Abstract
Hepatitis C virus (HCV) co-infection and biological sex may each affect response to antiretroviral therapy (ART), yet no studies have examined HIV-associated outcomes by both HCV status and sex. We conducted a cohort study of HIV-infected adults initiating ART in Kaiser Permanente California during 1996–2011. We used piecewise linear regression to assess CD4 changes by sex and HCV status over 5 years. We used Cox regression to estimate hazard ratios (HR) by sex and HCV status for HIV RNA <500 copies/mL over 1 year, and for AIDS and death over the follow-up period. Among 12,865 subjects, there were 154 HIV/HCV-co-infected women, 1000 HIV/HCV-co-infected men, 1088 HIV-mono-infected women, and 10,623 HIV-mono-infected men. CD4 increases were slower in the first year for HIV/HCV-co-infected women (75 cells/μL) and men (70 cells/μL) compared with HIV-mono-infected women (145 cells/μL) and men (120 cells/μL; p<0.001). After 5 years, women had higher CD4 than men in both HIV-mono-infected (598 vs. 562 cells/μL, p=0.003) and HIV/HCV-co-infected individuals (567 vs. 509 cells/μL, p=0.003). Regardless of sex, HIV/HCV co-infection was associated with 40% higher mortality [95% confidence interval (CI): 1.2–1.6] compared with HIV mono-infection, but was not associated with AIDS (HR 1.1, 95% CI: 0.9–1.3) or achieving HIV RNA <500 copies/mL (HR 1.0, 95% CI: 0.9–1.1). HIV/HCV-co-infected men and women have slower CD4 recovery after starting ART and have increased mortality compared with HIV-mono-infected men and women. HCV should be aggressively treated in HIV/HCV-co-infected adults, regardless of sex.
Introduction
Liver disease as a result of hepatitis C virus (HCV) infection is a leading cause of death among individuals infected with human immunodeficiency virus (HIV).1,2 HIV/HCV co-infection may also reduce the response to antiretroviral therapy (ART),3–9 and ultimately increase the risk of mortality among HIV-infected individuals.10–12 HIV/HCV-co-infected individuals are considered a priority population for HCV treatment due to more rapid liver disease progression in the setting of HIV infection.13 However, it is unclear if there are subgroups of HIV/HCV-co-infected individuals that may be at particular risk of the negative effects of HCV infection on HIV-associated outcomes.
One such subgroup may be defined by biological sex. The natural history of HCV infection varies by sex, with increased viral clearance and slower progression of liver disease in women compared with men, possibly resulting from a protective effect of estrogen on the liver.14–16 Sex may also modify the response to ART among HIV-infected individuals. While some studies have found no association of sex with response to ART,17–19 others have suggested that women have superior virologic, immunologic, and clinical outcomes compared with men.20–24 It is unknown if this is attributable to biological factors, such as pharmacokinetics,25,26 or behavioral or structural factors, such as adherence27 or access to or quality of care.28 Despite evidence that there may be sex differences in both the course of HCV disease and the response to ART among HIV-infected individuals, there have been no studies examining HIV-associated outcomes with respect to both sex and HCV status over time. Thus, the purpose of this study was to identify differences by sex and HCV status in response to ART among HIV-infected individuals, as measured by CD4 cell count, HIV RNA level, AIDS, and death after ART initiation.
Methods
Study design, setting, and population
The study population of HIV-infected subjects was ascertained from a previously described cohort.29 Briefly, the source cohort included 24,768 HIV-infected members of Kaiser Permanente (KP) Northern and Southern California (KPNC and KPSC, respectively), large integrated healthcare systems providing comprehensive medical services to over 30% of insured Californians.30 Subjects were identified and followed from 1996 through 2011. HIV status was determined using HIV registries that included all known cases of HIV/AIDS since the early 1980s for KPNC and 2000 for KPSC, with confirmation by review of medical charts or medical center case lists.
For the present study, subjects were selected from the overall cohort if they initiated their first combination ART regimen at KP, defined as a regimen containing three or more antiretroviral drugs,31 and had CD4 and HIV RNA measurements within 6 months prior to ART initiation. These subjects were followed from ART initiation until the earliest of death, loss to follow-up (i.e., leaving the health plan), or December 31, 2011. The outcomes of interest were change in CD4 count over 5 years; attainment of HIV RNA <500 copies/mL within 1 year; clinical AIDS diagnosis;32 and mortality from all causes, liver-related causes, AIDS-related causes, and other causes. Data on cause of death were only available through 2010; thus, analyses of mortality from liver-related causes, AIDS-related causes, and other causes were administratively censored at December 31, 2010.
The institutional review boards at KPNC and KPSC approved this study with a waiver of written informed consent.
Study measurements
HCV infection status
HCV status was based on laboratory data. Individuals were classified as HCV-infected if they had a positive HCV-antibody test or a positive qualitative or quantitative HCV RNA test from 2 years prior to baseline through the end of study follow-up. Individuals not tested for HCV were classified as HIV-mono-infected. Although this may have resulted in misclassification of individuals with undiagnosed HCV infection, rates of HCV testing among HIV-infected individuals in KP California are high (>98% in KPNC and >95% in KPSC), such that we expect this scenario to have been rare.
For the subset of KPNC subjects, data were available from the KP Viral Hepatitis Registry, which includes all known cases of HCV infection in KPNC since 1995. Cases were included in the registry if they had a documented HCV diagnosis [International Classification of Disease codes, version 9 (ICD-9): 070.54], positive HCV-antibody test, or positive qualitative or quantitative HCV RNA test. Individuals were excluded if they had a single positive HCV-antibody test followed by a negative HCV-antibody test, or a negative HCV RNA test or recombinant immunoblot assay following the most recent HCV-antibody test or HCV diagnosis, with no evidence of HCV treatment. To assess potential misclassification of HCV status in the main analysis, we conducted a sensitivity analysis of change in CD4 count in the subset of KPNC subjects, with HCV status defined by inclusion in the registry.
