Abstract
Objective
Fetal exposure to maternal prenatal stress hormones such as cortisol exerts influences on the developing nervous system that persist and include risk for internalizing symptoms later in life. Placental corticotropin-releasing hormone (pCRH) is a feto-placental stress signal that also shapes fetal neurodevelopment and may be a more direct indicator of the fetal experience than maternal stress hormones. The programming effects of pCRH on child development are unknown. The current investigation examined associations between prenatal maternal and placental stress hormone exposures (maternal cortisol and pCRH) and child self-reported internalizing symptoms at age 5.
Method
Maternal plasma cortisol and pCRH levels were measured at 15, 19, 25, 31, and 36 weeks’ gestation in a sample of 83 women and their 91 children (8 sibling pairs from separate pregnancies), who were born full-term. Child self-reported internalizing symptoms at age 5 were obtained using scales of the Berkeley Puppet Interview.
Results
Placental CRH profiles (including elevations in mid-gestation) were associated with higher levels of internalizing symptoms at age 5. This effect was not explained by critical prenatal or postnatal influences, including obstetric risk, concurrent maternal psychological state, and family socio-economic status. Prenatal maternal cortisol was not significantly associated with child self-reported internalizing symptoms.
Conclusions
Findings suggest that elevated exposures to the feto-placental stress signal pCRH exert programming effects on the developing fetal central nervous system, with lasting consequences for child mental health.
Keywords: fetal programming, prenatal, pregnancy, CRH, cortisol, internalizing problems
1. Introduction
The maternal endocrine stress system is profoundly altered during the course of human pregnancy. The maternal pituitary gland doubles in size, and the synthesis and release of stress peptides and hormones from the maternal hypothalamic-pituitary-adrenal (HPA) axis into the maternal circulation increases several-fold. However, it is the growth and development of a new transient endocrine organ, the placenta, that primarily is responsible for the profound changes in the maternal and fetal stress systems as gestation progresses. As early as 7 to 8 weeks’ gestation, the placenta begins to synthesize the stress hormone corticotropin-releasing hormone (CRH), and this active peptide is released into both the maternal and fetal circulation (Gitau et al., 2004; Goland et al., 1988; Karteris et al., 2001; Mastorakos & Ilias, 2003). Placental CRH (pCRH) is identical in structure and activity to hypothalamic CRH (hCRH) and increases dramatically in the maternal circulation over the course of human pregnancy, reaching levels only present in the hypothalamic portal system during stress (Lowry, 1993). Concentrations of circulating maternal CRH are almost exclusively of placental origin, because the minute quantities of hCRH that are released into the maternal circulation are rapidly degraded and largely undetectable (Mesiano, 2014).
The feto-placental unit derives information from maternal physiological signals, including stress signals conveying a response to environmental threats or challenges, and adapts its developmental program accordingly (Gluckman & Hanson, 2004; Sandman & Davis, 2012). Placental CRH is responsive to a range of these stress signals, including increased norepinephrine and epinephrine, reduced uterine blood flow, and infection (Herrmann et al., 2001; Petraglia et al., 1989; Wadhwa et al., 2001). Further, in contrast to its negative feedback regulation of hCRH, maternal cortisol stimulates the synthesis and release of CRH in the placenta. Placental CRH penetrates the fetal blood-brain barrier and stimulates fetal adrenal cortisol production, which in turn further stimulates pCRH production (Kastin & Akerstrom, 2002; Mesiano, 2014). Thus, placental CRH represents an integrative pathway through which diverse prenatal stressors might inform the fetus of the state of its environment and shape fetal developmental trajectories (Charil et al., 2010; O’Donnell et al., 2009; Sandman et al., 2011).
Increasing evidence suggests that anxiety and mood disorders have origins in prenatal experiences (Baram et al., 2012; Sandman & Davis, 2012). Perhaps one of the most consistent neurobehavioral findings is that fetal exposure to elevations in stress hormones is associated with subsequent increased risk for internalizing problems (Bergman et al., 2007; Davis et al., 2005, 2007; Davis & Sandman, 2012; de Weerth et al., 2003). Most research examining the role of prenatal stress hormone exposures on the programming of offspring internalizing symptoms has focused on maternal cortisol, which increases two to four fold over human gestation. Evidence from rodent and non-human primate studies indicates that fetal exposure to elevated glucocorticoids is associated with increased fearful behavior, depression-like behavior, and stress reactivity (Abe et al., 2007; Kapoor et al., 2006; Kapoor & Matthews, 2005; Seckl, 2008). In humans, fetal exposure to elevated concentrations of maternal cortisol is associated with increased fetal and neonatal reactivity to stimulation (Davis et al, 2011; Glynn & Sandman, 2012), increased fearful behavior in infancy (Bergman et al., 2010; Davis et al., 2007; de Weerth et al., 2003), higher levels of internalizing behavior problems in children (Davis & Sandman, 2012), and alterations in brain regions association with internalizing problems (Buss et al., 2012; for a review, see Zijlmans et al., 2015).
However, because the placenta is responsive to a variety of stress signals, including cortisol of both maternal and fetal origin (Mesiano, 2014), levels of circulating pCRH may be a more direct indicator of fetal exposure and response to stressors than maternal cortisol alone. In the only published study that evaluated the consequences of pCRH on the development of internalizing symptoms in humans, elevated pCRH levels at 25 weeks’ gestation predicted increased mother-reported infant fear and distress (Davis et al., 2005). Further, compelling evidence from experimental animal models indicates that exposure to stress-induced elevations in CRH or exogenous CRH early in life may alter the structure and function of brain regions involved in internalizing symptoms (Avishai-Eliner et al., 2002; Chen et al., 2010; Maras & Baram, 2012). These findings encourage further investigation of the role of pCRH in the programming of offspring internalizing symptoms.
