Abstract
Following myocardial infarction (MI), damaged myocytes are replaced by collagenous scar tissue, which serves an important mechanical function – maintaining integrity of the heart wall against enormous mechanical forces – but also disrupts electrical function as structural and electrical remodeling in the infarct and borderzone predispose to re-entry and ventricular tachycardia. Novel emerging regenerative approaches aim to replace this scar tissue with viable myocytes. Yet an alternative strategy of therapeutically modifying selected scar properties may also prove important, and in some cases may offer similar benefits with lower risk or regulatory complexity. Here, we review potential goals for such modifications as well as recent proof-of-concept studies employing specific modifications, including gene therapy to locally increase conduction velocity or prolong the refractory period in and around the infarct scar, and modification of scar anisotropy to improve regional mechanics and pump function. Another advantage of scar modification techniques is that they have applications well beyond MI. In particular, ablation treats electrical abnormalities of the heart by intentionally generating scar to block aberrant conduction pathways. Yet in diseases such as atrial fibrillation (AF) where ablation can be extensive, treating the electrical disorder can significantly impair mechanical function. Creating smaller, denser scars that more effectively block conduction, and choosing the location of those lesions by balancing their electrical and mechanical impacts, could significantly improve outcomes for AF patients. We review some recent advances in this area, including the use of computational models to predict the mechanical effects of specific lesion sets and gene therapy for functional ablation. Overall, emerging techniques for modifying scar properties represents a potentially important important set of tools for improving patient outcomes across a range of heart diseases, whether used in place of or as an adjunct to regenerative approaches.
Keywords: ablation, atrial fibrillation, cell therapy, gene therapy, myocardial infarction, scar
1. Introduction
Following injury to a region of the heart, damaged myocytes are gradually replaced by collagenous scar. In the setting of a myocardial infarction, this scar tissue plays an important mechanical role, protecting against rupture and limiting dyskinetic bulging of the healed infarct. However, the presence of scar also predisposes many patients to life-threatening arrhythmias. By contrast, a number of electrical abnormalities of the heart are treated by intentionally damaging a small region of the heart, promoting the formation of scar that blocks aberrant conduction pathways. Yet in diseases such as atrial fibrillation where ablation can be extensive, replacing a significant volume of myocardium with scar has important mechanical consequences. Many emerging therapies – including local polymer injection, gene therapy, and cell therapy – now offer the opportunity to modify the mechanical and electrical properties of cardiac scar as well as the cells in and around damaged regions. While much of the attention surrounding regenerative approaches has focused on replacing post-infarction scar with new myocytes, we propose that simply modifying scar properties appropriately could prove an equally important strategy, benefitting not only those who suffer infarction but also the increasing numbers of patients who require ablation for atrial fibrillation. In this review, we discuss emerging strategies for modifying cardiac scar tissue and the importance of balancing electrical and mechanical effects when re-engineering scar.
2. Myocardial Infarction
Over seven million people suffer a myocardial infarction (MI) every year worldwide, (White and Chew, 2008) and the majority of patients now survive the initial event. The acute injury triggers an inflammatory response, and damaged muscle is gradually resorbed over the following days and weeks. Unlike skeletal muscle, mammalian adult cardiac muscle has a limited natural capacity to regenerate; rather, lost muscle is replaced by collagenous scar tissue. This scar solves a mechanical problem, maintaining the integrity of the heart wall even when essentially all the muscle in an injured region is lost. Yet in many cases this same scar also creates an electrical problem, greatly increasing the risk of ventricular arrhythmias. In this section, we review the natural evolution of mechanical and electrical properties as scar forms during post-infarction healing. We then review emerging therapeutic options for modifying scar to improve mechanical and/or electrical function of the heart in patients with prior MI.
2.1. Evolution of Mechanical Properties Following Infarction
Within seconds following occlusion of the coronary artery supplying a region of the heart, the affected myocardium stops contracting with each heartbeat. (Tennant and Wiggers, 1935) Instead, the ischemic muscle stretches passively as the pressure in the heart rises during systole, and recoils as it falls. (Akaishi et al., 1986; Tyberg et al., 1974) This produces an immediate drop in systolic pump function, (Sunagawa et al., 1983) not only because the ischemic muscle no longer contributes, but also because some of the work done by remaining healthy muscle is wasted in stretching the ischemic region. Prolonged coronary occlusion induces permanent damage within the first hour, and by 6 hours the damaged region begins to stiffen, most likely due to edema. (Pirzada et al., 1976; Vokonas et al., 1976) The first few days post-infarction are a particularly vulnerable time from a mechanical point of view. Damaged myocardium is being resorbed and pre-existing collagen damaged by metalloproteases released by inflammatory cells, (Sato et al., 1983; Takahashi et al., 1990; Zamilpa and Lindsey, 2010) but fibroblasts have not yet infiltrated the infarct and begun depositing substantial amounts of new collagen. It is during this vulnerable window that infarct rupture can occur. This catastrophic complication is relatively rare in humans, but quite common in mouse models. (Gao et al., 2012)
By the end of the first week, fibroblast deposition of new collagen begins to dominate the healing process, and collagen content rises rapidly over the next 3-6 weeks. (Fomovsky and Holmes, 2010; Jugdutt and Amy, 1986; Jugdutt, 2003) Most studies show that scar stiffness increases in parallel with collagen content, (Fomovsky and Holmes, 2010; Holmes et al., 2005) but there have been some intriguing exceptions. Gupta et al. conducted ex vivo mechanical testing of sheep scar and found that stiffness peaked at 2-3 weeks and then fell, even as collagen content continued to increase. (Gupta et al., 1994) Similarly, McGarvey estimated scar stiffness in pigs using a finite-element model and in vivo MRI data and found that estimated scar stiffness peaked at 1 week and then decreased progressively until 12 weeks. (McGarvey et al., 2015) One of the most surprising recent discoveries about post-infarction scar is that collagen content and organization vary widely across different animal models (Figure 1). In some cases the collagen fibers in the scar become highly aligned in one direction and the scar is mechanically anisotropic (highly resistant to stretch in the fiber direction, while relatively compliant in other directions (Gupta et al., 1994; Holmes et al., 1997)); describing such scars as either ‘soft’ or ‘stiff’ is a substantial oversimplification. In other cases – such as the standard rat left coronary ligation model – the scar is structurally and mechanically isotropic. (Fomovsky and Holmes, 2010) Furthermore, collagen area fractions measured histologically in the standard rat model are only 30%, (Fomovsky and Holmes, 2010) compared to 60-70% in the dog and pig. (Clarke et al., 2015; Fomovsky and Holmes, 2010) Fomovsky et al. showed that the pattern of mechanical stretch in the infarct varies with infarct location in rats and proposed that stretch determines collagen orientation in healing infarcts, (Fomovsky et al., 2012b) but did not offer an explanation for the variation in collagen density among animal models. it is not yet clear whether or how collagen content, collagen alignment and mechanical properties vary with infarct location in humans.
Figure 1.
Illustration of differences in collagen content and alignment in histologic sections cut parallel to the epicardial surface at approximately 50% transmural depth in different experimental models and imaged under polarized light (Unpublished images of scars from studies by Fomovsky, (Fomovsky and Holmes, 2010) Holmes, (Holmes et al., 1997) and Clarke (Clarke et al., 2015)). A Rat, 3 weeks after ligation of the LAD. B Dog, 8 weeks after ligation of the left anterior descending (LAD) coronary artery. C Pig, 3 weeks after ligation of a branch of the left circumflex (LCx) coronary artery.
2.2. Evolution of Electrical Properties Following Infarction
Throughout the course of infarct healing, changes in the electrophysiological substrate lead to an increased propensity for development of malignant ventricular arrhythmias and sudden cardiac death through a variety of arrhythmogenic mechanisms (abnormal automaticity, triggered activity, and reentry). (Janse and Wit, 1989) Moreover, while there has been considerable emphasis on structural remodeling after MI, the electrophysiological changes that occur in the heart during infarct evolution are of critical importance as they contribute to potential arrhythmogenic complications for years after the initial event. (Qin et al., 1996)
Within minutes after the onset of ischemia, several electrophysiological changes occur in the ischemic myocardium including depolarization of the resting membrane potential, reduced cellular excitability, reduction in the action-potential (AP) upstroke velocity (slope of phase 0) and amplitude, and disruption of calcium handling (calcium overload). (Carmeliet, 1999; Janse and Wit, 1989) Significant electrical uncoupling of cardiomyocytes occurs followed by irreversible cell damage and the disruption of gap junctions. (Hoyt et al., 1990) The end result of these changes is an increased risk for development of malignant ventricular arrhythmias through all three basic arrhythmogenic mechanisms: 1) increased risk for reentry due to the conduction and repolarization changes; 2) increased risk of delayed after-depolarization (DADs) and triggered arrhythmias possibly due to myocardial stretch and calcium abnormalities; and 3) abnormal automaticity due to the depolarized resting membrane potential. The characteristic arrhythmia during acute ischemia is polymorphic ventricular tachycardia (VT), which may deteriorate into ventricular fibrillation and cardiac arrest and often may be the presenting symptom of ischemic heart disease. Ventricular arrhythmias are also frequent following resumption of coronary perfusion either spontaneously or due to clinical intervention. Several different processes may underlie these arrhythmias including generation of reactive oxygen species, intracellular calcium overload, activation of the sodium-calcium exchanger in the reverse mode, and conduction and repolarization heterogeneities caused by variable recovery of individual myocytes from ischemia. (Opie and Coetzee, 1988)
In the hours and days following an acute MI, before significant fibrosis is present, surviving cardiomyocytes demonstrate changes associated with partial electrophysiological and membrane recovery. (Gardner et al., 1985; Lue and Boyden, 1992) Abnormalities in several ionic currents result in depolarization of the resting membrane potential, leading to inactivation of sodium channels, lower action-potential amplitude and reduced upstroke velocity, and slowed conduction. In addition to the ionic abnormalities, this period is characterized by disruption of cell-cell contacts as gap junctions close and migrate away from cell borders, development of non-uniform anisotropy, and slowed conduction across the transverse orientation of cells in the infarct borderzone. (Dillon et al., 1988; Gardner et al., 1985) Arrhythmias in this period could of course be related to ongoing or repeated ischemic insults, but may also be related to the aforementioned electrophysiological and gap junction remodeling that may predispose to reentry, triggered activity, or abnormal automaticity.
Weeks to months after MI, the infarct scar border is now comprised of myocytes interdigitated with extracellular matrix. Whereas abnormal automaticity and triggered activity related to calcium overload in the form of after-depolarizations are the most common mechanisms for arrhythmia initiation during acute ischemia, disruptions in conductance and the facilitation of reentrant circuits is the clinical paradigm of chronic post-infarction ventricular arrhythmia. (Benito and Josephson, 2012) Reentry in the chronic phase of an MI originates from and relies on surviving myocardium within the scar, separated by scar tissue but connected through abnormal gap junctions and generally disordered intercellular coupling. (Lazzara and Scherlag, 2003; Peters et al., 1997) Fibrosis and abnormal cell communication can facilitate re-entry by slowing conduction, creating conduction blocks, and generating an alternate pathway for the electrical impulse to re-enter. Other electrophysiological changes that can also contribute to arrhythmogenesis during this stage include AP duration (APD) prolongation associated with post-infarction hypertrophy and remodeling, which may be related to a decrease in the repolarizing potassium currents. (Antzelevitch et al., 1991; Bénitah et al., 1993; Thollon et al., 1989) In addition, there are important differences in the time course of repolarization in the remodeled epicardial regions compared to endocardial regions. This creates regional heterogeneity (differences between the scar, borderzone, and healthy myocardium) as well as transmural dispersion of refractoriness which predispose patients to re-entrant arrhythmias. (Qin et al., 1996) APD prolongation can also facilitate early after-depolarizations that can generate triggered arrhythmias. (Habbab and El-Sherif, 1990)
2.3. Making Better Infarct Scar
In the first hours after MI, interventional therapies such as thrombolytics, angioplasty, and coronary stenting aim to limit the amount of muscle damaged by the infarction by reopening blocked arteries. However, after this initial window, current therapies do not actually treat the infarct. Cholesterol-lowering and anti-hypertensive drugs can reduce the risk of a second infarct in some patients. (White and Chew, 2008) Beta-blockers and angiotensin converting enzyme (ACE) inhibitors aim to slow the onset of post-infarction heart failure by limiting neurohormonal and geometric remodeling in the surviving myocardium. Radiofrequency catheter ablation (O'Donnell et al., 2002) (usually attempted in only a minority of the relevant patient population) aims to treat monomorphic re-entrant ventricular tachycardias by destroying surviving myocardium responsible for re-entrant circuits, which may be undesirable in patients with compromised contractile function. Implantable cardioverter-defibrillators (ICDs) sense and respond to arrhythmic events; although they prolong life, they may worsen quality of life (especially in the presence of inappropriate shocks), (Czosek et al., 2012) and are associated with various complication such as infection and lead and device malfunctions. (Gould and Krahn, 2006)
Several early attempts to directly treat the infarct region failed, arguably due to insufficient understanding of the complex evolution of infarct mechanical and electrical properties. One striking example was the administration of post-infarction steroids. Steroids were shown in animal studies to reduce damage during ischemia, but in a clinical study they unexpectedly increased infarct size and triggered life-threatening arrhythmias. (Roberts et al., 1976) Subsequent studies showed that not only steroids but also non-steroidal inflammatories such as ibuprofen and indomethacin could promote infarct expansion (dilation and thinning of the infarct region), (Brown et al., 1983; Hammerman et al., 1983a, 1983b; Mannisi et al., 1987) which increases wall stress in the infarct and border region and impairs LV function. Similarly, anti-arrhythmic drugs were shown to suppress inducibility and spontaneous initiation of ventricular arrhythmias related to previous myocardial infarction. (Garan et al., 1986) Yet in clinical trials, these drugs actually increased (Echt et al., 1991) or at best had a neutral effect (Singh et al., 1995) on mortality in post-infarction patients. One of the main reasons for this is that because these drugs act globally in the heart (not just at the abnormal substrate) and prolong APD, they themselves may be associated with the development of malignant arrhythmias (pro-arrhythmia). This arrhythmogenic risk stems both from triggered activity mechanisms (e.g. EADs) and from changes in the substrate such as APD prolongation, which can facilitate re-entry.