Electronic medical record (EMR)
Data on sex were obtained as self-reported gender from the clinical and administrative databases constituting KP's EMR. Other data extracted from the EMR included age at study entry; laboratory test results (CD4 counts and HIV RNA levels); health plan enrollment periods; inpatient or outpatient clinical diagnoses, including drug/alcohol abuse (ICD-9: 291, 292, 303-305.0, 305.2-305.5) and smoking/tobacco use (ICD-9: 305.1, V15, V65, 649, internal social history codes); and dates and causes of death from hospital records, California death certificates, and Social Security Administration datasets, with complete ascertainment of deaths regardless of KP membership status. Deaths were defined as liver-related if the underlying or a contributing cause of death was viral hepatitis, hepatic failure, hepatocellular carcinoma, or chronic liver disease. The remaining deaths were defined as AIDS-related if the underlying or a contributing cause of death was an AIDS-defining infection or malignancy, and otherwise were defined as related to other causes.
HIV registries
In addition to HIV status, HIV registries were used to obtain race/ethnicity, clinical AIDS diagnosis, HIV-transmission risk factor, dates of first ART use, and beginning of known HIV infection.
Statistical analysis
Variables for analyses included age (<40, 40–49, ≥50 years); sex; race/ethnicity (white, black/African American, Hispanic/Latino, other; with imputed data where missing for 3% of subjects);33,34 year of ART initiation (1996–2000, 2001–2005, 2006–2011); KP region (KPNC vs. KPSC); and drug abuse, alcohol abuse, and smoking, all of which were defined as ever/never from 2 years prior to baseline through the end of follow-up. We also evaluated any prior noncombination ART experience, duration of known HIV infection at study entry (≥10, 5–9.9, <5 years), HIV-transmission risk factor (IDU vs. other), CD4 count (<200, 200–499, ≥500 cells/μL), and HIV RNA level (≥10,000, 500–9999, <500 copies/mL). For HIV RNA, we used <500 copies/mL to account for changes in test cutoffs during study follow-up. Baseline CD4 counts and HIV RNA levels were defined as the most recent measurements within the 6 months prior to ART initiation.
We compared baseline characteristics of subjects, assessing differences by sex within HIV/HCV-co-infected and HIV-mono-infected individuals using the Pearson chi-square test for categorical variables and the t-test for continuous variables. We used linear regression to analyze the change in CD4 count up to 5 years following ART initiation in the four groups defined by sex and HCV status. Generalized estimating equations were used for estimation of regression parameters, accounting for within-individual correlation between repeated CD4 measurements. We constructed piecewise linear curves to account for a nonlinear pattern of CD4 change, allowing for a change in slope at 1 year. In addition to sex, HCV status, time, and corresponding interaction terms, adjusted models included variables for age, race/ethnicity, calendar era, KP region, prior ART use, HIV-transmission risk factor, years known to be HIV-infected, drug/alcohol abuse, smoking, and baseline HIV RNA level. For sensitivity analyses, we analyzed change in CD4 (1) in the subset of KPNC subjects, defining HCV status based on registry inclusion to assess potential misclassification of HCV status in the main analysis; (2) in the subset of ART-naïve subjects to remove a possible effect of prior antiretroviral experience on CD4 recovery; and (3) including only women who were postmenopausal (≥50 years of age) to exclude a possible protective effect of estrogen on the immune system.35
We then used Kaplan-Meier plots and log-rank tests to compare the cumulative incidence of attaining HIV RNA <500 copies/mL within 1 year by sex and HCV status, restricting the analysis to subjects with HIV RNA ≥500 at baseline. We compared the cumulative incidence of clinical AIDS diagnosis among subjects not previously diagnosed with AIDS, and the cumulative incidence of mortality from all causes, liver-related causes, AIDS-related causes, and other causes among all subjects, by sex and HCV status. Hazard ratios (HR) were obtained from Cox regression models comparing HIV/HCV-co-infected to HIV-mono-infected subjects, both overall and stratified by sex. Adjustment for confounders was performed as described for CD4 models, with the addition of a variable for baseline CD4 count, and interaction terms were used to assess differences across sex strata.
Analyses were conducted in SAS 9.1 (Cary, NC). Statistical tests were two-sided, and statistical significance was defined as p<0.05.
Results
Study population
Among 12,865 HIV-infected individuals initiating ART, there were 1088 HIV-mono-infected women, 10,623 HIV-mono-infected men, 154 HIV/HCV-co-infected women, and 1000 HIV/HCV-co-infected men (Table 1). Subjects differed by HCV status with respect to several demographic and clinical characteristics at baseline, although small differences among men may have been statistically significant only because of large sample size. Less than 20% of HIV-mono-infected and HIV/HCV-co-infected men were African American, while about 40% of HIV-mono-infected and HIV/HCV-co-infected women were African American. HIV/HCV-co-infected women and men were older than HIV-mono-infected subjects. A history of smoking was more common among HIV/HCV-co-infected than HIV-mono-infected subjects, as were alcohol and drug abuse. HIV/HCV-co-infected subjects more often reported IDU as their HIV-transmission risk factor, and had a longer duration of HIV infection, than HIV-mono-infected subjects. For all subjects, almost half reported a history of ART use, CD4 counts were approximately 300, and on average HIV RNA levels were in the detectable range.
Table 1.
