Abstract
Physiologic variations in perfusate composition have been identified as a new and important modulator of cardiac conduction velocity (CV), particularly when gap junctions (GJ) are reduced. We recently demonstrated in ex vivo hearts that perfusates with low sodium and high potassium preferentially slow ventricular CV in mice genetically engineered to express 50% less of the gap junction protein, connexin43 (Cx43). We also reported the possible role of calcium in modulating CV. In this review we discuss previous murine studies that explored the CV-GJ relationship in isolated mouse heart preparations with approximately 50% reduced Cx43. Studies were grouped according to the type of perfusate utilized, and CV during GJ uncoupling was compared.
Studies in Group A preferentially used perfusates with low sodium, high potassium and non-physiologic calcium, and found CV slows and arrhythmias increase in mouse hearts with reduced Cx43. Studies in Group B used solutions with high sodium, low potassium and physiologic calcium, and did not observe CV slowing nor increased arrhythmia risk with loss of Cx3. Studies in Group C used solutions with low sodium, low potassium, physiologic calcium, creatine, taurine, and insulin. CV slowing was not observed, nor was arrhythmia risk increased with loss of Cx43.
We suggest that perfusate ion composition may be a major determinant of whether CV slows when Cx43 is reduced. Furthermore, the review of these studies highlights important theoretical developments in the understanding of cardiac conduction and suggests that ionic milieu can conceal electrophysiologic remodeling secondary to reduced Cx43 expression as occurs in many cardiac diseases.
Keywords: Conduction, Conduction Reserve, Connexin43, Perfusate, Ephaptic Coupling
INTRODUCTION
“In 1960... first three causes of death alone - diseases of the heart, malignant neoplasms, and vascular lesions of the central nervous system - accounted for 66 percent of all deaths.” – Vital Statistics of the United States, 1960.
“CVD [cardiovascular disease] is the leading global cause of death, accounting for 17.3 million deaths per year, a number that is expected to grow to >23.6 million by 2030.” – Heart Disease and Stroke Statistics – 2015 Update. (Mozaffarian et al., 2015)
In 1960, the US Department of Health, Education and Welfare listed diseases of the heart as the leading cause of death and reported a death rate greater than two times that of the second highest cause of death in all studied groups – male and female, white and non-white. Today, the American Heart Association reports that cardiovascular disease still holds the position of leading cause of death, not only in the United States but worldwide, and the number of death due to CVD is projected to grow in 2030 despite decades of cardiovascular research.
Approximately 40-50% of CVD deaths today are attributable to sudden cardiac death. (Mehra, 2007) Sudden cardiac death is a result of abnormal and uncoordinated conduction of electrical impulses through the myocardium leading to a lack of synchronous contraction, inefficient pumping of blood to the body and tissue death. Conduction of electrical excitation is dependent on numerous factors like electrical coupling between the myocytes, tissue structure and excitability. (Kleber and Rudy, 2004) Of these determinants, electrical coupling between the myocytes through gap junctions, and their role in modulating conduction is a richly researched yet controversial area of cardiac arrhythmia research. (Eloff et al., 2001; Guerrero et al., 1997; Morley et al., 1999; Rohr et al., 1998; Stein et al., 2011; Stein et al., 2009; Thomas et al., 2000) Groups have reported either no change in conduction or conduction slowing secondary to an approximately 50% reduction of intercellular electrical coupling mediated by the principal ventricular gap junction protein connexin43 (Cx43). Perhaps, it is the existence of such controversies that have prevented the field from achieving greater success in preventing cardiac arrhythmias. The purpose of this review is to explore a common thread that may explain what can at first glance appear to be inconsistent results in mice with 50% Cx43. We will make the case that the apparent inconsistencies are in actuality clues to mechanisms that can conceal cardiac diseases. Understanding these clues may therefore reveal new therapies to prevent sudden cardiac death.
1. Gap Junctions in Cardiac Conduction
The myocardium was initially thought to be a syncytium, and the conduction of electrical excitation could be explained by a model of linear cable theory. (Weidmann, 1952) Later, it was discovered that the myocardium is composed of individual myocytes surrounded by a cell membrane that are connected to each other by channel forming gap junction proteins. (Dewey and Barr, 1962) Cx43 forms gap junction plaques within the intercalated discs, and gap junctions act as resistive pathways for the propagation of electrical impulses from one cell to the next. (Barr et al., 1965) Importantly, reduced Cx43 expression is found in numerous cardiac diseases. (Cascio et al., 2005; Celes et al., 2007; Poelzing and Rosenbaum, 2004) As a result, most contemporary studies seeking to explore changes in cardiac electrical propagation include some quantification of gap junction mRNA, protein expression, protein phosphorylation, protein distribution, and/or direct cell-to-cell conductance measurements. Even with this intense focus on gap junctions and cardiac conduction, and the wide-spread acknowledgement that gap junctions are crucial for intercellular communication, there are surprisingly diverse results and opinions on the degree of gap junctional uncoupling required to measurably slow conduction in whole-heart preparations.
Cardiac conduction velocity (CV) is a widely used metric of cardiac conduction, and this parameter has been extensively used to determine the functional consequence of gap junctional uncoupling by either pharmacologic and/or genetic means. (Eloff et al., 2001; Guerrero et al., 1997; Morley et al., 1999; Rohr et al., 1998; Stein et al., 2011; Stein et al., 2009; Thomas et al., 2000; van Rijen et al., 2004) Although murine models of cardiac electrophysiology are often criticized due to dissimilarities in repolarization from human cardiac electrophysiology, genetically manipulated mouse models nonetheless enable straightforward comparison of results between research groups. The mouse has been invaluable for elucidating the relationship between cardiac conduction and gap junctions.
Table 1 lists manuscripts with electrophysiologic data plotted in Figures 1-3. To facilitate direct comparisons between figures, studies conducted with identical senior authors are combined in the figures; values between studies averaged when appropriate, and references bolded to indicate the origin of the summary data. As can be seen in Table 1, there are a number of studies that utilized various mouse models with different degrees of homogeneously reduced Cx43. For the purpose of this review, we will focus our comments mainly on genetic mouse models of reduced Cx43 functional expression by approximately 50%. The studies outlined in black in Table 1 reported CV secondary to 50% reduced Cx43. The other studies in Table 1 are included in this review for the purpose of comparing other electrophysiologic parameters that can affect CV, despite the fact that these studies did not specifically report CV.
Table 1.
Conduction Velocity – Gap Junction Mice studies
| Study | PMID | Model | Electrical Uncoupling | CV-GJ Relationship |
|---|---|---|---|---|
| Guerrero-1997 | 9109444 | Cx43 HZ | 50% | Direct |
| Thomas-1998 | 9495305 | Cx43 HZ - Ventricles | 50% | Direct |
| Cx43 HZ - Atria | 50% | None | ||
| Johnson-1999 | 10515564 | Neonatal myocytes from Cx43 HZ | 50% | No Data |
| Morley-1999 | 10515561 | Cx43 HZ | 50% | None |
| Eloff-2001 | 11530101 | Cx43 HZ | 50% | Direct |
| van Rijen-2004 | 14967725 | Cx43Cre-ER(T)/fl | 50% | None |
| 95% | Direct | |||
| Danik-2004 | 15499029 | O-CKO | 59% | None |
| 82% | Direct | |||
| Danik-2008 | 17984180 | O-CKO | 59% Based on Danik 2004 | Direct |
| Kontogeorgis-2008 | 18757477 | Cx43 HZ | 66% | No Data |
| Stein-2009 | 19389723 | Cx43 HZ | 50% | None |
| Stein-2011 | 21673812 | Cx43 HZ | 50% | None |
| 90% | Direct | |||
| Lubkemeier-2013 | 23558439 | Cx43D378stop myocytes | 85% | No Data |
| George-2015 | 25771952 | Cx43 HZ | 50% | Perfusate Dependent |
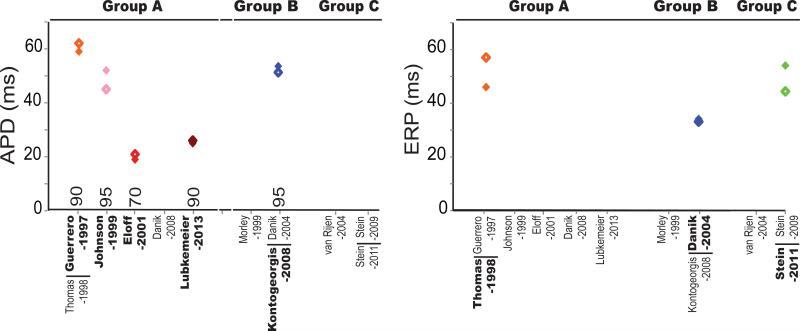
Figure 1. Electrophysiological characteristics of genetically manipulated mice.
