Abstract
Purpose
About a third of untreated, perinatally HIV-infected children reach adolescence. We evaluated the durability and effectiveness of non-nucleoside reverse transcriptase inhibitor (NNRTI)-based antiretroviral therapy (ART) in this population.
Methods
Data from perinatally HIV-infected, antiretroviral-naïve patients initiated on NNRTI-based ART aged 10–19 years who had ≥6 months of follow-up were analyzed. Competing risk regression was used to assess predictors of NNRTI substitution and clinical failure (WHO stage 3/4 event or death). Viral suppression was defined as a viral load <400 copies/ml.
Results
Data from 534 adolescents met our inclusion criteria (56.2% female; median age at treatment initiation 11.8 years). After five years of treatment, median height-for-age z-score increased from −2.3 to −1.6 and median CD4+ cell count increased from 131 to 580 cells/mm3. The proportion of patients with viral suppression after 6 months was 87.6% and remained >80% up to five years of follow-up. NNRTI substitution and clinical failure occurred at rates of 4.9 and 1.4 events per 100 patient-years, respectively. Not using cotrimoxazole prophylaxis at ART initiation was associated with NNRTI substitution (HR 1.5 versus using, 95%CI 1.0–2.2, p=0.05). Baseline CD4+ count ≤200 cells/mm3 (HR 3.3 versus >200, 95%CI 1.2–8.9, p=0.02) and not using cotrimoxazole prophylaxis at ART initiation (HR 2.1 versus using, 95%CI 1.0–4.6, p=0.05) were both associated with clinical failure.
Conclusions
Despite late ART initiation, adolescents achieved good rates of catch-up growth, CD4+ count recovery, and virological suppression. Earlier ART initiation and routine cotrimoxazole prophylaxis in this population may help to reduce current rates of NNRTI substitution and clinical failure.
Keywords: HIV, perinatal HIV-infection, antiretroviral therapy, non-nucleoside reverse transcriptase inhibitor, cotrimoxazole
Of the 35 million people living with HIV, an estimated 2.1 million are adolescents (aged 10 – 19 years) living in low- or middle-income countries and approximately 230,000 under 15 years of age are living in the Asia Pacific region.[1,2] Despite a perception in earlier stages of the HIV epidemic that very few perinatally HIV-infected (paHIV) children could survive to adolescence without treatment, data from sub-Saharan Africa indicates that about a third live beyond the age of 10 years.[3,4] Consequently, until early infant diagnosis and linkage to HIV care improves, the number of paHIV adolescents presenting for treatment is likely to continue increasing.
The World Health Organization (WHO) preferred first-line antiretroviral therapy (ART) regimen for adolescents includes two nucleoside reverse transcriptase inhibitors (NRTIs) and a non-nucleoside reverse transcriptase inhibitor (NNRTI).[5] Maximizing the durability of first-line ART in adolescents is crucial given the length of time they will require treatment and the high cost and poorer tolerability of second-line protease inhibitors. While NNRTI-based ART is generally very safe and efficacious, initiation and maintenance of HIV treatment may be complicated by the difficult physical, emotional, and social changes that take place during the transition to adulthood. Furthermore, prescribers must consider the pubertal delay and organ damage that is common in paHIV adolescents.[6–8] Studies from resource-limited areas have found adolescents tend to have high rates of poor treatment adherence, treatment failure and loss-to-follow-up.[9–12] A global effort is now underway to bring greater attention to this unique population, however, in order to improve the current situation we need to understand which treatment interventions are most likely to be effective.
This analysis evaluated the durability and effectiveness of NNRTI-based ART in paHIV adolescents receiving care in low- and middle-income countries in Asia. Specific outcomes evaluated included height-for-age recovery, immunological recovery, virological suppression, NNRTI substitution, and clinical failure.
Methods
The study population consisted of HIV-infected patients enrolled in the TREAT Asia Pediatric HIV Observational Database (TApHOD) which contributes to the International Epidemiologic Databases to Evaluate AIDS global consortium. Recruitment started in 2008 and as of March 2014, TApHOD included data from 5511 children and adolescents who had ever received care at one of 16 pediatric clinics in Cambodia (n=1), India (n=1), Indonesia (n=2), Malaysia (n=4), Thailand (n=5), or Vietnam (n=3). These sites are predominantly public or university-based pediatric HIV referral clinics. Ethics approval was obtained at the sites, TREAT Asia/amfAR (coordinating center), and the Kirby Institute (data management and statistical analysis center). Patient consent is deferred to the individual participating sites and their institutional review boards; some sites require informed consent and others do not.
Data collection in TApHOD is based on a standardized set of demographic, monitoring and treatment variables that have been described previously.[13] The distinction between behavioral and perinatal HIV infection is made by study site investigators based on the clinical information available to them at the time of clinic entry. In general, documentation of perinatal infection is based on a combination of the following criteria: infection identified in early childhood; known or suspected parental HIV; no history of blood transfusion; and no documented or suspected history of sexual abuse.
Data from PaHIV, ART-naive children who started NNRTI-based ART (defined as a triple regimen containing two NRTIs and an NNRTI) aged 10 to 19 years on or after January 1, 2003 and who had at least six months of follow-up on NNRTI-based ART were included in this analysis. Patients exposed to mono/dual therapy prior to starting triple therapy were excluded. Baseline date was the date of ART initiation. Treatment breaks less than 14 days and NRTI modifications were ignored.
The window period for baseline height, weight, CD4+ cell count, hemoglobin, and alanine aminotransferase measurements was within three months before or after ART initiation. For baseline viral load, the window period was between three months before and two weeks after treatment started. Measurements taken closest to the baseline date were used. Alanine aminotransferase upper limit of normal was consistent with the age and sex specific ranges defined by the Harriet Lane Handbook 20th Edition.[14] Severe anemia was defined as hemoglobin <7.5 g/dl.[15] HIV disease staging was based on the WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children.[16] The highest WHO stage documented prior to or at ART initiation was considered the baseline HIV disease severity.
