Abstract
We hypothesize that Anorexia Nervosa (AN) poses a physiological stress. Therefore, the way an individual copes with stress may affect AN vulnerability. Since prenatal stress (PNS) exposure alters stress responsivity in offspring this may increase their risk of developing AN. We tested this hypothesis using the activity based anorexia (ABA) rat model in control and PNS rats that were characterized by either proactive or passive stress-coping behavior.
We found that PNS passively coping rats ate less and lost more weight during the ABA paradigm. Exposure to ABA resulted in higher baseline corticosterone and lower insulin levels in all groups. However, leptin levels were only decreased in rats with a proactive stress-coping style. Similarly, ghrelin levels were increased only in proactively coping ABA rats. Neuropeptide Y (Npy) expression was increased and proopiomelanocortin (Pomc) expression was decreased in all rats exposed to ABA. In contrast, agouti-related peptide (Agrp) and orexin (Hctr) expression were increased in all but the PNS passively coping ABA rats. Furthermore, DNA methylation of the orexin gene was increased after ABA in proactive coping rats and not in passive coping rats.
Overall our study suggests that passive PNS rats have innate impairments in leptin and ghrelin in responses to starvation combined with prenatal stress associated impairments in Agrp and orexin expression in response to starvation. These impairments may underlie decreased food intake and associated heightened body weight loss during ABA in the passively coping PNS rats.
Keywords: anorexia nervosa, agouti-related peptide, orexin, stress-coping style, prenatal stress, leptin, ghrelin
1. Introduction
Anorexia Nervosa (AN) is a psychiatric disorder characterized by decreased voluntary energy intake, low body weight for age, sex and physiological health, fear of gaining weight and a disturbance in the way one’s body weight is perceived (DSM-V (Association, 2013). AN has the highest mortality rate of any psychiatric disorder (Arcelus et al., 2011) and is one of the most debilitating diseases in young women (Mathers et al., 2000). Unfortunately, AN has low rates of recovery and, for 25% of patients, AN becomes a chronic, relapsing condition (reviewed in (Berkman et al., 2007).
To allow investigation of the underlying neuroendocrine pathways explaining AN vulnerability, an animal model has been used that mimics symptoms of the disorder in rodents. The activity based anorexia (ABA) paradigm combines restricted food access with free access to wheel running, which results in hyperactivity, hypophagia when food is available, and severe body weight loss (Routtenberg and Kuznesof, 1967). Although this phenotype can be induced in most rodents, young female rodents are most vulnerable to weight loss in the ABA model consistent with the prevalence of AN in human.
It is still unclear what predicts susceptibility to AN. Human population-based twin studies fail to find evidence for high heritability, although genetic epidemiology of AN does demonstrate strong evidence for familial aggregation (Clarke et al., 2012). Environmental factors may play an important role in the onset of AN, and gene-environment interactions likely play a role as well. Epidemiological studies suggest that early life stress, such as child abuse, may predispose an individual to the development of AN (Favaro et al., 2003). In addition, low birth weight, preterm birth, and other perinatal complications have been associated with higher AN risk (Cnattingius et al., 1999; Foley et al., 2001). A study by Favaro and colleagues suggests an interaction between perinatal factors and child abuse on the risk of developing AN (Favaro et al., 2010). Several studies have shown that early life stress exposure, either prenatal or postnatal, alters stress responsivity (Henry et al., 1994; Koehl et al., 1999; Lehmann and Feldon, 2000). One may argue that food restriction induces a physiological stressor, and consequently if perinatal stress increases stress responsivity, it may also increase vulnerability to AN. To specifically study the effects of the prenatal environment, maternal exposure to mild chronic variable stress during gestation has been employed (Koenig et al., 2005). Rodents exposed to prenatal stress (PNS) were shown to have altered stress reactivity (reviewed in (Boersma et al., 2014a)).
Based on the hypothesis that food restriction poses a physiological stressor, the way an individual copes with stress, the stress-coping style, may also determine an individual’s vulnerability to weight loss during ABA. Two distinct stress-coping styles can be identified-passive and proactive stress-coping styles. The characteristics of these stress-coping styles have been extensively described by Koolhaas and colleagues (Koolhaas et al., 2011). Briefly, the proactive stress-coping style is characterized by an active approach towards a stressor; individuals with this stress-coping style will attempt to modulate their environment to reduce stress exposure. Passive stress-coping, on the other hand, is characterized by an avoidance of the stressor. To what extent stress-coping style plays a role in the vulnerability to weight loss during ABA remains to be determined.
In the current experiment we investigate how PNS and stress-coping style affect weight loss during ABA. PNS might signal adverse conditions to come, and prime the offspring to adapt to potential aversive conditions more strongly in the future. Furthermore, hyperactivity during ABA may be viewed as an adaptation to starvation that paradoxically leads to more weight loss. Therefore, we hypothesize that individuals exposed to PNS might be more vulnerable to weight loss during ABA, as they show stronger (mal)adaptive response to starvation. In line with this, we hypothesize that passively coping individuals within the PNS population will show most severe weight loss during the ABA paradigm, since these individuals have heightened HPA-axis responsivity and are more behaviorally flexible. To evaluate the mechanisms underlying vulnerability for weight loss during ABA we will measure plasma hormone levels, gene expression, and DNA methylation (DNAm) of several genes involved in energy balance regulation and stress. To control for effects of running wheel (RW) activity and weight loss, RW control and body weight matched control groups were included in the experiment.
2. Methods
2.1. Animals
Thirty-two pregnant female Sprague-Dawley rats were obtained on gestational day (GD)2 from Charles River (Kingston NY). Rats were individually housed in standard tub cages with food and water available ad libitum. Rats were fed a standard rodent chow diet (Harlan 2018). Animals were kept in a climate controlled room with a 12:12 hr light: dark cycle (Lights on at CT0). All animal procedures were approved by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine.
2.2. Experimental set-up
All rats were kept under standard husbandry conditions until GD15. Sixteen dams remained under standard conditions thereafter. The other 16 dams were exposed to chronic variable stress from GD15 until GD21. Each stressor was applied during the light cycle unless noted otherwise (Supplemental Table 1 (S1)). Body weight and food intake of the dams were measured daily during gestation. The day a litter was found was designated postnatal day 0 (PND0). On PND1 litters were culled to 10 pups per litter (5 male and 5 female). Pups were weaned on PND21 and housed with 2 or 3 same-sex littermates until PND36 then individually housed for the remainder of the study.
2.3. Defensive burying test
Stress-coping style was determined on PND37-39 using the defensive burying test. The rats were tested in a polycarbonate cage (40×21×26cm) with a hole ~2cm in diameter through which an electric prod (length 8.2 cm, diameter 1cm) could be inserted. Clean corn cob bedding covered the test cage floor. The rats were habituated to the test cage for at least 10 minutes before testing began. At the start of the test the electrified probe was inserted and the latency to touch the prod was recorded. Upon touching the probe the rats received a mild shock (2.5 mA). Behavior of the rats was scored for 5 minutes after the first shock using Hindsight behavioral scoring software. The following behaviors were scored: grooming, immobility, exploration of the cage, exploration of the probe, and burying the probe with bedding. The percentage time spent burying the prod was used as the criterion to categorize the rats as proactive or passive coping. Rats that spent 10% or less time burying were characterized as passive coping, rats that spent 20% or more time burying were characterized as proactive coping. These selection criteria were based on observations of defensive bury behavior within several different laboratory rat strains and have been previously used and published (Boersma et al., 2011; Boersma et al., 2010).
2.4. Activity-Based Anorexia (ABA) paradigm
Proactive coping and passive coping rats from each group (control vs. PNS) were selected for further experiments. Half of the rats from each group were housed in standard tub cages, the other half of the rats from each group were housed in Nalgene running wheel cages and revolutions were recorded by Vitalview software (Minimitter, Bend, OR). Running wheels were unlocked on PND40. After RW habituation for 10 days (PND50) the RW rats were further divided into running wheel controls (RW) (n=4 per group) and Activity-Based Anorexia (ABA) rats (n=8 per group). The RW control group remained in cages with a running wheel and had ad libitum access to food throughout the experiment. For ABA groups, food access was limited to 1.5 hr at the beginning of the dark cycle (CT12 -CT13.5) on PND50. Sedentary housed rats were divided into sedentary (SED) controls (n=20 per group) and body weight matched (BWM) controls (n=4 per group). The SED controls had ad libitum access to food. BWM rats received limited amounts of chow in order to match their body weight to ABA rats. This resulted in 16 experimental groups, which are summarized in supplemental table 2 (S2).