Mid-gestation is a period of rapid acceleration in maternal cortisol and pCRH trajectories (Sandman et al., 2006) and appears to be a sensitive window for effects on the fetal central nervous system (Sandman et al., 2015). Elevations in mid-gestational pCRH are linked to increased infant fear and distress (Davis et al., 2005), as well as a variety of other fetal and infant developmental outcomes related to maturation and body composition (Class et al., 2008; Ellman et al., 2008; Sandman et al., 2006; Stout et al., 2015). The fetal brain is rapidly developing at mid-gestation, such that it might be particularly susceptible to organizing and disorganizing programming influences at this time.
The aim of the current study is to test the hypothesis that accelerated prenatal trajectories of pCRH and maternal cortisol during mid-gestation are associated with child self-reported internalizing symptoms at age 5 in a sample of typically developing children born at full term. We chose to assess internalizing symptoms early in childhood because rates of depressive and anxious disorders begin to accelerate at this age (Luby et. al, 2002; Merikangas et al., 2010), and because early childhood symptoms are predictive of symptom trajectories and later psychiatric diagnosis (Ialongo et al., 2001). Unlike the vast majority of studies that have relied on parental report to examine the association between prenatal exposures and child temperament, we eliminated possible maternal bias and obtained unique insight into children’s symptoms by directly assessing child self-report of internalizing child behavior (Ialongo et al., 2001; Luby et al., 2007; Najman et al., 2001).
2. Method
2.1 Study Overview
Study participants were mother-child pairs from a prospective, longitudinal study of prenatal psychobiological risk and development. Women with intrauterine, singleton pregnancies less than 16 weeks’ gestational age were recruited at a large university medical center in Southern California and assessed over the course of gestation. Mother-child pairs were assessed when the child was 5 years of age.
2.2 Participants
The sample comprised 83 mothers and their 91 five-year-old, typically developing children (Mage = 5.13, 53% female). Eight of these women participated in the study twice for two separate pregnancies, with both children included in the study1. At recruitment, inclusion criteria were English-speaking, adult (> 18 years old) women with intrauterine, singleton pregnancies. Exclusion criteria at recruitment were the presence of uterine or cervical abnormalities; conditions such as endocrine, hepatic or renal disorders or use of corticosteroid medication; and self-reported abuse of tobacco, alcohol, or recreational drugs in pregnancy. Additional inclusion criteria for the current study were delivery of a full-term infant (gestational age at delivery ≥ 37 weeks; M = 39.64 weeks, SD = 1.14 weeks), maternal completion of at least 3 of 5 prenatal hormone assessments, and child completion of the internalizing symptoms scales of the Berkley Puppet Interview. Mothers were primarily Caucasian, non-Hispanic and Hispanic; had received greater than a high school level of education; and were cohabiting with the child’s father at the time of child assessment (see Table 1 for sample descriptives). Mothers provided written informed consent for all aspects of the protocol, which was approved by the university’s Institutional Review Board for protection of human subjects.
Table 1.
Participant Characteristics at Child Assessment (N = 91 children)
| M | SD | |
|---|---|---|
|
| ||
| Maternal age | 35.3 | 5.2 |
|
| ||
| Child age | 5.1 | 0.2 |
|
| ||
| Annual household income (USD) 87,746.3 | 87,746.3 | 55,675.5 |
|
| ||
| N | % | |
|
| ||
| Child sex | ||
| Female | 48 | 52.7 |
|
| ||
| Child birth order | ||
| First born | 46 | 50.5 |
|
| ||
| Maternal race | ||
| Caucasian, non-Hispanic | 47 | 51.6 |
| Latina | 22 | 24.2 |
| Asian | 9 | 9.9 |
| Other | 13 | 14.3 |
|
| ||
| Maternal education | ||
| High school or less | 9 | 9.9 |
| Associates or vocational degree | 29 | 31.9 |
| 4-year college degree | 29 | 31.9 |
| Graduate degree | 15 | 16.5 |
|
| ||
| Maternal cohabitation with child’s father | ||
| Yes | 75 | 82.4 |
| No | 16 | 17.6 |
2.3 Procedures
Maternal blood plasma samples were obtained for cortisol and pCRH analysis at 15 (M = 15.45 ± 0.93), 19 (M = 19.63 ± 1.10), 25 (M = 25.86 ± 1.03), 31 (M = 31.14 ± 0.93), and 36 (M = 36.78 ± 0.87) weeks’ gestation. Obstetric risk information was collected by detailed medical interviews at each prenatal visit, along with comprehensive examination of prenatal and delivery medical records. Child self-reported internalizing symptoms and maternal depressive symptoms were evaluated when children were 5 years of age.
2.4 Measures
2.4.1 Endocrine measures
Maternal blood samples (20/ml) were collected by antecubital venipuncture into EDTA (purple top) vacutainers and then immediately chilled on ice. Aprotinin (Sigma Chemical, St. Louis, MO) was added at 500 KIU/ml blood. Samples were centrifuged at 2,000 g for 15 minutes, decanted into polypropylene tubes, and stored at −80°C until assayed.
Total CRH concentrations were determined by a radioimmunoassay with reported sensitivity of 2.04 pg/ml (RIA; Bachem Peninsula Laboratories, San Carlos, CA). Plasma samples (1–2 ml) were extracted with three volumes of ice-cold methanol, mixed, allowed to stand for 10 minutes at 4°C, and centrifuged at 1700 g and 4°C for 20 minutes (Linton et al., 1995). Pellets were washed with 0.6 ml methanol, and the combined supernatants were dried down in a Savant SpeedVac concentrator. Reconstituted samples with assay buffer were incubated (100 μl/assay tube) with anti-CRH serum (100 μl) for 48 hours at 4°C, followed by a 24-hour incubation with 125I-CRH at 4°C. Labeled and unlabeled CRH were collected by immunoprecipitation with goat antirabbit IgG serum and normal rabbit serum after 90 minutes of incubation at room temperature. Samples were then centrifuged at 1700 g and 4°C for 20 minutes, after which the aspirated pellets were quantified with a gamma scintillation counter. This assay has less than 0.01% cross-reactivity with ovine CRH, 36% cross-reactivity with bovine CRH, and nondetectable reactivity with human ACTH. Intra-assay and interassay coefficients ranged from 5% to 15%, respectively. For additional details, see Glynn and Sandman (2014).