Weight and height measurements were converted into age- and sex-standardized z-scores. Height-for-age z-scores were calculated using the WHO 2007 child growth standards and macros (ages 5–19 years).[17] Weight-for-age z-scores were calculated using the WHO child growth standards and macros for 1977.[18] The 1977 standards were used because the WHO 2007 weight-for-age standards are only applicable to children ≤10 years old, and a previous TApHOD analysis found the 1977 and 2007 standards give similar results.[19] Patients were considered hepatitis B co-infected if they had any record of a positive hepatitis B surface antigen test, and hepatitis C co-infected if they had any record of a positive hepatitis C antibody test. Patients were considered to be using cotrimoxazole prophylaxis if they were using cotrimoxazole at ART initiation or if they started within three months of initiating ART. Country income status was defined according to World Bank categorizations.[20]
Although ART adherence data are not routinely collected in TApHOD, a 2010 survey of ten participating sites collected information on their standard practices.[21] All sites reported providing adherence counseling for children before starting ART, and eight sites indicated that they regularly use one or more methods to monitor adherence to treatment [unpublished results].
Endpoints
Poor follow-up height-for-age was defined as a z-score <−2, poor follow-up CD4+ cell count was defined as a measurement <500 cells/mm3 and viral suppression was defined as a viral load <400 copies/ml. NNRTI substitution was defined as the first instance of NNRTI cessation or addition of a further drug class. Switching to a different NNRTI was not considered an NNRTI substitution. Treatment failure was defined as: after six months of ART; a viral load >1000 copies/mL, CD4+ cell count <100 cells/mm3, CD4+ cell count below baseline, a new or recurrent WHO stage 3 or 4 event, or an NNRTI substitution that the treating physician indicated was due to treatment failure. Other reasons for NNRTI substitution were based on reports from the treating physician. Clinical failure was defined as a new or recurrent WHO stage 3 or 4 event, or death. Loss-to-follow-up was defined as not presenting to care for ≥12 months without documentation of transfer to another clinic. Causes of death were determined by clinic physicians, including designations for immediate and underlying causes. Each case was reviewed by two clinicians, who independently assigned the causes of death, and a final review panel of three clinicians confirmed the causes of death. The most recently documented CD4+ cell count, viral load, and hemoglobin values prior to death or loss-to-follow-up were considered the last known measurements if they were within six months of the final follow-up date.
Statistical Analysis
Generalized estimating equations [22] adjusted for duration of ART were used to investigate predictors of poor follow-up height-for-age, poor follow-up CD4+ cell count, and viral suppression. Height, CD4+, and viral load measurements were considered while patients were using NNRTI-based ART regardless of whether a baseline value was available. Proportions of adolescents meeting our definition of poor follow-up height-for-age, poor follow-up CD4+, and viral suppression were evaluated at 6-month (±3 months) intervals up to five years of follow-up. If, for any given time interval, multiple measurements were recorded for a patient, the value closest to the six-monthly time point was used.
Competing risk regression [23] was used to analyze predictors of NNRTI substitution and clinical failure. Time-to-NNRTI substitution was censored at the last clinic visit and competing events were death and loss-to-follow-up. Other NNRTI substitutions were considered an additional competing risk when time-to-NNRTI substitution due to treatment failure was evaluated. Time-to-clinical failure was censored at the last clinic visit or at the time of NNRTI substitution, and loss-to-follow-up was considered a competing event. Follow-up in all time-to-event analyses was left-truncated.[24]
Covariables were considered statistically significant in our multivariate models if one or more categories exhibited a p-value ≤0.05. Country income status was considered an important inclusion in all multivariate models regardless of significance. Patients with missing data were included in all analyses. However, hazard and odds ratios for missing categories are not reported.
Stata software version 13.1 was used for all statistical analysis.
Results
Patient Characteristics
Of 789 paHIV adolescents who started ART, 534 (67.7%) had at least six months of follow-up on first-line NNRTI-based ART and were eligible for this analysis. Baseline characteristics of the eligible population are shown in Table 1. Median follow-up time was 3.6 (IQR 2.1–6.3) years. For patients using cotrimoxazole prophylaxis at baseline, the median duration of their first cotrimoxazole regimen whilst on NNRTI-based ART was 1.2 (IQR 0.7–1.8) years.
Table 1.
Baseline Characteristics
| Characteristic | Total number of adolescents=534 |
|---|---|
| Age in years, median (IQR) | 11.8 (10.7–13.2) |
| Female | 300 (56.2%) |
| Orphan status, n=369 | |
| Both parents alive | 63 (11.8%) |
| Single parent alive | 111 (20.8%) |
| Neither parent alive | 195 (36.5%) |
| WHO category | |
| 1/2 | 281 (52.6%) |
| 3/4 | 253 (47.4%) |
| Height-for-age z-score, median (IQR), n=418 | −2.3 (−3.6 to −1.4) |
| Weight-for-age z-score, median (IQR), n=429 | −2.6 (−3.6 to −1.4) |
| CD4+ cell count in cells/mm3, median (IQR), n=457 | 131 (31–278) |
| HIV viral load in copies/mL, median (IQR), n=153 | 100,000 (40,900–250,000) |
| Hemoglobin in g/dl, median (IQR), n=407 | 11.2 (10.1–11.9) |
| Alanine transaminase in U/L, median (IQR), n=370 | 22 (15–37) |
| HBsAg positive, n(%tested), n=333 | 25 (7.5%) |
| Hepatitis C antibody positive, n(%tested), n=196 | 4 (2.0%) |
| Prior tuberculosis diagnosis | 104 (19.5%) |
| Using cotrimoxazole prophylaxis | 362 (67.8%) |
| Initial NNRTI-based regimen | |
| 3TC/AZT/EFV | 114 (21.3%) |
| 3TC/d4T/EFV | 70 (13.1%) |
| 3TC/AZT/NVP | 129 (24.2%) |
| 3TC/d4T/NVP | 202 (37.8%) |
| 3TC/TDF/EFV or NVP# | 19 (3.6%) |
| Country income status | |
| High-middle | 400 (74.9%) |
| Low/low-middle | 134 (25.1%) |
Values are n (%total) unless otherwise specified.
One patient was using 3TC/TDF/NVP in this group.
IQR; interquartile range, WHO; World Health Organization, HBsAg; hepatitis B surface antigen, NNRTI; non-nucleoside reverse transcriptase inhibitor, 3TC; lamivudine, AZT; zidovudine, EFV; efavirenz, d4T; stavudine, NVP; nevirapine, TDF; tenofovir.