All rats were sacrificed on PND56. Food was removed from all rats 5 hours prior to start of the dark cycle. Three hours later all rats were killed by rapid decapitation. Trunk blood was collected into EDTA-treated tubes, centrifuged and plasma was stored at −20 degrees °C for hormone analysis. Brains were rapidly removed, frozen on powdered dry ice, and stored at −80 degrees °C for mRNA expression and DNAm analysis.
To control for the effects of acute food restriction, a subset of the SED rats (n=6) was food restricted for 24hrs prior to sacrifice, while an additional subset was of SED rats (n=6) was ad lib. fed prior to sacrificed. Brains were collected according to the methods described above.
2.5. Brain processing
The prefrontal cortex (PFC), nucleus accumbens (NAC), and ventral tegmental area (VTA), paraventricular nucleus (PVN), arcuate nucleus (ARC), and lateral nucleus of the hypothalamus (LH) were microdissected from 200–600 µm thick frozen coronal sections using a Harris uni-core tissue puncher (ID 0.75 mm) (Ted Pella, Inc., Redding, CA, USA) based on the coordinates for adult rat brain (Paxinos, 1982). Tissue from the left side of each brain was used for mRNA expression analysis and stored in Qiazol reagent (Qiagen, Valencia, CA), the tissue from the right side was used for DNAm analysis and stored in Tissue and Cell Lysis Solution (Epicentre Biotechnologies, Madison, WI).
2.6. mRNA expression
The RNeasy Lipid Tissue Mini Kit with the Qiazol reagent (Qiagen, Valencia, CA) was used to isolate total RNA from the tissue punches. mRNA expression of Drd1, Drd2, Hrctr1, Npy, Agrp, Pomc, Hcrt, Slc6a3, Th, Crh and Actb was determined in relevant brain regions by RT-PCR. 200ng of RNA was used to generate cDNA for subsequent quantitative real-time PCR with a QuantiTect Reverse Transcription Kit (Qiagen, Valencia, CA). All reactions were carried out in triplicate using 1X Taqman master mix (Applied Biosystems, Foster City, CA), cDNA template, and 1X Taqman probes for each gene (Drd1, Drd2, Hrctr1, Npy, Agrp, Pomc, Hcrt, Slc6a3, Th, Crh and Actb) (Life technologies, Grand Island, NY) in a total volume of 20µL. Real-time reactions were performed with standard PCR conditions (50°C for 2 min; 95°C for 10 min; and 95°C for 15 sec and 60°C for 1 min for 40 cycles) on an Applied Biosystems 7900HT Fast Real-Time PCR System. Each set of triplicates was checked to ensure that the threshold cycle (Ct) values were all within 1 Ct of each other. Negative RT samples were used to control for possible contamination of gDNA. To determine relative expression values, the −ΔΔCt method (Applied Biosystems, Foster City, CA) was used, where triplicate Ct values for each tissue sample were averaged and subtracted from those derived from the housekeeping gene Betaactin (Actb). The average Ct difference for the control group (SED-CON-PRO rats) was subtracted from those of the test samples, and the resulting −ΔΔCt values were raised to a power of two to determine normalized relative expression.
For the comparison of 24hr-fasted, ad lib fed control, and ABA rats, 4 ABA samples with the median mRNA expression from each of the experimental groups were selected for each gene. These samples were re-run during the analysis of the fasted vs fed samples to allow for direct comparison. Fold changes for these samples were calculated by above described methods using the average Ct difference of the ad lib. fed control rats.
2.7. DNA methylation
Genomic DNA (gDNA) from rat PVN and ARC tissue punches was isolated using the Masterpure DNA Purification Kit (Epicentre Biotechnologies, Madison, WI) according to manufacturer’s protocol. The percentage of DNAm of the Pomc promoter region, the Crh promoter region, the Hcrt promoter, and the Hcrt CpG island region were determined using bisulfite pyrosequencing. The concentration of the gDNA was determined using a NanoDrop 1000 Spectrophotometer (Thermo Scientific, Rockford, IL). Bisulfite conversion of 500ng gDNA was performed according to the manufacturer's protocol (EZ DNA Methylation- Gold Kit; Zymo Research, Irvine, CA). For each initial PCR reaction 3.5 µL Bisulfite-treated DNA (25ng) was used. An additional nested PCR was performed with 4 µL of the previous PCR reaction and one biotinylated primer (other primer being unmodified). Amplification for both PCR steps consisted of 40 cycles (95°C for 1 min, 53°C for 2 minutes and 30s, 72°C for 1 min).
To assess DNAm of the Pomc promoter region, two sets of primers were designed to amplify a 378-bp region of rat Pomc: outside forward 5’-GATGGAGATAGATTGTATAAATTTATTTGT-3’; outside reverse 5’-CCTCCCRTTTAATCCCTATCACTCT-3’; nested forward 5’-AAAGGTAGTTTGTTTTGGGTTGTTATGA-3’; nested reverse 5’-TCTCTCTTCTTTTATACCTACAA-3’. To assess DNAm of the Crh promoter region, two sets of primers were designed to amplify a 251-bp region of rat Crh: outside forward 5’-TGGTTTGTATTTGGTTTATTATAGTAAGAG-3’; outside reverse 5’-CAACTCAAACAACACAAAATTAATAAC-3’; nested forward 5’-TGTTAATGGATAAGTTATAAGAAGTT-3’; nested reverse 5’-TCTCAAAATACCTCCTACAAATTTTCTT-3’.
To assesses DNAm of the Orexin (Hcrt) promoter region, two sets of primers were designed to amplify a 430-bp region of rat Hcrt: outside forward 5’-TAGGGGTTGAAGTAGTAGTTTGAGAA-3’; Outside reverse: 5’-ACTAATCTACACCAAAAACTTCATAACA-3’; nested forward 5’-GGATATTTAGTTGGAGATAATG-3’; nested reverse 5’-ATCACATCCTAAACACACAACTATCCCTA-3’.
To assesses DNAm of the Orexin (Hcrt) CpG island region, two sets of primers were designed to amplify a 441-bp region of rat Hcrt: outside forward 5’-GAGGAGAGGGGAAAGTTAGGAT-3’; Outside reverse: 5’-TACACCTTATCTCTATCCCTTTAAATTC-3’; nested forward 5’-GAATTTGATATAAAGATTAGTTATATT-3’; nested reverse 5’-TCAAAACCAACTAACTCTATAAAATAAAATCC-3’.
Pyro Gold reagents were used to prepare samples for pyrosequencing according to manufacturer's instructions. For each sample (in duplicate), biotinylated PCR product was mixed with streptavidin coated sepharose beads (GE Healthcare, Waukesha, WI), binding buffer, and Milli-Q water, and shaken at room temperature. A vacuum prep tool was used to isolate the sepharose bead-bound single-stranded PCR products. The attached DNAs were released into a PSQ HS 96-well plate containing pyrosequencing primers in annealing buffer. Pyrosequencing reactions were performed by PyroMark MD System (Qiagen, Valencia, CA). CpG methylation quantification was performed with Pyro Q-CpGt 1.0.9 software (Qiagen, Valencia, CA). An internal quality-control step was employed to disqualify any assays that contained unconverted DNA.
Percentage of methylation at each CpG as determined by pyrosequencing was compared between gDNA from control and PNS offspring. Specific CpG sites that were assayed are summarized in Supplemental Table 3 (S3).