Plasma cortisol levels were determined by a competitive binding solid-phase enzyme-linked immunosorbent assay with reported sensitivity of 0.22 μg/dl. Plasma samples (20 μl) and enzyme conjugate (200 μl) were thoroughly mixed in antibody-coated microtiter wells and incubated at room temperature for 60 minutes. Each well was then washed three times with wash solution (400 μl per well), followed by a 15-minute incubation at room temperature with substrate solution (100 μl). Absorbance units were measured at 450 nm within 10 minutes of adding stop solution. This assay has less than 9% cross-reactivity with progesterone and less than 2% cross-reactivity with other naturally-occurring steroid hormones (e.g., testosterone, estradiol). Interassay and intra-assay coefficients of variance were less than 8%.
2.4.2 Child self-reported internalizing symptoms
Child self-reported internalizing symptoms were assessed with the Berkeley Puppet Interview (Measelle et al., 1998), a validated semi-structured interview designed to measure young children’s perceptions of their functioning in a variety of academic and socio-emotional domains. The interviewer engaged the child with two identical hand puppets, which presented the child with contrasting statements of positive and negative valence, such as, “I’m a happy kid/I’m not a happy kid.” The child was then asked to indicate which puppet is most like him or her, with standardized prompts issued only if a response was ambiguous. For the current study, interviews were conducted by one of three research associates trained and certified in administration and coding of the BPI by the developers of the measure (Measelle et al., 1998). Children’s responses were videotaped and later coded independently by two trained research associates. Each response was rated based on how much emphasis was placed on an answer, using a seven-point scale ranging from 1 (amplified negative response) to 7 (amplified positive response). Inter-rater reliability across all scales administered was 96%.
For the current investigation, the Depression (7 items), Anxiety (7 items), and Separation Anxiety (6 items) subscales of the BPI were summed and averaged to create an internalizing symptoms broadband scale ranging from 1 to 7 (Ringoot et al., 2013). Ratings for each item were reverse scored; higher total scores on the internalizing symptoms broadband scale reflected higher self-reported internalizing symptoms. Of the 91 children included in analyses, 4 did not complete 90% or more of the full internalizing symptoms scale. The subset of items available for these 4 children (ranging from 4 to 11 items) were highly correlated with the full scale score (r = .79) in the total sample of children and were considered to be adequately representative of these children’s full scale scores. The internalizing symptoms broadband scale has previously demonstrated internal reliability and validity in 5 to 7-year-old children (Ringoot et al., 2013), and reliability in the current sample was acceptable (α = .73). Further, these internalizing symptom scales have been found to accurately discriminate between young children with and without a depressive disorder diagnosis (Luby et al., 2002; 2007) and between community and clinic-referred children (Ablow et al., 1999).
2.4.3 Maternal depressive symptoms
Maternal depressive symptoms were assessed with the 9-item form of the Center for Epidemiological Studies Depression Inventory (Santor & Coyne, 1997) when the child was 3 months old and 5 years of age and considered for potential association with child self-reported internalizing symptoms. Participants indicated how much they had experienced each symptom in the past week, using a 4-point Likert scale ranging from 1 (rarely or none of the time) to 4 (most or all of the time). This measure has been used extensively in previous research and has demonstrated excellent reliability and validity (Santor & Coyne, 1997). Internal consistency was good in the current sample (α = .82).
2.4.4 Obstetric medical risk
An extensive structured medical interview was performed by a research nurse at each prenatal visit to assess maternal health and pregnancy-related complications. Maternal and infant medical records were reviewed to assess pregnancy complications and birth outcome. The obstetric risk score accounted for prenatal infection, pregnancy-induced hypertension, gestational diabetes, oligohydramnios, polyhydramnios, preterm labor, vaginal bleeding, placenta previa, and anemia. A cumulative score assessing prenatal obstetric risk was derived from the sum of all present risk variables (Hobel, 1982). In this low risk sample, 71% of women had none of these risk factors, and no woman had more than two risk factors.
2.5 Statistical Analyses
Preliminary analyses included t-tests and correlations to identify demographic (maternal race/ethnicity, maternal education, maternal cohabitation with child’s father, annual household income), pregnancy (obstetric medical risk), maternal psychological state (postpartum and concurrent depressive symptoms), and child (age, sex, birth order) variables that might be associated with child self-reported internalizing symptoms. Only child sex was significantly associated with child self-reported internalizing symptoms, with girls reporting significantly more internalizing symptoms than boys, t(86.30) = 3.07, p = .003.
Multilevel modeling techniques (HLM6; Raudenbush et al., 2004) were conducted to evaluate associations between prenatal hormone trajectories and child self-reported internalizing symptoms. Multilevel modeling is advantageous in the case of repeated measures data, because it allows for hierarchical modeling of both within-dyad (prenatal hormone levels) and between-dyad (child self-reported internalizing symptoms) variance. Multilevel models produce robust estimates of missing values for the repeated measure. Models weight cases with complete data more heavily, but all cases are included in the estimation of effects. In the current sample, every woman completed at least 3 of 5 prenatal hormone assessments, with 44 women completing all 5 assessments. Women with incomplete prenatal hormone data were not significantly different from women with complete data on any demographic, maternal psychological state, or child variables. These women also did not significantly differ in prenatal hormone trajectories or child self-reported internalizing symptoms.
Initial testing indicated that a quadratic model best fit maternal plasma cortisol trajectories, and a cubic model best fit pCRH trajectories. For each prenatal hormone, a series of two-level models were computed to test differences in level (intercept) and instantaneous rate of change (slope) at 1-week intervals within the range of assessments available (13 – 39 weeks’ gestation). Positive differences in the instantaneous rate of change or slope reflected acceleration in the prenatal hormone trajectory at the interval tested. In each two-level model, level 1 (time-variant, within-dyad) variables were hormone levels at each study visit and the timing of these visits in gestational weeks. For cortisol analyses, time of sample collection was also added as a level 1 variable in all models (pCRH was not related to time of sample collection). Child self-reported internalizing symptoms and child sex were included as level 2 (time-invariant, between-dyad) variables in each model.
