Abstract
Although neurotensin (NT) analogs are known to produce antipsychotic-like effects, the therapeutic possibility of a brain penetrant NTS1 agonist in treating psychiatric disorders has not been well studied. Here, we examined whether PD149163, a brain-penetrant NTS1-specific agonist, displays antipsychotic-like effects in C57BL/6J mice by investigating the effect of PD149163 on amphetamine-mediated hyperactivity and amphetamine-induced disruption of prepulse inhibition. In addition, we assessed the effect of PD149163 on glycogen synthase kinase-3 (GSK-3) activity, a downstream molecular target of antipsychotics and mood stabilizers, using phospho-specific antibodies. PD149163 (0.1 and 0.5 mg/kg) inhibited amphetamine-induced hyperactivity in mice, indicating that NTS1 activation inhibits psychomotor agitation. PD149163 (0.5 mg/kg) also increased prepulse inhibition, suggesting that NTS1 activation reduces prepulse inhibition deficits which often co-occur with psychosis in humans. Interestingly, PD149163 increased the inhibitory serine phosphorylation on both GSK-3α and GSK-3β in a dose- and time-dependent manner in the nucleus accumbens and medial prefrontal cortex of the mice. Moreover, PD149163 inhibited GSK-3 activity in the nucleus accumbens and medial prefrontal cortex in the presence of amphetamine. Thus, like most current antipsychotics and mood stabilizers, PD149163 inhibited GSK-3 activity in cortico-striatal circuitry. Together, our findings indicate that PD149163 may be a novel antipsychotic.
Keywords: amphetamine, prepulse inhibition, locomotion, neurotensin type 1 receptor, glycogen synthase kinase 3
1. Introduction
Schizophrenia and mania share phenotypes and pathology. Psychosis, the hallmark of schizophrenia, may also occur during severe manic episodes. Both disorders could present with hyperactivity, and psychosis. Shared phenotypes may result from a common dysregulation in neurotransmitter systems and genetics. For example, elevated dopamine signaling has been proposed to lead to symptoms present in mania and schizophrenia [1, 2]. Furthermore, atypical antipsychotics, which typically inhibit dopamine D2 receptors (D2R), along with other receptor subtypes, are approved for the treatment of both schizophrenia and mania. Interestingly, genetic studies indicate that there are shared genetic factors that may increase the risk of development of bipolar disorder or schizophrenia [3]. Although a number of pharmacological agents are approved for the treatment of mania and schizophrenia, including antipsychotics, most current medications exhibit suboptimal efficacy, delayed onset of therapeutic action, and produce numerous side effects [4–6].
Increasing evidence suggests that neurotensin receptor type 1 (NTS1) is a promising therapeutic target for mania and schizophrenia. Patients with affective disorders, schizoaffective disorder, or schizophrenia were reported to exhibit reduced neurotensin (NT) receptor binding in the entorhinal cortex and decreased NT levels in the cerebrospinal fluid (CSF) [7–9]. It is possible that decreased NT signaling may lead to some of the symptoms in schizophrenia and mania. Moreover, increased NT signaling may underlie the therapeutic effects of antipsychotics [10]. Both acute and chronic antipsychotic administration increased NT levels in rodent brains [10, 11]. Consistently, several clinical studies showed that antipsychotic treatment increased NT levels in the CSF [9, 12]. The antipsychotic-induced increase in NT signaling may be involved in the therapeutic effects of antipsychotics. NT receptor antagonists were demonstrated to inhibit the therapeutic-like effects of antipsychotics on disruptions in prepulse inhibition (PPI) and amphetamine-induced hyperlocomotion in rodents [13, 14]. Furthermore, NT analogs have been shown to suppress psychomotor agitation and disruptions in PPI without causing extrapyramidal side effects, indicating that the effects of NT analogs more closely resemble that of atypical antipsychotics [15, 16]. Genetic and pharmacological manipulations suggest that NTS1 may mediate the antipsychotic-like effects of NT [17–19]. PD149163 is a brain permeable, NTS1 selective agonist. Thus, PD149163 has potential as a novel compound to specifically target hyperactivity, agitation, and psychosis in patients with schizophrenia or mania. PD149163 exhibits antipsychotic-like effects in rats, but whether PD149163 dose-dependently inhibits psychomotor agitation in mice is unclear [16, 18]. It has been suggested that the NT system in mice, as opposed to rats, more closely resembles the human NT system [20]. Furthermore, given the greater availability of techniques to manipulate the mouse genome, it is important to establish the effects of NTS1 activation in mice, which could pave the way for future circuitry-specific studies.
To examine whether PD149163 exerts antipsychotic-like effects, we examined the effect of PD149163 on amphetamine-mediated hyperactivity and amphetamine-induced disruption of PPI in mice. Acute amphetamine treatment is commonly used to model certain behavioral domains of mania and schizophrenia, and screen if novel drugs exert antipsychotic-like effects. The acute amphetamine model induces schizophrenia- and mania-like psychomotor agitation in rodents [21, 22]. Amphetamine also produces deficits in PPI of startle reactivity, modeling the disruption in PPI observed in humans with schizophrenia, and possibly mania [23–25]. Furthermore, amphetamine increases dopamine output, and dysregulated dopaminergic signaling has been hypothesized to explain some of the symptoms in mania and schizophrenia [1, 2]. The model is also commonly used since antipsychotics and other anti-manic drugs typically inhibit amphetamine-induced hyperactivity and disruptions in PPI [26–28].
One of the most well studied molecular targets of current antipsychotics and anti-manic drugs is glycogen synthase kinase-3 (GSK-3). GSK-3 is a ubiquitously expressed serine/threonine kinase with two isoforms, GSK-3α and GSK-3β. Antipsychotics and other anti-manic drugs are known to inhibit GSK-3 activity [29, 30]. GSK-3 inhibitors have been shown to produce antipsychotic-like effects, indicating that some of the effects of current medications could be through inhibition of GSK-3 [31, 32]. It is not known if activation of the G protein-coupled receptor, NTS1, also inhibits GSK-3 activity in cortico-striatal circuitry by increasing the inhibitory serine phosphorylation on GSK-3 (pGSK-3α Ser21 and pGSK-3β Ser9) like many existing antipsychotics. NT was found to inhibit GSK-3 activity in human colon cancer cells, suggesting that it is possible that NT may inhibit GSK-3 in the brain [33]. Therefore, in this study, we examined whether PD149163 exerts antipsychotic-like effects in mice by examining the effect of PD149163 on amphetamine-induced behaviors and GSK-3 phosphorylation in cortico-striatal circuitry.
2. Materials and methods
2.1. Animals
Male C57BL/6J mice (6 weeks old, Jackson Laboratories, Bar Harbor, ME) were group housed (4–5 mice per group) in standard plexiglass cages under a 12 h light/dark cycle with lights on at 6:00 AM. Behavioral experiments were carried out during the light phase. Food and water were provided ad libitum. Mice were used for the behavioral studies between 8–16 weeks of age. Animal care and handling procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committees in accordance with National Institutes of Health guidelines.
2.2. Drugs
PD149163 tetrahydrochloride hydrate (PD149163) and D-amphetamine hemisulfate salt (amphetamine) were purchased from Sigma-Aldrich (St. Louis, MO). Both drugs were diluted in saline. A dilution of 0.02 mg/mL of PD149163 was used for the 0.5 mg/kg dose. For the 0.05 and 0.1 mg/kg doses, PD149163 was injected at a concentration of 0.01 mg/mL. Amphetamine was administered at a dose of 2 mg/kg (0.2 mg/mL) or 10 mg/kg (1 mg/mL). Mice received intraperitoneal (i.p.) injections of amphetamine, PD149163, or an equal volume of saline.