2.9. Plasma analysis
Plasma corticosterone concentration was determined by commercially available radioimmunoassay kit for corticosterone (MP Biomedicals, Solon, OH, inter-assay variability: 6.5–7.1%, intra-assay variability: 4.4 –10.3%). Plasma leptin was analyzed using a commercially available ELISA kit (Millipore, Billerca, MA, inter-assay variability: 2.95–3.93%, intra-assay variability: 1.88–2.49%). Plasma insulin was determined by radioimmunoassay kit for insulin (Millipore, Billerca, MA: inter-assay variability: 2.2–3.7%, intra-assay variability 8.9–9.4%). Plasma ghrelin was analyzed using a commercially available ghrelin ELISA kit (Millipore, Billerca, MA, inter-assay variability: 1.99–3.24%, intra-assay variability: 1.11–1.67%).
2.10. Statistical analysis
Data are presented as mean ± standard error of the mean. Statistical analyses were performed using Statistica 7 (Systat, Tulsa, OK) software. Group differences in body weight, food intake and running wheel activity were assessed with repeated measures ANOVA with prenatal conditions (STRESS), stress-coping style (COPING) and activity based anorexia exposure (GROUP) as between subjects factors. Group differences in mRNA expression and plasma hormone levels were assessed with multivariate ANOVA with prenatal conditions (STRESS), stress-coping style (COPING), and activity based anorexia exposure (GROUP) as between subjects factors. Differences between specific experimental groups and time-points were assessed by Bonferonni post hoc analysis or planned comparison analysis where relevant. For all statistical analysis a confidence interval of 95% was used.
3. Results
3.1. Dams
There were no significant differences between control and PNS dams in body weight or food intake during gestation or lactation. There were no differences in litter weight, litter size or male:female ratio between control and PNS litters (S4).
3.2. Defensive burying behavior
A defensive burying test was performed to characterize the stress coping style of the rats. There were no significant differences between female control and PNS offspring in exploratory, burying, immobile or grooming behavior or the latency to touch the shock probe during the defensive burying test (data not shown). There were no significant differences in the percentage of individuals categorized as passive coping, proactive coping, or between-cut-off criteria in the control and PNS offspring populations (CON: passive 47%, proactive 43% and between-cut-off 10%; PNS: passive 50%, proactive 43% and between-cut-off 7%).
3.3. Body weight, food intake, water intake and running wheel activity
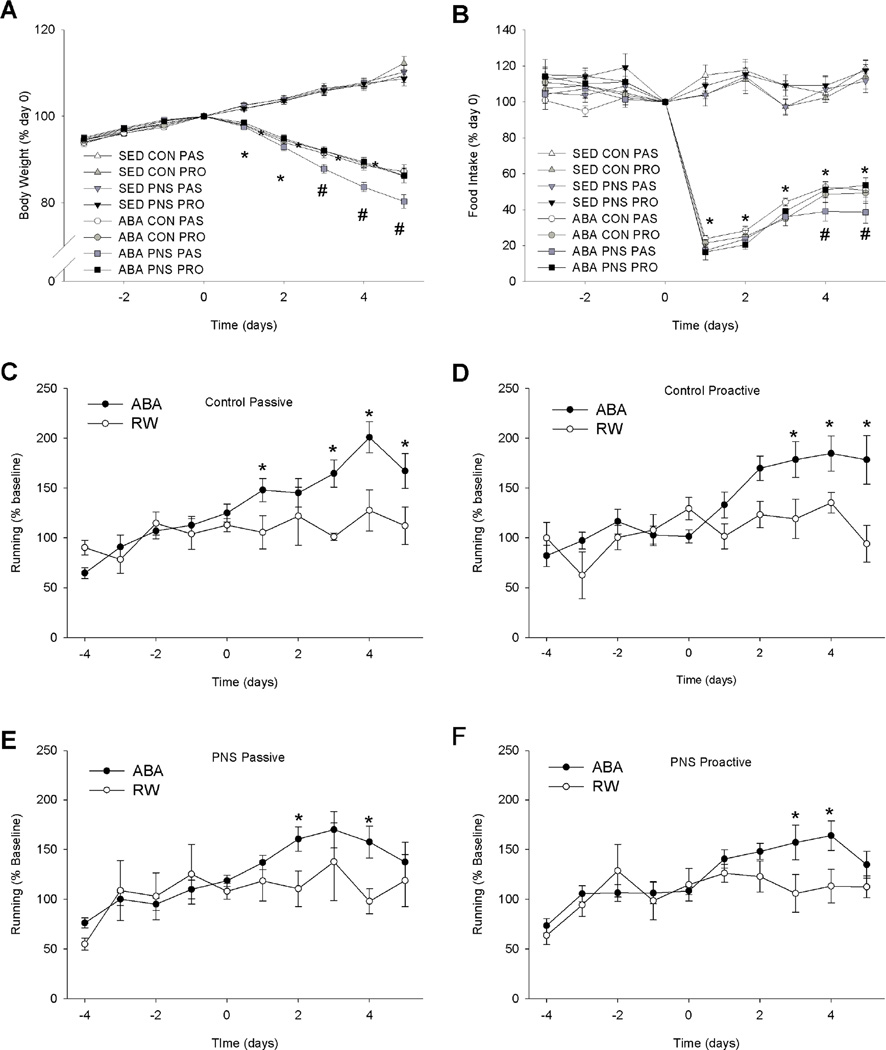
There were no significant differences in body weight or food intake between the experimental groups prior to ABA. The body weights of the rats in the ABA group and the BWM group were significantly lower than those in the SED and RW groups (S8) (F(3,77)=14.06, p<0.01). Furthermore, the ABA-PNS-PAS rats lost significantly more weight than ABA-PNSPRO, ABA-CON-PRO and ABA-CON-PAS rats on days 3, 4, and 5 of food restriction (Fig. 1A) (F(27,702)=1.80, p<0.01). Within the SED, RW and BWM groups there were no significant differences in body weight between the experimental groups.
Figure 1. Body weight, food intake, and running wheel activity of SED and ABA rats.
A: Body weight of control and ABA as expressed as a percentage of baseline body weight. * indicates a significant difference between ABA and control rats. # indicates a significant difference between passive-coping PNS ABA rats and all other groups p<0.05. B: Food intake of control and ABA rats as expressed as a percentage of baseline food intake. * indicates a significant difference between ABA and control rats. # indicates a significant difference between passive-coping PNS ABA rats and all other groups p<0.05. C: Running wheel activity in control passive ABA and RW rats. *indicates a significant difference between RW and ABA rats of the same group p<0.05. D: Running wheel activity in control proactive ABA and RW rats. *indicates a significant difference between RW and ABA rats of the same group p<0.05. E: Running wheel activity in prenatal stress passive ABA and RW rats. *indicates a significant difference between RW and ABA rats of the same group p<0.05. F: Running wheel activity in prenatal stress passive ABA and RW rats. *indicates a significant difference between RW and ABA rats of the same group p<0.05. There were no significant difference in running activity at t=0 (Proactive control ABA = 7577 ± 500, Proactive Control RW = 7801 ± 1656, Passive control ABA = 8547 ± 1159, Passive control RW = 10283 ± 1034, Proactive PNS ABA = 6928 ± 1166, Proactive PNS RW = 9978 ± 1142, Passive PNS ABA = 9578 ± 1032, and Passive PNS RW = 8153 ± 1121 revolutions/day).
Food intake of the ABA group was significantly lower than that of SED and RW groups (F3,77)=170.7, p<0.01). In order to achieve target body weight in the BWM group that was equivalent to that of ABA rats, food amount was restricted beyond that consumed by the ABA rats. ABA-PNS-PAS rats ate significantly less on days 4 and 5 than ABA-PNS-PRO, ABA-CONPRO and ABA-CON-PAS rats (Fig 1B) (F(27,702)=2.23, p<0.01). Within the SED, RW and BWM groups there were no differences in food intake between the experimental groups. Water intake was not affected by ABA, PNS or stress coping style (data not shown).
Running wheel activity was monitored as a measure of hyperactivity. At baseline there were no significant differences in RW activity among the groups. All rats exposed to ABA increased their RW activity during food restriction (Fig. 1C–F) (F(9,351)=5.68, p<0.001). Activity levels were however not correlated to body weight loss as within the ABA group there were no differences between the CON-PAS, CON-PRO, PNS-PAS and PNS-PRO groups (Fig. 1C–F).