Action potential duration and resting membrane potential reported in the discussed studies are classified into Groups A (Blue), B (Red) and C (Green) based on the perfusates used. The number above the x-axis in the left panel indicates the percent of repolarization used in APD measurements. Closed markers correspond to values reported in wild type mice and open markers correspond to values in mice with reduced Cx43. Data points are included only for manuscripts highlighted in Table 1.
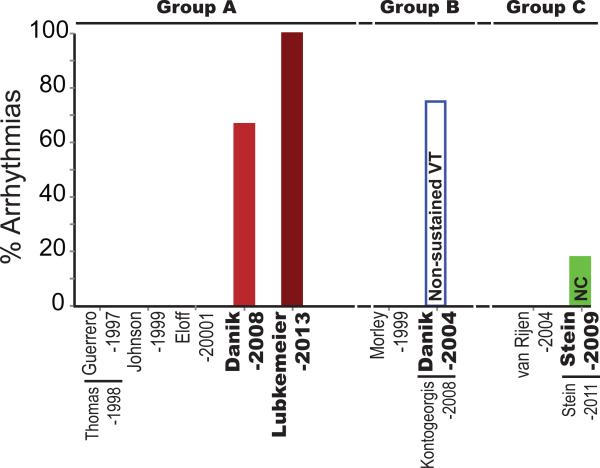
Figure 3. Arrhythmia susceptibility in genetically manipulated mice.
Percent of mice in which arrhythmias were inducible is plotted based on the perfusate classification into Groups A (Blue), B (Red) and C (Green). NC – not classified as sustained or non-sustained VT. Data points are included only for manuscripts highlighted in Table 1.
Even within this subset of the literature, the methods for genetically reducing Cx43 are varied among researchers. For example CV has been analyzed in mice with cardiac-restricted Cx43 deletion, conditional Cx43 knockout, and progressive conditional Cx43 knockout (Table 1). Though one might ascribe the varied results to the choice of mouse model, it is important to note that even groups studying mice from the same lineage and/or using similar Cx43 reduction techniques have produced results that can be generally divided into no CV slowing (Danik et al., 2008; Morley et al., 1999; Stein et al., 2011; Stein et al., 2009; Thomas et al., 2000) or CV slowing (Danik et al., 2004; Eloff et al., 2001; Guerrero et al., 1997) with a 50% loss of Cx43. Therefore, there may be other commonalities aside from choice of mouse model that link the groups who found a CV-GJ dependency and those who did not.
2. Ionic Composition in Cardiac Conduction
In recent reports, we provided evidence that differences in the choice of the artificial blood-like electrolyte solution (perfusate) circulated through the ex vivo heart, may underlie differences in results. Specifically, we demonstrated that altering extracellular sodium ([Na+]o) and potassium ([K+]o) disparately affected CV between the heterozygous Cx43 null mice (HZ) characterized in the Guerrero-1997 study (Guerrero et al., 1997) and their wild type (WT) littermates. (George et al., 2015) The CV changes we reported were also dependent on intercellular separation between cardiac myocytes within an intercalated disc microdomain called the perinexus, located adjacent to gap junctions. (Rhett and Gourdie, 2012) In brief, our data suggest that perfusate composition can determine whether or not a 50% loss of Cx43 will measurably slow conduction. The results of our study can be summarized as follows:
- CV was slower in both WT and HZ hearts when perinexal separation was large.
- Increased perinexal separation preferentially slowed CV in HZ hearts.
Increasing [Na+]o increased CV preferentially in HZ hearts.
- Increasing [K+]o slowed CV,
- Independent of Cx43 expression in hearts with wide perinexi.
- Dependent on both Cx43 expression and [Na+]o in hearts with narrow perinexi.
[Ca2+]o is inversely correlated with perinexal width.
Altogether, these results demonstrated that the relationship between Cx43 and CV is modulated by the ionic composition of the perfusate. In the above study, we briefly discussed our results in the context of previous directly comparable studies. However, we could not offer a more in-depth analysis of how perfusate composition may underlie the differences in other genetically manipulated mouse models of Cx43 down-regulation. Here, we will 1) briefly review the history of whole-heart ex vivo perfusates, 2) compare CV in genetic models with approximately 50% reduced Cx43 expression categorized by choice of perfusate, and 3) discuss how perfusate composition may determine how loss of Cx43 affects conduction. While we will focus the majority of our comments on the cationic composition of perfusates, we acknowledge that anions are equally important to electrophysiology.
3. History of Perfusion Solutions
The origin of salt perfusion solutions in science and medicine can be traced to the early 19th century when Indian Blue Cholera spread to the northern regions of England. In 1831, William Brooke O'Shaughnessy reported “injection of highly-oxygenated salts into the venous system” as a new method to treat cholera. This new discovery led several others to use their own versions of salt solutions to treat cholera. However, it was Thomas Latta, in 1832, who identified the first solution that was most similar to blood composition. About 50 years later in 1883, Sydney Ringer created what is now referred to as the original Ringer's solution (130 mM [Na+], 4 mM [K+], 1.5mM [Ca2+], 109mM [Cl−] and 28mM Lactate) to bathe explanted frog hearts. For a more complete discussion of the “History of 0.9% Saline” in humans we refer the reader to the elegant review by Awad et al. (Awad et al., 2008) Over the next century, the composition of solutions significantly diverged and the number of buffer solutions exploded because of the need to investigate single proteins, isolated cells, tissue cultures, organ preservation, organ perfusion and transplant.
In 1895, Oskar Langendorff developed the method of perfusing explanted mammalian hearts to study the amplitude and rate of contraction. (Broadley, 1979) The Langendorff method was then used to study the coronary vasculature and the effect of pharmacological interventions. Today, it has a wide range of applications in physiology, including the measurement of conduction velocity in explanted hearts. Tyrode's, Kreb's and Kreb's-Henseleit solutions are the most commonly used perfusates in explanted heart studies, and the individual solubilized components of these common buffers according to Cold Spring Harbor are listed in Table 2.
Table 2.
Ionic composition of common perfusates.
| Ions | Tyrode | Krebs | Krebs-Henseleit | Mouse Physiologic Range | Mouse Mean |
|---|---|---|---|---|---|
| Na+ | 149.2 | 152.2 | 143 | 140 - 160 | 150 |
| K+ | 2.7 | 2.5 | 5.9 | 5 - 7.5 | 6.25 |
| Mg2+ | 1 | 1.2 | 1.2 | NR | NR |
| Ca2+ | 1.8 | 2.5 | 1.25 | 1.7 - 2.5 | 2.1 |
| Cl− | 145.3 | 135.9 | 125.2 | 88 - 110 | 99 |
| H2PO4− | 0.2 | 1.2 | 1.2 | 1.8 - 3 | 2.4 |
| HCO3− | 12 | 25 | 25 | NR | NR |
| SO42− | 1.2 | NR | NR | ||
| Glucose | 5.5 | 11 | 3.4 - 9.8 | 6.6 |
The standard ionic composition (in mM) of commonly used perfusates in Langendorff preparations as published by Cold Spring Harbor Protocols is tabulated. Serum ion concentration in mice is also listed in the last column. NR – not reported.