Decades of work since those early studies now provide a much better understanding of infarct healing and the evolution of mechanical and electrical properties in the infarct region. Furthermore, sophisticated computational models that encapsulate current understanding allow in silico screening of potential modifications that facilitate designing and testing of novel interventions. Finally, emerging technologies such as gene therapy, cell therapy, and injectable biomaterials provide a much greater range of options for specifically targeting the infarct region. Together, these developments provide enormous potential for designing a new class of therapies that improve long-term survival following myocardial infarction by targeting the scar. Here, we review some of the emerging ideas for such therapies.
2.3.1. Encouraging Compaction
The single most important determinant of the mechanical impact of an infarct is its size. The degree of functional impairment, (Pfeffer et al., 1979) extent of adverse LV remodeling, (Masci et al., 2011) and likelihood of developing heart failure, (Zimmer et al., 1990) following infarction all correlate with infarct size. The recognition that reducing infarct size is arguably the most valuable single therapeutic intervention following MI has driven the development of protocols for rapid identification of coronary events and aggressive intervention to reopen blocked arteries. Yet it has long been thought that once the initial damage is done, infarct size either remains the same or increases through the processes of infarct extension and infarct expansion. (Hutchins and Bulkley, 1978) Infarct extension refers to necrosis of additional myocardium at the borders of the original infarct due to the local mismatch between elevated wall stresses and oxygen demand and reduced regional blood flow; by contrast, infarct expansion refers to radial thinning and stretching of the infarct in the circumferential-longitudinal plane, both of which can increase wall stresses and functional impairment even without the loss of additional myocardium.
Intriguing recent data suggest that in contrast to this conventional view, infarcts can compact spontaneously, reducing their circumferential and longitudinal dimensions and thereby effectively decreasing infarct size. Richardson and Holmes recently compiled data from more than 50 studies that reported quantitative data on changes in infarct dimensions over time. (Richardson and Holmes, 2015) They found while all reports showed infarct expansion over the first 24 hours, over the ensuing weeks only 50% documented further increase in circumferential or longitudinal dimensions consistent with infarct expansion, while 23% reported compaction (reduced dimensions) and the rest observed no significant change. Furthermore, measurements in unloaded hearts were less likely to show expansion, suggesting that in some cases increased dimensions are simply due to increased stretching under load rather than geometric rearrangement or remodeling.
The presence of spontaneous compaction in some healing infarcts suggests that intrinsic mechanisms exist that could be harnessed therapeutically. In an exciting potential step in this direction, multiple groups have now shown that they can encourage scar compaction by manipulating Wnt signaling in healing mouse infarcts. (Barandon et al., 2003; Kobayashi et al., 2009; Laeremans et al., 2011) Most recently, Laeremans treated infarcted mice for up to 5 weeks with a peptide that blocked binding of Wnt3a and Wnt5a to Frizzled-1 and -2 receptors. Treatment increased infarct thickness and myofibroblast density on histology, while reducing in-plane infarct dimensions relative to saline-treated controls. (Laeremans et al., 2011) Importantly, the peptide exerted similar effects even when treatment was delayed until 2 weeks post-MI, suggesting that the mechanism is independent of initial effects on infarct size. Together with earlier data from the same group showing that mouse strains with the highest infarct myofibroblast concentrations at 14 days experience the least thinning between 14 and 28 days, (van den Borne et al., 2009) these data suggest that increasing the number of myofibroblasts in the infarct may help encourage compaction.
2.3.2. Controlling Collagen Alignment
Immediately after infarction, stretching and bulging of the damaged region impairs systolic function of the heart. Therefore, one obvious strategy for improving post-infarction pump function would be to stiffen the infarct region. In fact, some recent studies have claimed to improve heart function by exactly this mechanism, injecting biomaterials to modulate infarct material properties. However, a closer inspection of the evidence shows clearly that while infarct stiffening can decrease cavity volumes – which may have the important benefit of limiting dilation – it does not improve pump function. By contrast, inspired by the finding that infarct scar can be highly anisotropic, Fomovsky and colleagues showed that selective reinforcement of large anterior infarcts in only the longitudinal direction does significantly improve function. (Fomovsky et al., 2012a, 2011) Thus, one way to improve post-infarction scar would be to direct collagen fiber alignment to optimize LV function.
In 1980, Bogen and colleagues used a simple computational model to explore the impact of changing infarct stiffness on left ventricular (LV) function. During acute ischemia, when the infarct material properties are similar to those of normal passive myocardium, they predicted a sharp drop in systolic function without any change in diastolic function. (Bogen et al., 1980) As they increased infarct stiffness, systolic performance (reflected in the end-systolic pressure-volume relationship, ESPVR) returned towards normal; however, diastolic compliance dropped, resulting in a steeper end-diastolic pressure-volume relationship (EDPVR). When they constructed simulated ventricular function curves, plotting predicted stroke volume against end-diastolic pressure, they found that the systolic benefits and diastolic impairment offset, producing very little change in stroke volume at most filling pressures over a wide range of infarct stiffness. This remarkably prescient study has stood the test of time: state-of-the-art finite-element models incorporating realistic three-dimensional geometry and fiber anatomy, nonlinear material properties, and other complex features of heart mechanics ignored by Bogen have all reached exactly the same conclusion: stiffening infarcts isotropically (equally in all directions) has almost no effect on predicted ventricular function curves. (Fomovsky et al., 2011; Wall et al., 2006)
Contradicting these remarkably consistent model predictions by multiple groups over more than three decades, numerous recent experimental studies claim improved LV function following injection of various biopolymers. We propose that much of the apparent difference between these modeling and experimental studies arises from the fact that most of the experimental studies relied on ejection fraction (EF) as their primary functional measure, rather than varying preload and constructing ventricular function curves. Unfortunately, EF is particularly difficult to interpret in the setting of myocardial infarction because end-diastolic volume (EDV), end-systolic volume (ESV), and hemodynamics are all changing at once. (Richardson et al., 2015) All of the modeling studies discussed above would predict increases in ejection fraction with infarct stiffening despite unchanged pump function, because predicted stroke volumes were unchanged while EDV and ESV decreased as the ESPVR and EDPVR shifted leftward. In support of this explanation, Ryan et al. reported absolute LV volumes immediately following injection of a stiff dermal filler into acute sheep infarcts; they showed that EDV and ESV decreased but cardiac output did not change. (Ryan et al., 2009) However, there is also a more optimistic explanation for some of the differences: the computational modeling studies considered only acute effects of infarct stiffening, while some polymer injection studies showed that infarct stiffening reduced adverse LV remodeling, which in turn prevented some of the functional deterioration observed in the untreated comparison group. (Landa et al., 2008; Leor et al., 2009; Ryan et al., 2009)
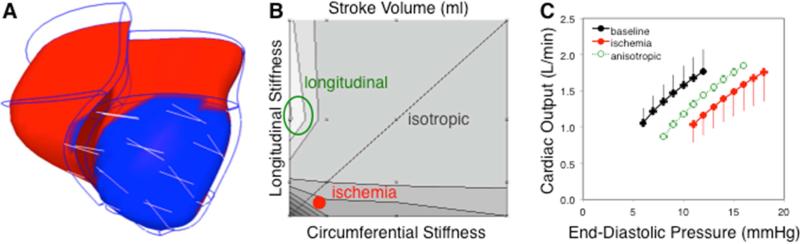
Recently, Fomovsky et al. revisited the inherent tradeoff between systolic and diastolic function originally discovered by Bogen. They realized that prior studies had treated infarcts as isotropic (having the same mechanical properties in all directions), while more recent data (see section 2.1) showed that infarct scar can be highly anisotropic. Therefore, Fomovsky repeated earlier computational studies of infarct stiffening, this time exploring the effects of many different combinations of circumferential and longitudinal stiffness in a realistic finite-element model of a canine heart with a large anteroapical infarct induced by LAD occlusion (Figure 2). (Fomovsky et al., 2011) To their surprise, the models showed that an infarct that was very stiff in the longitudinal direction but compliant in the circumferential direction – a combination never reported in naturally-occurring infarcts – could significantly enhance pump function, improving systolic performance with minimal impairment of filling. These investigators then went on to confirm the model prediction by surgically reinforcing acute anteroapical infarcts in dogs and measuring ventricular function curves over a range of filling pressures. As predicted by the model, cardiac output at matched EDP dropped by nearly half during acute ischemia, and selective reinforcement in only the longitudinal direction restored nearly half the deficit (Figure 2). (Fomovsky et al., 2012a)
Figure 2.
Impact of infarct anisotropy on LV function. A Image of a finite-element model of the infarcted canine heart employed by Fomovsky et al. (Fomovsky et al., 2011) to study the effect of anisotropy. B Contour plot of predicted stroke volume (SV) shows that isotropically increasing both circumferential and longitudinal stiffness (dotted diagonal line) provided little improvement in SV at matched EDP and ESP compared to acute ischemia (solid red circle). By contrast, selectively stiffening the infarct in the longitudinal direction substantially increased SV (green circle). C Surgically reinforcing large, acute anteroapical infarcts in only the longitudinal direction confirmed the model predictions: ischemia (filled red circles) shifted the cardiac output curve down and to the right as expected, while longitudinal reinforcement (open green circles) restored about 50% of the functional deficit. (Fomovsky et al., 2012a)
Although the study by Fomovsky and colleagues provided a proof-of-concept that infarct anisotropy can improve LV function, surgical reinforcement is likely too invasive to be viable as a therapeutic approach for generating and controlling anisotropy in patients. Rather, anisotropy represents one design goal for engineering “better” scar. Realizing this goal will require understanding the factors that govern collagen alignment in infarct scar, and devising methods to guide alignment based on that understanding. Rouillard et al. recently published a computational model that suggests progress in understanding the factors that determine scar collagen alignment. (Rouillard and Holmes, 2012) This agent-based model simulated fibroblast infiltration and remodeling of healing infarcts, and reproduced measured patterns of collagen alignment across multiple animal models, as well as reported transmural variations in infarct scar collagen structure. The key factors affecting collagen alignment in this model were the local orientation of pre-existing matrix and muscle fibers prior to infarction and the pattern of mechanical stretching during healing. However, their model integrated in vitro data on fibroblast responses to individual signals such as stretch and collagen alignment to predict in vivo behavior; it did not represent the signaling pathways and molecular biology underlying the fibroblast responses. Accordingly, the Rouillard model could be used to design therapies to control collagen alignment by modifying environmental cues such as stretch, but not to design therapies that reprogram cells at the molecular level to respond differently to a given environment. Thus, while scar anisotropy offers an intriguing therapeutic goal, much work remains to realize the goal of engineering anisotropy in situ.
2.3.3. Conceptual Framework for Preventing and Treating Post-Infarction Arrhythmias
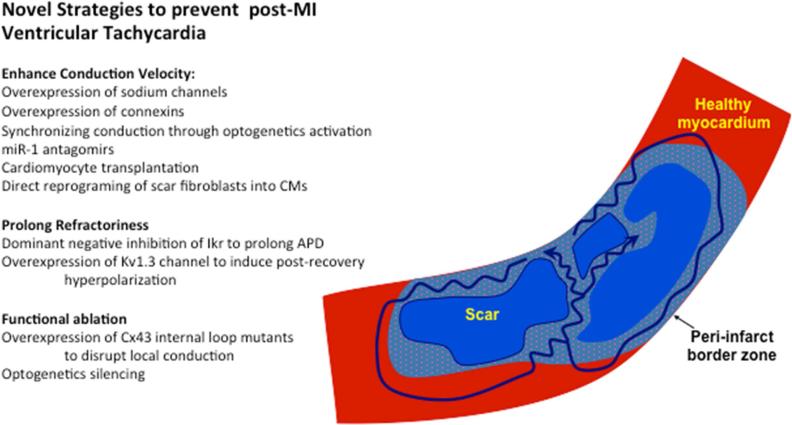
Numerous studies in both animal models and patients have demonstrated that post-MI ventricular arrhythmias are mainly due to reentry (de Bakker et al., 1988) and that the abnormal substrate at the infarct borderzone plays an important role in their development. (de Bakker et al., 1993, 1988; Dillon et al., 1988; Peters et al., 1997) The established concept is that areas of slow conduction in the surviving myocardium of the infarct borderzone allow for reentry. Consequently, as illustrated in the schematic representation in Figure 3, a properly timed premature ventricular beat may propagate through the slowly conduction surviving muscle fibers within the infarct. If this slowing of conduction leads to a delay in impulse propagation that is longer than the ventricular tissue refractory period, the impulse may re-excite the healthy ventricular tissue and then re-enter the scar, initiating a reentrant circuit and ventricular tachycardia (VT). Based on this understanding, three conceptual approaches can be devised to prevent post-infarction reentrant VT (Figure 3). These include: 1) eliminating the presence of slowly-conducting pathways within the infarct (as done for example by radiofrequency catheter ablation (O'Donnell et al., 2002)); 2) improving the conduction properties of the infarct borderzone; and 3) increasing or homogenizing refractoriness at the abnormal substrate area, thereby rendering this path incapable of supporting reentry. The result of enhancing conduction velocity (CV, measured in mm/ms) or prolonging the tissue's refractory period (RP, measured in ms) is an increase in a parameter termed the tissue wavelength (χ), which is the product of CV × RP. The wavelength (measured in mm) represents the minimal tissue path that can support and maintain reentry. Thus, any intervention that will increase the wavelength will decrease the likelihood for reentrant ventricular arrhythmias, while conditions that will reduce wavelength (decreasing CV or RP) will increase the probability for such arrhythmias.
Figure 3.
Mechanisms and novel strategies to prevent post-infarction re-entrant ventricular tachycardia (VT). The infarct borderzone consists of islands of viable myocytes (dotted blue) interspersed with collagenous scar tissue (blue). Healthy tissue outside the infarct is shown in red. Note that the reentrant VT circuits (identified by arrows) depend on the presence of slow conduction within the scar. Novel strategies to prevent/eliminate post MI can be grouped into interventions that augment conduction, prolong refractoriness, or induce functional ablation.