3.4. Plasma hormone levels
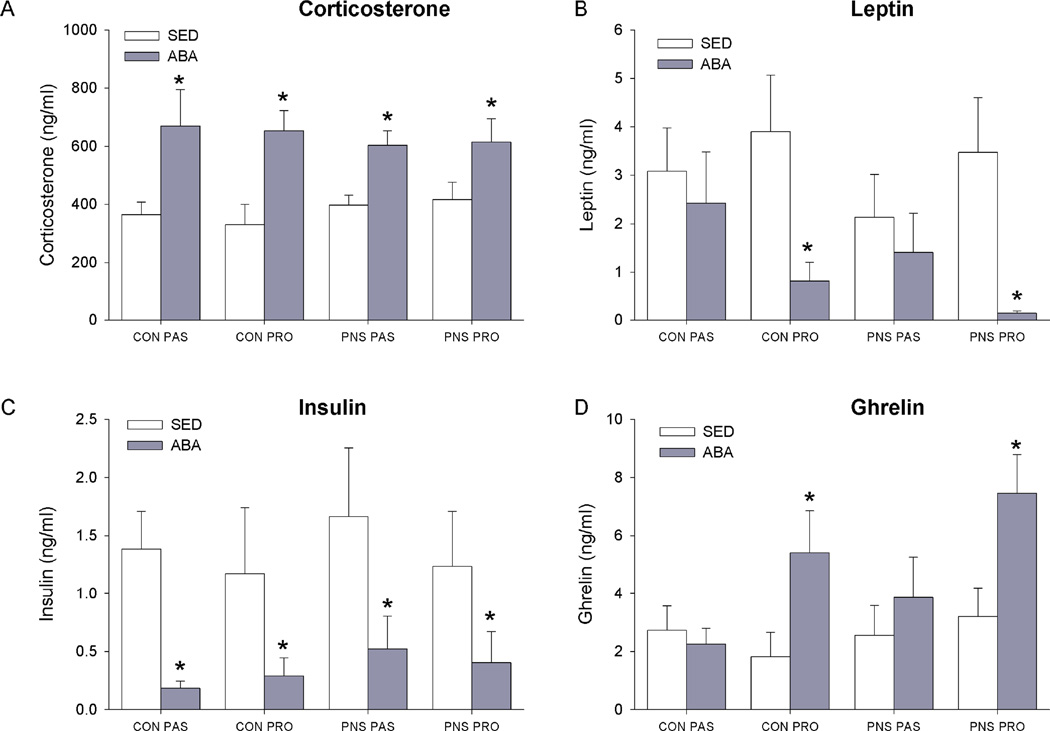
As a measure of HPA axis tone, corticosterone levels were measured at sacrifice. ABA, BWM and RW rats had higher corticosterone levels than SED rats (F(3,77)=18.55, p<0.01). Neither PNS nor stress-coping style affected plasma corticosterone levels (Fig. 2A, S5).
Figure 2. Plasma hormone levels in control and ABA rats.
A: Plasma corticosterone levels. B: Plasma leptin levels. C: plasma insulin levels. D: plasma ghrelin levels. * indicates a significant difference between SED and ABA rats p<0.05.
As measures of nutrient status plasma insulin, leptin, and ghrelin levels were assessed Plasma Insulin levels were significantly lower in the ABA, and BWM rats compared to control rats (F(3,77)=16.325, p<0.01) (Fig. 2C), S5). Plasma leptin levels were significantly lower in the ABA and BWM rats compared to the SED and RW control groups(F(3,77)= 10.40, p<0.01) (Fig. 2B, S5). Within proactive rats, ABA and BWM had significantly lower plasma leptin levels compared to RW and SED rats (F(3,77) = 4.06 p<0.05). In contrast, in the passive rats no difference between SED, RW, ABA, and BWM treatment condition were observed (S4).
There was a group effect in plasma ghrelin levels. Plasma ghrelin was significantly higher in the ABA and BWM groups compared to the SED and RW groups (F(3,77)=5.90, p<0.01)(Fig.2D, S5). Within the proactive coping rats, ABA and BWM rats had significantly higher ghrelin levels than SED rats (p<0.05). However, within the passive coping rats, no difference between SED and ABA or BWM rats was observed (F(3,77)=3.15, p<0.05).
3.5. Brain gene expression
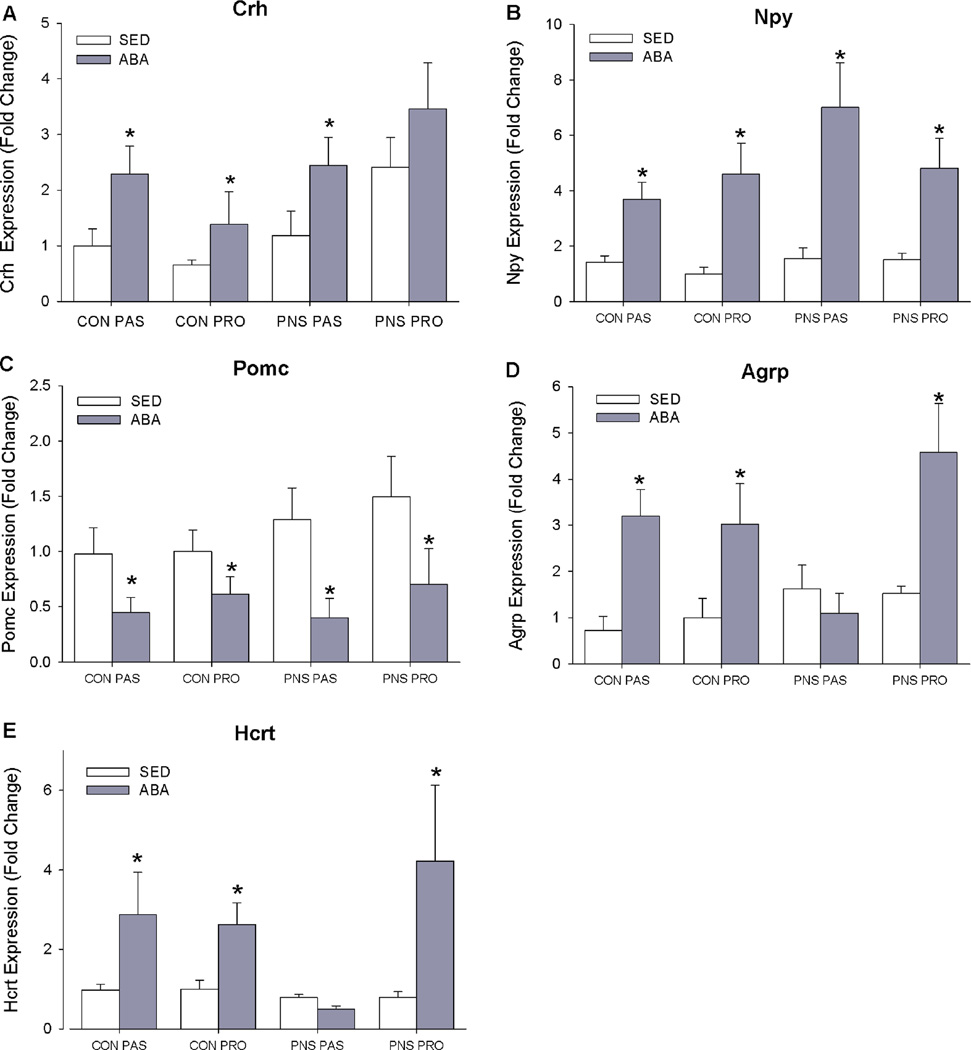
As a measure of HPA-axis activation, corticotrophin releasing hormone (Crh) mRNA expression was assessed. mRNA expression of Crh was significantly higher in the ABA, BWM and RW rats compared to control rats (F(3,77)= 4.20, p<0.05) (Fig. 3A). Within the SED rats, PNS-PAS rats had higher Crh levels than all other groups (p<0.05).
Figure 3. mRNA expression energy balance related peptides in the, paraventricular nucleus of the hypothalamus (PVN), arcuate nucleus of the hypothalamus (ARC) and lateral hypothalamus (LH) in control and ABA rats.