3. Results
3.1 Descriptives
As expected, a repeated measures ANOVA indicated that significant increases in maternal plasma levels of pCRH, F(1.71, 73.42) = 356.08, p < .001, and cortisol, F(3.56, 266.62) =118.56, p < .001, were observed over the course of pregnancy. After adjusting for gestational age and time at sample collection, concurrent pCRH and cortisol levels were significantly correlated only at the 36 weeks’ gestation assessment, r = .25, p = .02, consistent with data from previous examinations (Magiakou et al., 1996; Sandman et al., 2006). Placental CRH, F(1.74, 71.27) = 0.71, p = .48, and cortisol, F(3.57, 260.39) = 0.39, p = .79, trajectories did not differ by fetal sex. Children reported relatively low levels of internalizing symptoms, with scores ranging from 2.00 – 5.20 (M = 3.01 ± 0.70).
3.2 Associations with child self-reported internalizing symptoms
Because child sex was significantly associated with child self-reported internalizing symptoms, we initially included it as a covariate in all models tested. Child sex was not a significant level-2 variable at any of the gestational intervals tested for either prenatal hormone. Further, child sex was not a moderating variable; the magnitude and direction of associations were the same when testing the models separately for male and female children. Therefore, we did not adjust for child sex in the final models reported here. Profiles of placental CRH over gestation were associated with child self-reported internalizing symptoms (see Figure 1). Fetal exposure to an acceleration (instantaneous rate of change) in pCRH at 22–23 gestational weeks was associated with higher child internalizing symptoms (Coefficients: 3.04–3.47, SE = 1.51–1.72, ps < .05). This acceleration in placental CRH production resulted in exposure to higher levels of pCRH at 27–28 gestational weeks (Coefficients: 25.76–28.72, SE = 12.79–14.44, ps < .05), such that a 25.75–28.70 pg/ml increase in pCRH was associated with a 1unit increase in child self-reported internalizing symptoms. These data suggest that elevated fetal exposures to pCRH at mid-gestation represents a risk factor for increased internalizing symptoms in early childhood.
Figure 1.
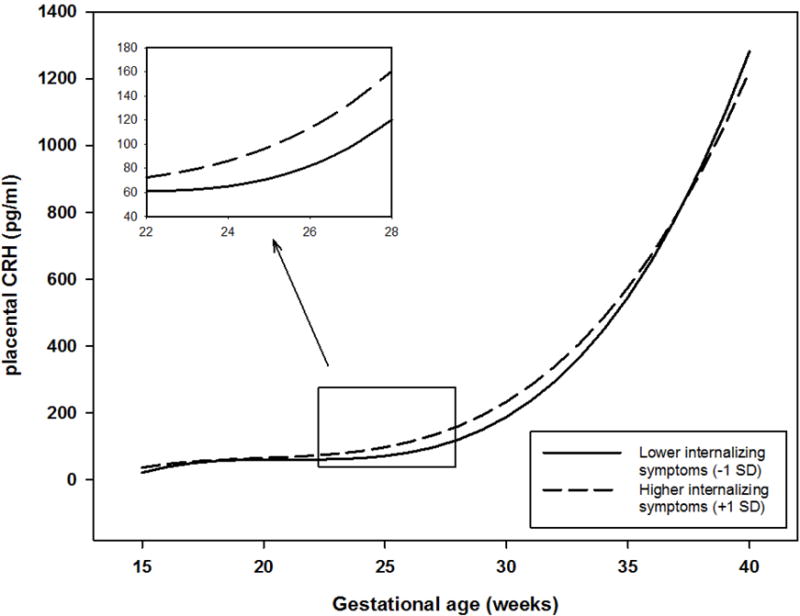
Fetal exposure to trajectories of placental CRH is associated with child report of internalizing symptoms at age 5. Child internalizing symptom scores were treated as a continuous variable for analyses. Trajectories of exposure to placental CRH for children with internalizing symptom scores above or below 1 standard deviation are plotted for illustrative purposes. Inset illustrates gestational window at which acceleration and level of placental CRH concentrations are positively associated with child internalizing symptoms.
Prenatal plasma cortisol trajectories were not related to self-reported internalizing symptoms in 5 year-old children, with no differences in slope (Coefficients: −0.12–0.08, SE = 0.05–0.22, ps > .10) or level (Coefficients: −1.07–0.15, SE = 0.56–1.42, ps > .10) at any gestational period.
4. Discussion
The current study provides evidence that pCRH, a stress signal of feto-placental origin, may be a pathway through which fetal programming influences risk for child internalizing symptoms. This prospective, longitudinal study in a unique cohort of children documents that fetal exposure to accelerated pCRH trajectories during mid-gestation is associated with child internalizing symptoms at 5 years of age. This effect was not explained by postnatal or other prenatal influences, including obstetric risk, postpartum or concurrent maternal psychological state, or family socio-economic status. Furthermore, this investigation included only children born full-term, so the consequences of prenatal pCRH exposures were independent of the well-documented effects of pCRH on preterm birth (Sandman et al., 2006; Smith et al., 2009) and of preterm birth on child internalizing symptoms (Bhutta et al., 2002). The association between increased pCRH and higher child self-reported internalizing symptoms is consistent with experimental animal research demonstrating that prenatal exogenous CRH exposure is linked to increased anxiety behaviors in offspring (Williams et al., 1995) and with human research linking elevated pCRH to altered profiles of fetal neurodevelopment (Class et al., 2008; Ellman et al., 2008) and increased infant fear and distress (Davis et al., 2005).