Height-for-age Recovery
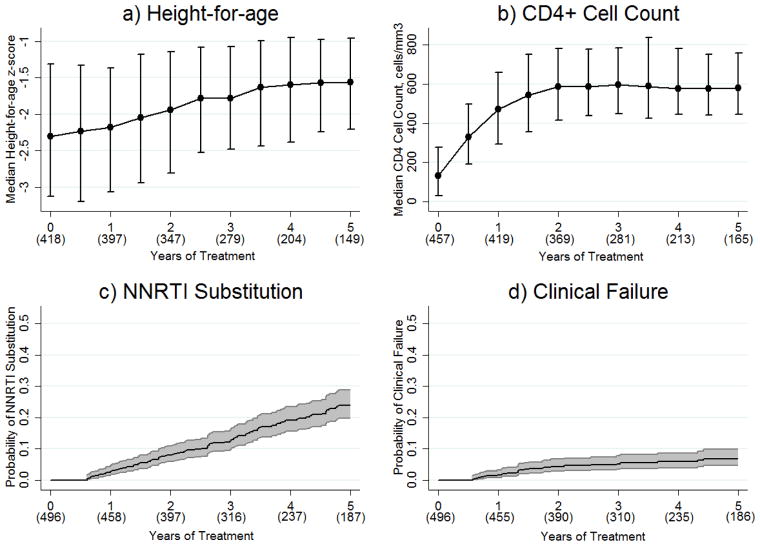
Median height-for-age z-score increased consistently from −2.3 at baseline to −1.6 after five years of treatment (Figure 1a). Baseline height-for-age (OR 41.5 for z-score ≤−2.5 versus >−2.5, 95%CI 25.8–66.5, p<0.01) and orphan status (OR 2.2 for neither parent alive versus both parents alive, 95%CI 1.0–4.3, p=0.04) were significantly associated with follow-up height-for-age z-score <−2. Baseline height-for-age was the only factor that remained significant when follow-up height-for-age was assessed as a continuous variable.
Figure 1. a) Height-for-age Recovery, b) CD4+ Cell Count Recovery, c) NNRTI Substitution, and d) Clinical Failure.
In a) and b), values in parentheses represent the number of patients with data available and error bars represent interquartile ranges. In c) and d), values in parentheses represent the number of patients at risk and shading represents the 95% confidence interval. NNRTI; non-nucleoside reverse transcriptase inhibitor.
CD4+ Cell Count Recovery
The median rate of CD4+ monitoring was 2.0 (IQR 1.7–2.5) tests/year. In high-middle and low/low-middle income countries the median rates were 2.0 (IQR 1.8–2.5) and 1.8 (1.4–2.7) tests/year, respectively. Median CD4+ cell count increased from 131 cells/mm3 at baseline to 330 cells/mm3 after six months, 470 cells/mm3 after 12 months, 585 cells/mm3 after 24 months, and thereafter remained above 575 cells/mm3 (Figure 1b). Baseline CD4+ cell count (OR 3.5 for ≤200 versus >200 cells/mm3, 95%CI 2.5–4.8, p<0.01) and older age (OR 1.1 for every year older, 95%CI 1.0–1.2, p=0.01) were significantly associated with follow-up CD4+ cell count <500 cells/cm3. Both covariates remained significant when follow-up CD4+ cell count was modelled as a continuous variable.
Viral Suppression
The overall median rate of viral load monitoring was 1.0 (IQR 0.5–1.5) test/year. In high-middle income countries the median rate was 1.1 (IQR 0.8–1.7) tests/year and in low/low-middle income countries the median rate was 0.0 (0.0–0.4) tests/year. At baseline, 153 patients had viral load data available. After 6, 12, 24, 36, 48 and 60 months the number of patients with viral load documented was 232, 173, 166, 148, 131, and 101, respectively. The proportion with viral suppression was 87.6% after six months of ART and remained >80% up to five years of follow-up. High baseline viral load was the only factor significantly associated with unsuppressed follow-up viral load (OR 3.9 for every log10 higher, 95%CI 1.9–8.0, p<0.01) and remained significant when follow-up viral load was modelled as a continuous variable.
NNRTI Substitution
Of 332 patients initiated on nevirapine, 44 (13.3%) switched to efavirenz during the course of NNRTI use. The primary reasons for switching from nevirapine to efavirenz included an adverse event (n=17, 38.6%), patient decision (n=10, 22.7%), unknown/other (n=10, 22.7%), drug interaction (n=5, 11.4%), and poor adherence (n=2, 4.6%). Of 202 patients initiated on efavirenz, 24 (11.9%) switched to nevirapine during the course of NNRTI use. The main reasons for switching from efavirenz to nevirapine were unknown/other (n=10, 41.7%), adverse event (n=7, 29.2%), patient decision (n=3, 12.5%), poor adherence (n=2, 8.3%), drug interaction (n=1, 4.2%), and drug stock out (n=1, 4.2%).
One hundred eight NNRTI substitutions occurred at a rate of 4.9 (95%CI 4.1–5.9) events per 100 patient-years (Figure 1c). Median time to NNRTI substitution was 2.9 (IQR 1.6–4.3) years and median CD4+ cell count at substitution was 278 (IQR 134–459) cells/mm3. Patients most commonly switched to protease inhibitor-based ART (n=84, 78%) or had a protease inhibitor added to their NNRTI-based regimen (n=2, 2%). Other switch regimens consisted of lamivudine monotherapy (n=10, 9%), dual therapy (n=8, 7%), or other monotherapy (n=4, 4%). Documented evidence of treatment failure was present for 97 substitutions (4.4 [95%CI 3.6–5.4] events per 100 patient-years); 79 treatment failures involved virological failure, 33 involved immunological failure, 14 involved a new or recurrent WHO stage 3 or 4 illness, and 19 were associated with both virological and immunological failure. Median time from the first documented evidence of treatment failure to NNRTI substitution was 8.5 (IQR 3.1–15.8) months. Among the 11 NNRTI substitutions that were not associated with evidence of treatment failure, three were due to patient/physician decision, one was due to toxicity, one was due to pregnancy, and six reasons were unknown. Not using cotrimoxazole prophylaxis at ART initiation was the only factor significantly associated with NNRTI substitution (HR 1.5 versus using cotrimoxazole, 95%CI 1.0–2.2, p=0.05; Table 2). Our models for NNRTI substitution due to treatment failure were very similar to those depicted in Table 2, although the use of cotrimoxazole prophylaxis became slightly less significant (see Supplementary Table 1).
Table 2.