2.3. Behavioral assays
2.3.1. Open-field
Spontaneous locomotor activity was measured during the light phase in open-field chambers (27 × 27 × 20.3 cm) equipped with infrared photobeams to record X-Y ambulatory movements at a 50 ms resolution (Med Associates Inc., St. Albans, VT; Vadnie et al, 2014). The chambers were located in brightly lit (500 lux), sound-attenuating cubicles. All mice were allowed to habituate to the room for 1 h prior to locomotor measurements. Mice were first injected with saline or PD149163 (0.05, 0.1 or 0.5 mg/kg, i.p.), then injected with saline or amphetamine (2 mg/kg, i.p.) after one hour. After the second injection, mice were immediately placed in the open-field and locomotor activity was recorded for 90 min. Activity was quantified as horizontal distance traveled (cm). Two mice were excluded from the open-field locomotor analysis due to malfunction of the sensors.
2.3.2. Prepulse inhibition (PPI)
One week after the open-field experiment, mice were randomly assigned to experimental groups for prepulse inhibition (PPI) testing. Sound-attenuating chambers were used to examine startle reactivity and PPI (SR-LAB, San Diego Instruments, San Diego, CA). Chambers were equipped with a house light and a loudspeaker. Each chamber contained a cylindrical plexiglass animal enclosure that rested on a platform with a piezoelectric accelerometer mounted below. The piezoelectric accelerometer converted vibrations of the mouse in the enclosure to analog signals that were stored on a computer. At the onset of the startle stimulus, 65 readings were recorded at 1 ms intervals to capture maximum startle amplitude. We used the maximum startle amplitude to determine the startle response.
Each session began with a 5-min acclimation period followed by two successive 120 dB stimulus alone trials. These two initial trials were excluded from the analysis. Four different trial types were then presented randomly: “no stimulus” (background, 65 dB), “startle pulse alone” (120 dB; 40 ms), “prepulse alone” (4, 8 or 16 dB above background; 20 ms) or “prepulse + startle pulse” (4, 8 or 16 dB prepulse given 100 ms before 120 dB startle pulse). The intertrial intervals varied randomly from 5 to 15 s. All trials were presented 5 times except for the “no stimulus” and “startle pulse alone” trials, which occurred 10 times. The average maximum amplitude vibrations from the “no stimulus” trials were subtracted from all startle response values to account for baseline movement in the chambers. The percentage of acoustic PPI was calculated as: % PPI = 1 – [(prepulse + startle pulse)/(startle pulse alone)] x 100.
To examine the effect of PD149163 and amphetamine on PPI, mice first received an injection of PD149163 (0.05, 0.1 or 0.5 mg/kg, i.p.) or saline, then amphetamine (10 mg/kg, i.p.) was administered 55 min later. Mice were placed in the PPI chambers 5 min after the injection of amphetamine. The rationale for injecting amphetamine 55 min after PD149163 stems from our previous observation that peak effect of PD149163 is reached within one hour [34].
2.4. Western blotting
Mice were anesthetized with carbon dioxide and the brains were quickly removed. The nucleus accumbens (NAc) and medial prefrontal cortex (mPFC) were dissected and immediately frozen on dry ice. Briefly, 0.5 mm zirconium oxide beads and 50 μl of lysis buffer [containing CelLytic MT lysis reagent (Sigma-Aldrich), complete protease inhibitor cocktail (Roche, Indianapolis, IN), and phosphatase inhibitor cocktails type II and III (Sigma-Aldrich)] were added to the tissues, which were magnetically homogenized in a Storm 24 Bullet Blender (Next Advance, Inc., Averill Park, NY). Homogenates were centrifuged at 16,400 RPM at 4°C for 15 min and supernatants were collected. Protein concentrations were determined by Bradford assays (Bio-Rad Laboratories, Hercules, CA). Proteins (30 μg) were loaded and separated on 4–12% NuPAGE™ Bis-Tris gels in MOPS buffer (Invitrogen, Carlsbad, CA) at 130 V for 2 hr. Subsequently, proteins were transferred onto PVDF membranes (Invitrogen) at 30 V for 1 hr. Membranes were incubated with antibodies against pGSK-3αβ Ser21/9 (9331, Cell Signaling, Boston, MA), GSK-3α (4337, Cell Signaling), GSK-3β (610201, BD Biosciences, San Jose, CA), pGSK-3β Tyr216 (612312, BD Biosciences) and GAPDH (MAB374, Millipore, Billerica, MA), as a loading control in 5% BSA with 0.1% Tween-20 in TBS at 4°C overnight. The monoclonal antibody against pGSK-3β Tyr216 also cross-reacts with pGSK-3α Tyr279 [35]. Immunoblots were then exposed to species-specific horseradish peroxidase-conjugated secondary antibodies (1:1000, Millipore). Blots were visualized with enhanced chemiluminescence detection (Thermo Scientific, Rockford, IL) and images were obtained on a Kodak Image Station 4000R scanner (New Haven, CT). Band optical density was quantified using NIH ImageJ software.
2.5. Statistical analysis
Data are presented as mean ± s.e.m. One-way analysis of variance (ANOVA) was used to analyze western blot and open-field data when more than two groups were compared. Unpaired two-tailed t-tests were used to assess the effect of amphetamine on locomotor activity and a single dose of PD149163 on GSK-3 signaling by western blot. Time-dependent open-field data were analyzed by two-way repeated measures (RM) ANOVA. PPI data were collapsed across prepulse values and analyzed by two-way ANOVA. ANOVA was followed by Tukey’s post-hoc tests for individual comparisons where appropriate. Results were considered statistically significant when p ≤ 0.05.
3. Results
3.1. PD149163 inhibited amphetamine-mediated hyperactivity
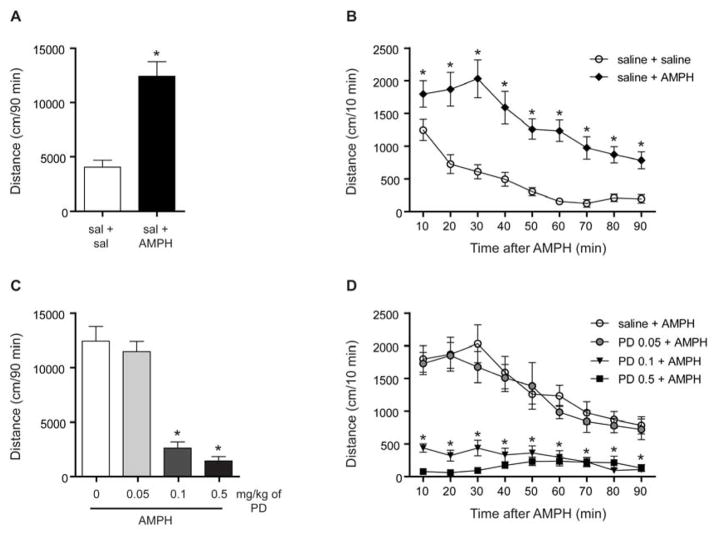
First, we examined the effect of amphetamine (2 mg/kg, i.p.) on the locomotor activity of C57BL/6J mice that were naïve to the open-field. Amphetamine increased the total distance traveled relative to the saline-treated mice [t(18) = 5.557, p < 0.001, n = 10; Fig. 1A]. Time-dependent analysis by two-way RM ANOVA detected a significant effect of amphetamine treatment [F(1,18) = 30.88, p < 0.001], time [F(8,144) = 24.47, p < 0.001] and an interaction [F(8,144) = 3.41, p < 0.01]. Individual group comparisons by Tukey’s post-hoc tests indicated that amphetamine-treated mice showed increased locomotor activity at each time point throughout the 90 min test session (Fig. 1B).
Fig. 1.