Baseline Characteristics of HIV-Infected Individuals Initiating ART, By Sex and HCV Status, Kaiser Permanente California, 1996–2011
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Characteristic | HIV mono-infected | HIV/HCV co-infected | p | HIV mono-infected | HIV/HCV co-infected | p |
| N | 1088 | 154 | 10,623 | 1000 | ||
| Year of ART initiation, % | 0.32 | <0.001 | ||||
| 1996–2000 | 25 | 31 | 24 | 31 | ||
| 2001–2005 | 32 | 31 | 29 | 32 | ||
| 2006–2011 | 43 | 38 | 47 | 37 | ||
| Kaiser Permanente region, % | <0.001 | <0.001 | ||||
| Northern California | 60 | 74 | 58 | 67 | ||
| Southern California | 40 | 26 | 42 | 33 | ||
| Mean age at baseline, years (SD) | 39 (10) | 45 (9) | <0.001 | 42 (10) | 45 (9) | <0.001 |
| Race/ethnicity, % | 0.08 | 0.002 | ||||
| White | 34 | 43 | 57 | 58 | ||
| Black/African American | 40 | 39 | 14 | 17 | ||
| Hispanic | 19 | 12 | 22 | 18 | ||
| Other/unknown | 8 | 6 | 8 | 7 | ||
| Ever smoked, % | 41 | 72 | <0.001 | 49 | 62 | <0.001 |
| Ever alcohol abuse, % | 8 | 16 | 0.003 | 11 | 22 | <0.001 |
| Ever drug abuse, % | 13 | 34 | <0.001 | 16 | 32 | <0.001 |
| HIV-transmission risk factor | <0.001 | <0.001 | ||||
| Men who have sex with men | 0 | 0 | 74 | 54 | ||
| Heterosexual transmission | 79 | 44 | 9 | 5 | ||
| Injection drug use | 4 | 40 | 4 | 25 | ||
| Other/unknown | 16 | 16 | 13 | 16 | ||
| Median years of known HIV infection (IQR) | 2 (0–6) | 5 (0–11) | 0.001 | 2 (0–8) | 4 (0–11) | <0.001 |
| Prior antiretroviral therapy use, % | 42 | 48 | 0.16 | 44 | 52 | <0.001 |
| Mean CD4 cells/μL (SD) | 341 (255) | 392 (273) | 0.020 | 362 (260) | 354 (256) | 0.34 |
| Mean HIV RNA log copies/mL (SD) | 8.7 (2.9) | 8.1 (3.1) | 0.037 | 8.7 (3.2) | 8.5 (3.1) | 0.06 |
ART, antiretroviral therapy; HCV, hepatitis C virus; HIV, human immunodeficiency virus; SD, standard deviation.
Immunologic response
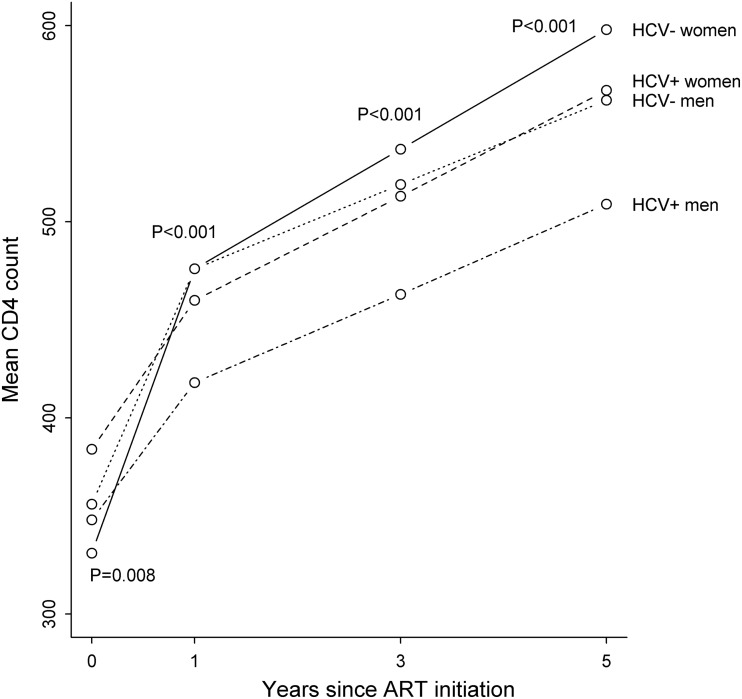
Changes in CD4 count over 5 years by sex and HCV status are shown in Fig. 1, with adjusted results shown in Table 2. There were small variations in CD4 count at the time of ART initiation, with an overall difference across groups (p=0.008); however, the only individual pairwise comparison that reached statistical significance was HIV/HCV-co-infected women compared with HIV-mono-infected men (384 vs. 356 cells/μL, p=0.022). Increases in CD4 count were slower in the first year for HIV/HCV-co-infected women (75 cells/μL) and men (70 cells/μL) compared with HIV-mono-infected women (145 cells/ μL) and men (120 cells/μL), with an overall significant difference in slopes in the first year (p<0.001). HIV/HCV-co-infected men had significantly slower increases in CD4 count in the first year compared with both HIV-mono-infected men (p<0.001) and HIV-mono-infected women (p<0.001), as did HIV/HCV-co-infected women compared with both HIV-mono-infected men (p=0.041) and HIV-mono-infected women (p=0.003). The increase in CD4 count for HIV-mono-infected women was significantly faster compared with HIV-mono-infected men (p=0.004). The slopes for HIV/HCV-co-infected women and men were similar (p=0.79).
FIG. 1.
Change in CD4 count over 5 years after ART initiation by sex and HCV status, Kaiser Permanente California, 1996–2011. ART, antiretroviral therapy; HCV, hepatitis C virus; HIV, human immunodeficiency virus. Mean CD4 counts were derived from a linear regression model including variables for HCV status, sex, time, and corresponding interaction terms. Generalized estimating equations accounted for within-individual correlation and piecewise linear curves allowed for a change in slope at 1 year. p Values test for differences in mean CD4 counts at baseline and 1, 3, and 5 years.
Table 2.