In general, conduction velocity can be influenced by interventions affecting the inherent excitability of the myocytes and specifically of the AP upstroke velocity (mediated by membrane ion channels such as sodium channels), the degree of cell-to-cell coupling (mediated by gap junction conduction and fibrosis) and the structural properties of the tissue. The refractory period of the tissue is governed by factors that affect AP repolarization, and can be influenced by altering the balance between outward repolarizing currents (potassium channels) and inward currents (sodium and calcium currents) during this period.
2.3.4. Increasing Conduction Velocity
Unfortunately, there are no clinically approved or effective pharmacological strategies that can improve myocardial conduction. However, compounds are being developed to augment gap junction function. One such compound, Rotigaptide (ZP123), was shown to reduce atrial fibrillation vulnerability in a canine model. (Guerra et al., 2006) While its method of action on gap junctions (thought to involve modulation of gap junction phosphorylation) is not completely understood, some studies have also demonstrated a ventricular antiarrhythmic effect, albeit primarily in animal models associated with active ischemia. (Hennan et al., 2006; Li et al., 2011)
In depolarized myocardial infarct borderzones, the cardiac sodium channel is largely inactivated. (Pu and Boyden, 1997) This reduction in sodium current density leads to a lower AP upstroke velocity (Vmax) and consequentially to slow conduction, which may precipitate the development of reentrant VT. In an initial attempt to overcome this cellular dysfunction, Lau et al. tested the hypothesis that myocardial excitability and conduction velocity could be improved and reentrant arrhythmias prevented by introduction of a sodium channel with activity at the relatively depolarized resting membrane potential found in damaged myocytes. (Lau et al., 2009) The investigators delivered the skeletal muscle sodium channel isoform, SkM1, which has a more positive voltage-dependence of inactivation than the cardiac variant (SCN5A), to the epicardial borderzone of a canine chronic infarct model using adenovirus. Epicardial mapping, programmed electrical stimulation, and microelectrode recordings demonstrated that overexpression of the SkM1 channel increased Vmax of the AP, improved conduction velocity, and suppressed VT inducibility when compared to control animals. (Lau et al., 2009) An alternative gene delivery strategy focuses on improving cell-to-cell coupling between cardiomyocytes in the infarct borderzone through overexpression of the major gap junction protein connexin43 (C×43). To this end, Greener et al. compared the effects of intracoronary adenoviral delivery of the C×43 transgene (AdC×43) to the infarcted area in a pig model of post-infarction VT to treatment with control vector (Adβgal), or no gene transfer. (Greener et al., 2012) The animals receiving AdC×43 had less electrogram fractionation and faster conduction velocity in the anterior-septal borderzone and a significant reduction in the inducibility for VT.
Finally, while the majority of gene transfer strategies aiming to modify electrophysiological function have directly overexpressed the gene of interest, recent studies have also attempted to affect regulators of translation such as microRNAs. For example, Yang and colleagues (Yang et al., 2007) noted an increase in miR-1 after myocardial infarction in both rats and humans that was associated with suppression of C×43 expression (resulting in slowing of conduction) and Kir2.1 expression (potassium channel responsible for the resting membrane potential-maintaining current IK1). By blocking miR-1 activity with specific antagomirs in the rat model, the authors could enhance conduction velocity and hyperpolarize the resting membrane potential and eventually suppress arrhythmia vulnerability.
Clinical translation of the exciting approaches described above has to overcome, however, some of the limitations associated with gene therapy, including the possible inflammatory response to viral vectors and the presence of neutralizing antibodies, the relatively transient transgene expression achieved by some vectors (adenoviral gene delivery systems), and unpredictable levels of transgene expression within the tissue. An alternative approach that can overcome some of the shortcomings of gene therapy may be the use of genetically modified cell grafts, which can be initially transfected ex vivo to express different ion channels or gap junction modulators and then transplanted to the in vivo heart. The general hypothesis is that the engineered non-myocyte cells (such as fibroblasts) would couple with host cardiomyocytes and influence the local myocardial electrophysiological properties (suppressing or augmenting excitability based on the type of channel expressed) via electrotonic interactions. To evaluate the potential of this strategy to improve conduction, Hofshi et al. established “molecular conducting-cables” by genetically modifying HEK cells to express the SCN5A-encoded sodium channel. (Hofshi et al., 2011) In this proof-of-concept co-culture study, SCN5A-expressing HEK cells were able to functionally couple with cultured cardiomyocytes and bridge conduction in an in vitro model of conduction block. A more recent study demonstrated that this effect can be recapitulated in a three-dimensional tissue-engineered model and that the effect may even be amplified by utilizing engineered cells that express not only sodium channels but also Kir2.1 potassium channels (to lower the resting membrane potential and thereby increase the amplitude of the resulting sodium current) as well as C×43 to increase gap junction coupling. (Kirkton and Bursac, 2011)
One potential caveat in using the gene therapy and combined gene and cell therapy strategies described above is the inability to externally control the function of the cells once genetically modified. A potentially elegant strategy to add external control on the modulation of excitability is the use of optogenetics. Optogenetics, using light to activate specific light-sensitive proteins, has revolutionized neuroscience by enabling activation or suppression of neural circuits both in vitro and in vivo with high spatial and temporal precision. (Boyden et al., 2005) The most widely used protein has been Channelorhodopsin-2 (ChR2), a non-selective light-sensitive cationic channel initially identified in the green-alga Chlamydomonas. (Nagel et al., 2003) More recently, the same strategy was transferred to the cardiac field where it was demonstrated that light activation of ChR2 overexpressed in cardiomyocytes can be used to pace the heart both in vitro and in vivo. (Bruegmann et al., 2010; Nussinovitch and Gepstein, 2015a) The ability to alter ventricular conduction using optogenetics was recently described, where AAV delivery of ChR2 to multiple sites in the rat ventricle combined with multisite illumination allowed the investigators to synchronize and shorten global ventricular activation time in an optogenetics-based implementation of cardiac resynchronization therapy (CRT). (Nussinovitch and Gepstein, 2015a) The same multisite optogenetics approach could theoretically be used to synchronize conduction within the infarct borderzone. The feasibility of this approach was recently demonstrated in an in vitro model of slow conduction and conduction block where diffuse illumination could restore synchronized electrical activity in the model. (Nussinovitch et al., 2014)
2.3.5. Prolonging Refractoriness
The most effective anti-arrhythmic drugs are those that block potassium channels and thus increase APD and refractoriness. Surprisingly these drugs were not associated with a survival benefit in post-infarction patients, probably due to their global cardiac action and significant pro-arrhythmic effects. Gene and cell therapies, in contrast, may have the potential to achieve the desired effect only at the area of interest, increasing the chances of therapeutic success while limiting potential side effects. The idea behind these strategies is to increase the probability of disrupting reentry at the treated areas by causing the activation wavefront to meet refractory tissues.
An elegant gene therapy approach to prolong refractoriness at the infarct borderzone was described by the Donahue group. (Sasano et al., 2006) This group hypothesized that overexpression of a transgene encoding for a dominant-negative mutation in the pore region of the KCNH2/HERG channel (KCNH2-G628S) would lead to loss of the IKr current in infected cardiomyocytes, leading to AP prolongation and prolongation of the refractory period. Impressively, adenoviral delivery of the KCNH2-G628S transgene to the infarcted area completely abolished the ability to induce VT in a reproducible pig model of post-MI VT. Interestingly, this gene therapy strategy was superior (potentially because of the resulting complete loss of IKr) to the results obtained in the same animal model through pharmacological blockade of the same KCNH2 potassium-channel with the antiarrhythmic drug dofetilide: the drug-treated animals still had inducible VTs and also developed proarrhythmic events, not seen in the gene therapy group.
To evaluate the alternative approach of using genetically modified cell-grafts to extend refractoriness, Feld et al. transfected fibroblasts to express the voltage-gated potassium channel Kv1.3. (Feld et al., 2002) In contrast to the dominant-negative approach described above, in which the aim was to prolong APD and RP by suppressing repolarizing potassium currents, Feld aimed paradoxically to augment potassium currents using a channel with unique biophysical properties. The Kv1.3 channel and specifically its mutated form H4031W are characterized by rapid inactivation on one hand and a prolonged tail current on the other hand. (Marom and Levitan, 1994) The hypothesis was that the engineered cells would prolong the refractory period in the coupled host cardiomyocytes through hyperpolarizing electrotonic currents acting at the immediate post-repolarization phase (early diastolic period), and would therefore prevent the initiation of a new action-potential (AP) during this period. Detailed computer modeling, (Feld et al., 2002) in vitro (co-culture), (Feld et al., 2002) and in vivo (Yankelson et al., 2008) studies in small and large animals demonstrated the validity of this concept by showing the development of localized conduction blocks or extension of the RP at the site of cell grafting, which were reversible upon the application of a specific Kv1.3 blocker.
A somewhat different approach attempts not to extend the RP at the infarct borderzone but rather to functionally completely dissociate the slow conducting tissue within the scar from the rest of the ventricle. For example, Kizana et al. employed a gene transfer strategy to suppress local gap junction communication using lentiviral vectors encoding for C×43 internal loop mutants. (Kizana et al., 2007) In an in vitro model, gap junction dye transfer studies and optical mapping experiments provided evidence for localized uncoupling at the sites of gene transfer. Optogenetics may also offer an alternative approach to silencing localized electrical activity through external control using light-sensitive proteins that induce hyperpolarizing currents. To test the feasibility of this concept Nussinovitch et al. generated cell grafts that express the light-sensitive proton pump Archorhodopsin. (Nussinovitch and Gepstein, 2015b) Activation of these cells by light in cardiomyocyte co-culture resulted in targeted localized suppression of the electrical activity. If the same concepts could be transferred to the in vivo setting, the ability to functionally isolate the slow conducting areas may prevent or terminate VT.
3. Ablation of Atrial Fibrillation
Atrial fibrillation (AF) affects more than 30 million individuals worldwide and is increasing in prevalence, particularly in Western countries with aging populations. (Andrade et al., 2014a; Chugh et al., 2014; Lloyd-Jones et al., 2004) A diagnosis of AF is associated with a doubling in all-cause mortality, a five-fold increase in stroke, an accelerated development of heart failure, and a substantially poorer quality of life. (McManus et al., 2012) Treatment options for AF include pharmacologic therapy, electric cardioversion, and ablation, which attempts to electrically isolate ectopic foci of electrical activity and/or interrupt aberrant conduction pathways by destroying myocardium using radiofrequency energy (RF ablation), freeze injury (cryoablation), or other techniques. Although ablation was initially reserved for severe cases of AF that did not respond to other approaches, the success of ablation relative to other options in preventing AF recurrence has led to a rapid increase in its use across the spectrum of AF patients. (Santangeli et al., 2014; Wright and Narayan, 2015) While AF ablation can in many cases restore normal conduction patterns, reducing the risk of stroke and other complications, there is emerging evidence that ablation can significantly impair long-term atrial mechanical function (Cochet et al., 2014) and induce other complications such as asymptomatic cerebral embolism. (Haines, 2013) Accordingly, the question of how to balance trade-offs between the risks and benefits when deciding when to perform ablation and how much damage to induce in individual patients is an important topic of active research and discussion.
3.1. Rationale for AF Ablation Procedures
There are two main strategies for the electrical management of AF, rhythm control and rate control. Several studies have shown non-inferiority of rhythm control compared to rate control in terms of survival benefit, (Carlsson et al., 2003; Hohnloser et al., 2000; Van Gelder et al., 2002; Wyse et al., 2002) and thus rhythm control is only indicated for patients who suffer from symptoms of AF, or those who cannot receive adequate rate control and suffer from tachycardia induced cardiomyopathy. (January et al., 2014) One of the potential reasons that rhythm control has not demonstrated superiority to rate control strategies is the ineffective and often unsafe nature of currently available anti-arrhythmic medications, which at best maintain sinus rhythm for 1 year in 50% of patients, and are associated with an increase in mortality. (Lafuente-Lafuente et al., 2007) When interrogating only patients who have achieved and maintained sinus rhythm, post-hoc analyses of rhythm vs. rate control strategies have shown a consistent pattern of improvement in quality of life measures. (Guédon-Moreau et al., 2010; Singh et al., 2006) In the PIAF and HOT CAFE trials for example, 6-minute walk times and maximal treadmill workloads were increased. (Hohnloser et al., 2000; Opolski et al., 2004) In the Canadian Trial of Atrial Fibrillation, quality of life measures were significantly improved at 3 months from baseline in patients who achieved sinus rhythm. (Dorian and Mangat, 2003) Thus, invasive catheter ablation has emerged as an alternate approach to achieving durable rhythm control.