Contrary to previous examinations that have relied on maternal report or laboratory observations of child behavior (Bergman et al., 2010; Bergman et al., 2007; Davis et al., 2007; Davis et al., 2011; Davis & Sandman, 2012; de Weerth et al., 2003), we did not observe an association between prenatal maternal cortisol and child internalizing symptoms. This may be related to differences between assessments of emotional and social adjustment from parental report and those determined directly from the child. In a large validation study of the child self-report measure of internalizing symptoms utilized in the current study, the child-report scale was weakly correlated with a parent-report measure of child internalizing symptoms, with the two scales sharing only 3% of their variance (Ringoot et al., 2013). While parent and child perspectives of children’s internalizing symptoms are clearly different, evidence suggests that both informants provide reliable and valid information in the characterization of these symptoms (Ablow et al., 1999; Ialongo et al., 2001; Jensen et al., 1999; Luby et al., 2002, 2007). We suggest that placental CRH exposures may explain a different, unique portion of variance in children’s internalizing symptoms, compared with the variance in symptoms that can be explained by prenatal maternal cortisol exposures. Indeed, the current study suggests that the aspects of children’s internalizing symptoms associated with pCRH exposures and not with maternal cortisol exposures are specifically those aspects captured by child report. Distinct physiological mechanisms may also be at play. For instance, exogenous CRH exposures in early-life have been shown to induce depression-like behavioral deficits in rats for which plasma glucocorticoids were clamped at low levels, indicating that CRH has programming effects independent of the effects of cortisol exposures (Brunson et al., 2001; Maras & Baram, 2012).
The rapid increase in pCRH during mid-gestation (Sandman et al., 2006) coincides with major developmental advances in the fetal nervous system, which may render the fetus particularly vulnerable at this time (de Graaf-Peters & Hadders-Algra, 2006; Sandman et al., 2015). By 28 weeks’ gestation, neuronal proliferation is 40% greater than in the adult brain (Becker et al., 1984; Bourgeois et al., 1994; Huttenlocher & Dabholkar, 1997). Cortical synapse formation accelerates between 24–26 weeks’ gestation, resulting in a six-fold increase in synaptic density from 28 weeks onward (de Graaf-Peters & Hadders-Algra, 2006; Levitt, 2003). Apoptosis also peaks at this time, which plays a fundamental role in determining the final number of neurons and glial cells present in the brain (de Graaf-Peters & Hadders-Algra, 2006).
Fetal exposure to elevated pCRH may confer risk for internalizing symptoms by programming of fetal brain regions and/or the fetal HPA axis. CRH is released from the placenta into fetal circulation and penetrates the immature fetal blood-brain barrier (Kastin & Akerstrom, 2002), where it may alter the trajectories of developing structures. Differentiated cortical neurons in the fetal brain express CRH receptors as early as 13 weeks’ gestation. Elevations in pCRH may regulate neurogenesis, synaptogenesis and apoptosis (Koutmani et al., 2013) specifically in CRH receptor-rich limbic regions linked to fear, anxiety, and depression, such as the amygdala and hippocampus (Avishai-Eliner et al., 2002; Charil et al., 2010; Cratty et al., 1995; Schumann et al., 2011). Rats exposed to exogenous CRH in the early postnatal period have been shown to exhibit progressive hippocampal cell loss and learning and memory dysfunctions distinctive to depression (Brunson et al., 2001). Additionally, hormone activity can be detected in the fetal HPA axis as early as 8–12 weeks’ gestation, with CRH regulating the growth of pituitary corticotrophs, adrenocortical differentiation, and steroidogenic maturation of the fetal HPA axis (Mesiano & Jaffe, 1997; Ng, 2000). The set points of the progressively activated fetal HPA axis may be permanently altered by increased pCRH in the fetal circulation (Goland et al., 1988; Mastorakos & Ilias, 2003; Petraglia et al., 1989; Sandman et al., 2011).
4.1 Limitations
The current study examined naturally occurring variations in prenatal stress hormone signals; without experimental manipulation, we cannot confirm independent effects of pCRH beyond other potential contributing factors such as genetic vulnerability. Notably, the effect observed in this study was not explained by maternal depressive symptomology at the time of child assessment. Additionally, our findings are consistent with experimental animal studies linking prenatal and early postnatal CRH exposures to offspring distress and depression-like dysfunction in which random assignment was possible (Brunson et al., 2001; Williams et al., 1995). Results of human studies involving children conceived by in vitro fertilization and not genetically related to their mothers also provide evidence that prenatal exposures are linked with negative child mental health outcomes, including internalizing symptomology (Rice et al., 2010). In addition, maternal cortisol levels were assessed at only one time during the day at each of the five assessment intervals, so we did not account for individual differences in diurnal variation of cortisol levels. However, we did adjust for time of day when modeling maternal cortisol trajectories across gestation.
4.2 Implications
Results of the current investigation are consistent with previous studies demonstrating the effects of prenatal stress on fetal central nervous system development. Placental CRH is a feto-placental signal independently responsive to a number of major stress signals and represents a plausible biological mechanism of prenatal programming of risk for anxiety and depression later in life. We assessed internalizing problems using child report, and there is some evidence that these reports may be more predictive than parent-report of later emergence of clinical levels of depression and anxiety (Ialongo et al., 2001; Luby et al., 2007). Further, our finding was observed in a sample of healthy, low-risk mother-child pairs, and thus it is possible that more extreme effects would be observed in higher risk populations.
The fetal period is a particularly sensitive window of development. The fetal central nervous system is in a state of unmatched growth and neuroplasticity, and the structure, function, and set-points of neural circuitry are primarily calibrated at this time. The fetal programming model of development (Barker, 1998) proposes that fetal exposures to signals of adversity such as pCRH are associated with later risk for disease. Our finding of increased internalizing symptoms in children with fetal exposure to elevated pCRH may support the programming model, because young children’s internalizing symptoms are predictive of psychiatric diagnosis in adolescence and adulthood (Copeland et al., 2013; Gregory et al., 2005; Ialongo et al., 2001; Roza et al., 2003). However, it also is possible that these effects represent an adaptive response in preparation for life after birth. It can be argued that fetal exposure to signals of adversity results in neurodevelopmental adjustments to prepare for a postnatal environment predicted to be hostile or threatening (Gluckman & Hanson, 2004). Internalizing symptoms could then reflect a survival strategy of increased vigilance in the face of threat or withdrawal from threat in an effort to conserve resources (Copeland et al., 2013; Gregory et al., 2005; Ialongo et al., 2001; Roza et al., 2003). Follow-up of the current study’s cohort into adolescence will allow for continued examination of the potentially long-term effects of pCRH exposures.