Change in CD4 Count Over 5 Years After ART Initiation by Sex and HCV Status, Kaiser Permanente California, 1996–2011
| Increase in CD4 cells/μL per year in year 1 | Increase in CD4 cells/μL per year in years 2–5 | |||
|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| HIV-mono-infected women | 145 (129–161) | 145 (129–161) | 30 (22–39) | 31 (23–39) |
| HIV/HCV-co-infected women | 75 (33–118) | 76 (33–118) | 27 (9–45) | 28 (10–46) |
| HIV-mono-infected men | 120 (115–125) | 120 (115–125) | 22 (19–24) | 22 (20–25) |
| HIV/HCV-co-infected men | 70 (56–83) | 69 (56–83) | 23 (16–29) | 24 (17–30) |
| p Value | <0.001 | <0.001 | 0.21 | 0.22 |
ART, antiretroviral therapy; HCV, hepatitis C virus; HIV, human immunodeficiency virus. Data are given as mean CD4 increases (95% confidence intervals) derived from linear regression models using generalized estimating equations and allowing for a change in slope at 1 year. Unadjusted models included variables for time, sex, HCV status, and corresponding interaction terms. Adjusted models additionally included variables for age, race/ethnicity, calendar era, Kaiser Permanente region, prior ART use, HIV-transmission risk factor, years known to be HIV-infected, drug abuse, alcohol abuse, smoking, and baseline HIV RNA level.
After the first year, the increase in CD4 count was 30 cells/μL per year for HIV-mono-infected women, followed by 27 cells/μL per year for HIV/HCV-co-infected women, 23 cells/μL per year for HIV/HCV-co-infected men, and 22 cells/μL per year for HIV-mono-infected men. The increase in CD4 count after the first year for HIV-mono-infected women was significantly faster compared with HIV-mono-infected men (p=0.040), but there were no other significant pairwise differences between slopes after the first year.
At 5 years, HIV-mono-infected women reached the highest mean CD4 count (598 cells/μL), followed by HIV/HCV-co-infected women (567 cells/μL), HIV-mono-infected men (562 cells/μL), and co-infected men (509 cells/μL; p<0.001 for an overall difference in means). The higher mean CD4 count at 5 years was statistically significant for women compared with men among both HIV-mono-infected (p=0.018) and HIV/HCV-co-infected individuals (p<0.001), and for HIV-mono-infected compared with HIV/HCV-co-infected among men (p<0.001). The pairwise comparison of mean CD4 counts at 5 years for HIV-mono-infected and co-infected women reached borderline significance (p=0.059). Results did not change after adjustment for potential confounders (Table 2). Results were also similar after additional adjustment for baseline CD4 count, in the subset of KPNC subjects with HCV status defined by registry inclusion, in the subset of ART-naïve subjects, and when including only postmenopausal women (data not shown).
Virologic response
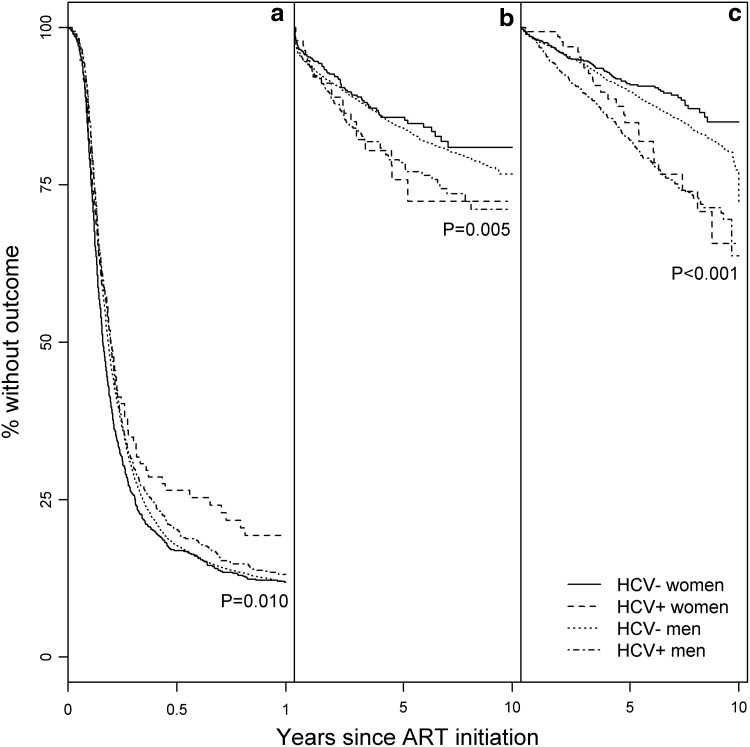
Among 8839 subjects with HIV RNA ≥500 copies/mL at the time of ART initiation (69% of all subjects), of whom 69% were ART-naïve, the cumulative incidence of attainment of HIV RNA <500 copies/mL within 1 year was 85% for HIV-mono-infected women, followed by 84% for HIV-mono-infected and HIV/HCV-co-infected men, and 79% for HIV/HCV-co-infected women (p=0.010; Fig. 2). HIV/HCV co-infection was not associated with achieving HIV RNA <500 copies/mL [HR 0.9, 95% confidence interval (CI): 0.9–1.0] (Table 3). Results did not differ by sex (p int=0.10), and were similar after adjustment (HR 1.0, 95% CI: 0.9–1.1).
FIG. 2.
Virologic and clinical outcomes after ART initiation by sex and HCV status, Kaiser Permanente California, 1996–2011. ART, antiretroviral therapy; HCV, hepatitis C virus. Kaplan-Meier curves show the cumulative proportion without (a) attainment of HIV RNA <500 copies/mL within 1 year (n=8839), (b) clinical AIDS diagnosis (n=11,559), and (c) all-cause mortality (n=12,865) after ART initiation, by sex and HCV status. p Values are from log-rank tests.
Table 3.