Baseline Factors Associated with NNRTI Substitution
| NNRTI substitutions | Patient years follow-up | Rate per 100 patient-years (95%CI) | Univariate HR (95%CI) | p | Multivariate HR (95%CI) | p | |
|---|---|---|---|---|---|---|---|
| Overall | 108 | 2197.9 | 4.9 (4.1 – 5.9) | ||||
|
| |||||||
| Cotrimoxazole prophylaxis⋄ | |||||||
| Yes | 68 | 1585.4 | 4.3 (3.4 – 5.4) | 1.0 | 1.0 | ||
| No | 40 | 612.5 | 6.5 (4.8 – 8.9) | 1.5 (1.0 – 2.3) | 0.03 | 1.5 (1.0 – 2.2) | 0.05 |
| Country income status⋄ | |||||||
| High-middle | 95 | 1810.4 | 5.2 (4.3 – 6.4) | 1.5 (0.9 – 2.7) | 0.14 | 1.4 (0.8 – 2.5) | 0.23 |
| Low/low-middle | 13 | 387.5 | 3.4 (1.9 – 5.8) | 1.0 | 1.0 | ||
| Age | |||||||
| Every one year older | 108 | 2197.9 | 4.9 (4.1 – 5.9) | 1.0 (0.9 – 1.1) | 0.79 | 1.0 (0.9 – 1.1) | 0.73 |
| Sex | |||||||
| Male | 44 | 907.0 | 4.9 (3.6 – 6.5) | 1.0 | 1.0 | ||
| Female | 64 | 1290.8 | 5.0 (3.9 – 6.3) | 1.0 (0.7 – 1.5) | 0.88 | 1.0 (0.7 – 1.5) | 0.85 |
| CD4+ cell count | |||||||
| >200 | 25 | 662.2 | 3.8 (2.6 – 5.6) | 1.0 | 1.0 | ||
| ≤200 | 59 | 1244.5 | 4.7 (3.7 – 6.1) | 1.2 (0.8 – 2.0) | 0.40 | 1.4 (0.9 – 2.3) | 0.15 |
| Missing | 24 | 291.1 | 8.2 (5.5 – 12.3) | - | - | ||
| Initial NNRTI-based regimen | |||||||
| 3TC/AZT/EFV | 22 | 386.3 | 5.7 (3.7 – 8.6) | 1.1 (0.7 – 1.9) | 0.65 | 1.2 (0.7 – 1.9) | 0.56 |
| 3TC/d4T/EFV | 14 | 375.7 | 3.7 (2.2 – 6.3) | 0.8 (0.4 – 1.4) | 0.36 | 0.7 (0.4 – 1.3) | 0.31 |
| 3TC/AZT/NVP | 25 | 483.6 | 5.2 (3.5 – 7.7) | 1.0 (0.6 – 1.6) | 0.94 | 0.9 (0.6 – 1.5) | 0.79 |
| 3TC/d4T/NVP | 47 | 920.7 | 5.1 (3.8 – 6.8) | 1.0 | 1.0 | ||
| 3TC/TDF/EFV or NVP | 0 | 31.5 | 0.0 (0.0 – 0.0) | nc | nc | ||
Multivariate HRs for variables not included in the final model show the effect of adjusting for included variables. HRs for missing categories are not shown.
Included in final multivariate model (cotrimoxazole prophylaxis, country income status).
NNRTI; non-nucleoside reverse transcriptase inhibitor, 95%CI; 95% confidence interval, HR; hazard ratio, 3TC; lamivudine, AZT; zidovudine, EFV; efavirenz, d4T; stavudine, NVP; nevirapine, TDF; tenofovir, nc; non-calculable.
Clinical Failure
Thirty clinical failures (including 17 deaths) occurred at a rate of 1.4 (95%CI 1.0–2.0) events per 100 patient-years (Figure 1d). One patient was enrolled into TApHOD after experiencing a WHO stage 3 event on ART but before undergoing NNRTI substitution, hence, this individual’s follow-up time was left-truncated out of our clinical failure analysis and but partly retained in our NNRTI substitution analysis. This is why 14 patients had an NNRTI substitution associated with a previous WHO stage 3 or 4 event but only 13 patients experienced clinical failure in the form of a WHO stage 3 or 4 event. Baseline CD4+ cell count ≤200 cells/mm3 (HR 3.3 versus >200 cells/mm3, 95%CI 1.2–8.9, p=0.02) and not using cotrimoxazole prophylaxis (HR 2.1 versus using cotrimoxazole, 95%CI 1.0–4.6, p=0.05) were significant baseline predictors of clinical failure (Table 3).
Table 3.
Baseline Factors Associated with Clinical Failure
| Clinical failures | Patient- years follow-up | Rate per 100 patient-years (95%CI) | Univariate HR (95%CI) | p | Multivariate HR (95%CI) | p | |
|---|---|---|---|---|---|---|---|
| Overall | 30 | 2176.1 | 1.4 (1.0 – 2.0) | ||||
|
| |||||||
| CD4+ cell count, cells/mm3⋄ | |||||||
| >200 | 5 | 655.4 | 0.8 (0.3 – 1.8) | 1.0 | 1.0 | ||
| ≤200 | 22 | 1230.3 | 1.8 (1.2 – 2.7) | 2.4 (0.9 – 6.4) | 0.07 | 3.3 (1.2 – 8.9) | 0.02 |
| Missing | 3 | 290.4 | 1.0 (0.3 – 3.2) | - | - | ||
| Cotrimoxazole prophylaxis⋄ | |||||||
| Yes | 19 | 1573.9 | 1.2 (0.8 – 1.9) | 1.0 | 1.0 | ||
| No | 11 | 602.2 | 1.8 (1.0 – 3.3) | 1.5 (0.7 – 3.1) | 0.33 | 2.1 (1.0 – 4.6) | 0.05 |
| Country income status⋄ | |||||||
| High-middle | 23 | 1801.6 | 1.3 (0.9 – 1.9) | 1.0 | 1.0 | ||
| Low/low-middle | 7 | 374.5 | 1.9 (0.9 – 3.9) | 1.3 (0.5 – 2.9) | 0.59 | 1.7 (0.7 – 4.3) | 0.24 |
| Age | |||||||
| Every one year older | 30 | 2176.1 | 1.4 (1.0 – 2.0) | 1.0 (0.8 – 1.2) | 0.93 | 1.0 (0.8 – 1.2) | 0.87 |