Highlights.
The feto-placental stress hormone pCRH is responsive to a variety of major stress signals and may be a direct indicator of fetal exposure to prenatal stress.
This prospective, longitudinal study documents an association between fetal exposure to pCRH and child self-reported internalizing symptoms at age 5.
pCRH is a plausible pathway of risk for prenatal programming of childhood internalizing symptoms.
Acknowledgments
This research was supported by National Institute of Health grants NS41298 and HD51852 to CAS and Conte Center award MH-96889. We are grateful for the expert assistance of compensated staff, Christina Canino, MA (subject recruitment, data collection) and Natalie Hernandez, BA (subject recruitment, data collection), and to the families who participated in our study.
Role of the funding source:
The funding sources did not contribute to the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Abbreviations
- pCRH
placental corticotropin-releasing hormone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Findings reported here did not change when removing one member of the sibling pair, so both siblings were included in analyses.
Conflict of Interest:
None of the authors have any financial interests to disclose.
Contributors:
Study concept and design: EPD, CAS, LMG. Acquisition of data: EPD, CAS, LMG, CC. Analysis and interpretation of data: MAH, CC, EPD, CAS, LMG. Drafting of the manuscript: MAH. Critical revision of the manuscript for important intellectual content: EPD, CAS, LMG, CC. Obtained funding: EPD, CAS, LMG.
References
- Abe H, Hidaka N, Kawagoe C, Odagiri K, Watanabe Y, Ikeda T, Ishida Y. Prenatal psychological stress causes higher emotionality, depression-like behavior, and elevated activity in the hypothalamo-pituitary-adrenal axis. Neurosci Res. 2007;59:145–151. doi: 10.1016/j.neures.2007.06.1465\. [DOI] [PubMed] [Google Scholar]
- Ablow JC, Measelle JR, Kraemer HC, Harrington R, Luby J, Smider N, Kupfer DJ. The MacArthur Three-City Outcome Study: Evaluating multi-informant measures of young children’s symptomatology. J Am Acad Child Psy. 1999;38:1580–1590. doi: 10.1097/00004583-199912000-00020. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero)? Trends Neurosci. 2002;25:518–524. doi: 10.1016/S0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Davis EP, Obenaus A, Sandman CA, Small SL, Solodkin A, Stern H. Fragmentation and unpredictability of early-life experience in mental disorders. Am J Psychiat. 2012;169:907–915. doi: 10.1176/appi.ajp.2012.11091347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. In utero programming of chronic disease. Clin Sci. 1998;95:115–128. [PubMed] [Google Scholar]
- Becker LE, Armstrong DL, Chan F, Wood MM. Dendritic development in human occipital cortical neurons. Brain Res. 1984;315:117–124. doi: 10.1016/0165-3806(84)90083-x. [DOI] [PubMed] [Google Scholar]
- Bergman K, Glover V, Sarkar P, Abbott DH, O’Connor TG. In utero cortisol and testosterone exposure and fear reactivity in infancy. Horm Behav. 2010;57:306–312. doi: 10.1016/j.yhbeh.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, O’Connor TG, Modi N, Glover V. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. J Am Acad Child Psy. 2007;46:1454–1463. doi: 10.1097/chi.0b013e31814a62f6. [DOI] [PubMed] [Google Scholar]
- Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJS. Cognitive and behavioral outcomes of school-aged children who were born preterm: A meta-analysis. J Amer Med Assoc. 2002;288:728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. P Natl Acad Sci USA. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. P Natl Acad Sci USA. 2012;109:E1312–1319. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charil A, Laplante DP, Vaillancourt C, King S. Prenatal stress and brain development. Brain Res Rev. 2010;65:56–79. doi: 10.1016/j.brainresrev.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Chen Y, Rex CS, Rice CJ, Dube CM, Gall CM, Lynch G, Baram TZ. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. P Natl Acad Sci USA. 2010;10:13123–13128. doi: 10.1073/pnas.1003825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Class QA, Buss C, Davis EP, Gierczak M, Pattillo C, Chicz-DeMet A, Sandman CA. Low levels of corticotropin-releasing hormone during early pregnancy are associated with precocious maturation of the human fetus. Dev Neurosci. 2008;30:419–426. doi: 10.1159/000191213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Adair CE, Smetanin P, Stiff D, Briante C, Colman I, Angold A. Diagnostic transitions from childhood to adolescence to early adulthood. J Child Psychol Psyc. 2013;54:791–799. doi: 10.1111/jcpp.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cratty MS, Ward HE, Johnson EA, Azzaro AJ, Birkle DL. Prenatal stress increases corticotropin-releasing factor (CRF) content and release in rat amygdala minces. Brain Res. 1995;675:297–302. doi: 10.1016/0006-8993(95)00087-7. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA. Corticotropin-releasing hormone during pregnancy is associated with infant temperament. Dev Neurosci. 2005;27:299–305. doi: 10.1159/000086709. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA. Prenatal exposure to maternal depression and cortisol influences infant temperament. J Am Acad Child Psy. 2007;46:737–746. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. J Child Psychol Psyc. 2011;52:119–129. doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Sandman CA. Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinology. 2012;37:1224–1233. doi: 10.1016/j.psyneuen.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf-Peters VB, Hadders-Algra M. Ontogeny of the human central nervous system: what is happening when? Early Hum Dev. 2006;82:257–266. doi: 10.1016/j.earlhumdev.2005.10.013. [DOI] [PubMed] [Google Scholar]