A major target for AF ablation has been the pulmonary veins, which generate ectopic beats that are frequent triggers for atrial fibrillation. These can be directly targeted and ablated with some long-term success. (Haïssaguerre et al., 1998) The procedure has evolved to target the proximal insertion points of the pulmonary veins to the left atrium (pulmonary vein isolation, PVI), with ongoing and rapid improvements in catheter and mapping technologies. However, this procedure predominantly targets and treats AF triggers, and thus has demonstrated the greatest benefit in patients with paroxysmal AF of short duration, absence of cardiac structural disease and comorbidities, and without signs of atrial remodeling such as dilatation or fibrosis. (Bhargava et al., 2009; Chang et al., 2007; Gaita et al., 2008; Medi et al., 2011; Ouyang et al., 2010; Pappone et al., 2003; Tzou et al., 2010) While AF invariably begins as isolated short-lived episodes (“paroxysmal” AF), each paroxysm accelerates atrial remodeling, thereby promoting longer-lasting “persistent” AF (i.e. “AF begets AF”). (Anné et al., 2007; Wijffels et al., 1995) In an effort to target the underlying substrate, many other ablation strategies have evolved, including the ablation of complex fractionated atrial electrograms (CFAEs), ablation of cardiac autonomic nerves, creating linear lesions that connect anatomical substrates, atrial debulking to reduce the amount of electrically viable atrial tissue, and mapping and ablation of cardiac rotors. (Earley and Schilling, 2006; Miller et al., 2014; Narayan et al., 2014)
3.2. Evolution of Electrical Properties Following AF Ablation
AF is associated with structural and electrophysiologic remodeling in a time-dependent process of adaptation. These changes can occur at the ionic and genomic level within 30 minutes, at the cellular level within 1 week, and at the cellular and extracellular matrix level over one month. (Casaclang-Verzosa et al., 2008) The result is a spectrum of adaptive and maladaptive processes including hypertrophy, necrosis, and apoptosis, as well as changes in the composition of the extracellular matrix, energy production, hormone regulation and ion channels. All of these have structural and functional consequences to the heart, many of which increase its propensity to develop and sustain AF. (Kojodjojo et al., 2007) There are many well described changes to the electrical system after the onset of episodes of AF, including shortening of the effective refractory period, shortening of the action potential duration, reductions in atrial conduction and maximum diastolic potential, and sympathetic hyperinnervation. (Everett et al., 2006, 2000; Mary-Rabine et al., 1983; Miyauchi et al., 2003; Morillo et al., 1995; Solti et al., 1989) Some of these changes can regress after restoration of sinus rhythm, as demonstrated by a gradual prolongation of the atrial effective refractory period and its rate adaptation after cardioversion, while others such as conduction velocity may not. (Yu et al., 1999) The hope of any strategy in a progressive disease such as AF would be to modify its course and reverse its trajectory.
Some evidence indicates that patients indeed demonstrate a reversal of the maladaptive electrophysiologic changes associated with AF after ablation. Case studies have demonstrated an increase in the voltages measured in the left atrium, and a corresponding decrease in the amount of low voltage areas typically associated with slowed conduction and reentry, after successful PVI, (Lo et al., 2006) but overall the evidence for beneficial electrical remodeling is limited and large studies are lacking. There is also a risk that the ablation will in fact promote arrhythmias by introducing heterogeneity in conduction velocities, incomplete lines predisposing to macroreentrant rhythms, and an increase in scar burden with the consequent risk of increased microreentrant rhythms. In animal models, ablation of ganglionated plexi is associated with an increase in the atrial effective refractory period in the short term, however in the long term nerve densities and the atrial effective refractory period returned to pre-ablation levels by 6 months. Interestingly, AF was more inducible in the treatment group compared to the control group at 6 and 12 months. (Wang et al., 2015) A second study targeting the epicardial ganglionated plexi in dogs similarly showed an increase in atrial arrhythmias, which was attributed to an overall decrease in the atrial effective refractory period and hyper-reinnervation of both the sympathetic and parasympathetic nerves. (Mao et al., 2014) By contrast, the sinus node seems to have the capability to remodel electrically after AF ablation, and has shown the potential to improve to normal function. Patients with pathophysiologic sinus pauses on termination of AF that underwent PVI demonstrated an improvement in mean, max and range of heart rate and a decrease in the time it takes for the sinus node to recover from overdrive suppression. (Hocini et al., 2003)
3.3. Evolution of Mechanical Properties Following AF Ablation
Surprisingly little is known about mechanical properties of human atrial tissue, and even less about how those properties change following ablation. Bellini and colleagues performed biaxial mechanical testing on porcine (Bellini and Di Martino, 2012) and human (Bellini et al., 2013) atrial tissue. They found that atrial tissue displays the nonlinear stress-strain behavior typical of most soft tissues in the body and is mechanically anisotropic (having different resistance to stretch in different directions). This anisotropy complicates interpretation of measures generated by ultrasound techniques such as shear-wave elastography and acoustic radiation force imaging. Nevertheless, multiple groups have now shown that deformability as indexed by these techniques drops immediately following RF ablation in vivo. (Bahnson et al., 2014; Kwiecinski et al., 2014) Interestingly, this shear stiffness is quite stable from 2-30 minutes after ablation in the center of the lesion, while the stiffness of adjacent myocardium increases more slowly; (Eyerly et al., 2015) Eyerly et al. suggested that the immediate response may reflect changes in tissue properties due to thermal damage, while the slower response in adjacent tissue reflects edema. (Eyerly et al., 2015) Less is known about the evolution of mechanical properties beyond the first few minutes post-ablation, other than the fact that scar tissue forms in damaged regions and is presumably stiffer than the surrounding myocardium.
The passive and active mechanical properties of the atrial myocardium are only two of the determinants of overall atrial function; atrial size, hemodynamic coupling to the pulmonary circulation and left ventricle, and physical interactions with surrounding structures in the chest are also important factors. (Moyer et al., 2015) Normally, the left atrium gradually fills with blood during LV systole, empties passively following mitral valve opening, reaches diastasis – during which blood flows from the pulmonary veins into the LV but atrial volume remains constant – and then contracts (“active emptying”) just prior to LV contraction. In young healthy subjects, active emptying plays a relatively minor role, accounting for just 20-25% of total emptying. (Alhogbani et al., 2010; Spencer et al., 2001) However, reliance on active emptying increases with age, accounting for 55% of emptying by age 60-70, (Alhogbani et al., 2010; Spencer et al., 2001) while the contribution from passive emptying decreases reciprocally. (Boyd et al., 2011) Reliance on active emptying may be even higher in AF patients; it contributed 61% of total emptying in a recent study of paroxysmal AF patients who were imaged in normal sinus rhythm immediately prior to AF ablation. (Moyer et al., 2015)
The effects of ablation on overall atrial function reflect a balance among three competing factors. First, during fibrillation active function is lost and passive filling/emptying are compromised; thus, function in patients with permanent/persistent AF who are successfully treated can only improve. Second, AF atria are typically enlarged, and this dilation imposes a mechanical disadvantage on the myocardium; thus, any reverse remodeling due to treatment should also augment atrial function. However, the third factor is that ablation destroys atrial myocardium and replaces it with scar tissue, which has the potential to disrupt both passive and active function. Possibly due to the different balance of these effects in different patient populations and ablation procedures, there is significant variability in the reported effects of ablation on atrial mechanical function. In patients with mild disease (“lone AF”), Donal reported reduced size and improved passive compliance and active contractility 1 year following successful ablation. (Donal et al., 2010) In a mixed population of patients with persistent and paroxysmal AF, Thomas found reduced atrial size in patients who remained in sinus rhythm 1 year following ablation, but these atria remained larger and achieved less active emptying than in control subjects. (Thomas et al., 2003) In a meta-analysis of 869 patients with a range of severities of AF, Jeevanantham concluded that patients without recurrence had a small but significant reduction in LA size with no significant change in atrial EF. (Jeevanantham et al., 2010) A more recent meta-analysis of 25 studies using echocardiography, cardiac CT and cardiac MR in 2040 patients with paroxysmal and persistent AF found similar overall trends (lower LA diameter and volumes without a change in EF); however, left atrial ejection fraction decreased significantly in patients with paroxysmal AF. (Xiong et al., 2015) Focusing on the most severe end of the disease spectrum, Cochet recently reported long-term followup (>5 years) of patients with persistent AF who required >2 procedures on average; in these patients, active emptying was strongly and negatively correlated with both the cumulative duration of RF pulses used in the ablation procedures and the amount of scar quantified from contrast-enhanced MRI. (Cochet et al., 2014)
3.4. Making Better Post-Ablation Scar
Ablation is now a mainstay treatment for patients with atrial fibrillation, but two aspects remain ripe for improvement. First, electrical pathways that are interrupted during the procedure often re-connect during recovery, leading to AF recurrence. Second, the destruction of myocardium during the procedure can impair subsequent mechanical function. More aggressive ablation patterns that target larger regions of the atrium may solve the first problem (electrical re-connection), but at the expense of exacerbating the second (impaired mechanics). Accordingly, some of the most exciting emerging therapies offer the possibility of circumventing this electrical-mechanical tradeoff by producing smaller, denser lesions that better interrupt conduction; designing novel lesion patterns that balance electrical and mechanical effects; or even locally altering the electrical properties of atrial myocytes without perturbing their mechanical function. Examples of these approaches are presented below.
3.4.1. Preventing Re-connection
The principle underlying ablative arrhythmia surgery is the creation of conduction block via endocardial or epicardial lesion sets that are continuous, linear, and transmural. Incomplete lesion sets can lead to recurrence, but can also be proarrhythmic by creating areas of slow conduction and diseased myocardium that have the potential to perpetuate re-entrant arrhythmias, or initiate focal tachycardias. For paroxysmal AF, the most important factor driving recurrence rates post-ablation is PV reconnection, and thus there has been a significant effort to improve ablation technology to create permanent and complete lesions. (Nishida et al., 2014) Technological improvements in the 1980s included replacing direct current radiofrequency (RF) ablation with continuous-wave unmodulated RF energy. RF creates tissue destruction through resistive heating of tissues to temperatures >50 degrees Celsius. (Andrade et al., 2014b) Many technologic improvements followed, including the use of active electrode cooling with continuous irrigation, and irrigated RF ablation has for some time been the standard of care for ablation of arrhythmias in the systemic circulation (such as AF and VT). (Macle et al., 2002) One of the most important determinants for lesion size, depth and durability is contact, which until recently has been gauged by several surrogate measures. The most recent innovation in RF ablation technology, the contact-sensing force catheter, allows for real time quantitative feedback and has been shown to improve procedural and outcome measures as well as safety profiles. (Neuzil et al., 2013; Reddy et al., 2012) However, there are remaining limitations to RF ablation, and thus novel energy sources have been sought.
Cryoablation was in fact the first alternative to the surgical dissection of arrhythmic substrates in the 1970s, and it now represents the most promising of the novel ablation energy sources for the treatment of AF. Cryothermal ablation induces both direct cellular damage secondary to hypothermia, and ischemic necrosis through its effects on the microcirculation. (Skanes et al., 2002) Experiments in ventricular tissue suggest that cryoablation produces dense homogeneous fibrosis that is generally nonarrhythmogenic, while preserving the underlying extracellular matrix and tensile strength. (Klein et al., 1979) Head-to-head trials of the dominant cryotherapy delivery system (the second-generation balloon) compared to contact-sensing force irrigated RF ablation catheters have generally shown similar results in terms of procedural safety and clinical anti-arrhythmic efficacy. (Jourda et al., 2015) Most recently, the use of adenosine to reveal viable but temporarily nonconducting tissue, which has been damaged by ablation but not destroyed (dormant conduction), has shown benefit in helping electrophysiologists further target areas at high risk of reconnection. (Macle et al., 2015)
3.4.2. Functional Ablation
As discussed above, extensive atrial ablation, while potentially preventing or reducing atrial fibrillation burden, may negatively impact the mechanical properties of the left atrium due to a combination of 1) significant loss of atrial tissue by the ablation lesions and 2) alteration of contraction pattern of the left atrium and electromechanical isolation of some atrial regions. A possible approach to limiting this mechanical impairment is to utilize some of the innovative cell and gene therapy strategies described above for treatment of post-MI VT to perform functional (rather than destructive) atrial ablation. One attractive approach is to use the localized gene therapy or combined cell-gene therapy strategies described in section 2.3.5 to modulate cellular potassium currents and extend local atrial refractoriness. The functional lesions generated would act as a low-pass filter, blocking rapid electrical activity during atrial fibrillation and preventing conduction of premature atrial beats. In contrast, at slow physiological rates (sinus rhythm) conduction would proceed without alteration, resulting in a physiological activation of the left atrium with potentially improved mechanical performance relative to ablation strategies.
In order to prolong local atrial APD and refractoriness, a number of investigators have attempted to reduce outward potassium currents through local gene delivery. In a similar manner to their work in the left ventricle, the Donahue group utilized the unique properties of the KCNH2-G628S mutant channel, a dominant-negative mutation that blocks within the ion channel pore region to suppress the IKr current. (Kikuchi et al., 2005) Using a focal atrial painting strategy, the authors were able to deliver the Ad.KCNH2-G628s virus to the atrial tissue of a pig model with pacemaker induced atrial fibrillation. (Kikuchi et al., 2005) Monophasic action potential (MAP) recordings demonstrated significant APD prolongation at the sites of transgene delivery. Importantly, global delivery of the transgene suppressed atrial fibrillation in this model. Using a different concept to prolong atrial APD, Burton et al. used naked DNA plasmids for focal atrial delivery of the human MIRP1-Q9E mutant transgene driven by a clarithromycin inducible promoter. (Burton et al., 2003) The MIRP1-Q9E mutations are known to cause long QT syndrome due to the effects of the mutated accessory potassium subunit on decreasing delayed rectifier currents. Clarithromycin administration produced site-specific prolongation of atrial MAPs. Beyond the ability to prolong atrial RP, this study also demonstrated the feasibility of external control (by a drug) on modulation of atrial electrical activity.
3.4.3. Reducing Mechanical Dysfunction
During AF ablation procedures, the clinician chooses a pattern of lesions based on both the overall experience of the cardiac electrophysiology (EP) community with various approaches and patient-specific information such as electrical mapping data acquired during the procedure. Much of the discussion of the various potential ablation patterns has centered on their electrical efficacy; however, as reviewed above, it now seems likely that more aggressive ablation approaches that generate more scar also impair long-term mechanical function relative to less aggressive approaches. Thus, there appears to be a broad tradeoff between the electrical benefits and mechanical detriments of more aggressive ablation lesions. There is active discussion in the EP community about this tradeoff and the merits of procedures that induce more or less damage. Here, we raise the possibility that emerging data on regional function in the left atrium might provide another way to address electrical-mechanical tradeoffs: design lesion patterns that target regions that contribute less to mechanical function while sparing those that contribute more.