An important factor to consider when using various perfusion solutions is that the concentration of solutes in serum varies from species to species. (Research Animals Resources, 2009) The physiological ranges of solutes in mouse serum, the species discussed in this review, are listed in Table 2. (Research Animals Resources, 2009) Many groups have modified the original solutions to resemble the serum concentrations of the particular species in use. It was the observation that different groups used different perfusate solutions that led us to hypothesize that perfusate composition may underlie the diverse CV-GJ relationships reported in the literature. Given the complex relationship between CV and even a single extracellular ion, as evidenced by the biphasic response of CV to [K+]oand pH, for example, (Kagiyama et al., 1982; Nygren and Giles, 2000) the question of precisely how perfusate composition alters CV becomes complicated.
The purpose of this review is to collate comparable studies and highlight the perfusate composition as a determinant of conduction slowing secondary to loss of Cx43. We further highlight the extracellular space as an under-investigated target for rescuing abnormal electrical conduction.
4. Classification by Ionic Composition
We categorized those studies in Table 1 that report approximately 50% reduction in Cx43 according to the effect we hypothesized each solution would have on the CV-GJ relationship (Table 3) based on our recent study. (George et al., 2015) To categorize studies by solution composition, we will discuss extracellular sodium, potassium, and calcium concentrations relative to the arithmetic mean of murine blood concentrations as reported in the University of Minnesota's Research Animal Resources document entitled, “Reference Values for Laboratory Animals” (Research Animals Resources, 2009) (Table 2).
Table 3.
Perfusate composition of mouse CV-GJ studies.
| Group A | Group B | Group C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Guerrero-1997 Thomas-1998 |
Johnson-1999 | Eloff-2001 | Danik-2008 | Lubkemeier-2013 | Morley-1999 | Kontogeorgis -2009 | Danik-2004 | van Rijen-2004 | Stein-2009 Stein- 2011 |
|
| Na+ | 143 | 137 | 147.3 | 140.2 | 143 | 155.2 | >140 | 155.2 | 109.2 | 109.2 |
| K+ | 5.9 | 4 | 6.1 | 4.6 | 5.9 | 4 | 4 | 4 | 4.52 | 4.52 |
| Ca2+ | 1.25 | 1.8 | 3.4 | 1.5 | 1.25 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 |
| Mg2+ | 1.2 | 143.6 | 1 | 0.7 | 1.2 | 1 | 1.1 | 1 | 0.92 | 0.92 |
| Cl− | 125.2 | 129.8 | 123 | 125.2 | 139.6 | 149.8 | 139.6 | 97.2 | 97.2 | |
| H2PO4− | 1.2 | 1.4 | 1.2 | 1.2 | 1.2 | 1.2 | 0.92 | 0.92 | ||
| SO42− | 1.2 | 1 | 1.2 | 0.92 | 0.92 | |||||
| HCO3− | 25 | 29 | 25 | 25 | 24 | 24 | 19.2 | 19.2 | ||
| HEPES | 10 | 10 | ||||||||
| Glucose | 11 | 11 | 10 | 10 | 11 | 5.6 | 10 | 5.6 | 22 | 22 |
| Creatin | 6 | 6 | ||||||||
| Taurin | 6 | 6 | ||||||||
| Insulin (μM) | 0.1 | 0.1 | ||||||||
| BDM | 10 | 15 | 15 | |||||||
The various studies discussed in this review are classified based on their sodium and potassium concentrations and its ionic composition is tabulated.
Group A-Perfusate: Perfusates with lower [Na+]o and higher [K+]o were classified as Group A. Many of the Group A perfusates were hypocalcemic with the exception of the Eloff-2001 (Eloff et al., 2001) perfusate, which was hypercalcemic.
Group B-Perfusate: Perfusates with higher [Na+]o and lower [K+]o were classified as Group B. [Ca2+]o was uniform at 1.8 mM within Group B, which fell in the lower physiologic bounds for mice.
Group C-Perfusate: Perfusates with the lowest [Na+]o, low [K+]o, and physiologic [Ca2+]o were classified as Group C. Importantly, these solutions differed from Group A and B in that Group C solutions included additional non-ionic solutes such as creatine, taurine and insulin.
This perfusate classification is based only on the differences in sodium, potassium and calcium ion concentrations and does not take into consideration other modulators of CV like the temperature of the perfusates or the use of electromechanical uncouplers. However, the classification applied in this review is robust independent of these confounders.
4.1 Gap Junctions
As mentioned previously, the methods to reduce Cx43 functional expression are many even for the subset of studies chosen for this review. Therefore, it is important to have a brief discussion of whether the mouse model confounds the simplistic categorization of studies by perfusate.
The Cx43 knockout mice (Cx43+/− and Cx43−/−) used in Guerrero-1997 (Guerrero et al., 1997) study were of the C57BL/6 background with a homogenous and approximate 50% reduction in Cx43. (Guerrero et al., 1997) Conduction in these mice was quantified in the Guerrero-1997, (Guerrero et al., 1997) Morley-1999, (Morley et al., 1999) Eloff-2001, (Eloff et al., 2001) and George-2015 (George et al., 2015) studies. As evidenced by Table 3, these studies are represented in both Groups A and B. Furthermore, the George-2015 study reported results from hearts perfused with solutions found in both Groups A and B to make the point that the different CV-GJ results in this particular Cx43 knockout model were due to perfusate composition.
The Kontogeorgis-2008 study (Kontogeorgis et al., 2008) used Cx43+/− mice maintained in a mixed background consisting of C57BL/6J, SV129 and FVB strains. The two Gutstein-2001 studies used mice with cardiac restricted Cx43 reduction (Gutstein et al., 2001a) (approximately 95%) and chimeric mice with a heterogeneous reduction in Cx43 (129SvJ background). (Gutstein et al., 2001b) While both studies were performed by the same group and would have been categorized in Group B, neither reported CV from a homogeneous 50% Cx43 reduction. Therefore, they are not represented in the figures, yet it would be interesting to know if altering the perfusate composition affects conduction in these models.
Progressively older cardiac-restricted Cx43 conditional knockout mice (O-CKO) were generated for the Danik-2004 study to produce a more gradual loss of Cx43 (59% at 25 days). (Danik et al., 2004) These mice were also used in the Danik-2008 study. (Danik et al., 2008) Importantly, the Danik-2008 study would be categorized as Group A, while the Danik-2004 study would be categorized as Group B based on published perfusate composition. As will be discussed below, only CV from hearts of 25 days old mice are included in this discussion to make comparisons between results due to similar Cx43 reduction (approximately 50%).
The van Rijen-2004, (van Rijen et al., 2004) Stein-2009, (Stein et al., 2009) and Stein-2011 (Stein et al., 2011) studies used Cx43Cre-ER(T)/fl mice (50% Cx43 reduction) with inducible deletion of Cx43 by application of 4-hydroxytamoxifen (95% Cx43 reduction). These mice were of a mixed background (129P2/OlaHsd-C57BL/6). Once again, these three studies were largely performed by the same group, and as a result used the same solution in all experiments – Group C.
Several of these groups have used mice models with varying degrees of Cx43 downregulation as indicated in Table 1. As stated earlier, in this review we have compared only those data that pertain to hearts with approximately 50% reduction in Cx43. It should be noted that the apparently discrepant findings from these manuscripts cannot be ascribed solely to differences in perfusate composition until the different perfusates are systematically tested in the various mouse models used. However, two mouse models have already been perfused with Group A and B solutions, suggesting that mouse models may not be the primary source of differing results.
4.2 Action Potential and Ionic Currents
Cardiac conduction is determined by more than just Cx43 functional expression, and Cx43 remodeling has been associated with altered functional expression of various intercalated disc proteins such as ion channels and mechanical junction proteins. (Agullo-Pascual et al., 2014; Delmar, 2012; Palatinus et al., 2011) As a result, ion channel remodeling in response to genetic down-regulation of Cx43 can alter CV. If this is the case, one might expect to observe concurrent changes in action potential (AP) morphology. Despite significant differences between mice and humans in the repolarization phase of the action potential, the effects of ion channel remodeling on the AP morphology should be apparent in genetically manipulated animals. This section will summarize reported alterations in ion channels, resulting AP remodeling, and any effects these changes may have on CV in Cx43 deficient mice.