| Sex | |||||||
| Male | 14 | 896.8 | 1.6 (0.9 – 2.6) | 1.0 | 1.0 | ||
| Female | 16 | 1279.3 | 1.3 (0.8 – 2.0) | 0.8 (0.4 – 1.7) | 0.63 | 0.9 (0.5 – 1.9) | 0.84 |
| Weight-for-age z-score | |||||||
| >−2.5 | 8 | 833.5 | 1.0 (0.5 – 1.9) | 1.0 | 1.0 | ||
| ≤−2.5 | 20 | 920.7 | 2.2 (1.4 – 3.4) | 2.3 (1.0 – 5.2) | 0.05 | 2.0 (0.9 – 4.5) | 0.09 |
| Missing | 2 | 421.9 | 0.5 (0.1 – 1.9) | - | - | ||
| Hemoglobin | |||||||
| Not severely anemic | 24 | 1636.1 | 1.5 (1.0 – 2.2) | 1.0 | 1.0 | ||
| Severely anemic | 2 | 42.1 | 4.8 (1.2 – 19.0) | 3.2 (0.8 – 13.3) | 0.11 | 2.7 (0.7 – 10.6) | 0.15 |
| Missing | 4 | 497.9 | 0.8 (0.3 – 2.1) | - | - | ||
| Initial NNRTI-based regimen | |||||||
| 3TC/AZT/EFV | 6 | 383.7 | 1.6 (0.7 – 3.5) | 1.3 (0.5 – 3.6) | 0.61 | 1.2 (0.4 – 3.3) | 0.72 |
| 3TC/d4T/EFV | 7 | 370.3 | 1.9 (0.9 – 4.0) | 1.9 (0.7 – 4.8) | 0.21 | 1.7 (0.7 – 4.3) | 0.27 |
| 3TC/AZT/NVP | 7 | 482.0 | 1.5 (0.7 – 3.1) | 1.3 (0.5 – 3.3) | 0.65 | 1.3 (0.5 – 3.3) | 0.63 |
| 3TC/d4T/NVP | 10 | 908.5 | 1.1 (0.6 – 2.1) | 1.0 | 1.0 | ||
| 3TC/TDF/EFV or NVP | 0 | 31.5 | 0.0 (0.0 – 0.0) | nc | nc | ||
Multivariate HRs for variables not included in the final model show the effect of adjusting for included variables. HRs for missing categories are not shown.
Included in final multivariate model (CD4 cell count, cotrimoxazole prophylaxis, country income status).
95%CI; 95% confidence interval, HR; hazard ratio, NNRTI; non-nucleoside reverse transcriptase inhibitor, 3TC; lamivudine, AZT; zidovudine, EFV; efavirenz, d4T; stavudine, NVP; nevirapine, TDF; tenofovir, nc; non-calculable.
Mortality and Loss-to-follow-up
Seventeen deaths occurred at a rate of 0.8 (95%CI 0.5–1.2) events per 100 patient-years. The median age, last CD4+ cell count and time on ART at death were 14.4 (IQR 12.5–16.6) years, 130 (15–328) cells/mm3 and 1.4 (1.0–3.1) years, respectively. Further details on the causes of death are provided in Table 4. Loss-to-follow-up occurred in 13 children at an overall rate of 0.6 (95%CI 0.3–1.0) events per 100 patient-years. The median age, last CD4+ cell count and time on ART at the last documented visit before being lost were 16.4 (IQR 15.6–18.5) years, 624 (341–725) cells/mm3 and 4.9 (2.6–7.0) years, respectively.
Table 4.
Causes of death
| Patient | Age in years at death | Sex | Last CD4+ cell count in cells/mm3 | Last viral load in copies/mL | Last hemoglobin in g/dl | Using cotrimoxazole at time of death | Last ART regimen | Years on ART at death | Underlying cause of death |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 17.3 | F | 468 | ? | 12.1 | No | 3TC/AZT/EFV | 7.0 | Encephalopathy |
| 2 | 16.6 | F | ? | <400 | ? | No | 3TC/AZT/NVP | 3.1 | Rapidly progressive glomerulonephritis |
| 3 | 19.3 | F | ? | ? | ? | No | 3TC/AZT/EFV | 8.7 | Unknown |
| 4 | 11.8 | F | 192 | <400 | 7.6 | No | 3TC/d4T/NVP | 1.0 | Lymphoma, B-cell |
| 5 | 21.4 | F | ? | ? | ? | No | 3TC/d4T/NVP | 9.4 | Wasting |
| 6 | 11.6 | M | 43 | ? | 6.4 | No | 3TC/d4T/NVP | 0.8 | Unknown |
| 7 | 13.7 | F | 11 | <400 | 13.1 | No | 3TC/d4T/NVP | 1.4 | Unknown |
| 8 | 16.6 | M | 389 | 53,800 | 13.0 | No | 3TC/AZT/NVP | 4.4 | Psychiatric disease |
| 9 | 15.1 | F | 328 | <400 | 12.9 | Yes | 3TC/d4T/EFV | 0.8 | Lactic acidosis |
| 10 | 13.9 | F | 130 | ? | 12.2 | Yes | 3TC/d4T/NVP | 1.4 | Drug side effect |
| 11 | 12.5 | F | ? | ? | ? | Yes | 3TC/d4T/NVP | 1.5 | Mastoiditis |
| 12 | 16.0 | M | ? | ? | 14.3 | No | 3TC/d4T/EFV | 1.3 | Meningitis |
| 13 | 15.0 | F | 10 | 75,238 | 4.5 | Yes | 3TC/d4T/NVP | 3.0 | Sepsis |
| 14 | 11.9 | M | ? | ? | ? | Yes | 3TC/ddI/EFV | 1.7 | Rhabdomyosarcoma |
| 15 | 14.4 | M | 178 | ? | ? | Yes | 3TC/AZT/EFV | 0.7 | Tuberculosis, disseminated |
| 16 | 10.8 | F | 53 | ? | ? | Yes | 3TC/AZT/NVP | 0.7 | Unknown |
| 17 | 14.1 | M | 15 | ? | 8.4 | Yes | 3TC/TDF/EFV | 1.0 | Pulmonary tuberculosis |
ART; antiretroviral therapy, F; female, M; male, ?; unknown value, 3TC; lamivudine, AZT; zidovudine, EFV; efavirenz, NVP; nevirapine, d4T; stavudine, ddI; didanosine, TDF; tenofovir.