A: Corticotrophin releasing hormone (Crh) expression in PVN. B: Neuropeptide Y (Npy) expression in ARC. C: Proopiomelanocortin (Pomc) expression in ARC. D: Agouti-related peptide (Agrp) expression in ARC. E: Orexin (Hcrt) expression in LH. * indicates a significant difference between SED and ABA rats p<0.05.
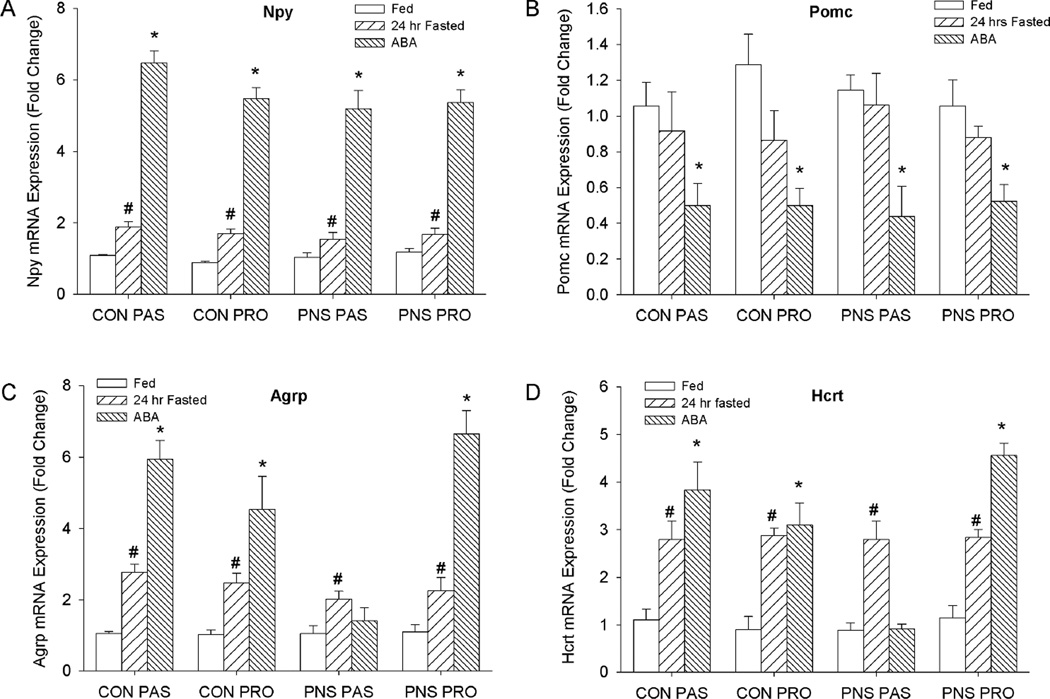
Food restriction or starvation typically causes expression of Npy, Agrp, and Orexin (Hcrt) to increase and Pomc to decrease. ARC Npy was significantly higher in ABA and BWM rats compared to SED and RW rats, and expression of ARC Pomc was significantly lower in ABA and BWM rats compared to SED and RW controls (Npy: (F(3,77)=9.35, p<0.01) (Pomc: (F(3,77)=5.92, p<0.01) (Fig. 3B, 3C). In contrast, ARC expression of agouti-related protein (Agrp) was significantly increased in ABA and BWM conditions in CON-PRO, PNS-CON and CON-PAS rats (p<0.01), whereas within the PNS-PAS rats no significant differences between rats in SED, RW, ABA and RW groups were observed (F(3,77)=3.05, p<0.05) (Fig. 3D). Consistent with Agrp, within the ABA condition the PNS-PAS rats had significantly lower Hcrt expression than CON-PRO, PNS-PR0 and CON-PAS rats (p<0.05)(Figure 4E). Hcrt expression was significantly increased in the ABA group compared to SED control in CON-PRO, PNS-PRO and CON-PAS rats, however, there were no differences in Hcrt expression between SED and ABA PNS-PAS rats(F(3,77)=4.44, p<0.01)(Fig. 4E). To control for the effects of acute food restriction on hypothalamic gene expression we compared rats food restricted for 24hr to ad lib fed controls. Expression of both Npy and Agrp in ARC was increased in 24hr-restricted rats compared to ad lib fed controls in all experimental groups (Agrp: F(1,72) = 13.18 p<0.01; Npy: F(1,72) = 7.17 p<0.05) (Fig 4A. Fig.4C). 24hr food restriction did not affect Pomc expression in any of the experimental groups (Fig. 4B). In all experimental groups LH expression of Hcrt was increased in 24hr-resticted rats compared to ad lib fed controls (Hcrt: F(1,72) = 9.14 p<0.05) (Fig 4D).
Figure 4. mRNA expression energy balance related peptides in the, paraventricular nucleus of the hypothalamus (PVN), arcuate nucleus of the hypothalamus (ARC) and lateral hypothalamus (LH) in ad lib. fed and 24hr food restricted rats.
A: Corticotrophin releasing hormone (Crh) expression in PVN. B: Neuropeptide Y (Npy) expression in ARC. C: Proopiomelanocortin (Pomc) expression in ARC. D: Agouti-related peptide (Agrp) expression in ARC. E: Orexin (Hcrt) expression in LH. * indicates a significant difference between ad lib. fed and 24hr food restricted rats p<0.05.
The behavior seen during ABA is reminiscent of behavior shown in individuals with disturbed reward seeking behavior like addiction. Therefore we assessed expression of dopamine related genes which are associated with reward. In the LH and Nucleus Accumbens (Nac), significantly lower Drd1 expression in ABA and BWM rats compared to SED rats was shown (LH: F(3,77)=3.70 p<0.05, Nac: F(3, 77)= 4.09, p<0.01;). Furthermore, Drd2 in the Nac was significantly lower in ABA or BWM rats compared to SED rats (F(3, 77)= 8.89, p<0.01). Finally, in the ventral tegmental area there were no significant differences between the experimental groups in expression of the dopamine transporter (Slc6a3), or Tyrosine hydroxylase (Th) an enzyme involved in dopamine synthesis (S7).
3.6. DNA methylation
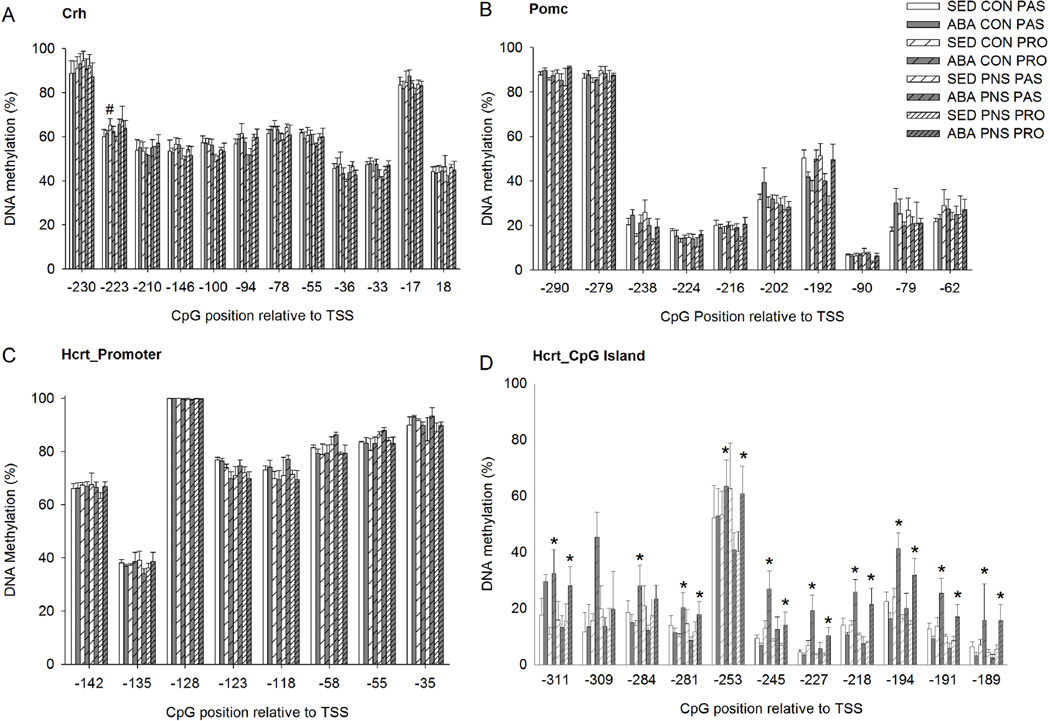
To understand the mechanism underlying altered gene expression after ABA we assessed DNA methylation (DNAm) in the hypothalamus. DNAm of Crh was affected by both stress coping style as well as PNS at specific positions. At CpG position TSS-223, there were overall effects of coping and PNS. PRO rats had significantly higher DNAm compared to PAS rats (p<0.05). And PNS rats had significantly higher DNAm at Crh CpG position TSS-223 than CON rats (p<0.05). Furthermore, there was a treatment-coping style interaction; SED-PRO rats had higher DNAm than SED-PAS rats (p<0.05), whereas no difference between ABA-PRO and ABA-PAS rats were observed (Fig. 5A). There were no significant differences between the experimental groups in the percentage of DNAm of the promoter region of the Pomc gene in the ARC (Fig. 5B). We analyzed DNAm in the LH for two different regions within the Orexin gene. No effects of ABA, PNS or stress coping were found on DNAm of the promoter region of the Hcrt gene (Fig. 5C). However the within PRO rats an increase in DNAm was observed in ABA compared to SED rats at several CpG positions within the CpG island region of the Hcrt gene (p<0.05). In contrast, within PAS rats, no differences in DNA methylation between ABA and SED groups were observed (Fig. 5D) (summarized in S9).