NaV1.5: The cardiac isoform of the voltage gated sodium channel (NaV1.5) localizes preferentially at the intercalated disc. (Makara et al., 2014; Palatinus et al., 2011; Sato et al., 2009; Veeraraghavan et al., 2015; Westenbroek et al., 2013) If reduction in Cx43 correlates with reduced expression of Nav1.5, (Lubkemeier et al., 2013) then the rate of rise of the action potential (dV/dtmax) should decrease. The parameter dV/dtmax is also used as an index of sodium channel peak conductance as well as gap junctional coupling, (Spach et al., 2000) both of which can affect CV. In cells isolated from neonatal mice hearts, the Guerrero-1998 (Guerrero et al., 1997) and the Johnson-1999 (Johnson et al., 1999) studies reported that dV/dtmax was not different between control and myocytes heterozygous for the Cx43 null mutation. The Johnson-1999 (Johnson et al., 1999) study also reported no change in sodium current (INa) characteristics. Additionally, the Johnson-1999, (Johnson et al., 1999) Stein-2009 (Stein et al., 2009) and Lubkemeier-2013 (Lubkemeier et al., 2013) studies reported no change in NaV1.5 expression when Cx43 was reduced. The only differences that were reported were in the mice with altered Cx43 C-termini. Specifically, the Lubkemeier-2013 (Lubkemeier et al., 2013) study reported reduced co-localization of NaV1.5 with N-Cadherin at the intercalated disc, a significant reduction of the peak INa, and prolongation of time course of inactivation in the Cx43D378stop compared to WT mice without any changes in voltage dependence of inactivation or time course of recovery.
Kir2.1: Intercalated disc localization of potassium channels such as the inward rectifier potassium channel (Kir2.1) has also been observed, and it has been suggested that there may be a relationship between Nav1.5 and Kir2.1. (Milstein et al., 2012) If genetic Cx43 remodeling alters Kir2.1 functional expression, one might expect alterations in resting membrane potential (RMP), action potential duration (APD), and possibly CV in mutant hearts. (Shaw and Rudy, 1997a; Veeraraghavan et al., 2013) However, the studies discussed here reported no significant changes in APD or RMP (Figure 1A) or in the effective refractory period (Figure 1B). The only exception is the Danik-2008 study (Danik et al., 2008) which reported increased RMP in the RV but not in the LV of O-CKO mice compared to WT. The authors of this study attributed raised RMP in the RV to altered sustained potassium currents (Isus) and IK1 in the O-CKO mice. Thus, potassium channel remodeling may have contributed to the CV changes observed in the Danik-2008 study. However, the Danik-2004 study, using the same mouse, but a different perfusate, found no significant conduction differences between mutant and WT mice.
In summary, it is unlikely that CV changes in Cx43 HZ mice reflect secondary changes in ion channel expression / function.
4.3 Conduction Velocity
Cardiac conduction depends on several factors including Cx43 expression and distribution, tissue excitability, cell size, interstitial volume and perfusate ion concentrations. (George et al., 2015; Kleber and Rudy, 2004) Modest changes in multiple factors together or a significant change in an individual parameter could result in conduction slowing. (George et al., 2015; Stein et al., 2011; Stein et al., 2009; van Rijen et al., 2004; Veeraraghavan et al., 2015) Here, we compare results from mice with similar structural and electrophysiological characteristics to explore the role of extracellular ions as modulators of the CV-GJ relationship.
In order to enable comparison of CV's from methodologically diverse studies (differences in conduction mapping systems, constant pressure versus flow systems, quantification techniques), we have homogenized the results. For instance, when studies quantified CV from the left and right ventricle in WT and mutant mice, we averaged the values and present a simplified version of CV in Figure 2. The reader is referred to the original studies for a more in-depth analysis of the CV-GJ relationship as each manuscript in this review offers insights into arrhythmia mechanisms greater than simply whether loss of Cx43 slows cardiac conduction.
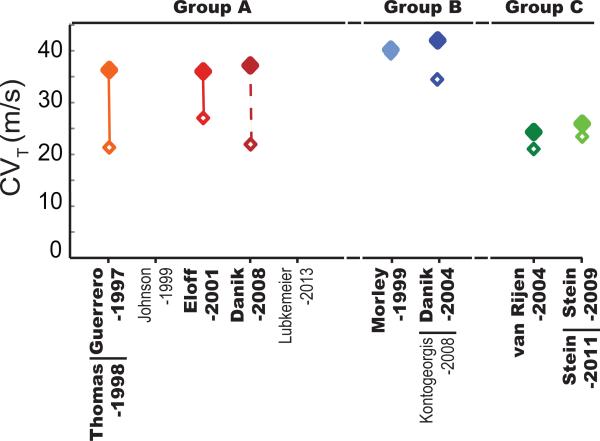
Figure 2. Conduction Velocity Classification.
Transverse CV reported in the discussed studies are classified into Groups A (Blue), B (Red) and C (Green) based on the perfusates used. Closed markers correspond to values reported in wild type mice and open markers correspond to values in mice with reduced Cx43. The lines connecting the open and closed markers indicates significant differences whereas absence of a connecting line indicates no significance. The dashed line between the Danik-2008 markers represents data that were not directly compared by statistical tests but report percent CV slowing similar to statistically significant groups. Data points are included only for manuscripts highlighted in Table 1.
Group A-Conduction: The Eloff-2001 (Eloff et al., 2001) and Guerrero-1998 (Guerrero et al., 1997) studies used similar mouse models and reported significant CV slowing (25 and 44% respectively) in Cx43+/− mice. Additionally, the Danik-2008 (Danik et al., 2008) study, reported a similar degree of CV slowing (43% average) (0.42 vs 0.23 m/s in RV and 0.35 vs 0.21 m/s in LV) in 25 day-old mice with approximately 50% reduced Cx43. The QRS duration, as a metric of ventricular depolarization time, has been used as a surrogate measure of CV. The Guerrero-1998 (Guerrero et al., 1997) and Lubkemeier-2013 (Lubkemeier et al., 2013) studies reported significant QRS prolongation in Cx43+/− mice compared to WT (11.5 vs 13.4 ms and approximately 10 to 23 ms). Overall, Group A studies are characterized by the observation of conduction slowing.
Group B-Conduction: Studies that were categorized into Group B – Danik-2004 (Danik et al., 2004) and Morley-1999 (Morley et al., 1999) – did not report a significant change in conduction in the longitudinal (CVL) or transverse direction (CVT) (19 and 2% CV reduction respectively) when Cx43 was reduced by approximately 50%. Furthermore, Group B studies rarely reported a change in the QRS duration, with the exception of the Danik-2004 study (Danik et al., 2004) where QRS duration was prolonged in 25 day old O-CKO mice (7.4 to 8.8 ms).
Group C-Conduction: The van Rijen-2004 (van Rijen et al., 2004) and Stein-2009,2011 (Stein et al., 2011; Stein et al., 2009) studies did not report CV slowing when Cx43 was reduced by approximately 50%. These studies used perfusates that contained the additional non-ionic compounds insulin, creatine and taurine that can additionally modulate normal electrophysiology. (Hernandez et al., 1984; Punske et al., 2004; Rozenshtraukh et al., 1990; Taniguchi et al., 1983) Interestingly, insulin, creatine, and taurine have all been reported to have anti-inflammatory properties in addition to their myriad effects on other cardiac and non-cardiac cellular processes. Just considering that these compounds may reduce extracellular volume expansion, the lack of observable CV slowing in the Group C studies are consistent with other results that reducing extracellular volume with non-ionic agents can conceal the pharmacologic (Veeraraghavan et al., 2015; Veeraraghavan et al., 2012) and genetic loss of Cx43. (George et al., 2015) Still, the effects of these compounds that can be found in blood requires additional and specific investigation as a potential confounder of the CV-GJ relationship.