Hazard Ratios for HIV/HCV Co-infection Associated with (1) Attainment of HIV RNA <500 Copies/mL Within 1 Year, (2) AIDS, and (3) All-Cause Mortality After ART Initiation, Overall and Stratified by Sex, Kaiser Permanente California, 1996–2011
| Attainment of HIV RNA <500 copies/mL | Clinical AIDS diagnosis | All-cause mortality | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | p | Adjusted | p | Unadjusted | p | Adjusted | p | Unadjusted | p | Adjusted | p | |
| Overall | 0.9 (0.9–1.0) | 0.07 | 1.0 (0.9–1.1) | 0.99 | 1.3 (1.1–1.6) | <0.001 | 1.1 (0.9–1.3) | 0.27 | 1.8 (1.5–2.1) | <0.001 | 1.4 (1.2–1.6) | <0.001 |
| Women | 0.8 (0.6–1.0) | 0.039 | 0.9 (0.7–1.1) | 0.28 | 1.5 (1.0–2.4) | 0.08 | 1.3 (0.7–2.2) | 0.37 | 1.9 (1.2–3.0) | 0.004 | 1.3 (0.7–2.4) | 0.43 |
| Men | 1.0 (0.9–1.0) | 0.26 | 1.0 (0.9–1.1) | 0.7 | 1.3 (1.1–1.6) | 0.002 | 1.1 (0.9–1.3) | 0.39 | 1.8 (1.5–2.1) | <0.001 | 1.4 (1.2–1.6) | <0.001 |
| p Int | 0.1 | 0.18 | 0.58 | 0.45 | 0.74 | 0.73 | ||||||
ART, antiretroviral therapy; HCV, hepatitis C virus; HIV, human immunodeficiency virus; p Int, p value for interaction across sex strata. Data are given as hazard ratios (95% confidence intervals) derived from Cox regression models. Adjusted models included variables for age, race/ethnicity, calendar era, Kaiser Permanente region, prior ART use, HIV-transmission risk factor, years known to be HIV-infected, drug abuse, alcohol abuse, smoking, baseline HIV RNA level, and baseline CD4 count. HIV RNA models were restricted to subjects with HIV RNA ≥500 copies/mL at the time of ART initiation, and AIDS models were restricted to subjects without a prior AIDS diagnosis.
Clinical outcomes
Among 11,559 subjects without a prior AIDS diagnosis (90% of all subjects), the cumulative incidence of AIDS was 15% for HIV/HCV-co-infected women and men, followed by 11% for HIV-mono-infected men and 10% for HIV-mono-infected women (p=0.005; Fig. 2). In unadjusted analysis, HIV/HCV co-infection was associated with a 1.3-fold greater incidence of AIDS overall (95% CI: 1.1–1.6), with no difference by sex (p int=0.58). After adjustment, HIV/HCV co-infection was no longer associated with an increased risk of AIDS (HR 1.1, 95% CI: 0.9–1.3), again with no difference by sex (p int=0.45).
The cumulative incidence of all-cause mortality was 18% for HIV/HCV-co-infected men, followed by 16% for HIV/HCV-co-infected women, 9.4% for HIV-mono-infected men, and 8.0% for HIV-mono-infected women (p<0.001; Fig. 2). In unadjusted models, HIV/HCV co-infection was associated with a 1.8-fold greater incidence of all-cause mortality overall (95% CI: 1.5–2.1), with no difference by sex (p int=0.74). After adjustment, HIV/HCV co-infection remained associated with a 1.4-fold increased risk of all-cause mortality (HR 1.4, 95% CI: 1.2–1.6), again with no difference by sex (p int=0.73).
The association of HIV/HCV co-infection with all-cause mortality was largely driven by liver-related deaths, with an unadjusted HR of 8.5 (95% CI: 5.9–12.3) and an adjusted HR of 6.0 (95% CI: 3.9–9.0) for deaths from liver-related causes. HIV/HCV co-infection was also associated with deaths from other causes, with an unadjusted HR of 1.8 (95% CI: 1.3–2.4) and an adjusted HR of 1.4 (95% CI: 1.0–2.0). In contrast, HIV/HCV-co-infected individuals were not at increased risk of AIDS-related mortality in unadjusted (HR 1.1, 95% CI: 0.9–1.4) or adjusted models (HR 0.9, 95% CI: 0.7–1.2). There were no differences by sex in the association of HIV/HCV co-infection with incidence of liver-related deaths, AIDS-related deaths, or deaths from other causes (data not shown).
Discussion
To our knowledge, this is the first study to examine response to ART over time by both HCV status and sex. In this large cohort of HIV-mono-infected and HIV/HCV-co-infected adults within the same healthcare system, HIV/HCV co-infection was associated with slower CD4 recovery in the first year after initiating combination ART in both women and men. After 5 years, women had attained higher CD4 counts than men regardless of HCV status, with HIV/HCV-co-infected men achieving the lowest CD4 counts. Despite these differences in immunologic response, we found no association of HCV status or sex with virologic response in the first year after ART initiation or with incidence of AIDS after adjustment for potential confounders. Rather, we found that HIV/HCV co-infection was associated with an increased risk of mortality, attributable mainly to deaths from liver-related causes, with no excess risk of AIDS-related mortality and no variation by sex.
Our study confirms several prior studies that found an association between HIV/HCV co-infection and a reduced immunologic, but not virologic, response to ART,3–9 as well as studies demonstrating improved immunologic response among women compared with men.20–24 Similar to the findings of a recent meta-analysis by Tsiara et al.,5 we found that the difference in immunologic response by HCV status was most pronounced shortly after ART initiation and diminished over time. Although several prior studies found no association between HIV/HCV co-infection and response to ART,36–38 these studies were limited by smaller sample sizes and lack of adjustment for risk factors that differ by HCV status, including drug and alcohol abuse. In our study, the association between HIV/HCV co-infection and reduced immunologic response to ART remained after adjustment for substance abuse, duration of known HIV infection, and other risk factors that differ by HCV status. By examining response to ART by both HCV status and sex, our study further expands on the existing literature by showing that women have improved immune reconstitution after ART initiation compared with men among both HIV-mono-infected and HIV/HCV-co-infected individuals.