Figure 5. DNA methylation in the Hypothalamus.
A: DNA methylation of the Crh promoter region in the paraventricular nucleus. # indicates a significant difference between proactive SED and Passive SED rats. B: DNA methylation of the Pomc promoter region in the arcuate nucleus. C: DNA methylation of the Hcrt promoter region in the lateral hypothalamus. D: DNA methylation of the Hcrt CpG-island region in the lateral hypothalamus. * indicates a significant difference between proactive SED and proactive ABA rats.
4. Discussion
The aim of this study was to test whether prenatal stress biases rats to lose more weight or lose weight faster in the activity-based anorexia (ABA) paradigm, a rat model of anorexia nervosa (AN). We further hypothesized that stress-coping style behavior would result in differential weight loss in the ABA paradigm. Our data show that PNS exposure facilitated weight loss in the ABA paradigm; however, this was only the case in rats characterized by a passive stress-coping style. An interesting finding was that ABA altered hypothalamic gene expression differentially in the context of environment stress (PNS) and stress coping strategy. In most rats exposed to ABA we observed up-regulation of genes known to stimulate food intake, such as Hcrt, Npy and Agrp, and down-regulation of anorexigenic genes, such as Pomc. However, our data suggest insufficient up-regulation of Agrp and orexin (Hcrt) in the passive coping PNS rats during ABA that may have facilitated weight loss and hypophagia.
The passive coping PNS rats who were body weight matched (BWM control group) to the ABA rats also lacked a significant up-regulation of Agrp compared to SED passive PNS rats, indicating that the phenomenon may be generalized to a condition of chronic food restriction and/or low body weight. Passive PNS rats did show an increase in Agrp levels after an acute 24hr food restriction, suggesting that their hypothalamic response to acute food restriction is not affected. Exercise alone (RW control group) did not affect Agrp expression in any of the groups. The potential of Agrp to mediate vulnerability for weight loss during ABA has previously been suggested. Hillebrand and colleagues showed that administration of Agrp during ABA increased food intake and attenuated body weight loss in rats (Hillebrand et al., 2006; Kas et al., 2003). In our study, the rats were all sacrificed after ABA, which has the limitation that we cannot conclude whether the lack of increase in Agrp mRNA expression was causal to or resulted from weight loss. The expression of hypothalamic neuropeptides is dynamic during the development of ABA, and it is therefore possible that the expression profile of the hypothalamic neuropeptides in vulnerable individuals may be different during the acquisition of the phenotype than in a state where severe body weight loss had already occurred. Furthermore, we did not measure protein levels of these peptide and changes in mRNA expression may not directly translate to changes in protein levels.
Further evidence in humans support a role for Agrp in AN. The Ala67Thr polymorphism in the Agrp gene has been associated with greater risk of developing AN (Vink et al., 2001). Functional analysis of this polymorphism in a cell line showed that this polymorphism did not lead to dysfunction of Agrp’s ability to inhibit alpha-MSH induced activation of the MC4 receptor (de Rijke et al., 2005b), suggesting that this polymorphism may indirectly affect food intake via other actions of Agrp signaling in AN patients. Our finding of altered Agrp expression in rats vulnerable to weight loss in the ABA paradigm may allow for mechanistic studies to determine the exact role Agrp has in AN.
In addition to a lack of up-regulation of Agrp following ABA, the passive PNS rats were also characterized by a lack of increased expression of orexin in the LH. Prior studies report conflicting results of ABA on orexin expression, both increased and not altered Orexin expression has been reported (Scheurink et al., 2010) (de Rijke et al., 2005a). Our current data suggest that whether or not LH orexin expression increases during ABA may depend on the stress-coping style of the rats used, which may explain some of the variable results reported in the literature.
Given the similarities in expression profiles of Agrp and orexin in response to starvation in the ABA-PNS-PAS rats one may speculate whether alteration in the expression of these two genes may have overlapping origins. Orexin 1 receptors are found on approximately 90% of Npy/Agrp expressing neurons (Backberg et al., 2002), suggesting that alterations in orexin activity may impact Agrp levels. In addition, melanocortin (MC) receptors are found in the LH, and although MC-4 receptors are not expressed on orexin neurons (Cui et al., 2012), administration of MTII, an MC-3/4-receptor agonist in the LH has been shown to increase c-fos activity in orexin expressing neurons (Cui et al., 2012), suggesting either a direct activation of MC-3 or MC-4 on these neurons or an indirect activation through MC-receptors expressed on other LH neurons leading to modulation of orexin activity by the melanocortin pathway. Taken together these data suggest that the Agrp and orexin systems may closely interact and that alterations of expression of either of both of these genes may underlie the ABA-vulnerable phenotype of the ABA-PNS-PAS rat.
There were no group differences in Pomc or Npy expression levels. Agrp and Npy are co-localized in arcuate nucleus neurons and both signal appetite and therefore changes in Npy expression in response to fasting are often concomitant with changes in Agrp expression(Hahn et al., 1998). Although in response to fasting, both Npy and Agrp expression are typically both increased, in response to acute stress differential effects on Npy and Agrp expression have been reported. Kas and colleagues showed an increase in Npy expression 2 hrs following acute foot-shock stress, whereas Agrp expression was decreased (Kas et al., 2005). Given previously reported differences in stress responsivity between control and PNS rats (Weinstock, 2007) as well as between passive and proactive rats (Koolhaas et al., 1999), it is reasonable that exposure to ABA may have induced a stronger stress response in the PNS-PAS rats and thereby reduced fasting induced Agrp over-expression in this group. It is however unlikely that increased corticosterone levels drive ABA vulnerability in the ABA PNS-PAS group, as corticosterone levels were increased to a similar degree in all ABA rats.
ABA has been shown to increase circulating corticosterone levels, at least at the end of the light phase prior to food access. At this time point Crh expression in the PVN was also increased in ABA rats, suggesting increased activation of the HPA-axis prior to food access. Whether ABA increases HPA-axis activity at other time period remains to be studied. However, changes in HPA-axis activity do not seem to predict vulnerability for weight loss during ABA, as no correlation between weight loss and either corticosterone or Crh expression levels was observed. We did show increased Crh expression in PNS-PAS rats, however, this elevation was however not related to ABA exposure.