In summary, perfusate composition emerges as a predictor of the CV-GJ relationship observed in transgenic mouse studies. Studies using Group A perfusates (low [Na+]o, high [K+]o and non-physiologic [Ca2+]o) report a direct correlation between CV and Cx43 expression while those using Group B perfusates (high [Na+]o, low [K+]o and physiologic [Ca2+]o), do not observe such a relationship.
4.3.1 Potential Mechanisms
Conduction Reserve: This question why CV is relatively insensitive up to a 50% loss of gap junctional coupling is not new. The prevailing theory to explain this lack of simple linear correlation between CV and GJ coupling was coined in the Van Rijen-2005 editorial as “conduction reserve”. (van Rijen et al., 2005) The concept of conduction reserve is largely based on the elegant computational models of discontinuous conduction presented in the Shaw-1997 study. Although Drs. Shaw and Rudy do not use this term explicitly, they discuss their findings as follows: “When coupling is reduced, less inward current is shunted downstream (less load), effectively increasing availability of inward current for local depolarization.” (Shaw and Rudy, 1997b) The rationale for the Shaw-1997 study derived from previous computational and experimental investigations of the CV-GJ relationship in canine, sheep, and humans, and the simulations proved rather prescient. Subsequent studies in transgenic Cx43 KO mice reported diverse CV-GJ relationships, lending credence to the hypothesis that “conduction reserve” may modulate the CV-GJ relationship.
Ephaptic Coupling: An additional theory to explain the lack of a simple linear CV-GJ relationship is called ephaptic coupling, which simply means non-synaptic and non-gap junctional coupling. Ephaptic coupling is perhaps better accepted in the neural literature. (Anastassiou et al., 2011; Arvanitaki, 1942; Bokil et al., 2001; Su et al., 2012; Van der Goes van Naters, 2013) In the cardiac literature, the preponderance of work into ephaptic coupling has been largely confined to theoretical simulations. (Lin and Keener, 2013; Lin and Keener, 2014; Mori et al., 2008) While the Sperelakis-2002 manuscript (Sperelakis and Ramasamy, 2002) summarizes six potential mechanisms of ephaptic coupling, computational studies often model it as the generation of significant extracellular fields in small clefts between neighboring myocytes. By definition, excitable cells like cardiomyocytes depolarize when the transmembrane potential Vm – the difference between the intracellular and extracellular potentials (Vm = Φi – Φo) – rises. Vm can rise by either increasing Φi or decreasing Φo. In turn, Φi can be elevated by charge transfer from a pre- to a post-junctional cell via GJ, while Φo can change in response to accumulation or depletion of charge within restricted extracellular clefts between myocytes. In cardiomyocytes, it has been proposed that ephaptic coupling can occur via activation of sodium ion channels in the actively depolarizing myocyte, inducing an inward flow of sodium ions into the cell, while simultaneously reducing the potential in the cleft between the myocytes. This decreases cleft potential (reduces Φo), raises Vm of the neighboring cell, which then activates the post-junctional sodium channels by depolarizing the membrane from the extracellular domain to initiate cellular depolarization. (Lin and Keener, 2013; Lin and Keener, 2014)
Our group recently provided experimental evidence for ephaptic coupling in both murine (George et al., 2015) and guinea pig (Veeraraghavan et al., 2015; Veeraraghavan et al., 2012) whole-heart preparations. Further support for this hypothesis comes from the identification of intercalated disc microdomains such as the connexome (Agullo-Pascual et al., 2013) and perinexus (Rhett and Gourdie, 2012) which meet the theoretically-predicted requirements of a cardiac ephapse: dense sodium channel localization in narrow intercellular clefts. We have demonstrated that CV slowing during pharmacologic and genetic reduction of gap junctions can be mitigated or exacerbated by altering the perinexus through interventions like altered [Ca2+]o, albumin, and mannitol. Broadly, we find that wide perinexi exacerbate the loss of GJs, while narrow perinexi can either conceal or exacerbate the loss of GJs depending on [Na+]o and [K+]o. Importantly, only computational models incorporating ephaptic coupling predict that increasing extracellular volume and/or conductivity decreases CV and exacerbates GJ uncoupling induced CV slowing. (Lin and Keener, 2014; Veeraraghavan et al., 2015)
With the exception of the Eloff-2001 study, Group A studies, which observed CV slowing in hearts with reduced Cx43, used perfusates with lower [Ca2+]o than studies in Groups B and C. (Danik et al., 2008; Guerrero et al., 1997; Lubkemeier et al., 2013) Since our study demonstrated an inverse correlation between [Ca2+]o and perinexal width, we hypothesized that these groups would be associated with increased perinexal separation and thereby CV slowing during GJ uncoupling. The Eloff-2011 study, like the Guerrero-1997 and Lubkemeier-2013 studies, used a higher [K+]o, which in turn could reduce sodium channel availability and compromise ephaptic coupling. Thus, Group A perfusates are characterized by one or more ionic concentrations that would weaken ephaptic coupling and increase conduction dependence on gap junctions.
Group B perfusates on the other hand are characterized by high [Na+]o, low [K+]o and physiologic [Ca2+]o which would promote greater cellular excitability and support nominally narrow and pro-ephaptic perinexal cleft widths, thereby reducing CV dependence on GJ coupling.
Studies in Group C are difficult to analyze at present without a greater understanding of the how creatine, taurine and insulin modulate cardiac conduction and intercalated disc ultrastructure. Still, low [Na+]o should decrease CV modestly, but the relatively low [K+]o should increase sodium channel availability even while increasing the differential between resting membrane potential and the sodium channel activation voltage, and the near physiologic [Ca2+]o should preserve narrow perinexi and thereby, ephaptic coupling. Without systematic testing, it is difficult to say which factor underlies concealment of the loss of Cx43 in this particular mouse model.
Sodium current: The changes in ionic concentrations discussed here could alter sodium channel kinetics and cellular excitability irrespective of the role of these sodium channels in maintaining ephaptic coupling between cells. Reducing GJ coupling could have a dual effect of 1. slowing CV by diminishing the electrotonic depolarizing current from upstream cells and increasing impulse delay at the gap junction and 2. enhancing CV within myocytes by reducing the sink. Reducing cellular excitability on the other hand has been demonstrated to simply reduce CV by attenuating the depolarizing current. (Shaw and Rudy, 1997b) Thus, reducing available sodium channels by increasing [K+]o should first increase CV by mechanisms of hyperexcitability and then decrease CV as more sodium channels are shifted into an inactivated state. (Kagiyama et al., 1982; Nygren and Giles, 2000) Thus, one might expect that the relationship between CV and [K+]o should be biphasic with a CV peak at some nominal levels of [K+]o, or linearly increasing. However, the relationship between [K+]o and CV in the studies discussed herein does not always follow either of these expectations. Specifically, CV in WT hearts from Group B (4.0mM [K+]o) is faster than studies from Group C (4.52 mM [K+]o) and group A (approximately 6mM [K+]o). While this is an intriguing post-hoc analysis, this discussion should be interpreted cautiously because none of the studies discussed here were conducted to directly compare CV to [K+]o. However, the comparisons suggest an additionally complex relationship between [K+]o, sodium channels, excitability, GJs and CV.
4.4 Arrhythmia Susceptibility
It is well established that slow CV is associated with increased risk of reentrant arrhythmias. (Veeraraghavan et al., 2012) Additionally, GJ uncoupling has also been associated with an altered source-sink relationship that supports propagation of ectopic beats. (Joyner et al., 1984; Morley et al., 2005) Therefore, if perfusate composition modifies the CV-GJ relationship, these same perfusates could also alter arrhythmia susceptibility. In this section, we compare arrhythmia incidence across studies using different perfusate compositions (figure 3). In the case of studies that reported arrhythmia incidence from both ventricles, the values were combined for comparison.
Group A-Arrhythmias: A few Group A studies, where CV slowing was observed, also investigated arrhythmia susceptibility. The Danik-2008 (Danik et al., 2008) and Lubkemeier-2013 (Lubkemeier et al., 2013) studies reported sustained VT in the majority of the mice with reduced Cx43 (18/27 vs 2/8 in WT). Furthermore, the Lubkemeier-2013 study reported 0% survival rate after tamoxifen injection to conditionally reduce Cx43 expression (compared to 100% without tamoxifen).