Recent data show that regional function in the left atrium is remarkably heterogeneous in both normal subjects and patients with AF who are imaged in sinus rhythm. Moyer et al. measured regional EF from cine-MRI and reported values ranging from nearly 40% in the inferior and lateral walls to approximately 20% in the posterior wall in healthy subjects; (Moyer et al., 2013) in patients with paroxysmal AF imaged in sinus rhythm prior to a scheduled ablation procedure, regional EF was depressed in all regions but remained highest in the inferior and lateral walls. Kuklik reported very similar findings in a separate MRI study: decreased mechanical function in patients with paroxysmal AF compared to controls, and significantly higher mechanical function of the anterior, septal and lateral regions as opposed to roof and posterior regions in both groups. (Kuklik et al., 2014) Speckle-tracking ultrasound gives similar values for strain in these regions, confirming regional variability in atrial function. (Vianna-Pinton et al., 2009)
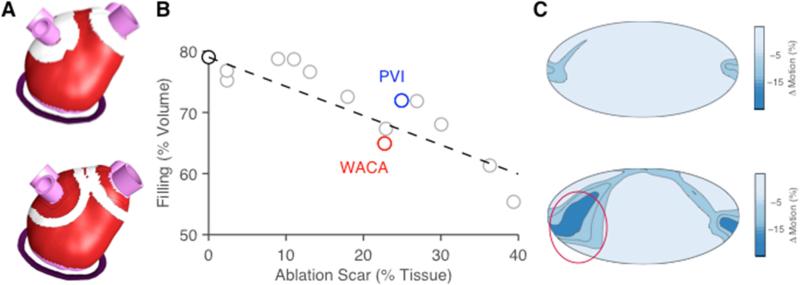
These observations raise the possibility that ablation lesions in some regions will impair mechanical function much more than ablation in others. Following this reasoning, Kuklik et al suggested that ablation in lateral, anterior and septal regions should be minimized since these regions move the most in both normal and paroxysmal AF atria. (Kuklik et al., 2014) Recently, Moyer et al. used a finite-element model of the left atrium to quantitatively evaluate the potential influence of the extent vs. location of ablation scar on atrial function (Figure 4). Starting with a model of the human left atrium validated against both invasive pressure-volume and non-invasive regional wall motion data, (Moyer et al., 2015) Moyer simulated distributions of scar in locations typical of standard pulmonary vein isolation (PVI) and wide area circumferential ablation (WACA) procedures. They assumed that scar material properties are similar to those of infarct scar, and varied the total scar volume by simply increasing or decreasing the width of the rings of simulated scar. They found that chamber compliance, total emptying, and active emptying all decrease with scar volume. (Moyer, 2013) In addition, they found that even at matched total scar volumes, WACA disrupted atrial function more than PVI, because it had a greater effect on the inferolateral wall of the atrium, where regional motion is normally greatest (Figure 4). Providing some support to these model results, Nori et al. reported that both total stroke volume and lateral wall radial excursion were decreased following ablation in paroxysmal AF patients. (Nori et al., 2009) The Moyer model reflects only the mechanical impact of ablation; however, together with recent state-of-the-art models of the electrophysiology of atrial fibrillation, these results suggest the intriguing possibility that models incorporating both electrical and mechanical consequences of ablation could be used to design novel lesion sets that optimize the balance of electrical benefits relative to functional impairment.
Figure 4.
Effect of pattern and location of simulated ablation scar on wall motion and chamber compliance during passive filling in a finite-element model of the human left atrium. (Moyer, 2013) A Located of simulated scar for pulmonary vein isolation (PVI, top) and wide area circumferential ablation (WACA, bottom) lesion sets; PVI lesion width was artificially increased to match total WACA scar volume. B Across a range of simulated lesions, chamber compliance dropped with scar volume; however even at matched scar volumes, WACA impaired function more than PVI. C Maps showing the change in regional wall motion due to ablation highlight the detrimental effects of WACA in the inferolateral wall (circled region).
4. Conclusions
It is clear from the evidence reviewed here that appropriate modification of scar properties has the potential to improve mechanical and electrical function of the left ventricle following MI and the left atrium in patients with AF; there is much exciting work in progress in this area, and much more remains to be done. In the Introduction, we posed scar modification as a potential alternative to regenerative approaches such as stem cell therapy, but in fact even successful regenerative approaches (discussed elsewhere in this special issue) may require scar modification as an adjunct or prologue. For example, data reviewed in Section 2.3.2 suggest that controlling scar anisotropy can improve LV function, but controlling ECM alignment may also be essential prior to regenerating myocytes, since the ECM orientation is likely to determine the orientation of the regenerating myocytes and thereby their efficacy in contributing useful mechanical work and propagating electrical signals. Similarly, while human pluripotent stem cell-derived myocytes have been shown to electrically couple with host cardiac cells, (Chong et al., 2014; Gepstein et al., 2010; Halbach et al., 2013; Kehat et al., 2004; Rubart et al., 2003; Shiba et al., 2012) this coupling could be either anti- or proarrhythmogenic. Engraftment of regenerated cardiomyocytes could facilitate the emergence of reentrant ventricular arrhythmias by generation of new slow conduction channels within the scar, or could reduce the chances of arrhythmia if sufficient regeneration and coupling occur to significantly improving conduction. The proarrhythmogenic risk associated with poor coupling of engrafted cells was already reported in the case of skeletal myoblast transplantation, (Gepstein et al., 2010; Roell et al., 2007) and contributed to the failure of this approach in clinical trials. (Menasché et al., 2003) Similar questions remain about the electrophysiological effects of reprogramming, wherein fibroblasts in the scar area could be converted into induced cardiomyocyte-like cells through ectopic expression of cardiac transcription factors (such as Gata4, Mef2c and Tb×5, GMT combination). (Qian et al., 2012; Song et al., 2012) Interestingly, while in the mouse (Roell et al., 2007) and guinea pig (Shiba et al., 2012) infarct models cardiomyocyte transplantation was associated with improved conduction and prevention of arrhythmias, the same strategy led to a transient episodes of VTs in a non-human primate model. (Chong et al., 2014) Thus, therapeutic modulation of electrical properties in remaining scar tissue may prove an important adjunct to various approaches to regeneration. We conclude that scar modification holds substantial potential for the future, both as a stand-alone approach and as an adjunct to surgical, pharmacologic, and regenerative therapies.
Acknowledgments
The authors acknowledge financial support from the National Institutes of Health HL116449 to JWH). The funding agencies had no role in the preparation of this review article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Akaishi M, Weintraub WS, Schneider RM, Klein LW, Agarwal JB, Helfant RH. Analysis of systolic bulging. Mechanical characteristics of acutely ischemic myocardium in the conscious dog. Circ. Res. 1986;58:209–17. doi: 10.1161/01.RES.58.2.209. [DOI] [PubMed] [Google Scholar]
- Alhogbani TM, Strohm O, Matthias F. Evaluation of normal atrial contribution to left ventricular filling. J. Cardiovasc. Magn. Reson. 2010;12:O91. doi: 10.1186/1532-429X-12-S1-O91. [DOI] [Google Scholar]
- Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: Relationships among clinical features, epidemiology, and mechanisms. Circ. Res. 2014a;114:1453–1468. doi: 10.1161/CIRCRESAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- Andrade JG, Rivard L, Macle L. The past, the present, and the future of cardiac arrhythmia ablation. Can. J. Cardiol. 2014b;30:S431–41. doi: 10.1016/j.cjca.2014.07.731. [DOI] [PubMed] [Google Scholar]
- Anné W, Willems R, Holemans P, Beckers F, Roskams T, Lenaerts I, Ector H, Heidbüchel H. Self-terminating AF depends on electrical remodeling while persistent AF depends on additional structural changes in a rapid atrially paced sheep model. J. Mol. Cell. Cardiol. 2007;43:148–158. doi: 10.1016/j.yjmcc.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Antzelevitch C, Sicouri S, Litovsky SH, Lukas A, Krishnan SC, Di Diego JM, Gintant GA, Liu DW. Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ. Res. 1991;69:1427–1449. doi: 10.1161/01.RES.69.6.1427. [DOI] [PubMed] [Google Scholar]
- Bahnson TD, Eyerly SA, Hollender PJ, Doherty JR, Kim Y-J, Trahey GE, Wolf PD. Feasibility of near real-time lesion assessment during radiofrequency catheter ablation in humans using acoustic radiation force impulse imaging. J. Cardiovasc. Electrophysiol. 2014;25:1275–83. doi: 10.1111/jce.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barandon L, Couffinhal T, Ezan J, Dufourcq P, Costet P, Alzieu P, Leroux L, Moreau C, Dare D, Duplàa C. Reduction of infarct size and prevention of cardiac rupture in transgenic mice overexpressing FrzA. Circulation. 2003;108:2282–9. doi: 10.1161/01.CIR.0000093186.22847.4C. [DOI] [PubMed] [Google Scholar]
- Bellini C, Di Martino ES. A mechanical characterization of the porcine atria at the healthy stage and after ventricular tachypacing. J. Biomech. Eng. 2012;134:021008. doi: 10.1115/1.4006026. [DOI] [PubMed] [Google Scholar]
- Bellini C, Di Martino ES, Federico S. Mechanical behaviour of the human atria. Ann. Biomed. Eng. 2013;41:1478–1490. doi: 10.1007/s10439-012-0699-9. [DOI] [PubMed] [Google Scholar]
- Bénitah JP, Gomez AM, Bailly P, Da Ponte JP, Berson G, Delgado C, Lorente P. Heterogeneity of the early outward current in ventricular cells isolated from normal and hypertrophied rat hearts. J. Physiol. 1993;469:111–138. doi: 10.1113/jphysiol.1993.sp019807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito B, Josephson ME. Ventricular tachycardia in coronary artery disease. Rev. Esp. Cardiol. 2012;65:939–955. doi: 10.1016/j.recesp.2012.03.027. [DOI] [PubMed] [Google Scholar]
- Bhargava M, Di Biase L, Mohanty P, Prasad S, Martin DO, Williams-Andrews M, Wazni OM, Burkhardt JD, Cummings JE, Khaykin Y, Verma A, Hao S, Beheiry S, Hongo R, Rossillo A, Raviele A, Bonso A, Themistoclakis S, Stewart K, Saliba WI, Schweikert RA, Natale A. Impact of type of atrial fibrillation and repeat catheter ablation on long-term freedom from atrial fibrillation: Results from a multicenter study. Heart Rhythm. 2009;6:1403–1412. doi: 10.1016/j.hrthm.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Bogen DK, Rabinowitz SA, Needleman A, McMahon TA, Abelmann WH. An analysis of the mechanical disadvantage of myocardial infarction in the canine left ventricle. Circ. Res. 1980;47:728–41. doi: 10.1161/01.res.47.5.728. [DOI] [PubMed] [Google Scholar]
- Boyd AC, Schiller NB, Leung D, Ross DL, Thomas L. Atrial dilation and altered function are mediated by age and diastolic function but not before the eighth decade. JACC Cardiovasc. Imaging. 2011;4:234–242. doi: 10.1016/j.jcmg.2010.11.018. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Brown EJ, Kloner RA, Schoen FJ, Hammerman H, Hale S, Braunwald E. Scar thinning due to ibuprofen administration after experimental myocardial infarction. Am. J. Cardiol. 1983;51:877–83. doi: 10.1016/s0002-9149(83)80148-9. [DOI] [PubMed] [Google Scholar]
- Bruegmann T, Malan D, Hesse M, Beiert T, Fuegemann CJ, Fleischmann BK, Sasse P. Optogenetic control of heart muscle in vitro and in vivo. Nat. Methods. 2010;7:897–900. doi: 10.1038/nmeth.1512. [DOI] [PubMed] [Google Scholar]
- Burton DY, Song C, Fishbein I, Hazelwood S, Li Q, DeFelice S, Connolly JM, Perlstein I, Coulter DA, Levy RJ. The incorporation of an ion channel gene mutation associated with the long QT syndrome (Q9E-hMiRP1) in a plasmid vector for site-specific arrhythmia gene therapy: in vitro and in vivo feasibility studies. Hum. Gene Ther. 2003;14:907–922. doi: 10.1089/104303403765701196. [DOI] [PubMed] [Google Scholar]
- Carlsson J, Miketic S, Windeler J, Cuneo A, Haun S, Micus S, Walter S, Tebbe U. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the strategies of treatment of atrial fibrillation (STAF) study. J. Am. Coll. Cardiol. 2003;41:1690–1696. doi: 10.1016/S0735-1097(03)00332-2. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Cardiac ionic currents and acute ischemia: from channels to arrhythmias. Physiol. Rev. 1999;79:917–1017. doi: 10.1152/physrev.1999.79.3.917. [DOI] [PubMed] [Google Scholar]
- Casaclang-Verzosa G, Gersh BJ, Tsang TSM. Structural and functional remodeling of the left atrium: clinical and therapeutic implications for atrial fibrillation. J. Am. Coll. Cardiol. 2008;51:1–11. doi: 10.1016/j.jacc.2007.09.026. [DOI] [PubMed] [Google Scholar]
- Chang SL, Tai CT, Lin YJ, Wongcharoen W, Lo LW, Tuan TC, Udyavar AR, Chang SH, Tsao HM, Hsieh MH, Hu YF, Chen YJ, Chen SA. Biatrial substrate properties in patients with atrial fibrillation. J. Cardiovasc. Electrophysiol. 2007;18:1134–1139. doi: 10.1111/j.1540-8167.2007.00941.x. [DOI] [PubMed] [Google Scholar]