A potential mechanism underlying altered gene expression is DNA methylation (DNAm). DNAm modulates gene transcription without altering the DNA sequence itself (Auclair and Weber, 2012) and prenatal stress has been shown to alter DNAm (Boersma et al., 2014b; Palacios-Garcia et al., 2015; Paternain et al., 2012; Schraut et al., 2014). We considered whether PNS exposure altered DNAm of the Agrp gene. However, the Agrp gene only has one CpG position making it an unlikely target for DNAm. Other epigenetic alterations, like histone modifications could play a role, but investigation of these epigenetic modifications is more complex and will require additional studies.
Investigation of DNAm of the orexin (Hcrt) gene in the LH revealed no differences between the experimental groups in DNAm at CpGs within the promoter region of the gene. However, several CpGs within a CpG island of the gene were differentially methylated in ABA compared to SED rats. Interestingly, these differences were selectively observed within proactive coping rats, whereas DNAm in passive rats was not altered by ABA. Increased DNAm is typically associated with decreases in mRNA expression. However, this is dependent on the nature of the factor binding at or around the CpG of interest. There is limited information on transcription factor binding within the Hcrt gene in rats. The Hcrt gene is highly conserved between human and rat, and thus we evaluated transcription factor binding within the human HCRT gene using ENCODE. Although several transcription factors bind at the region homologous to the CpG island in rat, one of the most interesting factors binding is transcription repressor CCCTC-binding factor (CTCF). The role of CTCF within the HCRT gene has not been studied, but this gene has been shown to repress insulin-like growth factor 2 (IGF2) gene expression (reviewed in (Ohlsson et al., 2001). Furthermore, this transcription factor localizes with cohesin across the genome and affects higher-order chromatin structure (Lee and Iyer, 2012). Based on this, one may speculate that increased DNAm at the CpG island induced by ABA in proactive rats may have inhibited binding of CTCF thereby increasing mRNA expression of the orexin gene.
Investigation of DNAm of the Pomc promoter region did not reveal differences associated with ABA, PNS exposure, or stress coping style. This was unexpected since clear ABA effects on gene expression were shown, and previous studies revealed differences in this region in response to dietary modulations (Marco et al., 2014). Similarly differences in Crh expression were not related to changes in DNAm of the Crh promoter region. It is possible that DNAm at other sites within in these genes may modulate gene expression. Alternatively, gene expression may have been altered through other mechanisms like histone modifications.
As expected, we observed higher levels of corticosterone and decreased insulin levels at sacrifice in all ABA rats. Furthermore, we also showed decreased leptin and increased ghrelin levels in ABA-PRO rats, consistent with their significant body weight loss. In contrast, passive coping ABA rats did not have altered leptin or ghrelin levels, suggesting impaired response to starvation in the passive coping rats. Nevertheless, these impaired leptin and ghrelin response to starvation do not lead to disrupted eating behavior, unless the rat was exposed to prenatal stress.
Since hyperactivity and subsequent hypophagia during ABA have been hypothesized to be related to reward pathways (Scheurink et al., 2010), we investigated differences in dopamine related gene expression in this model. Overall, we showed that ABA is associated with decreased mRNA expression of both Drd1 and Drd2 receptors in the nucleus accumbens. However, the decrease in dopamine receptor expression was not correlated to the degree of body weight loss during ABA. Furthermore, no differences in Th or Slc6a3 were observed in the VTA. This may suggest that exposure to ABA does not alter dopaminergic output from the VTA to its target areas. A limitation of this study is that only mRNA expression was measured. It is possible that weight loss vulnerability is associated with altered sensitivity of the dopamine receptors during ABA exposure. Food restriction for example has been shown to increase the behavioral response to a Drd2 agonist, suggesting increased receptor sensitivity under these conditions (Collins et al., 2008). Further research is needed to assess whether vulnerability to weight loss during ABA is associated with altered dopamine receptor sensitivity.
Taken together our data suggest that insufficient up-regulation of the orexin (Hcrt) and Agrp genes during ABA may have contributed to greater suppression of food intake and greater body weight loss specifically in ABA-PNS-PAS rats. Overall these data suggest that we can identify a vulnerable subpopulation with a characteristic behavioral and physiological profile. This model therefore has the potential to be used for the discovery of biomarkers of weight loss vulnerability and may help gain a better understanding of brain pathways involved in weight loss during AN. As such further studies using this model may facilitate early identification of susceptible individuals and the development of better targeted treatment options for AN patients.
Supplementary Material
Highlights.
Rats exposed to prenatal stress and characterized by passive stress coping are more vulnerable for weight loss during ABA
Passive coping rats do not increase leptin or ghrelin in response to ABA
Passive coping PNS rats lack up-regulation of Agrp and Orexin expression after ABA
The differences in mRNA expression during ABA may be mediate by DNA methylation differences.
Acknowledgments
We thank Alexander Moghadam, Leonard Marque, Patricia Timi, and Pique Choi for their technical assistance in these studies. The studies in this manuscript were supported by grants from NIH (MH090585 to K.L. Tamashiro) and the Klarman Family foundation (to T.H.Moran).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
Data in this manuscript have been presented at the annual meeting of the Society for the Study Ingestive Behavior 2015, Denver, CO, USA.
Contributor Information
Nu-Chu Liang, Email: jhuliang@gmail.com.
Richard S. Lee, Email: richardlee@jhmi.edu.
Jennifer D. Albertz, Email: jalbertz66@gmail.com.
Anneke Kastelein, Email: a.kastelein.1@student.rug.nl.
Laura A. Moody, Email: lmoody2@illinois.edu.
Shivani Aryal, Email: sxaryal@wesleyancollege.edu.
Timothy H. Moran, Email: tmoran@jhmi.edu.
Kellie L. Tamashiro, Email: ktamashiro@jhmi.edu.
References
- Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Archives of general psychiatry. 2011;68:724–731. doi: 10.1001/archgenpsychiatry.2011.74. [DOI] [PubMed] [Google Scholar]
- Association AP. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Auclair G, Weber M. Mechanisms of DNA methylation and demethylation in mammals. Biochimie. 2012;94:2202–2211. doi: 10.1016/j.biochi.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Backberg M, Hervieu G, Wilson S, Meister B. Orexin receptor-1 (OX-R1) immunoreactivity in chemically identified neurons of the hypothalamus: focus on orexin targets involved in control of food and water intake. Eur J Neurosci. 2002;15:315–328. doi: 10.1046/j.0953-816x.2001.01859.x. [DOI] [PubMed] [Google Scholar]
- Berkman ND, Lohr KN, Bulik CM. Outcomes of eating disorders: a systematic review of the literature. The International journal of eating disorders. 2007;40:293–309. doi: 10.1002/eat.20369. [DOI] [PubMed] [Google Scholar]
- Boersma GJ, Bale TL, Casanello P, Lara HE, Lucion AB, Suchecki D, Tamashiro KL. Long-term impact of early life events on physiology and behaviour. Journal of neuroendocrinology. 2014a doi: 10.1111/jne.12153. [DOI] [PubMed] [Google Scholar]
- Boersma GJ, Benthem L, van Dijk G, Scheurink AJW. Individual variation in the (patho)physiology of energy balance. Physiol Behav. 2011;103:89–97. doi: 10.1016/j.physbeh.2010.12.026. [DOI] [PubMed] [Google Scholar]