Group B-Arrhythmias: Among Group B studies, where no CV slowing was observed, only Danik-2004 (Danik et al., 2004) reported arrhythmia susceptibility. Specifically, they found that non-sustained VT was inducible in 5/10 WT and 7/10 mice with approximately 50% Cx43 at 25 days of age. Interestingly, as the mice aged and Cx43 and CV decreased, arrhythmia susceptibility was higher in the O-CKO mice relative to control.
Group C-Arrhythmias: The Stein-2009 (Stein et al., 2009) study from Group C reports similar arrhythmia induction rates with programmed electrical stimulation in WT (1/17) and reduced Cx43 (5/14) hearts. The nature of each of these arrhythmias (sustained vs non-sustained VT) was not specified.
While this is not a comprehensive analysis of arrhythmia susceptibility in mice with genetic down-regulation of Cx43, the studies considered as a whole suggest that arrhythmias were more prevalent and severe when reduced Cx43 expression was associated with conduction slowing. Thus, these results suggest an important role for perfusate composition in determining arrhythmia risk given a background of Cx43 downregulation.
5. Conclusions
Variations in perfusate composition within the physiologic range have been identified as a new and important modulator of CV, particularly when GJ coupling is reduced. We recently demonstrated that low [Na+]o and high [K+]o perfusates are associated with slower CV in mice. (George et al., 2015) We also demonstrated that [Ca2+]o may modulate perinexal spacing. Based on these findings, we categorized previous studies of cardiac conduction in genetically modified mice with approximately 50% reduction in Cx43.
In summary, studies that used solutions with relatively low [Na+]o, high [K+]o and non-physiologic [Ca2+]o (Group A) reported significant conduction slowing and elevated arrhythmia risk in hearts with reduced Cx43 expression. In contrast, studies that used solutions with relatively high [Na+]o, low [K+]o and physiologic [Ca2+]o (Group B) did not observe differences in CV or arrhythmia risk between hearts with reduced Cx43 and wild type controls. Under these conditions, no significant CV changes were observed when Cx43 was reduced by approximately 50%. Neither was arrhythmia risk significantly higher relative to control groups. Finally, studies in Group C behaved similarly to studies in Group B. However, the perfusate used in the studies categorized in Group C are more difficult to discuss in context of simple ionic composition because the solutions contained non-ionizable solutes which may have additional effects on electrophysiology beyond the scope of this review. Importantly, the CV-GJ relationship and arrhythmia susceptibility is likely dependent on perfusate composition, and the above discussion is consistent with our previous murine studies.(George et al., 2015)
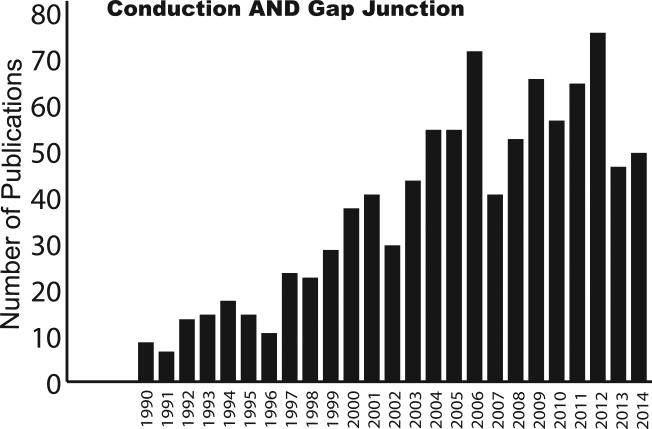
The CV-GJ relationship has been a subject of intense research for many years. The trend plot in Figure 4 illustrates the number of scientific studies published that are based on conduction and Cx43 in the heart. This review not only highlights the different CV-GJ relationships reported from only genetically manipulated mice, but also identifies a potential source for these differences – perfusate ion composition.
Figure 4. Conduction – Gap Junction Trend Plot.
The number of studies that were identified by Pubmed with the search term “Conduction AND Gap Junction” since 1990 is plotted.
The suggestion that perfusate ion composition may be a major determinant of whether CV slowing will be observed due to loss of Cx43 is important in its own right. However, this review suggests an additional important topic for consideration. Specifically, if ionic milieu can conceal electrophysiologic remodeling secondary to reduced Cx43 expression, it also suggests that ionic milieu can also conceal cardiac diseases, and this finding would be a valuable new tool in cardiac arrhythmia therapy. More plainly, rather than targeting the biophysical properties of specific sarcolemmal ion channels, pumps, receptors or exchangers, as is the main pharmacologic approach used in medicine to prevent sudden cardiac death, monitoring and balancing salt in the extracellular spaces may be an equally important method to prevent arrhythmias. Thus the source of the CV-GJ controversy – salt - may also be the future remedy for diseases associated with a loss of functional connexins.
Reproducibility versus Serendipity
Perfusates and buffers are used in every area of biological science. Ionic differences within physiological limits have been demonstrated to have a dramatic effect on physiology and this effect is further heightened during disease. In spite of the vast resources spent over the past decades, science still has irreproducible results with few explanations for the discrepancies. In this review we identify perfusate composition as a common experimental difference that could likely lead to CV-GJ controversies. Importantly, we do not claim that perfusate composition is the only or most important mechanism leading to disparate results. Instead, we hope to point out that a common experimental methodology as simple and easily overlooked as the perfusate composition can provide answers to controversies faced by various scientific fields, and that standardization of buffers and techniques may be the key to resolving them.
While this review points out the importance of standardizing buffers, at least within species, we acknowledge the difficulty that such a task poses, due to natural fluctuations in serum ion concentrations due to diet, circadian rhythms, diseases, ages and genders. However, reproducibility of results is still essential in order to prevent unnecessary duplication of science. Further, some metric of standardization, such as the categorization system proposed in this review, could be considered in future discussions of inconsistent results.
Conversely, this review also underscores the importance of scientific diversity as an essential tool for new discoveries. Many serendipitous findings have greatly advanced science, knowledge, and technology. This rich diversity of scientific inquiry has been key to the exploration of conduction reserve and alternative intercellular coupling modes such as ephaptic coupling. Thus, scientific diversity may be as crucial to advancing science as standardization.
This brings us back to the question of what is more important – reproducibility or serendipity? We think that this is yet another open ended question like that of the chicken and the egg. Both are essential components of science and this review highlights the importance of these two arms of scientific advancement.
Acknowledgments
FUNDING SOURCES: This work was supported by an R01-HL102298 awarded to SP, and a VTCRI Medical Research Scholar Award, an American Heart Association Pre-doctoral fellowship, and the David W Francis and Lillian Francis Scholarship Fund awarded to SG.