PD149163 (PD) inhibited amphetamine (AMPH)-mediated hyperactivity. (A) Total distance traveled in 90 min of mice placed in the open-field immediately after receiving an injection of amphetamine (2 mg/kg, i.p.). Amphetamine induced hyperactivity relative to the saline-treated mice. *p < 0.05 by unpaired two-tailed t-test. n = 10. (B) Data were also analyzed by distance traveled per 10 min in the open-field. Amphetamine resulted in increased activity at each examined time point relative to the saline-treated mice. *p < 0.05 by Tukey’s tests. (C) The two higher doses of PD149163 (0.1, 0.5 mg/kg, i.p.) inhibited amphetamine-induced locomotor activity. *p < 0.05 by Tukey’s tests relative to the saline + amphetamine-treated mice. n = 10 for saline + AMPH, n = 10 for 0.05 PD + AMPH, n = 11 for PD 0.1 + AMPH, n = 12 for PD 0.5 + AMPH. (D) Time-dependent analysis revealed that both 0.1 and 0.5 mg/kg of PD inhibited amphetamine-mediated locomotor activity at each time point. * indicates that PD 0.1 + AMPH- and PD 0.5 + AMPH-treated mice showed reduced activity relative to mice treated with saline + AMPH. Individual comparisons were assessed by Tukey’s tests and significance is indicated where p < 0.05. Data are presented as mean ± s.e.m.
We then examined the ability of various doses of PD149163 to inhibit the hyperactivity of the amphetamine-treated mice. Pretreatment with the two higher doses of PD149163 (0.1 and 0.5 mg/kg, i.p.) inhibited the locomotor activity of the amphetamine-treated mice, as identified by Tukey’s tests following analysis by one-way ANOVA [F(3, 39) = 46.79, p < 0.001, n = 10–12; Fig. 1C]. We also analyzed the data in 10 min time increments (Fig. 1D). Analysis by two-way RM ANOVA detected an effect of PD149163 treatment [F(3, 39) = 46.79, p < 0.001], time [F(8, 312) = 17.61, p < 0.001] and an interaction [F(24, 312) = 5.16, p < 0.001]. Tukey’s post-hoc tests indicated that the two higher doses of PD149163 (0.1 and 0.5 mg/kg) inhibited amphetamine-mediated hyperactivity at each time point throughout the test session.
3.2. PD149163 suppressed amphetamine-induced disruption of PPI
Next, we examined the effect of PD149163 on amphetamine-mediated disruption of PPI. Since the lower doses of PD149163 (0.05 and 0.1 mg/kg) did not increase PPI in amphetamine-treated mice we focused on examining the effect of 0.5 mg/kg of PD149163 on PPI in the presence and absence of amphetamine (Fig. S1). There was an effect of PD149163 [F(1, 47) = 15.74, p < 0.001; Fig. 2A] and amphetamine [F(1, 47) = 5.57, p < 0.05] by two-way ANOVA without an interaction [F(1, 47) = 0.01, p = 0.9]. As expected, amphetamine (10 mg/kg) reduced PPI and PD149163 increased PPI. Two-way ANOVA revealed no effect of amphetamine on startle reactivity to the startle pulse alone [F(1, 47) = 0.02, p = 0.9; Fig. 2B]. Interestingly, PD149163 decreased the startle response to the startle pulse alone [F(1, 47) = 30.34, p < 0.001] without an interaction [F(1, 47) = 1.96, p = 0.17]. Since 0.5 mg/kg of PD149163 suppressed startle reactivity we examined whether these effects may be dissociable by analyzing the PPI data by the median split in startle reactivity of the PD-treated mice (Fig. S1), which indicated that PD149163 increased PPI in mice with both higher and lower startle reactivity responses.
Fig. 2.
Amphetamine (AMPH) impaired PPI, where PD149163 (PD) increased PPI. (A) Mice that were treated with amphetamine (10 mg/kg, i.p.) exhibited impaired PPI. PD149163 (0.5 mg/kg) increased PPI. *p < 0.05 by two-way ANOVA. n = 22 for saline + saline, n = 11 for saline + AMPH, n = 8 for PD + saline, n = 10 for PD + AMPH. (B) amphetamine-treatment had no effect on the overall startle magnitude. PD149163 reduced the response to the startle pulse alone. *p < 0.05 by two-way ANOVA. Data are presented as mean ± s.e.m.
3.3. PD149163 increased the inhibitory serine phosphorylation on GSK-3
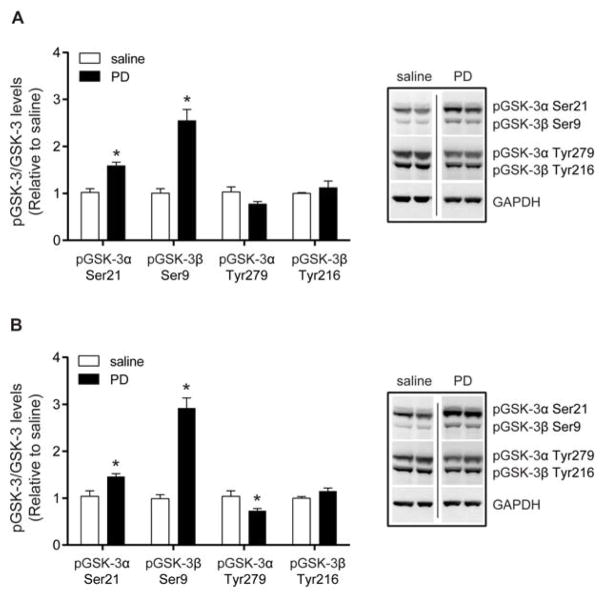
Since antipsychotics inhibit GSK-3 activity and GSK-3 polymorphisms were found to be associated with schizophrenia and bipolar disorder, we examined the effect of PD149163 treatment on GSK-3 phosphorylation [29, 30, 36, 37]. We focused on the NAc and mPFC, two brain regions that have been implicated in mania and schizophrenia in humans [38–40]. Treatment with the same dose of PD149163 (0.5 mg/kg, i.p.) at the same time point (1 h after injection) that inhibited amphetamine-induced hyperactivity and disruption of PPI, increased pGSK-3α Ser21 [t(10) = 5.069, p < 0.001, n = 6] and pGSK-3β Ser9 [t(10) = 5.950, p < 0.001] in the NAc, relative to total kinase levels (Fig. 3A and S2). PD149163 did not affect the tyrosine phosphorylation of GSK-3α/β in the NAc, which is associated with increased GSK-3 activity [41]. Moreover, administration of PD149163 also increased pGSK-3α Ser21 in the mPFC [t(10) = 3.176, p < 0.01, n = 6] and pGSK-3β Ser9 [t(10) = 8.420, p < 0.001; Fig. 3B]. There was a decrease in pGSK-3α Tyr279 [t(10) = 2.396, p < 0.05], but no change in pGSK-3β Tyr216 in the mPFC after PD149163 treatment.
Fig. 3.
The effects of PD149163 (PD) on GSK-3 phosphorylation in the NAc and mPFC. Mice were injected with 0.5 mg/kg, i.p., of PD149163 and brains were dissected 1 h after the injection. (A) In the NAc, PD increased the inhibitory serine phosphorylation on GSK-3α (Ser21) and GSK-3β (Ser9) relative to the saline-treated mice. PD149163 treatment did not alter tyrosine phosphorylation on GSK-3α (Tyr279) or GSK-3β (Tyr216) relative to the saline-treated mice. (B) In the mPFC, PD149163 also increased pGSK-3α Ser21 and pGSK-3β Ser9 relative to the saline-treated mice. PD149163 treatment reduced pGSK-3α Tyr279, but had no effect on pGSK-3β Tyr216. Phosphorylation levels are presented relative to total GSK-3α or GSK-β. Expression levels were normalized to GAPDH and the saline-treated mice. *p < 0.05 by unpaired two-tailed t-tests. n = 6 per treatment. Images were cropped from the same blot. Data are presented as mean ± s.e.m.