- de Weerth C, van Hees Y, Buitelaar JK. Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Hum Dev. 2003;74:139–151. doi: 10.1016/S0378-3782(03)00088-4. [DOI] [PubMed] [Google Scholar]
- Ellman LM, Schetter CD, Hobel CJ, Chicz-Demet A, Glynn LM, Sandman CA. Timing of fetal exposure to stress hormones: Effects on newborn physical and neuromuscular maturation. Dev Psychobiol. 2008;50:232–241. doi: 10.1002/dev.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitau R, Fisk NM, Glover V. Human fetal and maternal corticotrophin releasing hormone responses to acute stress. Arch Dis Child-Fetal. 2004;89:F29–32. doi: 10.1136/fn.89.1.F29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Developmental origins of disease paradigm: a mechanistic and evolutionary perspective. Pediatr Res. 2004;56:311–317. doi: 10.1203/01.PDR.0000135998.08025.FB. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Sandman CA. Sex moderates associations between prenatal glucocorticoid exposure and human fetal neurological development. Developmental Sci. 2012;15:601–610. doi: 10.1111/j.1467-7687.2012.01159.x. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Sandman CA. Evaluation of the association between placental corticotrophin-releasing hormone and postpartum depressive symptoms. Psychosom Med. 2014;76:355–362. doi: 10.1097/Psy.0000000000000066. [DOI] [PubMed] [Google Scholar]
- Goland RS, Wardlaw SL, Blum M, Tropper PJ, Stark RI. Biologically-active corticotropin-releasing hormone in maternal and fetal plasma during pregnancy. Am J Obstet Gynecol. 1988;159:884–890. doi: 10.1016/s0002-9378(88)80162-5. [DOI] [PubMed] [Google Scholar]
- Gregory AM, Caspi A, Eley TC, Moffitt TE, O’Connor TG, Poulton R. Prospective longitudinal associations between persistent sleep problems in childhood and anxiety and depression disorders in adulthood. J Abnorm Child Psych. 2005;33:157–163. doi: 10.1007/s10802-005-1824-0. [DOI] [PubMed] [Google Scholar]
- Herrmann TS, Siega-Riz AM, Hobel CJ, Aurora C, Dunkel-Schetter C. Prolonged periods without food intake during pregnancy increase risk for elevated maternal corticotropin-releasing hormone concentrations. Am J Obstet Gynecol. 2001;185:403–412. doi: 10.1067/mob.2001.115863. [DOI] [PubMed] [Google Scholar]
- Hobel CJ. Identifying the patient at risk. In: Bolognese SRH, Schneider RJ, editors. Perinatal medicine: Management of the high risk fetus and neonate. Baltimore, MA: Williams & Wilkins; 1982. pp. 3–28. [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Ialongo NS, Edelsohn G, Kellan SG. A further look at the prognostic power of young children’s reports of depressed mood and feelings. Child Dev. 2001;72:736–747. doi: 10.1111/1467-8624.00312. [DOI] [PubMed] [Google Scholar]
- Jensen PS, Rubio-Stipec M, Canino G, Bird HR, Dulcan MK, Schwab-Stone ME, Lahey BB. Parent and child contributions to diagnosis of mental disorder: are both informants always necessary? J Am Acad Child Psy. 1999;38:1569–1579. doi: 10.1097/00004583-199912000-00019. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: Prenatal stress and glucocorticoids. J Physiol-London. 2006;572:31–44. doi: 10.1113/jphysiol.2006.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Matthews SG. Short periods of prenatal stress affect growth, behaviour and hypothalamo-pituitary-adrenal axis activity in male guinea pig offspring. J Physiol-London. 2005;566:967–977. doi: 10.1113/jphysiol.2005.090191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karteris E, Grammatopoulos DK, Randeva HS, Hillhouse EW. The role of corticotropin-releasing hormone receptors in placenta and fetal membranes during human pregnancy. Mol Genet Metab. 2001;72:287–296. doi: 10.1006/mgme.2001.3159. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V. Differential interactions of urocortin/corticotropin-releasing hormone peptides with the blood-brain barrier. Neuroendocrinology. 2002;75:367–374. doi: 10.1159/000059433. [DOI] [PubMed] [Google Scholar]
- Koutmani Y, Politis PK, Elkouris M, Agrogiannis G, Kemerli M, Patsouris E, Karalis KP. Corticotropin-releasing hormone exerts direct effects on neuronal progenitor cells: Implications for neuroprotection. Mol Psychiatr. 2013;18:300–307. doi: 10.1038/mp.2012.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt P. Structural and functional maturation of the developing primate brain. J Pediatr. 2003;143:S35–45. doi: 10.1067/S0022-3476(03)00400-1. [DOI] [PubMed] [Google Scholar]
- Linton EA, Perkins AV, Hagan P, Poole S, Bristow AF, Tilders F, Wolfe CD. Corticotrophin-releasing hormone (CRH)-binding protein interference with CRH antibody binding: Implications for direct CRH immunoassay. J Endocrinol. 1995;146:45–53. doi: 10.1677/joe.0.1460045. [DOI] [PubMed] [Google Scholar]
- Lowry PJ. Corticotropin-releasing factor and its binding-protein in human plasma. Ciba F Symp. 1993;172:108–128. [PubMed] [Google Scholar]
- Luby JL, Heffelfinger AK, Mrakotsky C, Hessler MJ, Brown KA, Hildebrand T. Preschool major depressive disorder: Preliminary validation for developmentally modified DSM-IV criteria. J Am Acad Child Psy. 2002;41:928–937. doi: 10.1097/00004583-200208000-00011. [DOI] [PubMed] [Google Scholar]
- Luby JL, Belden A, Sullivan J, Spitznagel E. Preschoolers’ contribution to their diagnosis of depression and anxiety: Uses and limitations of young child self-report of symptoms. Child Psychiat Hum D. 2007;38:321–338. doi: 10.1007/s10578-007-0063-8. [DOI] [PubMed] [Google Scholar]
- Magiakou MA, Mastorakos G, Rabin D, Margioris AN, Dubbert B, Calogero AE, Chrousos GP. The maternal hypothalamic-pituitary-adrenal axis in the third trimester of human pregnancy. Clin Endocrinol. 1996;44:419–428. doi: 10.1046/j.1365-2265.1996.683505.x. [DOI] [PubMed] [Google Scholar]