Discussion
ART should be started during infancy in paHIV children.[5] However, early diagnosis of perinatal infection was a major challenge in Asia during the early years of the HIV epidemic, which has led to a survival cohort of adolescents with slow-progressing HIV who frequently present for care with advanced disease and severe immunosuppression. Since our study group represented a sample of this population, it was not surprising that almost 50% of patients were categorized as WHO stage 3/4 at ART initiation, and that median baseline CD4+ cell count was well below the threshold of ≤500 cells/mm3 currently recommended for treatment initiation by the WHO.[5] Clearly, there is a need to more rapidly identify and link infants and children to HIV care in Asia. Further work is also required to determine the proportion of paHIV adolescents that comprise the adolescent HIV population as current data does not distinguish between perinatally and behaviorally infected individuals.
Height-for-age is an important marker of nutritional status in children. Stunting, frequently defined as a height-for-age z-score <−2, can be a stigmatizing feature of pediatric HIV infection that distinguishes paHIV adolescents from their uninfected peers and those infected behaviorally.[7,25] Fortunately, children generally experience catch-up growth once started on ART.[26,27] The rate of height-for-age increase in our analysis was very similar to that reported for an HIV-infected cohort of 5–15 year old children using ART in China.[27] We also found that adolescents with the lowest baseline height-for-age had the greatest difficulty achieving a normal z-score which is consistent with previous studies in children.[26,27] Interestingly, double orphans were more likely to have a follow-up height-for-age z-score <−2 compared to non-orphans. This association should be interpreted with caution, as it became non-significant when follow-up height-for-age was modelled as a continuous variable. However, it is consistent with a study from Kenya which showed orphans experienced identical weight and height gains as non-orphans for the first 70 weeks of ART before both measurements begin to drop in orphans.[28] Bhattacharya et al (2010) have hypothesized that the poor nutritional status amongst HIV-infected orphans in India when compared to HIV-infected non-orphans may be associated with guardians being more likely than parents to become overwhelmed by their caregiving duties.[29]
Maximizing the durability and effectiveness of ART in adolescents is crucial given the length of time they will require treatment. However, current literature from Africa suggests HIV-infected adolescents experience difficulty achieving immunological recovery and remaining in care.[9–12] Shroufi et al (2013) reported that almost 25% of paHIV adolescents in southern Africa had a CD4+ cell count <200 cells/mm3 after five years on ART, and that the rate of loss-to-follow-up was 4.8 per 100 patient-years.[12] Moreover, Bakanda et al (2011) found that adolescents in a Ugandan HIV cohort had an overall mortality rate of 3.65 per 100 patient-years on ART.[9] In comparison, data from Asia, including our own, indicate that HIV-infected adolescents in the region have a better immune response to ART and lower rates of attrition and death.[30–32] Importantly, these differences do not appear to be attributable to regional variation in adherence as a recent meta-analysis reported that adolescents in Africa and Asia both exhibit an average ART adherence of approximately 84%.[10] The findings from our study suggest that an alternative explanation might be regional variation in the timing of ART initiation and use of cotrimoxazole prophylaxis.
Higher viral load at ART initiation was associated with higher odds of unsuppressed viral load on treatment, and baseline CD4+ cell count ≤200 cells/mm3 predicted both follow-up CD4+ count <500 cells/mm3 and clinical failure. Numerous adult and pediatric studies support these results,[12,30,33] including the recent START study which was terminated ahead of schedule after the interim analysis revealed that delaying ART initiation in asymptomatic adults until CD4+ cell count drops to 350 cells/mm3 significantly increased the risk of an AIDS-related event, serious non-AIDS-related event, or death.[33] Although the PREDICT trial reported no difference in AIDS-free survival between early and deferred ART in children older than 1 year of age, the authors themselves concluded that the lower than expected event rate in their study resulted in their analysis being underpowered to detect a significant difference.[34]
We also found that not using cotrimoxazole prophylaxis at ART initiation was associated with an increased rate of NNRTI substitution and clinical failure. The WHO currently recommends that cotrimoxazole prevention therapy is provided for all HIV-infected children living in resource-limited areas with high HIV prevalence for the prevention of Pneumocystis pneumonia, toxoplasmosis, bacterial infections, and malaria.[5] Further, cotrimoxazole has been shown to slow growth retardation when ART is not available,[35] and to substantially reduce the risk of HIV-associated mortality.[36,37] Given cotrimoxazole prophylaxis at ART initiation was only documented for 67.8% of our study population, increased use amongst paHIV adolescents in Asia should be strongly encouraged.
Notably, over 20% of adolescents in this analysis that switched from their NNRTI-based regimen were initiated on mono or dual therapy. Whilst such an approach is not widely recommended, financial and structural constraints in resource-limited areas often limit access to new drugs and drug-sparing regimens may help to improve treatment adherence. Furthermore, recent evidence suggests that, in paHIV children with virological treatment failure, switching to a drug-sparing therapy produces similar CD4% increases and viral load reductions as switching to a new ART regimen.[38]
There were several limitations to this study. Most centers involved in TApHOD are urban referral centers and therefore our results may not be generalizable to rural settings in low/middle income Asia. Given this was an analysis of observational program data we had to adjust for confounding factors using statistical methods. Importantly, the rate of viral load monitoring at sites from high-middle income countries was much higher than that of sites from low/low-middle income countries. As ART modifications occur more frequently at clinics that regularly monitor treatment [39] and viral failure tends to occur before immunological or clinical failure,[40] the difference in viral load testing by country income status may have driven the non-significant associations we observed between high-middle income status and NNRTI substitution, and low/low-middle income status and clinical failure. This highlights the importance of including income status in our models. Unfortunately however, our sample size was insufficient to adjust for more subtle differences in monitoring practices such as those that may have existed between sites or over the course of the study period. We were also missing some important information; more complete data on ART adherence, HIV viral load, drug toxicity, and social history would have helped to provide greater certainty to some of our findings.
PaHIV adolescents using NNRTI-based ART in our Asian cohort achieved good height-for-age and CD4+ cell count recovery, and high rates of virological suppression. Our results indicate that earlier ART initiation and wider use of cotrimoxazole prophylaxis in this population may help to reduce the current rates of NNRTI substitution and clinical failure.
Supplementary Material
Implication and Contributions Statement.
Little is known about how perinatally HIV-infected, ART-naïve adolescents respond to treatment. This work shows they achieve good height-for-age and CD4+ recovery, and a high rate of virological suppression. Earlier ART initiation and routine concomitant cotrimoxazole prophylaxis may help reduce current rates of NNRTI substitution and clinical failure.