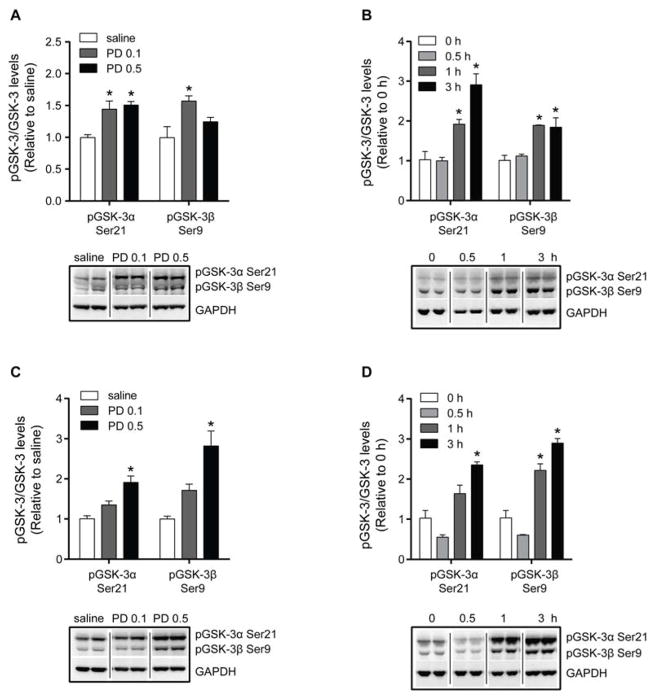
In a separate dose-dependent experiment, we also examined the effect of 0.1 mg/kg of PD149163 on the inhibitory serine phosphorylation on GSK-3, since this dose also inhibited amphetamine-induced hyperactivity (Fig. 4A and S3). One-way ANOVA identified an effect of PD149163 treatment on pGSK-3α Ser21 in both the NAc [F(2, 9) = 10.67, p < 0.01, n = 4] and mPFC [F(2, 9) = 15.34, p < 0.01]. Similarly, an effect of PD141963 administration was found on pGSK-3β Ser9 in the NAc [F(2, 9) = 6.10, p < 0.05] and mPFC [F(2, 9) = 15.53, p < 0.01]. Interestingly, PD149163 at a dose of 0.1 mg/kg increased pGSK-3α Ser21 and pGSK-3β Ser9 in the NAc (Fig. 4A), but not in the mPFC (Fig. 4C). As previously observed, 0.5 mg/kg of PD149163 increased pGSK-3α Ser21 in the NAc and mPFC. The higher dose of PD149163 (0.5 mg/kg) again increased pGSK-3β Ser9 in the mPFC, but there was no significant increase in pGSK-3β Ser9 in the NAc. Together, our data indicate that PD149163 inhibits GSK-3 activity in the NAc and mPFC.
Fig. 4.
Dose- and time-dependent effects of PD149163 (PD) on inhibitory serine phosphorylation on GSK-3 in the NAc and mPFC. In the dose-dependent studies (panels A and C), mice received saline, 0.1 mg/kg, or 0.5 mg/kg of PD149163. Brain regions were dissected 1 h after injections. In the time-dependent studies (panels B and D), mice received 0.5 mg/kg of PD149163 and the NAc and mPFC were dissected at various times after the injection. (A) In the NAc, 0.1 mg/kg of PD149163 resulted in increased pGSK-3α Ser21 and pGSK-3β Ser9. pGSK-3β Ser9 was also increased 1 h after administration of 0.5 mg/kg of PD149163 in the NAc. (B) PD149163 increased pGSK-3α Ser21 and pGSK-3β Ser9 in the NAc 1 and 3 h after administration. (C) In the mPFC, only 0.5 mg/kg of PD149163 increased pGSK-3α Ser21 and pGSK-3β Ser9 after 1 h. (D) PD149163 increased pGSK-3α Ser21 after 3 h, but pGSK-3β Ser9 after 1 and 3 h in the mPFC. Phosphorylation levels are presented relative to total GSK-3α or GSK-β. Expression levels were normalized to GAPDH and the expression in the control mice. *p < 0.05 by Tukey’s tests. n = 4 per treatment. Images were cropped from the same blot. Data are presented as mean ± s.e.m.
3.4. Time-dependent effects of PD149163 on the inhibitory serine phosphorylation on GSK-3
We then investigated the effect of PD149163 (0.5 mg/kg) on pGSK-3α/β Ser21/9 in the NAc and mPFC at various time points (0, 0.5, 1 and 3 h) after drug administration. One-way ANOVA detected an effect of time on pGSK-3α Ser21 [F(3, 12) = 23.30, p < 0.001, n = 4; Fig. 4B] and pGSK-3β Ser9 [F(3, 12) = 11.66, p < 0.001] in the NAc. There was also a time-dependent effect of PD149163 treatment on pGSK-3α Ser21 [F(3, 12) = 26.87, p < 0.001, n = 4; Fig. 4D] and pGSK-3β Ser9 [F(3, 12) = 60.38, p < 0.001] in the mPFC. As previously found, 0.5 mg/kg of PD149163 increased pGSK-3α Ser21 and pGSK-3β Ser9 in the NAc 1 h after administration. In the mPFC, PD149163 increased pGSK-3β Ser9 1 h after treatment, but there was no significant increase in pGSK-3α Ser21. Tukey’s post-hoc tests also indicated that the increase in pGSK-3α/β Ser21/9 in the NAc and mPFC lasted at least 3 h after PD149163 treatment. There was no effect of 0.5 mg/kg of PD149163 on GSK-3 phosphorylation 0.5 h after treatment in the NAc or mPFC.
3.5. Effects of PD149163 on the inhibitory serine phosphorylation on GSK-3 in amphetamine-treated mice
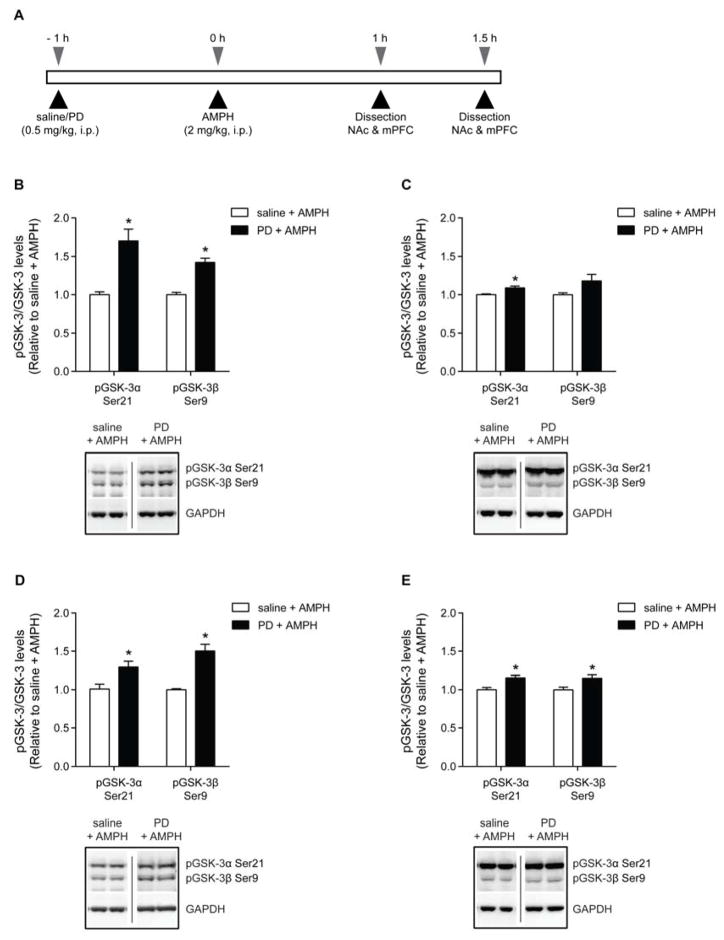
Finally, we wanted to examine whether PD149163 (0.5 mg/kg, i.p.) inhibits the amphetamine-mediated increase in GSK-3 activity, which has been previously reported to occur in the striatum after 1.5 h [31]. Here, mice received an injection of saline or PD149163 (0.5 mg/kg, i.p.), and then an injection of saline or amphetamine 1 h later (Fig. 5A). The NAc and mPFC were isolated 1 or 1.5 h after the last injection. Although we were not able to demonstrate that amphetamine results in a significant reduction in pGSK-3α/β Ser21/9 in the NAc or mPFC (Fig. S4), interestingly, we did find significant effects of PD149163 treatment on pGSK-3α/β Ser21/9 in amphetamine-treated mice. In the NAc, when amphetamine-treated mice were pretreated with PD149163, there was an increase in pGSK-3α Ser21 at both 1 h [t(8) = 4.544, p < 0.01, n = 5; Fig. 5B] and 1.5 h [t(8) = 3.689, p < 0.01, n = 5; Fig. 5C] after the last injection. In the NAc, there was also an increase in pGSK-3β Ser9 with PD149163 pretreatment in amphetamine-treated mice that were dissected 1 h after the last injection [t(8) = 6.854, p < 0.001, n = 5], but not after 1.5 h. In the mPFC, PD149163 pretreatment increased both pGSK-3α Ser21 [t(8) = 2.911, p < 0.05; n = 5; Fig. 5D] and pGSK-3β Ser9 [t(8) = 5.602, p < 0.001; n = 5] at the 1 h time point. PD149163 pretreatment also resulted in increased pGSK-3α Ser21 [t(8) = 3.625, p < 0.01, n = 5; Fig. 5E] and pGSK-3β Ser9 [t(7) = 2.511, p < 0.05, n = 4–5] at the 1.5 h time point. Thus, PD149163 treatment also increased the inhibitory serine phosphorylation on GSK-3 in the presence of amphetamine.