- Maras PM, Baram TZ. Sculpting the hippocampus from within: stress, spines, and CRH. Trends Neurosci. 2012;35:315–324. doi: 10.1016/j.tins.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann NY Acad Sci. 2003;997:136–149. doi: 10.1196/annals.1290.016. [DOI] [PubMed] [Google Scholar]
- Measelle JR, Ablow JC, Cowan PA, Cowan CP. Assessing young children’s views of their academic, social, and emotional lives: An evaluation of the self-perception scales of the Berkeley puppet interview. Child Dev. 1998;69:1556–1576. doi: 10.1111/j.1467-8624.1998.tb06177.x. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A) J Am Acad Child Psy. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesiano S. The endocrinology of human pregnancy and fetal-placental neuroendocrine development. In: Strauss JF, Barbieri R, editors. Yen and Jaffe’s reproductive endocrinology: Physiology, pathophysiology, and clinical management. Philadelphia, PA: Saunders; 2014. pp. 243–271. [Google Scholar]
- Mesiano S, Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev. 1997;18:378–403. doi: 10.1210/edrv.18.3.0304. [DOI] [PubMed] [Google Scholar]
- Najman JM, Williams GM, Nikles J, Spence S, Bor W, O’Callaghan M, Shuttlewood GJ. Bias influencing maternal reports of child behaviour and emotional state. Soc Psych Psych Epid. 2001;36:186–194. doi: 10.1007/s001270170062. [DOI] [PubMed] [Google Scholar]
- Ng PC. The fetal and neonatal hypothalamic-pituitary-adrenal axis. Arch Dis Child-Fetal. 2000;82:F250–254. doi: 10.1136/fn.82.3.F250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell K, O’Connor TG, Glover V. Prenatal stress and neurodevelopment of the child: Focus on the HPA axis and role of the placenta. Dev Neurosci. 2009;31:285–292. doi: 10.1159/000216539. [DOI] [PubMed] [Google Scholar]
- Petraglia F, Sutton S, Vale W. Neurotransmitters and peptides modulate the release of immunoreactive corticotropin-releasing factor from cultured human placental cells. Am J ObstetGynecol. 1989;160:247–251. doi: 10.1016/0002-9378(89)90130-0. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS, Congdon R. HLM 6 for Windows. Skokie, IL: Scientific Software International, Inc; 2004. [Google Scholar]
- Rice F, Harold GT, Boivin J, van den Bree M, Hay DF, Thapar A. The links between prenatal stress and offspring development and psychopathology: Disentangling environmental and inherited influences. Psychol Med. 2010;40:335–345. doi: 10.1017/S0033291709005911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringoot AP, Jansen PW, Steenweg-de Graaff J, Measelle JR, van der Ende J, Raat H, Tiemeier H. Young children’s self-reported emotional, behavioral, and peer problems: The Berkeley Puppet Interview. Psychol Assessment. 2013;25:1273–1285. doi: 10.1037/a0033976. [DOI] [PubMed] [Google Scholar]
- Roza SJ, Hofstra MB, van der Ende J, Verhulst FC. Stable prediction of mood and anxiety disorders based on behavioral and emotional problems in childhood: A 14-year follow-up during childhood, adolescence, and young adulthood. Am J Psychiat. 2003;160:2116–2121. doi: 10.1176/appi.ajp.160.12.2116. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Buss C, Head K, Davis EP. Fetal exposure to maternal depressive symptoms is associated with cortical thickness in late childhood. Biol Psychiat. 2015;77:324–334. doi: 10.1016/j.biopsych.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Davis EP. Neurobehavioral risk is associated with gestational exposure to stress hormones. Expert Rev of Endocrinol Metab. 2012;7:445–459. doi: 10.1586/eem.12.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Davis EP, Buss C, Glynn LM. Prenatal programming of human neurological function. Int J Pept. 2011;2011:837596. doi: 10.1155/2011/837596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, Hobel C. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): Priming the placental clock. Peptides. 2006;27:1457–1463. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Santor DA, Coyne JC. Shortening the CES-D to improve its ability to detect cases of depression. Psychol Assessment. 1997;9:233–243. doi: 10.1037//1040-3590.9.3.233. [DOI] [Google Scholar]
- Schumann CM, Bauman MD, Amaral DG. Abnormal structure or function of the amygdala is a common component of neurodevelopmental disorders. Neuropsychologia. 2011;49:745–759. doi: 10.1016/j.neuropsychologia.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckl JR. Glucocorticoids, developmental ‘programming’ and the risk of affective dysfunction. Prog Brain Res. 2008;167:17–34. doi: 10.1016/S0079-6123(07)67002-2. [DOI] [PubMed] [Google Scholar]
- Smith R, Smith JI, Shen X, Engel PJ, Bowman ME, McGrath SA, Smith DW. Patterns of plasma corticotropin-releasing hormone, progesterone, estradiol, and estriol change and the onset of human labor. J Clin Endocr Metab. 2009;94:2066–2074. doi: 10.1210/jc.2008-2257. [DOI] [PubMed] [Google Scholar]
- Stout SA, Espel EV, Sandman CA, Glynn LM, Davis EP. Fetal programming of children’s obesity risk. Psychoneuroendocrinology. 2015;53:29–39. doi: 10.1016/j.psyneuen.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa PD, Culhane JF, Rauh V, Barve SS. Stress and preterm birth: Neuroendocrine, immune/inflammatory, and vascular mechanisms. Matern Child Health J. 2001;5:119–125. doi: 10.1023/A:1011353216619. [DOI] [PubMed] [Google Scholar]
- Williams MT, Hennessy MB, Davis HN. CRF administered to pregnant rats alters offspring behavior and morphology. Pharmacol Biochem Be. 1995;52:161–167. doi: 10.1016/0091-3057(95)00082-8. [DOI] [PubMed] [Google Scholar]
- Zijlmans MA, Riksen-Walraven JM, de Weerth C. Associations between maternal prenatal cortisol concentrations and child outcomes: A systematic review. Neurosci Biobehav R. 2015;53:1–24. doi: 10.1016/j.neubiorev.2015.02.015. [DOI] [PubMed] [Google Scholar]