Acknowledgments
Sources of Funding: The TREAT Asia Pediatric HIV Observational Database is an initiative of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Cancer Institute as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA; U01AI069907), the AIDS Life Association, and ViiV Healthcare. The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, UNSW Australia.
The TREAT Asia Pediatric HIV Network: CV Mean, V Saphonn*, and S Sarun, National Centre for HIV/AIDS, Dermatology and STDs, Phnom Penh, Cambodia; J Tucker, New Hope for Cambodian Children, Phnom Penh, Cambodia; FJ Zhang, Beijing Ditan Hospital, Capital Medical University, Beijing, China; N Kumarasamy*, S Saghayam, and E Chandrasekaran, YRGCARE Medical Centre, CART CRS, Chennai, India; DK Wati*, LPP Atmikasari, and IY Malino, Sanglah Hospital, Udayana University, Bali, Indonesia; N Kurniati*, and D Muktiarti, Cipto Mangunkusumo General Hospital, Jakarta, Indonesia; SM Fong*,‡, M Thien, M Lim, and F Daut, Hospital Likas, Kota Kinabalu, Malaysia; NK Nik Yusoff*, and P Mohamad, Hospital Raja Perempuan Zainab II, Kelantan, Malaysia; KA Razali*, TJ Mohamed, NF Abdul Rahman, and NADR Mohammed, Pediatric Institute, Hospital Kuala Lumpur, Kuala Lumpur, Malaysia; R Nallusamy*, and KC Chan, Penang Hospital, Penang, Malaysia; T Sudjaritruk*, V Sirisanthana, L Aurpibul, and P Oberdorfer, Department of Pediatrics, Faculty of Medicine, Chiang Mai University and Research Institute for Health Sciences, Chiang Mai, Thailand; R Hansudewechakul*, S Denjanta, W Srisuk, and A Kongphonoi, Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand; P Lumbiganon*, P Kosalaraksa, P Tharnprisan, and T Udomphanit, Department of Pediatrics, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand; G Jourdain, PHPT-IRD UMI 174 (Institut de recherche pour le développement and Chiang Mai University), Chiang Mai, Thailand; T Bunupuradah*, T Puthanakit, W Prasitsuebsai, and W Chanthaweethip, HIV-NAT, Thai Red Cross AIDS Research Centre, Bangkok, Thailand; K Chokephaibulkit*, K Lapphra, W Phongsamart, and S Sricharoenchai, Department of Pediatrics, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand; KH Truong*,†, QT Du, and CH Nguyen, Children’s Hospital 1, Ho Chi Minh City, Vietnam; VC Do*, TM Ha, and VT An Children’s Hospital 2, Ho Chi Minh City, Vietnam; LV Nguyen*, DTK Khu, AN Pham, and LT Nguyen, National Hospital of Pediatrics, Hanoi, Vietnam; ON Le, Worldwide Orphans Foundation, Ho Chi Minh City, Vietnam; AH Sohn*, N Durier, and C Sethaputra, TREAT Asia/amfAR -- The Foundation for AIDS Research, Bangkok, Thailand; DA Cooper, MG Law*, and A Kariminia, The Kirby Institute, UNSW Australia, Sydney, Australia;
Abbreviations
- 95%CI
95% confidence interval
- ART
antiretroviral therapy
- AZT
zidovudine
- d4T
stavudine
- EFV
efavirenz
- HBsAg
hepatitis B surface antigen
- HR
hazard ratio
- IQR
interquartile range
- nc
non-calculable
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- NRTI
nucleoside reverse transcriptase inhibitor
- NVP
nevirapine
- OR
odds ratio
- paHIV
perinatally HIV infected
- PI
protease inhibitor
- TApHOD
TREAT Asia Pediatric HIV Observational Study
- TDF
tenofovir
- WHO
World Health Organization
Footnotes
TApHOD Steering Committee member
Current Steering Committee Chair;
co-Chair
Conflicts of Interest: None to disclose.
The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the governments or institutions mentioned above.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UNAIDS. [Accessed 13 April 2015];Global report - UNAIDS report on the global AIDS epidemic. 2013 http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Global_Report_2013_en_1.pdf.
- 2.UNAIDS. [Accessed 21 Feb 2014];HIV in Asia and the Pacific: UNAIDS report 2013. http://www.unaids.org/en/resources/documents/2013/name,89768,en.asp.
- 3.Ferrand RA, Corbett EL, Wood R, et al. AIDS among older children and adolescents in Southern Africa: projecting the time course and magnitude of the epidemic. Aids. 2009;23:2039–2046. doi: 10.1097/QAD.0b013e32833016ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marston M, Zaba B, Salomon JA, et al. Estimating the net effect of HIV on child mortality in African populations affected by generalized HIV epidemics. Journal of acquired immune deficiency syndromes. 2005;38:219–227. doi: 10.1097/00126334-200502010-00015. [DOI] [PubMed] [Google Scholar]
- 5.WHO ART Guidelines Committee. [Accessed 03 January 2014];Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2013 Jun; http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf?ua=1. [PubMed]
- 6.Ferrand RA, Desai SR, Hopkins C, et al. Chronic lung disease in adolescents with delayed diagnosis of vertically acquired HIV infection. Clin Infect Dis. 2012;55:145–152. doi: 10.1093/cid/cis271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrand RA, Munaiwa L, Matsekete J, et al. Undiagnosed HIV infection among adolescents seeking primary health care in Zimbabwe. Clin Infect Dis. 2010;51:844–851. doi: 10.1086/656361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller RF, Kaski JP, Hakim J, et al. Cardiac disease in adolescents with delayed diagnosis of vertically acquired HIV infection. Clin Infect Dis. 2013;56:576–582. doi: 10.1093/cid/cis911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakanda C, Birungi J, Mwesigwa R, et al. Survival of HIV-infected adolescents on antiretroviral therapy in Uganda: findings from a nationally representative cohort in Uganda. PloS one. 2011;6:e19261. doi: 10.1371/journal.pone.0019261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SH, Gerver SM, Fidler S, et al. Adherence to antiretroviral therapy in adolescents living with HIV: systematic review and meta-analysis. Aids. 2014 doi: 10.1097/QAD.0000000000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nachega JB, Hislop M, Nguyen H, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. Journal of acquired immune deficiency syndromes. 2009;51:65–71. doi: 10.1097/QAI.0b013e318199072e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shroufi A, Gunguwo H, Dixon M, et al. HIV-infected adolescents in southern Africa can achieve good treatment outcomes: results from a retrospective cohort study. Aids. 2013;27:1971–1978. doi: 10.1097/QAD.0b013e32836149ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kariminia A, Chokephaibulkit K, Pang J, et al. Cohort profile: the TREAT Asia pediatric HIV observational database. Int J Epidemiol. 2011;40:15–24. doi: 10.1093/ije/dyp358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engorn B, Flerlage J. The Harriet Lane handbook. 20. Philadelphia, PA: Elsevier Saunders; 2014. Johns Hopkins Hospital. Children’s Medical and Surgical Center. [Google Scholar]
- 15.United States NIH Division of AIDS (DAIDS) [Accessed 19 Jan 2015];DAIDS table for grading the severity of adult and pediatric adverse events version 1.0. 2004 Dec; Clarification August 2009. http://rcc.tech-res.com/safetyandpharmacovigilance/gradingtables.aspx.