- Chong JJH, Yang X, Don CW, Minami E, Liu Y-W, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem H-P, Laflamme MA, Murry CE. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–7. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJL. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SA, Goodman NC, Ailawadi G, Holmes JW. Effect of scar compaction on the therapeutic efficacy of anisotropic reinforcement following myocardial infarction in the dog. J. Cardiovasc. Transl. Res. 2015;8:353–61. doi: 10.1007/s12265-015-9637-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochet H, Scherr D, Zellerhoff S, Sacher F, Derval N, Denis A, Knecht S, Komatsu Y, Montaudon M, Laurent F, Pieske BM, Hocini M, Haïssaguerre M, Jaïs P. Atrial structure and function 5 years after successful ablation for persistent atrial fibrillation: an MRI study. J. Cardiovasc. Electrophysiol. 2014;25:671–679. doi: 10.1111/jce.12449. [DOI] [PubMed] [Google Scholar]
- Czosek RJ, Bonney WJ, Cassedy A, Mah DY, Tanel RE, Imundo JR, Singh AK, Cohen MI, Miyake CY, Fawley K, Marino BS. Impact of cardiac devices on the quality of life in pediatric patients. Circ. Arrhythmia Electrophysiol. 2012;5:1064–1072. doi: 10.1161/CIRCEP.112.973032. [DOI] [PubMed] [Google Scholar]
- De Bakker JM, van Capelle FJ, Janse MJ, Tasseron S, Vermeulen JT, de Jonge N, Lahpor JR. Slow conduction in the infarcted human heart. “Zigzag” course of activation. Circulation. 1993;88:915–926. doi: 10.1161/01.CIR.88.3.915. [DOI] [PubMed] [Google Scholar]
- De Bakker JM, van Capelle FJ, Janse MJ, Wilde AA, Coronel R, Becker AE, Dingemans KP, van Hemel NM, Hauer RN. Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation. Circulation. 1988;77:589–606. doi: 10.1161/01.CIR.77.3.589. [DOI] [PubMed] [Google Scholar]
- Dillon SM, Allessie MA, Ursell PC, Wit AL. Influences of anisotropic tissue structure on reentrant circuits in the epicardial border zone of subacute canine infarcts. Circ. Res. 1988;63:182–206. doi: 10.1161/01.RES.63.1.182. [DOI] [PubMed] [Google Scholar]
- Donal E, Ollivier R, Veillard D, Hamonic S, Pavin D, Daubert J-C, Mabo P. Left atrial function assessed by trans-thoracic echocardiography in patients treated by ablation for a lone paroxysmal atrial fibrillation. Eur. J. Echocardiogr. 2010;11:845–852. doi: 10.1093/ejechocard/jeq074. [DOI] [PubMed] [Google Scholar]
- Dorian P, Mangat I. Quality of life variables in the selection of rate versus rhythm control in patients with atrial fibrillation: Observations from the Canadian Trial of Atrial Fibrillation. Card. Electrophysiol. Rev. 2003;7:276–279. doi: 10.1023/B:CEPR.0000012395.33292.cd. [DOI] [PubMed] [Google Scholar]
- Earley MJ, Schilling RJ. Catheter and surgical ablation of atrial fibrillation. Heart. 2006;92:266–274. doi: 10.1136/hrt.2005.067389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N. Engl. J. Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- Everett TH, Li H, Mangrum JM, McRury ID, Mitchell MA, Redick JA, Haines DE. Electrical, morphological, and ultrastructural remodeling and reverse remodeling in a canine model of chronic atrial fibrillation. Circulation. 2000;102:1454–1460. doi: 10.1161/01.CIR.102.12.1454. [DOI] [PubMed] [Google Scholar]
- Everett TH, Wilson EE, Verheule S, Guerra JM, Foreman S, Olgin JE. Structural atrial remodeling alters the substrate and spatiotemporal organization of atrial fibrillation: a comparison in canine models of structural and electrical atrial remodeling. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H2911–H2923. doi: 10.1152/ajpheart.01128.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyerly SA, Vejdani-Jahromi M, Dumont DM, Trahey GE, Wolf PD. The evolution of tissue stiffness at radiofrequency ablation sites during lesion formation and in the peri-ablation period. J. Cardiovasc. Electrophysiol. 2015 doi: 10.1111/jce.12709. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feld Y, Melamed-Frank M, Kehat I, Tal D, Marom S, Gepstein L. Electrophysiological modulation of cardiomyocytic tissue by transfected fibroblasts expressing potassium channels: a novel strategy to manipulate excitability. Circulation. 2002;105:522–529. doi: 10.1161/hc0402.102661. [DOI] [PubMed] [Google Scholar]
- Fomovsky GM, Clark SA, Parker KM, Ailawadi G, Holmes JW. Anisotropic reinforcement of acute anteroapical infarcts improves pump function. Circ. Hear. Fail. 2012a;5:515–522. doi: 10.1161/CIRCHEARTFAILURE.111.965731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomovsky GM, Holmes JW. Evolution of scar structure, mechanics, and ventricular function after myocardial infarction in the rat. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H221–8. doi: 10.1152/ajpheart.00495.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomovsky GM, Macadangdang JR, Ailawadi G, Holmes JW. Model-based design of mechanical therapies for myocardial infarction. J. Cardiovasc. Transl. Res. 2011;4:82–91. doi: 10.1007/s12265-010-9241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomovsky GM, Rouillard AD, Holmes JW. Regional mechanics determine collagen fiber structure in healing myocardial infarcts. J. Mol. Cell. Cardiol. 2012b;52:1083–1090. doi: 10.1016/j.yjmcc.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaita F, Caponi D, Scaglione M, Montefusco A, Corleto A, Di Monte F, Coin D, Di Donna P, Giustetto C. Long-term clinical results of 2 different ablation strategies in patients with paroxysmal and persistent atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2008;1:269–275. doi: 10.1161/CIRCEP.108.774885. [DOI] [PubMed] [Google Scholar]
- Gao X-M, White DA, Dart AM, Du X-J. Post-infarct cardiac rupture: recent insights on pathogenesis and therapeutic interventions. Pharmacol. Ther. 2012;134:156–179. doi: 10.1016/j.pharmthera.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Garan H, Stavens CS, McGovern B, Kelly E, Ruskin JN. Reproducibility of ventricular tachycardia suppression by antiarrhythmic drug therapy during serial electrophysiologic testing in coronary artery disease. Am. J. Cardiol. 1986;58:977–980. doi: 10.1016/S0002-9149(86)80022-4. [DOI] [PubMed] [Google Scholar]
- Gardner PI, Ursell PC, Fenoglio JJ, Wit AL. Electrophysiologic and anatomic basis for fractionated electrograms recorded from healed myocardial infarcts. Circulation. 1985;72:596–611. doi: 10.1161/01.CIR.72.3.596. [DOI] [PubMed] [Google Scholar]
- Gepstein L, Ding C, Rehemedula D, Wilson EE, Yankelson L, Caspi O, Gepstein A, Huber I, Olgin JE. In vivo assessment of the electrophysiological integration and arrhythmogenic risk of myocardial cell transplantation strategies. Stem Cells. 2010;28:2151–2161. doi: 10.1002/stem.545. [DOI] [PubMed] [Google Scholar]
- Gould P, Krahn A. Complications associated with implantable cardioverterdefibrillator replacement in response to device advisories. JAMA. 2006;295:1907–1911. doi: 10.1001/jama.295.16.1907. [DOI] [PubMed] [Google Scholar]
- Greener ID, Sasano T, Wan X, Igarashi T, Strom M, Rosenbaum DS, Donahue JK. Connexin43 gene transfer reduces ventricular tachycardia susceptibility after myocardial infarction. J. Am. Coll. Cardiol. 2012;60:1103–1110. doi: 10.1016/j.jacc.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guédon-Moreau L, Capucci A, Denjoy I, Morgan CC, Périer A, Leplège A, Kacet S. Impact of the control of symptomatic paroxysmal atrial fibrillation on health-related quality of life. Europace. 2010;12:634–642. doi: 10.1093/europace/euq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra JM, Everett TH, Lee KW, Wilson E, Olgin JE. Effects of the gap junction modifier rotigaptide (ZP123) on atrial conduction and vulnerability to atrial fibrillation. Circulation. 2006;114:110–118. doi: 10.1161/CIRCULATIONAHA.105.606251. [DOI] [PubMed] [Google Scholar]
- Gupta KB, Ratcliffe MB, Fallert MA, Edmunds LH, Bogen DK. Changes in passive mechanical stiffness of myocardial tissue with aneurysm formation. Circulation. 1994;89:2315–26. doi: 10.1161/01.CIR.89.5.2315. [DOI] [PubMed] [Google Scholar]
- Habbab MDA, El-Sherif N. Drug-induced torsades de pointes: role of early afterdepolarizations and dispersion of repolarization. Am. J. Med. 1990;89:241–246. doi: 10.1016/0002-9343(90)90307-Y. [DOI] [PubMed] [Google Scholar]
- Haines DE. Asymptomatic cerebral embolism and atrial fibrillation ablation: What price victory? Circ. Arrhythmia Electrophysiol. 2013;6:455–457. doi: 10.1161/CIRCEP.113.000539. [DOI] [PubMed] [Google Scholar]
- Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998;339:659–666. doi: 10.1097/00045415-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Halbach M, Peinkofer G, Baumgartner S, Maass M, Wiedey M, Neef K, Krausgrill B, Ladage D, Fatima A, Saric T, Hescheler J, Müller-Ehmsen J. Electrophysiological integration and action potential properties of transplanted cardiomyocytes derived from induced pluripotent stem cells. Cardiovasc. Res. 2013;100:432–440. doi: 10.1093/cvr/cvt213. [DOI] [PubMed] [Google Scholar]
- Hammerman H, Kloner RA, Hale S, Schoen FJ, Braunwald E. Dose-dependent effects of short-term methylprednisolone on myocardial infarct extent, scar formation, and ventricular function. Circulation. 1983a;68:446–52. doi: 10.1161/01.CIR.68.2.446. [DOI] [PubMed] [Google Scholar]
- Hammerman H, Kloner RA, Schoen FJ, Brown EJ, Hale S, Braunwald E. Indomethacin-induced scar thinning after experimental myocardial infarction. Circulation. 1983b;67:1290–5. doi: 10.1161/01.CIR.67.6.1290. [DOI] [PubMed] [Google Scholar]
- Hennan JK, Swillo RE, Morgan GA, Keith JC, Schaub RG, Smith RP, Feldman HS, Haugan K, Kantrowitz J, Wang PJ, Abu-Qare A, Butera J, Larsen BD, Crandall DL. Rotigaptide (ZP123) prevents spontaneous ventricular arrhythmias and reduces infarct size during myocardial ischemia/reperfusion injury in open-chest dogs. J. Pharmacol. Exp. Ther. 2006;317:236–243. doi: 10.1124/jpet.105.096933.Coronel. [DOI] [PubMed] [Google Scholar]
- Hocini M, Sanders P, Deisenhofer I, Jaïs P, Hsu LF, Scavée C, Weerasoriya R, Raybaud F, Macle L, Shah DC, Garrigue S, Le Metayer P, Clémenty J, Haïssaguerre M. Reverse remodeling of sinus node function after catheter ablation of atrial fibrillation in patients with prolonged sinus pauses. Circulation. 2003;108:1172–1175. doi: 10.1161/01.CIR.0000090685.13169.07. [DOI] [PubMed] [Google Scholar]
- Hofshi A, Itzhaki I, Gepstein A, Arbel G, Gross GJ, Gepstein L. A combined gene and cell therapy approach for restoration of conduction. Heart Rhythm. 2011;8:121–130. doi: 10.1016/j.hrthm.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation--Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. Lancet. 2000 doi: 10.1016/S1062-1458(01)00270-7. [DOI] [PubMed] [Google Scholar]
- Holmes JW, Borg TK, Covell JW. Structure and mechanics of healing myocardial infarcts. Annu. Rev. Biomed. Eng. 2005;7:223–53. doi: 10.1146/annurev.bioeng.7.060804.100453. [DOI] [PubMed] [Google Scholar]
- Holmes JW, Nuñez JA, Covell JW. Functional implications of myocardial scar structure. Am. J. Physiol. 1997;272:H2123–30. doi: 10.1152/ajpheart.1997.272.5.H2123. [DOI] [PubMed] [Google Scholar]
- Hoyt RH, Cohen ML, Corr PB, Saffitz JE. Alterations of intercellular junctions induced by hypoxia in canine myocardium. Am. J. Physiol. 1990;258:H1439–H1448. doi: 10.1152/ajpheart.1990.258.5.H1439. [DOI] [PubMed] [Google Scholar]
- Hutchins GM, Bulkley BH. Infarct expansion versus extension: two different complications of acute myocardial infarction. Am. J. Cardiol. 1978;41:1127–1132. doi: 10.1016/0002-9149(78)90869-X. [DOI] [PubMed] [Google Scholar]
- Janse MJ, Wit AL. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol. Rev. 1989;69:1049–1169. doi: 10.1152/physrev.1989.69.4.1049. [DOI] [PubMed] [Google Scholar]
- January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW, American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2014;64:e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Jeevanantham V, Ntim W, Navaneethan SD, Shah S, Johnson AC, Hall B, Shah A, Hundley WG, Daubert JP, Fitzgerald D. Meta-analysis of the effect of radiofrequency catheter ablation on left atrial size, volumes and function in patients with atrial fibrillation. Am. J. Cardiol. 2010;105:1317–1326. doi: 10.1016/j.amjcard.2009.12.046. [DOI] [PubMed] [Google Scholar]
- Jourda F, Providencia R, Marijon E, Bouzeman A, Hireche H, Khoueiry Z, Cardin C, Combes N, Combes S, Boveda S, Albenque J-P. Contact-force guided radiofrequency vs. second-generation balloon cryotherapy for pulmonary vein isolation in patients with paroxysmal atrial fibrillation-a prospective evaluation. Europace. 2015;17:225–31. doi: 10.1093/europace/euu215. [DOI] [PubMed] [Google Scholar]
- Jugdutt BI. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation. 2003;108:1395–403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- Jugdutt BI, Amy RWM. Healing after myocardial infarction in the dog: changes in infarct hydroxyproline and topography. J. Am. Coll. Cardiol. 1986;7:91–102. doi: 10.1016/S0735-1097(86)80265-0. [DOI] [PubMed] [Google Scholar]
- Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, Huber I, Satin J, Itskovitz-Eldor J, Gepstein L. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat. Biotechnol. 2004;22:1282–1289. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, McDonald AD, Sasano T, Donahue JK. Targeted modification of atrial electrophysiology by homogeneous transmural atrial gene transfer. Circulation. 2005;111:264–270. doi: 10.1161/01.CIR.0000153338.47507.83. [DOI] [PubMed] [Google Scholar]
- Kirkton RD, Bursac N. Engineering biosynthetic excitable tissues from unexcitable cells for electrophysiological and cell therapy studies. Nat. Commun. 2011;2:300. doi: 10.1038/ncomms1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizana E, Chang CY, Cingolani E, Ramirez-Correa GA, Sekar RB, Abraham MR, Ginn SL, Tung L, Alexander IE, Marbán E. Gene transfer of connexin43 mutants attenuates coupling in cardiomyocytes: novel basis for modulation of cardiac conduction by gene therapy. Circ. Res. 2007;100:1597–1604. doi: 10.1161/CIRCRESAHA.106.144956. [DOI] [PubMed] [Google Scholar]
- Klein GJ, Harrison L, Ideker RF, Smith WM, Kasell J, Wallace AG, Gallagher JJ. Reaction of the myocardium to cryosurgery: electrophysiology and arrhythmogenic potential. Circulation. 1979;59:364–372. doi: 10.1161/01.CIR.59.2.364. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Luo M, Zhang Y, Wilkes DC, Ge G, Grieskamp T, Yamada C, Liu T-C, Huang G, Basson CT, Kispert A, Greenspan DS, Sato TN. Secreted Frizzled-related protein 2 is a procollagen C proteinase enhancer with a role in fibrosis associated with myocardial infarction. Nat. Cell Biol. 2009;11:46–55. doi: 10.1038/ncb1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojodjojo P, Peters NS, Davies DW, Kanagaratnam P. Characterization of the electroanatomical substrate in human atrial fibrillation: the relationship between changes in atrial volume, refractoriness, wavefront propagation velocities, and AF burden. J. Cardiovasc. Electrophysiol. 2007;18:269–275. doi: 10.1111/j.1540-8167.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- Kuklik P, Molaee P, Podziemski P, Ganesan AN, Brooks AG, Worthley SG, Sanders P. Quantitative description of the 3D regional mechanics of the left atrium using cardiac magnetic resonance imaging. Physiol. Meas. 2014;35:763–775. doi: 10.1088/0967-3334/35/5/763. [DOI] [PubMed] [Google Scholar]
- Kwiecinski W, Provost J, Dubois R, Sacher F, Haïssaguerre M, Legros M, Nguyen-Dinh A, Dufait R, Tanter M, Pernot M. Quantitative evaluation of atrial radio frequency ablation using intracardiac shear-wave elastography. Med. Phys. 2014;41:112901. doi: 10.1118/1.4896820. [DOI] [PubMed] [Google Scholar]
- Laeremans H, Hackeng TM, van Zandvoort MAMJ, Thijssen VLJL, Janssen BJA, Ottenheijm HCJ, Smits JFM, Blankesteijn WM. Blocking of frizzled signaling with a homologous peptide fragment of wnt3a/wnt5a reduces infarct expansion and prevents the development of heart failure after myocardial infarction. Circulation. 2011;124:1626–35. doi: 10.1161/CIRCULATIONAHA.110.976969. [DOI] [PubMed] [Google Scholar]
- Lafuente-Lafuente C, Mouly S, Longas-Tejero MA, Bergmann JF. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst. Rev. 2007 doi: 10.1002/14651858.CD005049.pub2. [DOI] [PubMed] [Google Scholar]
- Landa N, Miller L, Feinberg MS, Holbova R, Shachar M, Freeman I, Cohen S, Leor J. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117:1388–96. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- Lau DH, Clausen C, Sosunov EA, Shlapakova IN, Anyukhovsky EP, Danilo P, Rosen TS, Kelly C, Duffy HS, Szabolcs MJ, Chen M, Robinson RB, Lu J, Kumari S, Cohen IS, Rosen MR. Epicardial border zone overexpression of skeletal muscle sodium channel SkM1 normalizes activation, preserves conduction, and suppresses ventricular arrhythmia an in silico, in vivo, in vitro study. Circulation. 2009;119:19–27. doi: 10.1161/CIRCULATIONAHA.108.809301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzara R, Scherlag BJ. Mechanisms of monomorphic ventricular tachycardia in coronary artery disease. J. Interv. Card. Electrophysiol. 2003;8:87–92. doi: 10.1023/A:1023651231389. [DOI] [PubMed] [Google Scholar]
- Leor J, Tuvia S, Guetta V, Manczur F, Castel D, Willenz U, Petneházy O, Landa N, Feinberg MS, Konen E, Goitein O, Tsur-Gang O, Shaul M, Klapper L, Cohen S. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in swine. J. Am. Coll. Cardiol. 2009;54:1014–23. doi: 10.1016/j.jacc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Li JS, Zhong JQ, Zeng QX, Liu HZ, Su GY, Zhang Y. Effect of ZP123, a gap junction modifier, on prolonged ventricular fibrillation in swine. Cardiology. 2011;118:147–152. doi: 10.1159/000328016. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D'Agostino RB, Massaro JM, Beiser A, Wolf PA, Benjamin EJ. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- Lo LW, Tai CT, Lin YJ, Chang SL, Wongcharoen W, Chen SA. Reverse atrial electrical remodeling after catheter ablation of persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2006;17:798–799. doi: 10.1111/j.1540-8167.2006.00470.x. [DOI] [PubMed] [Google Scholar]
- Lue WM, Boyden PA. Abnormal electrical properties of myocytes from chronically infarcted canine heart. Alterations in Vmax and the transient outward current. Circulation. 1992;85:1175–1188. doi: 10.1161/01.CIR.85.3.1175. [DOI] [PubMed] [Google Scholar]
- Macle L, Jaïs P, Weerasooriya R, Hocini M, Shah DC, Choi K-J, Scavée C, Raybaud F, Clémenty J, Haïssaguerre M. Irrigated-tip catheter ablation of pulmonary veins for treatment of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2002;13:1067–1073. doi: 10.1046/j.1540-8167.2002.01067.x. [DOI] [PubMed] [Google Scholar]
- Macle L, Khairy P, Weerasooriya R, Novak P, Verma A, Willems S, Arentz T, Deisenhofer I, Veenhuyzen G, Scavée C, Jaïs P, Puererfellner H, Levesque S, Andrade JG, Rivard L, Guerra PG, Dubuc M, Thibault B, Talajic M, Roy D, Nattel S, ADVICE trial investigators Adenosine-guided pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation: an international, multicentre, randomised superiority trial. Lancet (London, England) 2015;386:672–9. doi: 10.1016/S0140-6736(15)60026-5. [DOI] [PubMed] [Google Scholar]
- Mannisi JA, Weisman HF, Bush DE, Dudeck P, Healy B. Steroid administration after myocardial infarction promotes early infarct expansion. A study in the rat. J. Clin. Invest. 1987;79:1431–9. doi: 10.1172/JCI112971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Yin X, Zhang Y, Yan Q, Dong J, Ma C, Liu X. Ablation of epicardial ganglionated plexi increases atrial vulnerability to arrhythmias in dogs. Circ. Arrhythm. Electrophysiol. 2014;7:711–7. doi: 10.1161/CIRCEP.113.000799. [DOI] [PubMed] [Google Scholar]
- Marom S, Levitan IB. State-dependent inactivation of the Kv3 potassium channel. Biophys. J. 1994;67:579–589. doi: 10.1016/S0006-3495(94)80517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mary-Rabine L, Albert A, Pham TD, Hordof A, Fenoglio JJ, Malm JR, Rosen MR. The relationship of human atrial cellular electrophysiology to clinical function and ultrastructure. Circ. Res. 1983;52:188–199. doi: 10.1161/01.RES.52.2.188. [DOI] [PubMed] [Google Scholar]
- Masci PG, Ganame J, Francone M, Desmet W, Lorenzoni V, Iacucci I, Barison A, Carbone I, Lombardi M, Agati L, Janssens S, Bogaert J. Relationship between location and size of myocardial infarction and their reciprocal influences on post-infarction left ventricular remodelling. Eur. Heart J. 2011;32:1640–8. doi: 10.1093/eurheartj/ehr064. [DOI] [PubMed] [Google Scholar]
- McGarvey JR, Mojsejenko D, Dorsey SM, Nikou A, Burdick JA, Gorman JH, Jackson BM, Pilla JJ, Gorman RC, Wenk JF. Temporal changes in infarct material properties: an in vivo assessment using magnetic resonance imaging and finite element simulations. Ann. Thorac. Surg. 2015 doi: 10.1016/j.athoracsur.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus DD, Rienstra M, Benjamin EJ. An update on the prognosis of patients with atrial fibrillation. Circulation. 2012;126:e143–6. doi: 10.1161/CIRCULATIONAHA.112.129759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medi C, Sparks PB, Morton JB, Kistler PM, Halloran K, Rosso R, Vohra JK, Kumar S, Kalman JM. Pulmonary vein antral isolation for paroxysmal atrial fibrillation: results from long-term follow-up. J. Cardiovasc. Electrophysiol. 2011;22:137–141. doi: 10.1111/j.1540-8167.2010.01885.x. [DOI] [PubMed] [Google Scholar]
- Menasché P, Hagège AA, Vilquin J-T, Desnos M, Abergel E, Pouzet B, Bel A, Sarateanu S, Scorsin M, Schwartz K, Bruneval P, Benbunan M, Marolleau J-P, Duboc D. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J. Am. Coll. Cardiol. 2003;41:1078–1083. doi: 10.1016/S0735-1097(03)00092-5. [DOI] [PubMed] [Google Scholar]
- Miller JM, Kowal RC, Swarup V, Daubert JP, Daoud EG, Day JD, Ellenbogen KA, Hummel JD, Baykaner T, Krummen DE, Narayan SM, Reddy VY, Shivkumar K, Steinberg JS, Wheelan KR. Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: multicenter FIRM registry. J. Cardiovasc. Electrophysiol. 2014 doi: 10.1111/jce.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi Y, Zhou S, Okuyama Y, Miyauchi M, Hayashi H, Hamabe A, Fishbein MC, Mandel WJ, Chen LS, Chen PS, Karagueuzian HS. Altered atrial electrical restitution and heterogeneous sympathetic hyperinnervation in hearts with chronic left ventricular myocardial infarction: implications for atrial fibrillation. Circulation. 2003;108:360–366. doi: 10.1161/01.CIR.0000080327.32573.7C. [DOI] [PubMed] [Google Scholar]
- Morillo CA, Klein GJ, Jones DL, Guiraudon CM. Chronic rapid atrial pacing. Structural, functional, and electrophysiological characteristics of a new model of sustained atrial fibrillation. Circulation. 1995;91:1588–1595. doi: 10.1161/01.CIR.91.5.1588. [DOI] [PubMed] [Google Scholar]
- Moyer CB. PhD Dissertation. University of Virginia; 2013. Mechanical function of the left atrium. [Google Scholar]
- Moyer CB, Helm PA, Clarke CJ, Budge LP, Kramer CM, Ferguson JD, Norton PT, Holmes JW. Wall-motion based analysis of global and regional left atrial mechanics. IEEE Trans. Med. Imaging. 2013;32:1765–1776. doi: 10.1109/TMI.2013.2264062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer CB, Norton PT, Ferguson JD, Holmes JW. Changes in global and regional mechanics due to atrial fibrillation: insights from a coupled finite-element and circulation model. Ann. Biomed. Eng. 2015;43:1600–13. doi: 10.1007/s10439-015-1256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. U. S. A. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan SM, Baykaner T, Clopton P, Schricker A, Lalani GG, Krummen DE, Shivkumar K, Miller JM. Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared with trigger ablation alone: extended follow-up of the CONFIRM trial (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) J. Am. Coll. Cardiol. 2014;63:1761–1768. doi: 10.1016/j.jacc.2014.02.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuzil P, Reddy VY, Kautzner J, Petru J, Wichterle D, Shah D, Lambert H, Yulzari A, Wissner E, Kuck KH. Electrical reconnection after pulmonary vein isolation is contingent on contact force during initial treatment: results from the EFFICAS I study. Circ. Arrhythmia Electrophysiol. 2013;6:327–333. doi: 10.1161/CIRCEP.113.000374. [DOI] [PubMed] [Google Scholar]
- Nishida K, Datino T, Macle L, Nattel S. Atrial fibrillation ablation: translating basic mechanistic insights to the patient. J Am Coll Cardiol. 2014;65:560–569. doi: 10.1016/j.jacc.2014.06.1172. [DOI] [PubMed] [Google Scholar]
- Nori D, Raff G, Gupta V, Gentry R, Boura J, Haines DE. Cardiac magnetic resonance imaging assessment of regional and global left atrial function before and after catheter ablation for atrial fibrillation. J. Interv. Card. Electrophysiol. 2009;26:109–117. doi: 10.1007/s10840-009-9409-4. [DOI] [PubMed] [Google Scholar]
- Nussinovitch U, Gepstein L. Optogenetics for in vivo cardiac pacing and resynchronization. Nat. Biotechnol. 2015a;33:750–4. doi: 10.1038/nbt.3268. [DOI] [PubMed] [Google Scholar]
- Nussinovitch U, Gepstein L. Optogenetics for suppression of cardiac electrical activity in human and rat cardiomyocyte cultures. Neurophotonics. 2015b;2:031204. doi: 10.1117/1.NPh.2.3.031204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussinovitch U, Shinnawi R, Gepstein L. Modulation of cardiac tissue electrophysiological properties with light-sensitive proteins. Cardiovasc. Res. 2014;102:176–187. doi: 10.1093/cvr/cvu037. [DOI] [PubMed] [Google Scholar]
- O'Donnell D, Bourke JP, Anilkumar R, Simeonidou E, Furniss SS. Radiofrequency ablation for post infarction ventricular tachycardia. Report of a single centre experience of 112 cases. Eur. Heart J. 2002;23:1699–1705. doi: 10.1053/euhj.2001.3230. [DOI] [PubMed] [Google Scholar]
- Opie LH, Coetzee WA. Role of calcium ions in reperfusion arrhythmias: relevance to pharmacologic intervention. Cardiovasc. Drugs Ther. 1988;2:623–636. doi: 10.1007/BF00054202. [DOI] [PubMed] [Google Scholar]