The slower CD4 increases among HIV/HCV-co-infected compared with HIV-mono-infected individuals may be attributable to higher T-cell activation in the setting of HIV/HCV co-infection.39,40 This chronic immune activation may lead to immune dysfunction and cytokine production, resulting in lower CD4 counts;41 indeed, suppression of HCV infection with therapy has been shown to reduce immune activation.40 Slower CD4 recovery among HIV/HCV-co-infected individuals could also be attributable to a longer duration of HIV infection, but our findings were similar after adjustment for this factor.
Women attained higher CD4 counts than men regardless of HCV status, a finding that may be caused by differences by sex in behavioral factors such as substance use42 or biological factors such as pharmacokinetics.25,26 This finding may also be attributable to differences in sex hormones, with prior studies suggesting a protective effect of estrogen on the immune system;35 however, our CD4 results did not change when we included only women >50 years of age, who are likely to be menopausal. It may also be that women have higher CD4 counts on average compared with men, regardless of HIV status.43 The results of our CD4 analysis were similar after adjustment for demographic and behavioral risk factors, suggesting that the variations we observed in response to ART were related to biological differences by HCV status and sex.
Despite differences in immunologic response to ART, we found that HIV/HCV co-infection was not associated with a higher incidence of AIDS diagnosis after adjustment for risk factors, with no variation in these results by sex. Our results are in concordance with a recent meta-analysis by Chen et al., which included 30 studies with over 100,000 individuals and found no association of HIV/HCV co-infection with AIDS-defining events in the combination ART era.12 In our study, we observed a significantly elevated risk of AIDS diagnosis prior to adjustment for risk factors that differed by HCV status, such as duration of known HIV infection and IDU status. This suggests that results may vary across studies as a result of differences in the distribution of or adjustment for potential confounders. We found that HIV/HCV co-infection was not associated with the incidence of AIDS, either overall or by sex, after adjustment. It may be that the variations in CD4 count over time by sex and HCV status, while statistically significant, were not large enough to have a clinical impact on the incidence of AIDS.
Our finding of increased all-cause mortality among HIV/HCV-co-infected individuals is consistent with several prior studies.10–12 We found that adjustment for demographic, behavioral, and HIV-specific risk factors attenuated but did not remove the association, consistent with an independent effect of HCV infection on mortality. While the multi-cohort CASCADE study found that HIV/HCV co-infection was associated with AIDS-related mortality,11 the meta-analysis by Chen et al. found that HIV/HCV co-infection was associated with mortality but not progression to AIDS in the combination ART era.12 Similarly, we observed an increased risk of liver-related but not AIDS-related death among HIV/HCV-co-infected individuals in our study, suggesting that the HCV-associated increase in mortality is driven by liver disease rather than HIV disease progression.
There are limitations to our study. First, individuals who cleared their HCV infection before initiating ART may have been misclassified as HIV/HCV-co-infected. However, our study was conducted prior to the introduction of highly effective therapies for HCV infection, when treatment response among HIV-infected individuals was poor;44 women may have been especially unlikely to have been treated, given that HCV treatment is not recommended during pregnancy.13 Furthermore, we expect spontaneous clearance of HCV infection to be uncommon among HIV-infected individuals.45 We also conducted a sensitivity analysis of change in CD4 count in the subset of KPNC subjects, defining HCV status using a registry with rigorous inclusion and exclusion criteria, and found results similar to those in the main analysis.
A second limitation is that, given a clinical practice setting without serial testing, we could not identify the exact timing of HCV infection. Some individuals in the HIV/HCV-co-infected group may have acquired HCV infection after initiating ART, potentially biasing our results toward the null.
Third, we used the EMR to collect risk factors such as smoking and drug/alcohol abuse, which may have resulted in some misclassification and limited our ability to analyze these variables with a high level of detail. Fourth, we did not evaluate ART adherence, interruptions, or discontinuation by exposure group; ART adherence may be lower among HIV/HCV-co-infected individuals,46 and ART use patterns vary with pregnancy and the postpartum period among women.47 However, the lack of association of sex and HCV status with virologic response to ART suggests that these factors did not confound the immunologic results we observed. Finally, our subjects were mostly men, potentially limiting our ability to detect differences in outcomes by sex.
Our study also has several strengths. First, to our knowledge, this is the first study to directly compare response to ART by both HCV status and sex. Second, our study population was a large, well-characterized cohort of HIV-mono-infected and HIV/HCV-co-infected individuals from the same healthcare system. Third, the EMR and HIV registries allowed for near-perfect case ascertainment for mortality and HIV infection, as well as the identification of important covariates such as history of IDU, drug/alcohol abuse, and duration of known HIV infection. Fourth, because all KP members have comprehensive medical insurance coverage, differences in outcomes were unlikely to be attributable to differential access to care. Finally, the KP membership mirrors the age, sex, and race/ethnicity distributions of the California statewide population,30,48 and the demographics of HIV-infected KP members are comparable to those of reported AIDS cases in California.49 Thus, our results are likely to be generalizable to other individuals with access to healthcare.
In summary, while the association of HCV infection with AIDS, death, and virologic response to ART does not vary by sex, short-term immunologic response is poorer among HIV/HCV-co-infected compared with HIV-mono-infected individuals, and women attain higher CD4 counts in the long term, irrespective of HCV status. As HIV/HCV co-infection is associated with an increased risk of death overall, HCV infection should be aggressively treated among HIV-infected individuals as recommended,13 regardless of sex. Future research is needed to determine if our findings persist in the new era of highly effective therapies for HCV infection.
Acknowledgments
This research was supported by a research grant from Pfizer Pharmaceuticals. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; or decision to submit the article for publication.
Funding: This research was supported by a research grant from Pfizer Pharmaceuticals. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; or decision to submit the article for publication.
Author Disclosure Statement
DBK and PCT report no conflicts. WAL and LX received research grant support from Pfizer. JLM received research grant support from Merck. MAH, CPQ, MJS, and CRC received research grant support from Pfizer and Merck. WJT received research grant support from Pfizer, Merck, Gilead, Bristol-Myers Squibb, ViiV Healthcare, and Vertex.