- Boersma GJ, Benthem L, Van Dijk G, Steimer TJ, Scheurink AJW. Coping style predicts the (in)sensitivity for developing hyperinsulinemia on a high fat diet in rats. Physiol Behav. 2010;100:401–407. doi: 10.1016/j.physbeh.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Boersma GJ, Lee RS, Cordner ZA, Ewald ER, Purcell RH, Moghadam AA, Tamashiro KL. Prenatal stress decreases expression and increases methylation of Bdnf exon IV in rats. Epigenetics : official journal of the DNA Methylation Society. 2014b;9:437–447. doi: 10.4161/epi.27558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TK, Weiss AR, Berrettini WH. The genetics of anorexia nervosa. Clinical pharmacology and therapeutics. 2012;91:181–188. doi: 10.1038/clpt.2011.253. [DOI] [PubMed] [Google Scholar]
- Cnattingius S, Hultman CM, Dahl M, Sparen P. Very preterm birth, birth trauma, and the risk of anorexia nervosa among girls. Archives of general psychiatry. 1999;56:634–638. doi: 10.1001/archpsyc.56.7.634. [DOI] [PubMed] [Google Scholar]
- Collins GT, Calinski DM, Newman AH, Grundt P, Woods JH. Food restriction alters N'-propyl-4,5,6,7-tetrahydrobenzothiazole-2,6-diamine dihydrochloride (pramipexole)-induced yawning, hypothermia, and locomotor activity in rats: evidence for sensitization of dopamine D2 receptor-mediated effects. The Journal of pharmacology and experimental therapeutics. 2008;325:691–697. doi: 10.1124/jpet.107.133181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Sohn JW, Gautron L, Funahashi H, Williams KW, Elmquist JK, Lutter M. Neuroanatomy of melanocortin-4 receptor pathway in the lateral hypothalamic area. The Journal of comparative neurology. 2012;520:4168–4183. doi: 10.1002/cne.23145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rijke CE, Hillebrand JJ, Verhagen LA, Roeling TA, Adan RA. Hypothalamic neuropeptide expression following chronic food restriction in sedentary and wheel-running rats. Journal of molecular endocrinology. 2005a;35:381–390. doi: 10.1677/jme.1.01808. [DOI] [PubMed] [Google Scholar]
- de Rijke CE, Jackson PJ, Garner KM, van Rozen RJ, Douglas NR, Kas MJ, Millhauser GL, Adan RA. Functional analysis of the Ala67Thr polymorphism in agouti related protein associated with anorexia nervosa and leanness. Biochemical pharmacology. 2005b;70:308–316. doi: 10.1016/j.bcp.2005.04.033. [DOI] [PubMed] [Google Scholar]
- Favaro A, Ferrara S, Santonastaso P. The spectrum of eating disorders in young women: a prevalence study in a general population sample. Psychosomatic medicine. 2003;65:701–708. doi: 10.1097/01.psy.0000073871.67679.d8. [DOI] [PubMed] [Google Scholar]
- Favaro A, Tenconi E, Santonastaso P. The interaction between perinatal factors and childhood abuse in the risk of developing anorexia nervosa. Psychological medicine. 2010;40:657–665. doi: 10.1017/S0033291709990973. [DOI] [PubMed] [Google Scholar]
- Foley DL, Thacker LR, 2nd, Aggen SH, Neale MC, Kendler KS. Pregnancy and perinatal complications associated with risks for common psychiatric disorders in a population-based sample of female twins. American journal of medical genetics. 2001;105:426–431. [PubMed] [Google Scholar]
- Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nature neuroscience. 1998;1:271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- Henry C, Kabbaj M, Simon H, Le Moal M, Maccari S. Prenatal stress increases the hypothalamo-pituitary-adrenal axis response in young and adult rats. Journal of neuroendocrinology. 1994;6:341–345. doi: 10.1111/j.1365-2826.1994.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Hillebrand JJ, Kas MJ, Scheurink AJ, van Dijk G, Adan RA. AgRP(83-132) and SHU9119 differently affect activity-based anorexia. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2006;16:403–412. doi: 10.1016/j.euroneuro.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Kas MJ, Bruijnzeel AW, Haanstra JR, Wiegant VM, Adan RA. Differential regulation of agouti-related protein and neuropeptide Y in hypothalamic neurons following a stressful event. Journal of molecular endocrinology. 2005;35:159–164. doi: 10.1677/jme.1.01819. [DOI] [PubMed] [Google Scholar]
- Kas MJ, van Dijk G, Scheurink AJ, Adan RA. Agouti-related protein prevents self-starvation. Molecular psychiatry. 2003;8:235–240. doi: 10.1038/sj.mp.4001206. [DOI] [PubMed] [Google Scholar]
- Koehl M, Darnaudery M, Dulluc J, Van Reeth O, Le Moal M, Maccari S. Prenatal stress alters circadian activity of hypothalamo-pituitary-adrenal axis and hippocampal corticosteroid receptors in adult rats of both gender. J Neurobiol. 1999;40:302–315. [PubMed] [Google Scholar]
- Koenig JI, Elmer GI, Shepard PD, Lee PR, Mayo C, Joy B, Hercher E, Brady DL. Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia. Behavioural brain research. 2005;156:251–261. doi: 10.1016/j.bbr.2004.05.030. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, de Boer SF, Coppens CM, Buwalda B. Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front Neuroendocrinol. 2011;31:307. doi: 10.1016/j.yfrne.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, de Boer SF, van dV, Van Reenen CG, Hopster H, De JI, Ruis MA, Blokhuis HJ. Coping styles in animals: current status in behaviour and stress-physiology. Neurosci.Biobehav.Rev. 1999;23:925. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Lee BK, Iyer VR. Genome-wide studies of CCCTC-binding factor (CTCF) and cohesin provide insight into chromatin structure and regulation. The Journal of biological chemistry. 2012;287:30906–30913. doi: 10.1074/jbc.R111.324962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing? Reviews in the neurosciences. 2000;11:383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- Marco A, Kisliouk T, Tabachnik T, Meiri N, Weller A. Overweight and CpG methylation of the Pomc promoter in offspring of high-fat-diet-fed dams are not "reprogrammed" by regular chow diet in rats. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014 doi: 10.1096/fj.14-255620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers CD, Vos ET, Stevenson CE, Begg SJ. The Australian Burden of Disease Study: measuring the loss of health from diseases, injuries and risk factors. The Medical journal of Australia. 2000;172:592–596. doi: 10.5694/j.1326-5377.2000.tb124125.x. [DOI] [PubMed] [Google Scholar]
- Ohlsson R, Renkawitz R, Lobanenkov V. CTCF is a uniquely versatile transcription regulator linked to epigenetics and disease. Trends in genetics : TIG. 2001;17:520–527. doi: 10.1016/s0168-9525(01)02366-6. [DOI] [PubMed] [Google Scholar]
- Palacios-Garcia I, Lara-Vasquez A, Montiel JF, Diaz-Veliz GF, Sepulveda H, Utreras E, Montecino M, Gonzalez-Billault C, Aboitiz F. Prenatal stress downregulates Reelin expression by methylation of its promoter and induces adult behavioral impairments in rats. PloS one. 2015;10:e0117680. doi: 10.1371/journal.pone.0117680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternain L, Batlle MA, De la Garza AL, Milagro FI, Martinez JA, Campion J. Transcriptomic and Epigenetic Changes in the Hypothalamus Are Involved in an Increased Susceptibility to a High-Fat-Sucrose Diet in Prenatally Stressed Female Rats. Neuroendocrinology. 2012 doi: 10.1159/000341684. [DOI] [PubMed] [Google Scholar]
- Paxinos GWC. The Rat Brain in Stereotaxic Coordinates, 6 ed. Sydney: Academic Press; 1982. [Google Scholar]
- Routtenberg A, Kuznesof AW. Self-starvation of rats living in activity wheels on a restricted feeding schedule. J.Comp Physiol Psychol. 1967;64:414. doi: 10.1037/h0025205. [DOI] [PubMed] [Google Scholar]
- Scheurink AJ, Boersma GJ, Nergardh R, Sodersten P. Neurobiology of hyperactivity and reward: agreeable restlessness in anorexia nervosa. Physiol Behav. 2010;100:490. doi: 10.1016/j.physbeh.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Schraut KG, Jakob SB, Weidner MT, Schmitt AG, Scholz CJ, Strekalova T, El Hajj N, Eijssen LM, Domschke K, Reif A, Haaf T, Ortega G, Steinbusch HW, Lesch KP, Van den Hove DL. Prenatal stress-induced programming of genome-wide promoter DNA methylation in 5-HTT-deficient mice. Translational psychiatry. 2014;4:e473. doi: 10.1038/tp.2014.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink T, Hinney A, van Elburg AA, van Goozen SH, Sandkuijl LA, Sinke RJ, Herpertz-Dahlmann BM, Hebebrand J, Remschmidt H, van Engeland H, Adan RA. Association between an agouti-related protein gene polymorphism and anorexia nervosa. Molecular psychiatry. 2001;6:325–328. doi: 10.1038/sj.mp.4000854. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochemical research. 2007;32:1730–1740. doi: 10.1007/s11064-007-9339-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.