Fig. 5.
The effects of PD149163 (PD) on the inhibitory serine phosphorylation on GSK-3 in the NAc and mPFC in amphetamine-treated mice. (A) Schematic illustrating the experimental design. Mice were pretreated with saline or PD149163 (0.5 mg/kg, i.p.) 1 h before a second injection of amphetamine (AMPH, 2 mg/kg, i.p.). The NAc and mPFC were then dissected 1 h (panels B and D) or 1.5 h (panels C and E) after the last injection. (B) In the NAc, PD149163 increased pGSK-3α/β Ser21/9 1 h after the last injection. (C) PD149163 also increased pGSK-3α Ser21 in the NAc isolated 1.5 h after amphetamine administration. (D) In the mPFC, PD149163 increased pGSK-3α/β Ser21/9 1 h after the last injection. (E) PD149163 also increased pGSK-3α/β Ser21/9 1.5 h after the amphetamine injection. *p < 0.05 by unpaired two-tailed t-tests. n = 4–5 per treatment. Data are presented as mean ± s.e.m.
4. Discussion
Here we demonstrated that the NTS1-selective agonist, PD149163, dose-dependently reduces amphetamine-mediated hyperactivity and increases PPI. We also showed that PD149163 increased the inhibitory serine phosphorylation on GSK-3 in the NAc and mPFC of mice at the same time after drug administration and dose at which antipsychotic-like effects were observed. Our study supports previous findings indicating that PD149163 may be a novel antipsychotic.
The inhibitory effect of PD149163 on amphetamine-induced hyperactivity in mice is consistent with previous findings in rats [18, 42]. Our findings are also in agreement with our previous work demonstrating that PD149163 inhibits hyperactivity induced by the D1R agonist SKF-81297 and the D2R agonist bromocriptine [34]. Interestingly, PD149163 at a low dose of 0.05 mg/kg inhibited bromocriptine-, but not SKF-81297-induced hyperactivity, indicating that PD149163 more potently inhibits D2R-mediated hyperlocomotion. Bromocriptine has been reported to produce mania- and schizophrenia-like behaviors in humans, and thus our previous work also suggests that PD149163 produces antipsychotic-like effects [43]. As reported previously, we also found that PD149163 increased PPI [44]. Although it is possible that the PD149163-mediated reduction in startle reactivity may affect PPI, in this study and others the effect of PD149163 on startle reactivity was dissociable from the effect on PPI [18]. Here, the effect of PD149163 on PPI did not appear to be dependent upon the magnitude of the effect of PD149163 on startle response to the startle pulse alone (Fig. S1). Furthermore, antipsychotics also suppress startle reactivity and this effect was shown to dissociate from the effects on PPI [45]. Therefore, the effect of PD149163 on startle reactivity does not likely confound the PPI findings. Our findings build upon previous studies examining the antipsychotic-like effects of PD149163, and indicate that NTS1 agonists could be useful antipsychotics.
We also revealed a potential mechanism through which PD149163 may produce antipsychotic-like effects. We found that 1 h after PD149163 (0.5 mg/kg) treatment pGSK-3α Ser21 and pGSK-3β Ser9 were increased in the NAc and mPFC, suggesting that NTS1 activation inhibits GSK-3 activity in these brain regions. This effect persisted for at least 3 h. In the time-dependent and dose-dependent experiments, PD149163 (0.5 mg/kg) did not significantly increase pGSK-3α Ser21 in the mPFC and pGSK-3β Ser9 in the NAc 1 h after administration, respectively, which may have been due to the smaller sample size of four mice per group. In the dose-dependent study, we found that 0.1 mg/kg of PD149163 increased pGSK-3α/β Ser21/9 in the NAc, but not the mPFC. Interestingly, 0.1 mg/kg of PD149163 inhibited amphetamine-induced locomotor activity, but not the disruption in PPI. It is possible that lower doses of PD149163 suppress hyperactivity through inhibition of GSK-3 in the NAc, whereas higher doses of PD149163 suppress hyperactivity and increase PPI through inhibition of GSK-3 in both the NAc and mPFC. Consistently, previous work has implicated the NAc in the antipsychotic-like effects of NTS1 agonists. For example, activating NT receptors in the NAc reduced basal locomotion, inhibited dopamine-mediated hyperactivity, and increased PPI [34, 46, 47]. Microinjection of NT or NT analogs in the mPFC had no effect on basal locomotion and modulated dopamine-mediated activity [34, 48]. Although it is hypothesized that the mPFC plays a role in the effects of NT on PPI, the role of mPFC NTS1 in sensory motor gating is still unclear and warrants future investigation.
GSK-3 has been shown to positively regulate basal and dopamine-mediated locomotor activity. Mice with constitutively active GSK-3 are hyperactive and more sensitive to amphetamine-induced hyperactivity [49]. Conversely, loss or inhibition of GSK-3 suppresses hyperactivity [31, 50]. Amphetamine has been shown to decrease pGSK-3α/β Ser21/9 through dopamine D2 receptors (D2R). Activation of D2R enhances GSK-3 activity by inducing the formation of a complex with β-arrestin, Akt, and protein phosphatase 2A (PP2A) [51]. PP2A dephosphorylates Akt, inactivating it, and therefore prevents the phosphorylation and inhibition of GSK-3. D2R-mediated inhibition of GSK-3 potentially underlies the behavioral effects of amphetamine, since loss of GSK-3β in D2R-, but not D1R-expressing cells, reduced amphetamine-mediated hyperactivity and deficits in PPI [52]. However, we did not find a significant reduction in pGSK-3 after amphetamine treatment. One possible explanation may be that our euthanasia protocol produced a floor effect on pGSK-3α/β Ser21/9 levels. Here, mice were euthanized by exposure to carbon dioxide and subsequent decapitation. Hypoxia has been shown to rapidly reduce pGSK-3α/β Ser21/9 [53]. Furthermore, amphetamine was demonstrated to reduce pGSK-3α/β Ser21/9 in the striatum after 90 min when mice were killed by decapitation and brains were quickly frozen by immersion in liquid nitrogen [31]. Thus, it is possible that amphetamine-mediated reduction in pGSK-3α/β Ser21/9 may only be apparent in brains immediately frozen following a rapid euthanasia. However, we did demonstrate that PD149163 treatment increased pGSK-3α/β Ser21/9 in the NAc and mPFC of amphetamine-treated mice, indicating that PD149163-induced inhibition of GSK-3 may explain the effects of PD149163 on amphetamine-induced behaviors.