References
- 1.Weber R, Sabin CA, Friis-Moller N, et al. . Liver-related deaths in persons infected with the human immunodeficiency virus: The D:A:D study. Arch Intern Med 2006;166:1632–1641 [DOI] [PubMed] [Google Scholar]
- 2.HIV/AIDS and Viral Hepatitis. <http://www.cdc.gov/hepatitis/Populations/HIV.htm> (Last accessedOctober29, 2014)
- 3.Antonucci G, Girardi E, Cozzi-Lepri A, et al. . Role of hepatitis C virus (HCV) viremia and HCV genotype in the immune recovery from highly active antiretroviral therapy in a cohort of antiretroviral-naive HIV-infected individuals. Clin Infect Dis 2005;40:e101–e109 [DOI] [PubMed] [Google Scholar]
- 4.Carmo RA, Guimaraes MD, Moura AS, et al. . The influence of HCV co-infection on clinical, immunological and virological responses to HAART in HIV-patients. Braz J Infect Dis 2008;12:173–179 [DOI] [PubMed] [Google Scholar]
- 5.Tsiara CG, Nikolopoulos GK, Dimou NL, et al. . Effect of hepatitis C virus on immunological and virological responses in HIV-infected patients initiating highly active antiretroviral therapy: A meta-analysis. J Viral Hepat 2013;20:715–724 [DOI] [PubMed] [Google Scholar]
- 6.Weis N, Lindhardt BO, Kronborg G, et al. . Impact of hepatitis C virus co-infection on response to highly active antiretroviral therapy and outcome in HIV-infected individuals: A nationwide cohort study. Clin Infect Dis 2006;42:1481–1487 [DOI] [PubMed] [Google Scholar]
- 7.De Luca A, Bugarini R, Lepri AC, et al. . Co-infection with hepatitis viruses and outcome of initial antiretroviral regimens in previously naive HIV-infected subjects. Arch Intern Med 2002;162:2125–2132 [DOI] [PubMed] [Google Scholar]
- 8.Miller MF, Haley C, Koziel MJ, Rowley CF. Impact of hepatitis C virus on immune restoration in HIV-infected patients who start highly active antiretroviral therapy: A meta-analysis. Clin Infect Dis 2005;41:713–720 [DOI] [PubMed] [Google Scholar]
- 9.Shmagel KV, Saidakova EV, Korolevskaya LB, et al. . Influence of hepatitis C virus co-infection on CD4+ T cells of HIV-infected patients receiving HAART. AIDS 2014;28:2381–2388 [DOI] [PubMed] [Google Scholar]
- 10.Hernando V, Perez-Cachafeiro S, Lewden C, et al. . All-cause and liver-related mortality in HIV positive subjects compared to the general population: Differences by HCV co-infection. J Hepatol 2012;57:743–751 [DOI] [PubMed] [Google Scholar]
- 11.van der Helm J, Geskus R, Sabin C, et al. . Effect of HCV infection on cause-specific mortality after HIV seroconversion, before and after 1997. Gastroenterology 2013;144:751–760 e2 [DOI] [PubMed] [Google Scholar]
- 12.Chen TY, Ding EL, Seage GR, Iii, Kim AY. Meta-analysis: Increased mortality associated with hepatitis C in HIV-infected persons is unrelated to HIV disease progression. Clin Infect Dis 2009;49:1605–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Recommendations for Testing, Managing, and Treating Hepatitis C. American Association for the Study of Liver Diseases and the Infectious Diseases Society of America, 2014 [Google Scholar]
- 14.Baden R, Rockstroh JK, Buti M. Natural history and management of hepatitis C: Does sex play a role? J Infect Dis 2014;209 Suppl 3:S81–S85 [DOI] [PubMed] [Google Scholar]
- 15.Collazos J, Carton JA, Asensi V. Gender differences in liver fibrosis and hepatitis C virus-related parameters in patients co-infected with human immunodeficiency virus. Curr HIV Res 2011;9:339–345 [DOI] [PubMed] [Google Scholar]
- 16.Di Martino V, Lebray P, Myers RP, et al. . Progression of liver fibrosis in women infected with hepatitis C: Long-term benefit of estrogen exposure. Hepatology 2004;40:1426–1433 [DOI] [PubMed] [Google Scholar]
- 17.Thorsteinsson K, Ladelund S, Jensen-Fangel S, et al. . Impact of gender on response to highly active antiretroviral therapy in HIV-1 infected patients: A nationwide population-based cohort study. BMC Infect Dis 2012;12:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore AL, Kirk O, Johnson AM, et al. . Virologic, immunologic, and clinical response to highly active antiretroviral therapy: The gender issue revisited. J Acquir Immune Defic Syndr 2003;32:452–461 [DOI] [PubMed] [Google Scholar]
- 19.Nicastri E, Angeletti C, Palmisano L, et al. . Gender differences in clinical progression of HIV-1-infected individuals during long-term highly active antiretroviral therapy. AIDS 2005;19:577–583 [DOI] [PubMed] [Google Scholar]
- 20.Barber TJ, Geretti AM, Anderson J, et al. . Outcomes in the first year after initiation of first-line HAART among heterosexual men and women in the UK CHIC Study. Antivir Ther 2011;16:805–814 [DOI] [PubMed] [Google Scholar]
- 21.Collazos J, Asensi V, Carton JA. Sex differences in the clinical, immunological and virological parameters of HIV-infected patients treated with HAART. AIDS 2007;21:835–843 [DOI] [PubMed] [Google Scholar]
- 22.Zaragoza-Macias E, Cosco D, Nguyen ML, Del Rio C, Lennox J. Predictors of success with highly active antiretroviral therapy in an antiretroviral-naive urban population. AIDS Res Hum Retroviruses 2010;26:133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giordano TP, Wright JA, Hasan MQ, White AC, Jr, Graviss EA, Visnegarwala F. Do sex and race/ethnicity influence CD4 cell response in patients who achieve virologic suppression during antiretroviral therapy? Clin Infect Dis 2003;37:433–437 [DOI] [PubMed] [Google Scholar]
- 24.Maskew M, Brennan AT, Westreich D, McNamara L, MacPhail AP, Fox MP. Gender differences in mortality and CD4 count response among virally suppressed HIV-positive patients. J Womens Health (Larchmt) 2013;22:113–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ofotokun I, Chuck SK, Hitti JE. Antiretroviral pharmacokinetic profile: A review of sex differences. Gend Med 2007;4:106–119 [DOI] [PubMed] [Google Scholar]