ABBREVIATIONS
- [Na+]o
Extracellular sodium ion concentration
- [K+]o
Extracellular potassium ion concentration
- WT
Wild type
- HZ
Heterozygous
- [Ca2+]o
Extracellular calcium ion concentration
- Cx43
Connexin43
- dV/dtmax
Maximum rate of rise of the action potential
- INa
Sodium current
- APD
Action potential duration
- RMP
Resting membrane potential
- CVL
Longitudinal conduction velocity
- CVT
Transverse conduction velocity
- Vm
Transmembrane potential
- Φo
Extracellular potential
- Φi
Intracellular potential
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agullo-Pascual E, Lin X, Leo-Macias A, Zhang M, Liang FX, Li Z, Pfenniger A, Lubkemeier I, Keegan S, Fenyo D, Willecke K, Rothenberg E, Delmar M. Super-resolution imaging reveals that loss of the C-terminus of connexin43 limits microtubule plus-end capture and NaV1.5 localization at the intercalated disc. Cardiovasc Res. 2014;104:371–81. doi: 10.1093/cvr/cvu195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agullo-Pascual E, Reid DA, Keegan S, Sidhu M, Fenyo D, Rothenberg E, Delmar M. Super-resolution fluorescence microscopy of the cardiac connexome reveals plakophilin-2 inside the connexin43 plaque. Cardiovasc Res. 2013;100:231–40. doi: 10.1093/cvr/cvt191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastassiou CA, Perin R, Markram H, Koch C. Ephaptic coupling of cortical neurons. Nat Neurosci. 2011;14:217–23. doi: 10.1038/nn.2727. [DOI] [PubMed] [Google Scholar]
- Arvanitaki A. Effects Evoked in an Axon by teh Activity of a Contiguous one. Journal of Neurophysiology. 1942;5:89–108. [Google Scholar]
- Awad S, Allison SP, Lobo DN. The history of 0.9% saline. Clin Nutr. 2008;27:179–88. doi: 10.1016/j.clnu.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Barr L, Dewey MM, Berger W. Propagation of Action Potentials and the Structure of the Nexus in Cardiac Muscle. J Gen Physiol. 1965;48:797–823. doi: 10.1085/jgp.48.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokil H, Laaris N, Blinder K, Ennis M, Keller A. Ephaptic interactions in the mammalian olfactory system. J Neurosci. 2001;21:RC173. doi: 10.1523/JNEUROSCI.21-20-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadley KJ. The Langendorff heart preparation - Reappraisal of its role as a research and teaching model for coronary vasoactive drugs. Journal of Pharmacological Methods. 1979;2:143–156. [Google Scholar]
- Cascio WE, Yang H, Muller-Borer BJ, Johnson TA. Ischemia-induced arrhythmia: the role of connexins, gap junctions, and attendant changes in impulse propagation. J Electrocardiol. 2005;38:55–9. doi: 10.1016/j.jelectrocard.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Celes MR, Torres-Duenas D, Alves-Filho JC, Duarte DB, Cunha FQ, Rossi MA. Reduction of gap and adherens junction proteins and intercalated disc structural remodeling in the hearts of mice submitted to severe cecal ligation and puncture sepsis. Crit Care Med. 2007;35:2176–85. doi: 10.1097/01.ccm.0000281454.97901.01. [DOI] [PubMed] [Google Scholar]
- Danik SB, Liu F, Zhang J, Suk HJ, Morley GE, Fishman GI, Gutstein DE. Modulation of cardiac gap junction expression and arrhythmic susceptibility. Circ Res. 2004;95:1035–41. doi: 10.1161/01.RES.0000148664.33695.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danik SB, Rosner G, Lader J, Gutstein DE, Fishman GI, Morley GE. Electrical remodeling contributes to complex tachyarrhythmias in connexin43-deficient mouse hearts. FASEB J. 2008;22:1204–12. doi: 10.1096/fj.07-8974com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmar M. Desmosome-ion channel interactions and their possible role in arrhythmogenic cardiomyopathy. Pediatr Cardiol. 2012;33:975–9. doi: 10.1007/s00246-012-0257-0. [DOI] [PubMed] [Google Scholar]
- Dewey MM, Barr L. Intercellular Connection between Smooth Muscle Cells: the Nexus. Science. 1962;137:670–2. doi: 10.1126/science.137.3531.670-a. [DOI] [PubMed] [Google Scholar]
- Eloff BC, Lerner DL, Yamada KA, Schuessler RB, Saffitz JE, Rosenbaum DS. High resolution optical mapping reveals conduction slowing in connexin43 deficient mice. Cardiovasc Res. 2001;51:681–90. doi: 10.1016/s0008-6363(01)00341-8. [DOI] [PubMed] [Google Scholar]
- George SA, Sciuto KJ, Lin J, Salama ME, Keener JP, Gourdie RG, Poelzing S. Extracellular sodium and potassium levels modulate cardiac conduction in mice heterozygous null for the Connexin43 gene. Pflugers Arch. 2015b doi: 10.1007/s00424-015-1698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero PA, Schuessler RB, Davis LM, Beyer EC, Johnson CM, Yamada KA, Saffitz JE. Slow ventricular conduction in mice heterozygous for a connexin43 null mutation. J Clin Invest. 1997;99:1991–8. doi: 10.1172/JCI119367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, Chien KR, Stuhlmann H, Fishman GI. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res. 2001a;88:333–9. doi: 10.1161/01.res.88.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutstein DE, Morley GE, Vaidya D, Liu F, Chen FL, Stuhlmann H, Fishman GI. Heterogeneous expression of Gap junction channels in the heart leads to conduction defects and ventricular dysfunction. Circulation. 2001b;104:1194–9. doi: 10.1161/hc3601.093990. [DOI] [PubMed] [Google Scholar]
- Hernandez J, Artillo S, Serrano MI, Serrano JS. Further evidence of the antiarrhythmic efficacy of taurine in the rat heart. Res Commun Chem Pathol Pharmacol. 1984;43:343–6. [PubMed] [Google Scholar]
- Johnson CM, Green KG, Kanter EM, Bou-Abboud E, Saffitz JE, Yamada KA. Voltage-gated Na+ channel activity and connexin expression in Cx43-deficient cardiac myocytes. J Cardiovasc Electrophysiol. 1999;10:1390–401. doi: 10.1111/j.1540-8167.1999.tb00195.x. [DOI] [PubMed] [Google Scholar]
- Joyner RW, Overholt ED, Ramza B, Veenstra RD. Propagation through electrically coupled cells: two inhomogeneously coupled cardiac tissue layers. Am J Physiol. 1984;247:H596–609. doi: 10.1152/ajpheart.1984.247.4.H596. [DOI] [PubMed] [Google Scholar]
- Kagiyama Y, Hill JL, Gettes LS. Interaction of acidosis and increased extracellular potassium on action potential characteristics and conduction in guinea pig ventricular muscle. Circ Res. 1982;51:614–23. doi: 10.1161/01.res.51.5.614. [DOI] [PubMed] [Google Scholar]
- Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431–88. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- Kontogeorgis A, Li X, Kang EY, Feig JE, Ponzio M, Kang G, Kaba RA, Wit AL, Fisher EA, Morley GE, Peters NS, Coetzee WA, Gutstein DE. Decreased connexin43 expression in the mouse heart potentiates pacing-induced remodeling of repolarizing currents. Am J Physiol Heart Circ Physiol. 2008;295:H1905–16. doi: 10.1152/ajpheart.590.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Keener JP. Ephaptic coupling in cardiac myocytes. IEEE Trans Biomed Eng. 2013;60:576–82. doi: 10.1109/TBME.2012.2226720. [DOI] [PubMed] [Google Scholar]
- Lin J, Keener JP. Microdomain effects on transverse cardiac propagation. Biophys J. 2014;106:925–31. doi: 10.1016/j.bpj.2013.11.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubkemeier I, Requardt RP, Lin X, Sasse P, Andrie R, Schrickel JW, Chkourko H, Bukauskas FF, Kim JS, Frank M, Malan D, Zhang J, Wirth A, Dobrowolski R, Mohler PJ, Offermanns S, Fleischmann BK, Delmar M, Willecke K. Deletion of the last five C-terminal amino acid residues of connexin43 leads to lethal ventricular arrhythmias in mice without affecting coupling via gap junction channels. Basic Res Cardiol. 2013;108:348. doi: 10.1007/s00395-013-0348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makara MA, Curran J, Little SC, Musa H, Polina I, Smith SA, Wright PJ, Unudurthi SD, Snyder J, Bennett V, Hund TJ, Mohler PJ. Ankyrin-G coordinates intercalated disc signaling platform to regulate cardiac excitability in vivo. Circ Res. 2014;115:929–38. doi: 10.1161/CIRCRESAHA.115.305154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra R. Global public health problem of sudden cardiac death. J Electrocardiol. 2007;40:S118–22. doi: 10.1016/j.jelectrocard.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Milstein ML, Musa H, Balbuena DP, Anumonwo JM, Auerbach DS, Furspan PB, Hou L, Hu B, Schumacher SM, Vaidyanathan R, Martens JR, Jalife J. Dynamic reciprocity of sodium and potassium channel expression in a macromolecular complex controls cardiac excitability and arrhythmia. Proc Natl Acad Sci U S A. 2012;109:E2134–43. doi: 10.1073/pnas.1109370109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y, Fishman GI, Peskin CS. Ephaptic conduction in a cardiac strand model with 3D electrodiffusion. Proc Natl Acad Sci U S A. 2008;105:6463–8. doi: 10.1073/pnas.0801089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley GE, Danik SB, Bernstein S, Sun Y, Rosner G, Gutstein DE, Fishman GI. Reduced intercellular coupling leads to paradoxical propagation across the Purkinje-ventricular junction and aberrant myocardial activation. Proc Natl Acad Sci U S A. 2005;102:4126–9. doi: 10.1073/pnas.0500881102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley GE, Vaidya D, Samie FH, Lo C, Delmar M, Jalife J. Characterization of conduction in the ventricles of normal and heterozygous Cx43 knockout mice using optical mapping. J Cardiovasc Electrophysiol. 1999;10:1361–75. doi: 10.1111/j.1540-8167.1999.tb00192.x. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB, American Heart Association Statistics, C. and Stroke Statistics, S. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Nygren A, Giles WR. Mathematical simulation of slowing of cardiac conduction velocity by elevated extracellular. Ann Biomed Eng. 2000;28:951–7. doi: 10.1114/1.1308489. [DOI] [PubMed] [Google Scholar]
- Palatinus JA, O'Quinn MP, Barker RJ, Harris BS, Jourdan J, Gourdie RG. ZO-1 determines adherens and gap junction localization at intercalated disks. Am J Physiol Heart Circ Physiol. 2011;300:H583–94. doi: 10.1152/ajpheart.00999.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelzing S, Rosenbaum DS. Altered connexin43 expression produces arrhythmia substrate in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1762–70. doi: 10.1152/ajpheart.00346.2004. [DOI] [PubMed] [Google Scholar]
- Punske BB, Rossi S, Ershler P, Rasmussen I, Abel ED. Optical mapping of propagation changes induced by elevated extracellular potassium ion concentration in genetically altered mouse hearts. J Electrocardiol. 2004;37(Suppl):128–34. doi: 10.1016/j.jelectrocard.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Research Animals Resources U.o.M. Reference Values for Laboratory Animals. Normal Hematology Values., in: Editor (Ed.)^(Eds.), Book Reference Values for Laboratory Animals. Normal Hematology Values., City. 2009.