Our findings indicate that NTS1-mediated inhibition of both GSK-3α and GSK-3β activity in the NAc and mPFC may play a role in the antipsychotic-like effects of PD149163. Corroborating our brain region-specific results, microinjection studies indicate that GSK-3 in the mPFC and NAc regulates locomotor activity [54, 55]. However, it is unclear whether inhibition of both GSK-3α and GSK-3β induces antipsychotic-like effects. The kinase domains of GSK-3α and GSK-3β are 98% homologous, and thus isoform-specific GSK-3 inhibitors are not available [56]. Both isoforms have been implicated in regulating locomotion and PPI [50, 57–59]. More work should be devoted to understanding the isoform-specific roles in psychiatric disorders.
The pathway underlying NTS1/GSK-3 signaling is unclear. It is well established that NTS1 inhibits D2R signaling [60]. However, we did not find consistent and robust increases in Akt phosphorylation with PD149163 treatment, suggesting that the NTS1/D2R interaction is not the only mechanism underlying PD149163-mediated inhibition of GSK-3. In congruence, PD149163 has been shown to inhibit hyperactivity and disruptions in PPI mediated by pharmacological agents targeting other neurotransmitter receptors [61]. Numerous signaling pathways converge onto GSK-3, and it is possible that NTS1 activation may inhibit mania- and schizophrenia-like behaviors induced by various signaling mechanisms through suppressing GSK-3 activity. Interestingly, it appears that atypical antipsychotics have a greater effect on β-arrestin/Akt/GSK-3 signaling than on D2R-induced G protein signaling [52]. It is thought that adenylyl cyclase/PKA signaling plays a greater role in the extrapyramidal side effects induced by typical antipsychotics. PD149163 does not induce catalepsy, mimics the effects of atypical antipsychotics, and, as shown here, inhibits GSK-3 activity [62]. Atypical antipsychotics may more strongly inhibit GSK-3 activity since they weakly interact with multiple receptors that affect GSK-3 signaling. This strategy could produce an additive effect on GSK-3, but could also increase unwanted side effects. An alternative strategy for treating mania or schizophrenia could be GSK-3 inhibitors, but GSK-3 is ubiquitously expressed, and prolonged inhibition of GSK-3 in all cells may have serious consequences. NTS1 is found in brain regions implicated in mania and schizophrenia, and thus targeting NTS1 may be a novel approach to effectively inhibit GSK-3 activity with minimal side effects [38–40, 63].
Our finding that activation of NTS1 inhibits GSK-3 activity suggests that NTS1 agonists may be beneficial for treating mania and schizophrenia. GSK-3 has been implicated in bipolar disorder and schizophrenia in humans [36, 37]. Furthermore, there is some evidence to support that the therapeutic effects of antipsychotics and other anti-manic drugs may result from inhibition of GSK-3 [30, 31, 64]. PD149163 effectively suppresses hyperactivity and increases PPI, indicating that NTS1 agonists may reduce schizophrenia- and mania-like behaviors [18, 34, 61]. However, we recognize the limitations of the amphetamine model used in this study. There are other symptoms in schizophrenia and mania that are not represented by the amphetamine model. Also, acute amphetamine treatment cannot represent the chronic symptoms of schizophrenia and bipolar disorder. Thus, it will be important to examine the effects of PD149163 in other rodent models of mania- and schizophrenia-like behaviors [65, 66].
We also found that PD149163 nonspecifically increased PPI, which is not different from other studies that have reported that PD149163 alone increases PPI [16, 67]. In addition, previously we found that PD149163 dose-dependently (0.05–0.5 mg/kg, i.p.) inhibited the locomotor activity of untreated mice that are naïve to the open-field [34]. Interestingly, in our study tolerance developed to the hypolocomotor effect of PD149163 in C57BL/6J mice, but it has been reported that tolerance does not develop to the antipsychotic-like effects of PD149163 in rats [34, 68]. Thus, in future studies it will be important to carefully investigate the effects of chronic PD149163 treatment on various behaviors and GSK-3 activity since antipsychotics are given chronically to humans. It is possible that tolerance may develop to the potential side effects of PD149163, but not to the amphetamine-related or antipsychotic-like effects of PD149163.
Our study adds to the growing literature indicating that PD149163 may be a novel antipsychotic that could be beneficial for the treatment of mania in bipolar disorder and schizophrenia. This work adds to our understanding of the functional role of NTS1 in the brain, and we hope will provoke further research investigating the therapeutic potential of NTS1 agonists for the treatment of psychiatric disorders.
Supplementary Material
HIGHLIGHTS.
Acknowledgments
Role of the funding source
This project was funded by the Samuel C. Johnson Genomics of Addiction Program, the Ulm Foundation, the David Lehr Research Award from American Society for Pharmacology and Experimental Therapeutics, and by a grant from the National Institutes of Health (AA018779). The funding sources had no involvement in the study design or preparation of the manuscript. CAV was supported by fellowship funding from Mayo Graduate School.
We thank YuBin Choi for her help in analyzing data in initial studies associated with this work.
Footnotes
Author contributions
CAV, OAA, MJH and DSC contributed to the design of the studies. CAV, JAR, AO, SC and OAA performed experiments. CAV performed the statistical analyses. All authors contributed to and approved the final manuscript.