- Opolski G, Torbicki A, Kosior DA, Szulc M, Wozakowska-Kapłon B, Kołodziej P, Achremczyk P. Rate control vs rhythm control in patients with nonvalvular persistent atrial fibrillation: The results of the Polish How to Treat Chronic Atrial Fibrillation (HOT CAFE) study. Chest. 2004;126:476–486. doi: 10.1378/chest.126.2.476. [DOI] [PubMed] [Google Scholar]
- Ouyang F, Tilz R, Chun J, Schmidt B, Wissner E, Zerm T, Neven K, Köktürk B, Konstantinidou M, Metzner A, Fuernkranz A, Kuck KH. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation. 2010;122:2368–2377. doi: 10.1161/CIRCULATIONAHA.110.946806. [DOI] [PubMed] [Google Scholar]
- Pappone C, Rosanio S, Augello G, Gallus G, Vicedomini G, Mazzone P, Gulletta S, Gugliotta F, Pappone A, Santinelli V, Tortoriello V, Sala S, Zangrillo A, Crescenzi G, Benussi S, Alfieri O. Mortality, morbidity, and quality of life after circumferential pulmonary vein ablation for atrial fibrillation: outcomes from a controlled nonrandomized long-term study. J. Am. Coll. Cardiol. 2003;42:185–197. doi: 10.1016/S0735-1097(03)00577-1. [DOI] [PubMed] [Google Scholar]
- Peters NS, Coromilas J, Severs NJ, Wit AL. Disturbed connexin43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation. 1997;95:988–996. doi: 10.1161/01.CIR.95.4.988. [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Pfeffer JM, Fishbein MC, Fletcher PJ, Spadaro J, Kloner RA, Braunwald E. Myocardial infarct size and ventricular function in rats. Circ. Res. 1979;44:503–12. doi: 10.1161/01.res.44.4.503. [DOI] [PubMed] [Google Scholar]
- Pirzada FA, Ekong EA, Vokonas PS, Apstein CS, Hood WB. Experimental myocardial infarction. XIII. Sequential changes in left ventricular pressure-length relationships in the acute phase. Circulation. 1976;53:970–5. doi: 10.1161/01.CIR.53.6.970. [DOI] [PubMed] [Google Scholar]
- Pu J, Boyden PA. Alterations of Na+ currents in myocytes from epicardial border zone of the infarcted heart. A possible ionic mechanism for reduced excitability and postrepolarization refractoriness. Circ. Res. 1997;81:110–119. doi: 10.1161/01.RES.81.1.110. [DOI] [PubMed] [Google Scholar]
- Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu J, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012 doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin D, Zhang ZH, Caref EB, Boutjdir M, Jain P, El-Sherif N. Cellular and ionic basis of arrhythmias in postinfarction remodeled ventricular myocardium. Circ. Res. 1996;79:461–473. doi: 10.1161/01.RES.79.3.461. [DOI] [PubMed] [Google Scholar]
- Reddy VY, Shah D, Kautzner J, Schmidt B, Saoudi N, Herrera C, Jaïs P, Hindricks G, Peichl P, Yulzari A, Lambert H, Neuzil P, Natale A, Kuck KH. The relationship between contact force and clinical outcome during radiofrequency catheter ablation of atrial fibrillation in the TOCCATA study. Heart Rhythm. 2012;9:1789–1795. doi: 10.1016/j.hrthm.2012.07.016. [DOI] [PubMed] [Google Scholar]
- Richardson WJ, Clarke SA, Holmes JW. Physiological implications of myocardial scar structure. Compr. Physiol. 2015;5:1877–1909. doi: 10.1002/cphy.c140067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson WJ, Holmes JW. Why is infarct expansion such an elusive therapeutic target? J. Cardiovasc. Transl. Res. 2015;8:421–430. doi: 10.1007/s12265-015-9652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R, DeMello V, Sobel BE. Deleterious effects of methylprednisolone in patients with myocardial infarction. Circulation. 1976;53:I204–6. [PubMed] [Google Scholar]
- Roell W, Lewalter T, Sasse P, Tallini YN, Choi B-R, Breitbach M, Doran R, Becher UM, Hwang S-M, Bostani T, von Maltzahn J, Hofmann A, Reining S, Eiberger B, Gabris B, Pfeifer A, Welz A, Willecke K, Salama G, Schrickel JW, Kotlikoff MI, Fleischmann BK, Figures S. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature. 2007;450:819–824. doi: 10.1038/nature06321. [DOI] [PubMed] [Google Scholar]
- Rouillard AD, Holmes JW. Mechanical regulation of fibroblast migration and collagen remodelling in healing myocardial infarcts. J. Physiol. 2012;590:4585–602. doi: 10.1113/jphysiol.2012.229484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubart M, Pasumarthi KBS, Nakajima H, Soonpaa MH, Nakajima HO, Field LJ. Physiological coupling of donor and host cardiomyocytes after cellular transplantation. Circ. Res. 2003;92:1217–1224. doi: 10.1161/01.RES.0000075089.39335.8C. [DOI] [PubMed] [Google Scholar]
- Ryan LP, Matsuzaki K, Noma M, Jackson BM, Eperjesi TJ, Plappert TJ, St John-Sutton MG, Gorman JH, Gorman RC. Dermal filler injection: a novel approach for limiting infarct expansion. Ann. Thorac. Surg. 2009;87:148–55. doi: 10.1016/j.athoracsur.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangeli P, Di Biase L, Natale A. Ablation versus drugs: what is the best first-line therapy for paroxysmal atrial fibrillation? Antiarrhythmic drugs are outmoded and catheter ablation should be the first-line option for all patients with paroxysmal atrial fibrillation: pro. Circ. Arrhythm. Electrophysiol. 2014;7:739–46. doi: 10.1161/CIRCEP.113.000629. [DOI] [PubMed] [Google Scholar]
- Sasano T, McDonald AD, Kikuchi K, Donahue JK. Molecular ablation of ventricular tachycardia after myocardial infarction. Nat. Med. 2006;12:1256–1258. doi: 10.1038/nm1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Ashraf M, Millard RW, Fujiwara H, Schwartz A. Connective tissue changes in early ischemia of porcine myocardium: an ultrastructural study. J. Mol. Cell. Cardiol. 1983;15:261–75. doi: 10.1016/0022-2828(83)90281-x. [DOI] [PubMed] [Google Scholar]
- Shiba Y, Fernandes S, Zhu W-Z, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H, Van Biber B, Dardas T, Mignone JL, Izawa A, Hanna R, Viswanathan M, Gold JD, Kotlikoff MI, Sarvazyan N, Kay MW, Murry CE, Laflamme MA. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012 doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SN, Fletcher RD, Fisher SG, Singh BN, Lewis HD, Deedwania PC, Massie BM, Colling C, Lazzeri D. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure. The New England journal of medicine. 1995 doi: 10.1056/NEJM199507133330201. [DOI] [PubMed] [Google Scholar]
- Singh SN, Tang XC, Singh BN, Dorian P, Reda DJ, Harris CL, Fletcher RD, Sharma SC, Atwood JE, Jacobson AK, Lewis HD, Lopez B, Raisch DW, Ezekowitz MD. Quality of life and exercise performance in patients in sinus rhythm versus persistent atrial fibrillation. A Veterans Affairs Cooperative Studies Program Substudy. J. Am. Coll. Cardiol. 2006;48:721–730. doi: 10.1016/j.jacc.2006.03.051. [DOI] [PubMed] [Google Scholar]
- Skanes AC, Yee R, Krahn AD, Klein GJ. Cryoablation of atrial arrhythmias. Card. Electrophysiol. Rev. 2002;6:383–388. doi: 10.1023/A:1021128207078. [DOI] [PubMed] [Google Scholar]
- Solti F, Vecsey T, Kékesi V, Juhász-Nagy A. The effect of atrial dilatation on the genesis of atrial arrhythmias. Cardiovasc. Res. 1989;23:882–886. doi: 10.1093/cvr/23.10.882. [DOI] [PubMed] [Google Scholar]
- Song K, Nam Y-J, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KT, Mor-Avi V, Gorcsan J, DeMaria AN, Kimball TR, Monaghan MJ, Perez JE, Weinert L, Bednarz J, Edelman K, Kwan OL, Glascock B, Hancock J, Baumann C, Lang RM. Effects of aging on left atrial reservoir, conduit, and booster pump function: a multi-institution acoustic quantification study. Heart. 2001;85:272–277. doi: 10.1136/heart.85.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunagawa K, Maughan WL, Sagawa K. Effect of regional ischemia on the left ventricular end-systolic pressure-volume relationship of isolated canine hearts. Circ. Res. 1983;52:170–8. doi: 10.1161/01.res.52.2.170. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Barry A, Factor S. Collagen degradation in ischaemic rat hearts. Biochem. J. 1990;265:233–241. doi: 10.1042/bj2650233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R, Wiggers CJ. The effect of coronary occlusion on myocardial contraction. Am. J. Physiol. 1935;112:351–361. [Google Scholar]
- Thollon C, Kreher P, Charlon V, Rossi A. Hypertrophy induced alteration of action potential and effects of the inhibition of angiotensin converting enzyme by perindopril in infarcted rat hearts. Cardiovasc. Res. 1989;23:224–230. doi: 10.1093/cvr/23.3.224. [DOI] [PubMed] [Google Scholar]
- Thomas L, Boyd A, Thomas SP, Schiller NB, Ross DL. Atrial structural remodelling and restoration of atrial contraction after linear ablation for atrial fibrillation. Eur. Heart J. 2003;24:1942–1951. doi: 10.1016/j.ehj.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Tyberg JV, Forrester JS, Wyatt HL, Goldner SJ, Parmley WW, Swan HJ. An analysis of segmental ischemic dysfunction utilizing the pressure-length loop. Circulation. 1974;49:748–54. doi: 10.1161/01.CIR.49.4.748. [DOI] [PubMed] [Google Scholar]
- Tzou WS, Marchlinski FE, Zado ES, Lin D, Dixit S, Callans DJ, Cooper JM, Bala R, Garcia F, Hutchinson MD, Riley MP, Verdino R, Gerstenfeld EP. Long-term outcome after successful catheter ablation of atrial fibrillation. Circ. Arrhythmia Electrophysiol. 2010;3:237–242. doi: 10.1161/CIRCEP.109.923771. [DOI] [PubMed] [Google Scholar]
- Van den Borne SWM, van de Schans VAM, Strzelecka AE, Vervoort-Peters HTM, Lijnen PM, Cleutjens JPM, Smits JFM, Daemen MJAP, Janssen BJA, Blankesteijn WM. Mouse strain determines the outcome of wound healing after myocardial infarction. Cardiovasc. Res. 2009;84:273–82. doi: 10.1093/cvr/cvp207. [DOI] [PubMed] [Google Scholar]
- Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, Said SA, Darmanata JI, Timmermans AJM, Tijssen JGP, Crijns HJGM. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N. Engl. J. Med. 2002 doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- Vianna-Pinton R, Moreno CA, Baxter CM, Lee KS, Tsang TSM, Appleton CP. Two-dimensional speckle-tracking echocardiography of the left atrium: feasibility and regional contraction and relaxation differences in normal subjects. J. Am. Soc. Echocardiogr. 2009;22:299–305. doi: 10.1016/j.echo.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Vokonas PS, Pirzada F, Hood WB. Experimental myocardial infarction: XII. Dynamic changes in segmental mechanical behavior of infarcted and non-infarcted myocardium. Am. J. Cardiol. 1976;37:853–9. doi: 10.1016/0002-9149(76)90109-0. [DOI] [PubMed] [Google Scholar]
- Wall ST, Walker JC, Healy KE, Ratcliffe MB, Guccione JM. Theoretical impact of the injection of material into the myocardium: a finite element model simulation. Circulation. 2006;114:2627–35. doi: 10.1161/CIRCULATIONAHA.106.657270. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang M, Zhang Y, Xie X, Wang W, Li Z, Gao M, Wang Z, Hou Y. Long-term effects of ganglionated plexi ablation on electrophysiological characteristics and neuron remodeling in target atrial tissues in a canine model. Circ. Arrhythm. Electrophysiol. 2015 doi: 10.1161/CIRCEP.114.002554. [DOI] [PubMed] [Google Scholar]
- White HD, Chew DP. Acute myocardial infarction. Lancet. 2008;372:570–584. doi: 10.1016/S0140-6736(08)61237-4. [DOI] [PubMed] [Google Scholar]
- Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. a study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.CIR.92.7.1954. [DOI] [PubMed] [Google Scholar]
- Wright M, Narayan SM. Ablation of atrial fibrillation. Trends Cardiovasc. Med. 2015;25:409–419. doi: 10.1016/j.tcm.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD. A comparison of rate control and rhythm control in patients with atrial fibrillation. N. Engl. J. Med. 2002 doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- Xiong B, Li D, Wang J, Gyawali L, Jing J, Su L. The effect of catheter ablation on left atrial size and function for patients with atrial fibrillation: an updated meta-analysis. PLoS One. 2015;10:e0129274. doi: 10.1371/journal.pone.0129274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, Zhang Y, Xu C, Bai Y, Wang H, Chen G, Wang Z. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat. Med. 2007;13:486–491. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- Yankelson L, Feld Y, Bressler-Stramer T, Itzhaki I, Huber I, Gepstein A, Aronson D, Marom S, Gepstein L. Cell therapy for modification of the myocardial electrophysiological substrate. Circulation. 2008;117:720–731. doi: 10.1161/CIRCULATIONAHA.106.671776. [DOI] [PubMed] [Google Scholar]
- Yu WC, Lee SH, Tai CT, Tsai CF, Hsieh MH, Chen CC, Ding YA, Chang MS, Chen SA. Reversal of atrial electrical remodeling following cardioversion of long-standing atrial fibrillation in man. Cardiovasc. Res. 1999;42:470–476. doi: 10.1016/S0008-6363(99)00030-9. [DOI] [PubMed] [Google Scholar]
- Zamilpa R, Lindsey ML. Extracellular matrix turnover and signaling during cardiac remodeling following MI: causes and consequences. J. Mol. Cell. Cardiol. 2010;48:558–563. doi: 10.1016/j.yjmcc.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer HG, Gerdes AM, Lortet S, Mall G. Changes in heart function and cardiac cell size in rats with chronic myocardial infarction. J. Mol. Cell. Cardiol. 1990;22:1231–43. doi: 10.1016/0022-2828(90)90060-f. [DOI] [PubMed] [Google Scholar]