- 16.WHO. [Accessed 29 Jul 2015];WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. 2006 http://www.who.int/hiv/pub/guidelines/hivstaging/en/
- 17.WHO. [Accessed 29 Jul 2014];WHO 2007 child growth standards and macros (ages 5–19yrs) http://www.who.int/growthref/en/
- 18.WHO. WHO child growth standards and macros. 1977. [Google Scholar]
- 19.Hansudewechakul R, Sirisanthana V, Kurniati N, et al. Antiretroviral therapy outcomes of HIV-infected children in the TREAT Asia pediatric HIV observational database. Journal of acquired immune deficiency syndromes. 2010;55:503–509. doi: 10.1097/QAI.0b013e3181f5379a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The World Bank. [Accessed 02 Feb 2015];Countries and Economies. http://data.worldbank.org/country.
- 21.IeDEA Pediatric Working Group. A survey of paediatric HIV programmatic and clinical management practices in Asia and sub-Saharan Africa--the International epidemiologic Databases to Evaluate AIDS (IeDEA) Journal of the International AIDS Society. 2013;16:17998. doi: 10.7448/IAS.16.1.17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang K, SLZ Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 23.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 24.Tsai W, NPJ, Wang M. A note on the product-limit estimator under right censoring and left truncation. Biometrika. 1987;74:883–886. [Google Scholar]
- 25.Majaliwa ES, Mohn A, Chiarelli F. Growth and puberty in children with HIV infection. Journal of endocrinological investigation. 2009;32:85–90. doi: 10.1007/BF03345686. [DOI] [PubMed] [Google Scholar]
- 26.Gsponer T, Weigel R, Davies MA, et al. Variability of growth in children starting antiretroviral treatment in southern Africa. Pediatrics. 2012;130:e966–977. doi: 10.1542/peds.2011-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y, Li C, Sun X, et al. Mortality and treatment outcomes of China’s National Pediatric antiretroviral therapy program. Clin Infect Dis. 2013;56:735–744. doi: 10.1093/cid/cis941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nyandiko WM, Ayaya S, Nabakwe E, et al. Outcomes of HIV-infected orphaned and non-orphaned children on antiretroviral therapy in western Kenya. Journal of acquired immune deficiency syndromes. 2006;43:418–425. doi: 10.1097/01.qai.0000243122.52282.89. [DOI] [PubMed] [Google Scholar]
- 29.Bhattacharya M, Rajeshwari K, Saxena R. Demographic and clinical features of orphans and nonorphans at a pediatric HIV centre in North India. Indian journal of pediatrics. 2010;77:627–631. doi: 10.1007/s12098-010-0076-3. [DOI] [PubMed] [Google Scholar]
- 30.Chokephaibulkit K, Kariminia A, Oberdorfer P, et al. Characterizing HIV manifestations and treatment outcomes of perinatally infected adolescents in Asia. The Pediatric infectious disease journal. 2014;33:291–294. doi: 10.1097/INF.0b013e3182a18223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee KH, Ho TS, Shen CF, et al. Clinical and laboratory characteristics of human immunodeficiency virus-infected adolescents: experience from a single medical center. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 2012;45:329–336. doi: 10.1016/j.jmii.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Narkbunnam T, Boon-yasidhi V, Tarugsa J, et al. Characteristics of perinatal HIV-infected adolescents at Siriraj Hospital, Mahidol University (abstract number 43. 020) International Journal of Infectious Diseases. 2012;165:e188. [Google Scholar]
- 33.Insight Start Study Group. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015 doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed]
- 34.Puthanakit T, Saphonn V, Ananworanich J, et al. Early versus deferred antiretroviral therapy for children older than 1 year infected with HIV (PREDICT): a multicentre, randomised, open-label trial. The Lancet infectious diseases. 2012;12:933–941. doi: 10.1016/S1473-3099(12)70242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prendergast A, Walker AS, Mulenga V, et al. Improved growth and anemia in HIV-infected African children taking cotrimoxazole prophylaxis. Clin Infect Dis. 2011;52:953–956. doi: 10.1093/cid/cir029. [DOI] [PubMed] [Google Scholar]
- 36.Bwakura-Dangarembizi M, Kendall L, Bakeera-Kitaka S, et al. A randomized trial of prolonged co-trimoxazole in HIV-infected children in Africa. N Engl J Med. 2014;370:41–53. doi: 10.1056/NEJMoa1214901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chintu C, Bhat GJ, Walker AS, et al. Co-trimoxazole as prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): a double-blind randomised placebo-controlled trial. Lancet. 2004;364:1865–1871. doi: 10.1016/S0140-6736(04)17442-4. [DOI] [PubMed] [Google Scholar]
- 38.Fairlie L, Karalius B, Patel K, et al. CD4+ and viral load outcomes of antiretroviral therapy switch strategies after virologic failure of combination antiretroviral therapy in perinatally HIV-infected youth in the United States. Aids. 2015;29:2109–2119. doi: 10.1097/QAD.0000000000000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wright S, Boyd MA, Yunihastuti E, et al. Rates and factors associated with major modifications to first-line combination antiretroviral therapy: results from the Asia-Pacific region. PloS one. 2013;8:e64902. doi: 10.1371/journal.pone.0064902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartlett JA, Shao JF. Successes, challenges, and limitations of current antiretroviral therapy in low-income and middle-income countries. The Lancet infectious diseases. 2009;9:637–649. doi: 10.1016/S1473-3099(09)70227-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.