Conflict of Interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cousins DA, Butts K, Young AH. The role of dopamine in bipolar disorder. Bipolar Disord. 2009;11:787–806. doi: 10.1111/j.1399-5618.2009.00760.x. [DOI] [PubMed] [Google Scholar]
- 2.Guillin O, Abi-Dargham A, Laruelle M. Neurobiology of dopamine in schizophrenia. Int Rev Neurobiol. 2007;78:1–39. doi: 10.1016/S0074-7742(06)78001-1. [DOI] [PubMed] [Google Scholar]
- 3.Craddock N, O’Donovan MC, Owen MJ. Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophr Bull. 2006;32:9–16. doi: 10.1093/schbul/sbj033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fountoulakis KN, Kasper S, Andreassen O, Blier P, Okasha A, Severus E, et al. Efficacy of pharmacotherapy in bipolar disorder: a report by the WPA section on pharmacopsychiatry. Eur Arch Psychiatry Clin Neurosci. 2012;262(Suppl 1):1–48. doi: 10.1007/s00406-012-0323-x. [DOI] [PubMed] [Google Scholar]
- 5.Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951–62. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- 6.Gao K, Kemp DE, Ganocy SJ, Gajwani P, Xia G, Calabrese JR. Antipsychotic-induced extrapyramidal side effects in bipolar disorder and schizophrenia: a systematic review. J Clin Psychopharmacol. 2008;28:203–9. doi: 10.1097/JCP.0b013e318166c4d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf SS, Hyde TM, Saunders RC, Herman MM, Weinberger DR, Kleinman JE. Autoradiographic characterization of neurotensin receptors in the entorhinal cortex of schizophrenic patients and control subjects. J Neural Transm Gen Sect. 1995;102:55–65. doi: 10.1007/BF01276565. [DOI] [PubMed] [Google Scholar]
- 8.Hamid EH, Hyde TM, Egan MF, Wolf SS, Herman MM, Nemeroff CB, et al. Neurotensin receptor binding abnormalities in the entorhinal cortex in schizophrenia and affective disorders. Biol Psychiatry. 2002;51:795–800. doi: 10.1016/s0006-3223(01)01325-7. [DOI] [PubMed] [Google Scholar]
- 9.Sharma RP, Janicak PG, Bissette G, Nemeroff CB. CSF neurotensin concentrations and antipsychotic treatment in schizophrenia and schizoaffective disorder. Am J Psychiatry. 1997;154:1019–21. doi: 10.1176/ajp.154.7.1019. [DOI] [PubMed] [Google Scholar]
- 10.Kinkead B, Shahid S, Owens MJ, Nemeroff CB. Effects of acute and subchronic administration of typical and atypical antipsychotic drugs on the neurotensin system of the rat brain. J Pharmacol Exp Ther. 2000;295:67–73. [PubMed] [Google Scholar]
- 11.Radke JM, MacLennan AJ, Beinfeld MC, Bissette G, Nemeroff CB, Vincent SR, et al. Effects of short- and long-term haloperidol administration and withdrawal on regional brain cholecystokinin and neurotensin concentrations in the rat. Brain Res. 1989;480:178–83. doi: 10.1016/0006-8993(89)91580-1. [DOI] [PubMed] [Google Scholar]
- 12.Garver DL, Bissette G, Yao JK, Nemeroff CB. Relation of CSF neurotensin concentrations to symptoms and drug response of psychotic patients. Am J Psychiatry. 1991;148:484–8. doi: 10.1176/ajp.148.4.484. [DOI] [PubMed] [Google Scholar]
- 13.Binder EB, Kinkead B, Owens MJ, Kilts CD, Nemeroff CB. Enhanced neurotensin neurotransmission is involved in the clinically relevant behavioral effects of antipsychotic drugs: evidence from animal models of sensorimotor gating. J Neurosci. 2001;21:601–8. doi: 10.1523/JNEUROSCI.21-02-00601.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casti P, Marchese G, Casu G, Ruiu S, Pani L. Blockade of neurotensin receptors affects differently hypo-locomotion and catalepsy induced by haloperidol in mice. Neuropharmacology. 2004;47:128–35. doi: 10.1016/j.neuropharm.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Boules MM, Fredrickson P, Muehlmann AM, Richelson E. Elucidating the role of neurotensin in the pathophysiology and management of major mental disorders. Behav Sci. 2014;4:125–53. doi: 10.3390/bs4020125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feifel D, Melendez G, Shilling PD. Reversal of sensorimotor gating deficits in Brattleboro rats by acute administration of clozapine and a neurotensin agonist, but not haloperidol: a potential predictive model for novel antipsychotic effects. Neuropsychopharmacology. 2004;29:731–8. doi: 10.1038/sj.npp.1300378. [DOI] [PubMed] [Google Scholar]
- 17.Caceda R, Kinkead B, Owens MJ, Nemeroff CB. Virally mediated increased neurotensin 1 receptor in the nucleus accumbens decreases behavioral effects of mesolimbic system activation. J Neurosci. 2005;25:11748–56. doi: 10.1523/JNEUROSCI.4282-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feifel D, Melendez G, Murray RJ, Tina Tran DN, Rullan MA, Shilling PD. The reversal of amphetamine-induced locomotor activation by a selective neurotensin-1 receptor agonist does not exhibit tolerance. Psychopharmacology. 2008;200:197–203. doi: 10.1007/s00213-008-1197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang Y, Boules M, Li Z, Williams K, Miura T, Oliveros A, et al. Hyperactivity of the dopaminergic system in NTS1 and NTS2 null mice. Neuropharmacology. 2010;58:1199–205. doi: 10.1016/j.neuropharm.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smits SM, Terwisscha van Scheltinga AF, van der Linden AJ, Burbach JP, Smidt MP. Species differences in brain pre-pro-neurotensin/neuromedin N mRNA distribution: the expression pattern in mice resembles more closely that of primates than rats. Brain Res Mol Brain Res. 2004;125:22–8. doi: 10.1016/j.molbrainres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Machado-Vieira R, Kapczinski F, Soares JC. Perspectives for the development of animal models of bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:209–24. doi: 10.1016/j.pnpbp.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Marcotte ER, Pearson DM, Srivastava LK. Animal models of schizophrenia: a critical review. J Psychiatry Neurosci. 2001;26:395–410. [PMC free article] [PubMed] [Google Scholar]
- 23.Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–58. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- 24.Perry W, Minassian A, Feifel D, Braff DL. Sensorimotor gating deficits in bipolar disorder patients with acute psychotic mania. Biol Psychiatry. 2001;50:418–24. doi: 10.1016/s0006-3223(01)01184-2. [DOI] [PubMed] [Google Scholar]
- 25.Ludewig K, Geyer MA, Vollenweider FX. Deficits in prepulse inhibition and habituation in never-medicated, first-episode schizophrenia. Biol Psychiatry. 2003;54:121–8. doi: 10.1016/s0006-3223(02)01925-x. [DOI] [PubMed] [Google Scholar]
- 26.Arnt J. Differential effects of classical and newer antipsychotics on the hypermotility induced by two dose levels of D-amphetamine. Eur J Pharmacol. 1995;283:55–62. doi: 10.1016/0014-2999(95)00292-s. [DOI] [PubMed] [Google Scholar]
- 27.Borison RL, Sabelli HC, Maple PJ, Havdala HS, Diamond BI. Lithium prevention of amphetamine-induced ‘manic’ excitement and of reserpine-induced ‘depression’ in mice: possible role of 2-phenylethylamine. Psychopharmacology. 1978;59:259–62. doi: 10.1007/BF00426631. [DOI] [PubMed] [Google Scholar]
- 28.Ong JC, Brody SA, Large CH, Geyer MA. An investigation of the efficacy of mood stabilizers in rodent models of prepulse inhibition. J Pharmacol Exp Ther. 2005;315:1163–71. doi: 10.1124/jpet.105.090845. [DOI] [PubMed] [Google Scholar]
- 29.De Sarno P, Li X, Jope RS. Regulation of Akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium. Neuropharmacology. 2002;43:1158–64. doi: 10.1016/s0028-3908(02)00215-0. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Rosborough KM, Friedman AB, Zhu W, Roth KA. Regulation of mouse brain glycogen synthase kinase-3 by atypical antipsychotics. Int J Neuropsychopharmacol. 2007;10:7–19. doi: 10.1017/S1461145706006547. [DOI] [PubMed] [Google Scholar]