- Rhett JM, Gourdie RG. The perinexus: a new feature of Cx43 gap junction organization. Heart Rhythm. 2012;9:619–23. doi: 10.1016/j.hrthm.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr S, Kucera JP, Kleber AG. Slow conduction in cardiac tissue, I: effects of a reduction of excitability versus a reduction of electrical coupling on microconduction. Circ Res. 1998;83:781–94. doi: 10.1161/01.res.83.8.781. [DOI] [PubMed] [Google Scholar]
- Rozenshtraukh LV, Witt R, Rozanski G. [Electrophysiological aspects of the effect of creatine phosphate on myocardial cellular activity in the normal state and in ischemia] Kardiologiia. 1990;30:97–101. [PubMed] [Google Scholar]
- Sato PY, Musa H, Coombs W, Guerrero-Serna G, Patino GA, Taffet SM, Isom LL, Delmar M. Loss of plakophilin-2 expression leads to decreased sodium current and slower conduction velocity in cultured cardiac myocytes. Circ Res. 2009;105:523–6. doi: 10.1161/CIRCRESAHA.109.201418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RM, Rudy Y. Electrophysiologic effects of acute myocardial ischemia: a theoretical study of altered cell excitability and action potential duration. Cardiovasc Res. 1997a;35:256–72. doi: 10.1016/s0008-6363(97)00093-x. [DOI] [PubMed] [Google Scholar]
- Shaw RM, Rudy Y. Ionic mechanisms of propagation in cardiac tissue. Roles of the sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling. Circ Res. 1997b;81:727–41. doi: 10.1161/01.res.81.5.727. [DOI] [PubMed] [Google Scholar]
- Spach MS, Heidlage JF, Dolber PC, Barr RC. Electrophysiological effects of remodeling cardiac gap junctions and cell size: experimental and model studies of normal cardiac growth. Circ Res. 2000;86:302–11. doi: 10.1161/01.res.86.3.302. [DOI] [PubMed] [Google Scholar]
- Sperelakis N, Ramasamy L. Modeling electric field transfer of excitation at cell junctions. IEEE Eng Med Biol Mag. 2002;21:130–43. doi: 10.1109/memb.2002.1175149. [DOI] [PubMed] [Google Scholar]
- Stein M, van Veen TA, Hauer RN, de Bakker JM, van Rijen HV. A 50% reduction of excitability but not of intercellular coupling affects conduction velocity restitution and activation delay in the mouse heart. PLoS One. 2011;6:e20310. doi: 10.1371/journal.pone.0020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M, van Veen TA, Remme CA, Boulaksil M, Noorman M, van Stuijvenberg L, van der Nagel R, Bezzina CR, Hauer RN, de Bakker JM, van Rijen HV. Combined reduction of intercellular coupling and membrane excitability differentially affects transverse and longitudinal cardiac conduction. Cardiovasc Res. 2009;83:52–60. doi: 10.1093/cvr/cvp124. [DOI] [PubMed] [Google Scholar]
- Su CY, Menuz K, Reisert J, Carlson JR. Non-synaptic inhibition between grouped neurons in an olfactory circuit. Nature. 2012;492:66–71. doi: 10.1038/nature11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi J, Noma A, Irisawa H. Modification of the cardiac action potential by intracellular injection of adenosine triphosphate and related substances in guinea pig single ventricular cells. Circ Res. 1983;53:131–9. doi: 10.1161/01.res.53.2.131. [DOI] [PubMed] [Google Scholar]
- Thomas SP, Bircher-Lehmann L, Thomas SA, Zhuang J, Saffitz JE, Kleber AG. Synthetic strands of neonatal mouse cardiac myocytes: structural and electrophysiological properties. Circ Res. 2000;87:467–73. doi: 10.1161/01.res.87.6.467. [DOI] [PubMed] [Google Scholar]
- Van der Goes van Naters W. Inhibition among olfactory receptor neurons. Front Hum Neurosci. 2013;7:690. doi: 10.3389/fnhum.2013.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijen HV, de Bakker JM, van Veen TA. Hypoxia, electrical uncoupling, and conduction slowing: Role of conduction reserve. Cardiovasc Res. 2005;66:9–11. doi: 10.1016/j.cardiores.2005.02.003. [DOI] [PubMed] [Google Scholar]
- van Rijen HV, Eckardt D, Degen J, Theis M, Ott T, Willecke K, Jongsma HJ, Opthof T, de Bakker JM. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation. 2004;109:1048–55. doi: 10.1161/01.CIR.0000117402.70689.75. [DOI] [PubMed] [Google Scholar]
- Veeraraghavan R, Larsen AP, Torres NS, Grunnet M, Poelzing S. Potassium channel activators differentially modulate the effect of sodium channel blockade on cardiac conduction. Acta Physiol (Oxf) 2013;207:280–9. doi: 10.1111/j.1748-1716.2012.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraraghavan R, Lin J, Hoeker GS, Keener JP, Gourdie RG, Poelzing S. Sodium channels in the Cx43 gap junction perinexus may constitute a cardiac ephapse: an experimental and modeling study. Pflugers Arch. 2015 doi: 10.1007/s00424-014-1675-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraraghavan R, Salama ME, Poelzing S. Interstitial volume modulates the conduction velocity-gap junction relationship. Am J Physiol Heart Circ Physiol. 2012;302:H278–86. doi: 10.1152/ajpheart.00868.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann S. The electrical constants of Purkinje fibres. J Physiol. 1952;118:348–60. doi: 10.1113/jphysiol.1952.sp004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek RE, Bischoff S, Fu Y, Maier SK, Catterall WA, Scheuer T. Localization of sodium channel subtypes in mouse ventricular myocytes using quantitative immunocytochemistry. J Mol Cell Cardiol. 2013;64:69–78. doi: 10.1016/j.yjmcc.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]