, when averaged over the 10 year period. (Fig. S3) (Supplementary material is available in the online article at http://www.liebertonline.com/cap)
Discussion
We document significant use of atypical antipsychotics in children ≤6 years of age on Medicaid in Kentucky from 2001 to 2010. The rates of use for atypical antipsychotic medication for preschoolers in our study were similar to other reports examining very young children on Medicaid. Rates for atypical antipsychotic use among children 2–4 years of age in Kentucky averaged 2.14/1000 over the years 2001–2010. This compares to an increase in antipsychotic use from 1/1000 to 5/1000 in a Medicaid population of same-age youth in a mid-Atlantic state for the years 1997–2006 (Zito et al. 2013). Utilization of psychotropic drugs in preschool children has been shown to be higher in Medicaid than in managed care health maintenance organization (HMO) populations, and in our study 16.66/1000 of 5-year-olds received antipsychotics over the study interval compared with 3.04/1000 5-year-olds on private insurance in 2007 (Zito et al. 2007; Olfson et al. 2010).
Our results found risperidone to be the most common drug prescribed from 2001 to 2010, and by 2007, aripiprazole supplanted quetiapine as the second most common agent used in the population. The use of olanzapine particularly slowed starting in 2004. These findings are similar to those in other reports, and reflect the body of evidence for risperidone and aripiprazole in disruptive behavior, current FDA pediatric indications, professional guidelines, and formulary authorization procedures (Olfson et al. 2010, 2012).
Our study showed mood disorders (74.6%) to be the most common diagnosis listed as an indication for use. This may reflect the well-documented increase in the diagnosis of pediatric bipolar disorders in children (Moreno et al. 2007). In contrast to previous reports, autism and disruptive behaviors disorders (ODD and ADHD) in our study only accounted for 5.2% and 3.2%, respectively (Olfson et al. 2010). Again, 72% of our data set did not have diagnostic information, and verification of the codes listed by providers was not possible; therefore, questions exist about the legitimacy of the study's diagnostic data.
The estimated use of and duration of exposure to atypical antipsychotics in young children was greater in males and increased with age in our study. This suggests that guidelines for tapering the medication after a period of improvement and for reassessing the role of other interventions are not consistently being followed (American Academy of Child and Adolescent Psychiatry 2009).
The low percentage (32.1%) of prescriptions for atypical antipsychotics written by psychiatrists is a cause for concern regarding the mental healthcare of preschoolers. This agrees with a previous report in which <50% of very young children treated with antipsychotics received any mental health assessment or had psychotherapy visits (Olfson et al. 2010). Because <50% of United States counties have a psychiatrist or mental health specialist, increased integration with providers other than child psychiatrists in mental health systems may provide access to missing services (Pidano and Honigfeld 2012).
We found significant geographic disparities across our study map. In Kentucky, the state Medicaid population receives services from a network of community mental health centers (CMHC). The counties in our study associated with the most elevated prescription rates are situated prominently within 1–2 CMHC regions in Western Kentucky, suggesting that provider habits and/or a lack of alternative services may be factors requiring further study.
Many issues may help explain the increased use of antipsychotics in preschoolers. Overall, the rate of visits for mental health issues for children and adolescents is increasing at a much faster rate than for adults, and disruptive behavior is now the most common mental disorder diagnosis in youth (Olfson et al. 2014) Antipsychotics have been shown to reduce aggression, and practice guidelines support the appropriate use of atypical antipsychotics in youth (Knapp, et al. 2012, Scotto et al. 2012) However, payment systems, lack of access to alternative services, and marketing practices are also factors involved in the trend (Olfson et al. 2012).
Little evidence supports the use of antipsychotics in very young children. The lack of information on developmental pharmacokinetics, pharmacodynamics, and sensitivity to adverse effects prevents full understanding of the safety and efficacy in the preschool population (Gleason et al. 2007). Very young children may be at higher risk for known extrapyramidal side effects, alterations in prolactin levels, and metabolic changes, with uncertain long-term implications (Bobo et al. 2013) The impact of psychotropic medications on the developing child central nervous system is unknown, but the potential of long-term effects on brain structure and function has been suggested by animal studies (Andersen and Navalta 2011).
There are limitations to this study. During the study period, the data from the heavily populated metropolitan Louisville area and surrounding counties were not available. Mental health services in this region were provided by a Medicaid health plan separate from state Medicaid. This is important comparative data to include in future studies, as current data reflect a more rural population. Also, our data did not allow the measurement of intraclass polypharmacy; therefore, attempts at cross-titration were not detected. Race and socioeconomic status data were not collected in our study, but disparities in mental healthcare are important considerations for future research.
Conclusions
The rate of prescribing atypical antipsychotics to young children in Kentucky, although declining slightly, remains substantial. Furthermore, the rising proportion of prescriptions written by providers other than psychiatrists may indicate lack of access to needed specialists in underserved areas of the state. Further study is needed to identify etiologies behind significantly higher prescription rates in some regions of the state.