- 31.Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, et al. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci USA. 2004;101:5099–104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gould TD, Einat H, Bhat R, Manji HK. AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int J Neuropsychopharmacol. 2004;7:387–90. doi: 10.1017/S1461145704004535. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q, Zhou Y, Evers BM. Neurotensin phosphorylates GSK-3alpha/beta through the activation of PKC in human colon cancer cells. Neoplasia. 2006;8:781–7. doi: 10.1593/neo.06259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vadnie CA, Hinton DJ, Choi S, Choi Y, Ruby CL, Oliveros A, et al. Activation of neurotensin receptor type 1 attenuates locomotor activity. Neuropharmacology. 2014;85:482–92. doi: 10.1016/j.neuropharm.2014.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markou T, Cullingford TE, Giraldo A, Weiss SC, Alsafi A, Fuller SJ, et al. Glycogen synthase kinases 3alpha and 3beta in cardiac myocytes: regulation and consequences of their inhibition. Cell Signal. 2008;20:206–18. doi: 10.1016/j.cellsig.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Rowe MK, Wiest C, Chuang DM. GSK-3 is a viable potential target for therapeutic intervention in bipolar disorder. Neurosci Biobehav Rev. 2007;31:920–31. doi: 10.1016/j.neubiorev.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emamian ES. AKT/GSK3 signaling pathway and schizophrenia. Front Mol Neurosci. 2012;5:33. doi: 10.3389/fnmol.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carlson PJ, Singh JB, Zarate CA, Jr, Drevets WC, Manji HK. Neural circuitry and neuroplasticity in mood disorders: insights for novel therapeutic targets. NeuroRx. 2006;3:22–41. doi: 10.1016/j.nurx.2005.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10:105–16. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- 40.Grace AA. Gating of information flow within the limbic system and the pathophysiology of schizophrenia. Brain Res Brain Res Rev. 2000;31:330–41. doi: 10.1016/s0165-0173(99)00049-1. [DOI] [PubMed] [Google Scholar]
- 41.Hughes K, Nikolakaki E, Plyte SE, Totty NF, Woodgett JR. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. Embo J. 1993;12:803–8. doi: 10.1002/j.1460-2075.1993.tb05715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Norman C, Beckett SR, Spicer CH, Ashton D, Langlois X, Bennett GW. Effects of chronic infusion of neurotensin and a neurotensin NT1 selective analogue PD149163 on amphetamine-induced hyperlocomotion. J Psychopharmacol. 2008;22:300–7. doi: 10.1177/0269881107083838. [DOI] [PubMed] [Google Scholar]
- 43.Wang F, Zheng SQ, Zhang YH. Serious Bromocriptine-induced Psychosis: Suicide in the Treatment of Prolactinoma. Neurosurg Quart. 2013;23:249–51. [Google Scholar]
- 44.Feifel D, Reza TL, Wustrow DJ, Davis MD. Novel antipsychotic-like effects on prepulse inhibition of startle produced by a neurotensin agonist. J Pharmacol Exp Ther. 1999;288:710–3. [PubMed] [Google Scholar]
- 45.Ouagazzal AM, Jenck F, Moreau JL. Drug-induced potentiation of prepulse inhibition of acoustic startle reflex in mice: a model for detecting antipsychotic activity? Psychopharmacology. 2001;156:273–83. doi: 10.1007/s002130100763. [DOI] [PubMed] [Google Scholar]
- 46.Ervin GN, Birkemo LS, Nemeroff CB, Prange AJ., Jr Neurotensin blocks certain amphetamine-induced behaviours. Nature. 1981;291:73–6. doi: 10.1038/291073a0. [DOI] [PubMed] [Google Scholar]
- 47.Feifel D, Minor KL, Dulawa S, Swerdlow NR. The effects of intra-accumbens neurotensin on sensorimotor gating. Brain Res. 1997;760:80–4. doi: 10.1016/s0006-8993(97)00306-5. [DOI] [PubMed] [Google Scholar]
- 48.Radcliffe RA, Erwin VG. Alterations in locomotor activity after microinjections of GBR-12909, selective dopamine antagonists or neurotensin into the medial prefrontal cortex. J Pharmacol Exp Ther. 1996;277:1467–76. [PubMed] [Google Scholar]
- 49.Polter A, Beurel E, Yang S, Garner R, Song L, Miller CA, et al. Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology. 2010;35:1761–74. doi: 10.1038/npp.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaidanovich-Beilin O, Lipina TV, Takao K, van Eede M, Hattori S, Laliberte C, et al. Abnormalities in brain structure and behavior in GSK-3alpha mutant mice. Mol Brain. 2009;2:35. doi: 10.1186/1756-6606-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–73. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 52.Urs NM, Snyder JC, Jacobsen JP, Peterson SM, Caron MG. Deletion of GSK3beta in D2R-expressing neurons reveals distinct roles for beta-arrestin signaling in antipsychotic and lithium action. Proc Natl Acad Sci USA. 2012;109:20732–7. doi: 10.1073/pnas.1215489109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roh MS, Eom TY, Zmijewska AA, De Sarno P, Roth KA, Jope RS. Hypoxia activates glycogen synthase kinase-3 in mouse brain in vivo: protection by mood stabilizers and imipramine. Biol Psychiatry. 2005;57:278–86. doi: 10.1016/j.biopsych.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 54.Yen YC, Gassen NC, Zellner A, Rein T, Landgraf R, Wotjak CT, et al. Glycogen synthase kinase-3β inhibition in the medial prefrontal cortex mediates paradoxical amphetamine action in a mouse model of ADHD. Frontiers in Behavioral Neuroscience. 2015;9 doi: 10.3389/fnbeh.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim WY, Jang JK, Lee JW, Jang H, Kim JH. Decrease of GSK3beta phosphorylation in the rat nucleus accumbens core enhances cocaine-induced hyper-locomotor activity. J Neurochem. 2013;125:642–8. doi: 10.1111/jnc.12222. [DOI] [PubMed] [Google Scholar]
- 56.Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor A. Embo J. 1990;9:2431–8. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mines MA. Hyperactivity: glycogen synthase kinase-3 as a therapeutic target. Eur J Pharmacol. 2013;708:56–9. doi: 10.1016/j.ejphar.2013.02.055. [DOI] [PubMed] [Google Scholar]
- 58.King MK, Pardo M, Cheng Y, Downey K, Jope RS, Beurel E. Glycogen synthase kinase-3 inhibitors: Rescuers of cognitive impairments. Pharmacol Ther. 2014;141:1–12. doi: 10.1016/j.pharmthera.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amar S, Jones BC, Nadri C, Kozlovsky N, Belmaker RH, Agam G. Genetic correlational analysis of glycogen synthase kinase-3 beta and prepulse inhibition in inbred mice. Genes Brain Behav. 2004;3:178–80. doi: 10.1111/j.1601-183X.2004.00065.x. [DOI] [PubMed] [Google Scholar]
- 60.Binder EB, Kinkead B, Owens MJ, Nemeroff CB. Neurotensin and dopamine interactions. Pharmacol Rev. 2001;53:453–86. [PubMed] [Google Scholar]
- 61.Chou S, Davis C, Jones S, Li M. Repeated effects of the neurotensin receptor agonist PD149163 in three animal tests of antipsychotic activity: assessing for tolerance and cross-tolerance to clozapine. Pharmacol Biochem Behav. 2015;128:78–88. doi: 10.1016/j.pbb.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holly EN, Ebrecht B, Prus AJ. The neurotensin-1 receptor agonist PD149163 inhibits conditioned avoidance responding without producing catalepsy in rats. Eur Neuropsychopharmacol. 2011;21:526–31. doi: 10.1016/j.euroneuro.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fassio A, Evans G, Grisshammer R, Bolam JP, Mimmack M, Emson PC. Distribution of the neurotensin receptor NTS1 in the rat CNS studied using an amino-terminal directed antibody. Neuropharmacology. 2000;39:1430–42. doi: 10.1016/s0028-3908(00)00060-5. [DOI] [PubMed] [Google Scholar]
- 64.O’Brien WT, Huang J, Buccafusca R, Garskof J, Valvezan AJ, Berry GT, et al. Glycogen synthase kinase-3 is essential for beta-arrestin-2 complex formation and lithium-sensitive behaviors in mice. J Clin Invest. 2011;121:3756–62. doi: 10.1172/JCI45194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Young JW, Henry BL, Geyer MA. Predictive animal models of mania: hits, misses and future directions. Br J Pharmacol. 2011;164:1263–84. doi: 10.1111/j.1476-5381.2011.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abulseoud OA, Gawad NA, Mohamed K, Vadnie C, Camsari UM, Karpyak V, et al. Sex differences in mania phenotype and ethanol consumption in the lateral hypothalamic kindled rat model. Transl Psychiatry. 2015;5:e534. doi: 10.1038/tp.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feifel D, Pang Z, Shilling PD, Melendez G, Schreiber R, Button D. Sensorimotor gating in neurotensin-1 receptor null mice. Neuropharmacology. 2010;58:173–8. doi: 10.1016/j.neuropharm.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feifel D, Melendez G, Priebe K, Shilling PD. The effects of chronic administration of established and putative antipsychotics on natural prepulse inhibition deficits in Brattleboro rats. Behav Brain Res. 2007;181:278–86. doi: 10.1016/j.bbr.2007.04.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.