- 26.Umeh OC, Currier JS, Park JG, Cramer Y, Hermes AE, Fletcher CV. Sex differences in lopinavir and ritonavir pharmacokinetics among HIV-infected women and men. J Clin Pharmacol 2011;51:1665–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore DM, Zhang W, Yip B, et al. . Non-medically supervised treatment interruptions among participants in a universally accessible antiretroviral therapy programme. HIV Med 2010;11:299–307 [DOI] [PubMed] [Google Scholar]
- 28.Carter A, Min JE, Chau W, et al. . Gender inequities in quality of care among HIV-positive individuals initiating antiretroviral treatment in British Columbia, Canada (2000–2010). PLoS One 2014;9:e92334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silverberg MJ, Chao C, Leyden WA, et al. . HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomarkers Prev 2011;20:2551–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon N. 2006. How Does the Adult Kaiser Permanente Membership in Northern California Compare with the Larger Community? <http://www.dor.kaiser.org/external/uploadedFiles/content/research/mhs/_2011_Revised_Site/Documents_Special_Reports/comparison_kaiser_vs_nonKaiser_adults_kpnc(1).pdf> (Last accessed February18, 2015)
- 31.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. <http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf> (Last accessed December4, 2014)
- 32.Schneider E, Whitmore S, Glynn KM, Dominguez K, Mitsch A, McKenna MT. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged<18 months and for HIV infection and AIDS among children aged 18 months to <13 years–United States, 2008. MMWR Recomm Rep 2008;57:1–12 [PubMed] [Google Scholar]
- 33.Elliott MN, Fremont A, Morrison PA, Pantoja P, Lurie N. A new method for estimating race/ethnicity and associated disparities where administrative records lack self-reported race/ethnicity. Health Serv Res 2008;43:1722–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiscella K, Fremont AM. Use of geocoding and surname analysis to estimate race and ethnicity. Health Serv Res 2006;41:1482–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gameiro CM, Romao F, Castelo-Branco C. Menopause and aging: Changes in the immune system—A review. Maturitas 2010;67:316–320 [DOI] [PubMed] [Google Scholar]
- 36.Fuping G, Wei L, Yang H, et al. . Impact of hepatitis C virus co-infection on HAART in HIV-infected individuals: Multicentric observation cohort. J Acquir Immune Defic Syndr 2010;54:137–142 [DOI] [PubMed] [Google Scholar]
- 37.Sulkowski MS, Moore RD, Mehta SH, Chaisson RE, Thomas DL. Hepatitis C and progression of HIV disease. JAMA 2002;288:199–206 [DOI] [PubMed] [Google Scholar]
- 38.Rockstroh JK, Mocroft A, Soriano V, et al. . Influence of hepatitis C virus infection on HIV-1 disease progression and response to highly active antiretroviral therapy. J Infect Dis 2005;192:992–1002 [DOI] [PubMed] [Google Scholar]
- 39.Kovacs A, Al-Harthi L, Christensen S, Mack W, Cohen M, Landay A. CD8(+) T cell activation in women co-infected with human immunodeficiency virus type 1 and hepatitis C virus. J Infect Dis 2008;197:1402–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez VD, Falconer K, Blom KG, et al. . High levels of chronic immune activation in the T-cell compartments of patients co-infected with hepatitis C virus and human immunodeficiency virus type 1 and on highly active antiretroviral therapy are reverted by alpha interferon and ribavirin treatment. J Virol 2009;83:11407–11411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Operskalski EA, Kovacs A. HIV/HCV co-infection: Pathogenesis, clinical complications, treatment, and new therapeutic technologies. Curr HIV/AIDS Rep 2011;8:12–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baum MK, Rafie C, Lai S, Sales S, Page JB, Campa A. Alcohol use accelerates HIV disease progression. AIDS Res Hum Retroviruses 2010;26:511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maini MK, Gilson RJ, Chavda N, et al. . Reference ranges and sources of variability of CD4 counts in HIV-seronegative women and men. Genitourin Med 1996;72:27–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGovern BH. Hepatitis C in the HIV-infected patient. J Acquir Immune Defic Syndr 2007;45:S47–S56; discussion S66–S67 [DOI] [PubMed] [Google Scholar]
- 45.Stenkvist J, Nystrom J, Falconer K, Sonnerborg A, Weiland O. Occasional spontaneous clearance of chronic hepatitis C virus in HIV-infected individuals. J Hepatol 2014;61:957–961 [DOI] [PubMed] [Google Scholar]
- 46.Braitstein P, Justice A, Bangsberg DR, et al. . Hepatitis C co-infection is independently associated with decreased adherence to antiretroviral therapy in a population-based HIV cohort. AIDS 2006;20:323–331 [DOI] [PubMed] [Google Scholar]
- 47.Gertsch A, Michel O, Locatelli I, et al. . Adherence to antiretroviral treatment decreases during postpartum compared to pregnancy: A longitudinal electronic monitoring study. AIDS Patient Care STDS 2013;27:208–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koebnick C, Langer-Gould AM, Gould MK, et al. . Sociodemographic characteristics of members of a large, integrated health care system: Comparison with US Census Bureau data. Perm J 2012;16:37–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.2010. 2010. Quarterly HIV/AIDS Statistics. California Department of Public Health, Office of AIDS <http://www.cdph.ca.gov/data/statistics/Pages/OA2010QtrlyStats.aspx> (Last accessed November18, 2014)