Clinical Significance
This is the first study to examine the use of atypical antipsychotics to preschoolers on Medicaid in the State of Kentucky. The findings confirm important clinical trends about the use of atypical antipsychotics in this population, and support the need for national and state initiatives to improve their judicious use.
Supplementary Material
Acknowledgments
We thank Dr. Tom Badgett and Kurt Godshall with Kentucky Cabinet for Health and Family Services for making the data available to us; Dr. Joshua Honaker for his involvement in developing the idea and initial protocol; and Dr. Janice E. Sullivan, the Kosair Charities Pediatric Clinical Research Unit staff, and Kendra Sikes for assistance with study design and regulatory assistance.
Disclosures
No competing financial interests exist.
References
- American Academy of Child and Adolescent Psychiatry: Practice parameter on the use of psychotropic medication in children and adolescents. J Am Acad Child Adolesc Psychiatry 48:961–973, 2009 [DOI] [PubMed] [Google Scholar]
- Andersen SL, Navalta CP: Annual Research Review: New frontiers in developmental neuropharmacology: Can long-term therapeutic effects of drugs be optimized through carefully timed early intervention? J Child Psychol Psychiatry 52:476–503, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobo WV, Cooper WO, Stein CM, Olfson M, Graham D, Daugherty J, Fuchs DC, Ray WA: Antipsychotics and the risk of type 2 diabetes mellitus in children and youth. JAMA Psychiatry 70:1067–1075, 2013 [DOI] [PubMed] [Google Scholar]
- Cooper WO, Hickson GB, Fuchs C, Arbogast PG, Ray WA: New users of antipsychotic medications among children enrolled in TennCare. Arch Pediatr Adolesc Med 158: 753–759, 2004 [DOI] [PubMed] [Google Scholar]
- Correll CU: Antipsychotic use in children and adolescents: Minimizing adverse effects to maximize outcomes. J Am Acad Child Adolesc Psychiatry 47:9–20, 2008 [DOI] [PubMed] [Google Scholar]
- Gleason MM, Egger HL, Emslie GJ, Greenhill LL, Kowatch RA, Lieberman AF, Luby JL, Owens J, Scahill LD, Scheeringa MS, Stafford B, Wise B, Zeanah CH: Psychopharmacological treatment for very young children: Contexts and guidelines. J Am Acad Child Adolesc Psychiatry 46:1532–1572, 2007 [DOI] [PubMed] [Google Scholar]
- Knapp P, Chait A, Pappadopulos E, Crystal S, Jensen PS: Treatment of maladaptive aggression in youth: CERT guidelines I. Engagement, assessment, and management. Pediatrics 129: e1562–1576, 2012 [DOI] [PubMed] [Google Scholar]
- Moreno C, Laje G, Blanco C, Jiang H, Schmidt AB, Olfson M: National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry 64:1032–1039, 2007 [DOI] [PubMed] [Google Scholar]
- Olfson M, Blanco C, Liu SM, Wang S, Correll CU: National trends in the office-based treatment of children, adolescents, and adults with antipsychotics. Arch Gen Psychiatry 69:1247–1256, 2012 [DOI] [PubMed] [Google Scholar]
- Olfson M, Blanco C, Wang S, Laje G, Correll CU: “National trends in the mental health care of children, adolescents, and adults by office-based physicians. JAMA Psychiatry 71:81–90, 2014 [DOI] [PubMed] [Google Scholar]
- Olfson M, Crystal S, Huang C, Gerhard T: Trends in antipsychotic drug use by very young, privately insured children. J Am Acad Child Adolesc Psychiatry 49:13–23, 2010 [DOI] [PubMed] [Google Scholar]
- Pidano AE, Honigfeld L: Pediatric psychopharmacology: context, model programs, and considerations for care. Psychiatr Serv 63:929–934, 2012 [DOI] [PubMed] [Google Scholar]
- Scotto Rosato N, Correll CU, Pappadopulos E, Chait A, Crystal S, Jensen PS: Treatment of maladaptive aggression in youth: CERT guidelines II. Treatments and ongoing management. Pediatrics 129: e1577–1586, 2012 [DOI] [PubMed] [Google Scholar]
- Zito JM, Burcu M, Ibe A, Safer DJ, Magder LS: Antipsychotic use by medicaid-insured youths: impact of eligibility and psychiatric diagnosis across a decade. Psychiatr Serv 64:223–229, 2013 [DOI] [PubMed] [Google Scholar]
- Zito JM, Safer DJ, Valluri S, Gardner JF, Korelitz JJ, Mattison DR: Psychotherapeutic medication prevalence in Medicaid-insured preschoolers. J Child Adolesc Psychopharmacol 17:195–203, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.