Abstract
Objective
Characterize postural responses to forward and backward external perturbations in people with multiple sclerosis (PwMS), and relate performance to commonly-used clinical outcomes.
Design
Cross-sectional study. Postural responses were tested during large “stepping” and smaller “feet-in-place” perturbations in forward and backward directions.
Setting
University research laboratory
Participants
54 PwMS and 21 age-matched controls
Intervention
Not applicable
Main outcome measures
Center of mass displacement, step latency
Results
PwMS exhibited larger center of mass displacement and step latency than control participants in response to “stepping” perturbations (p=0.003 and p=0.028, respectively). Stepping deficits were more pronounced during backward stepping and were significantly correlated to increased severity on clinical measures (European Database for Multiple Sclerosis Disability Score and Timed 25-Foot Walk).
Conclusions
Compensatory stepping is impaired in PwMS, and correlates with clinical disability. Measurement of backward compensatory stepping may be more effective at identifying postural dysfunction in PwMS. Prolonged step latencies, large anticipatory postural adjustments, and multiple compensatory steps are especially altered in PwMS suggesting possible targets for neurorehabilitation.
Keywords: multiple sclerosis, compensatory stepping, postural perturbations
INTRODUCTION
Approximately 50% of people with multiple sclerosis (PwMS) fall each year, resulting in significant morbidity and associated socioeconomic costs1. Imbalance contributes to falls and is due, in part, to distorted and slowed conduction of electrical signals between the muscles and brain2. The slowed conduction is especially problematic when PwMS are exposed to external perturbations, which require quick postural responses to avoid falls. Depending on their size and speed, external perturbations can elicit feet-in-place postural responses (e.g. hip or ankle strategy), or change in support responses which include reactive, compensatory steps. These postural responses require quick, complex integration of somatosensory feedback and descending cortical control. Previous research has shown that during ‘smaller, feet-in-place’ postural responses, PwMS exhibit delayed muscle activity3, 4, and these delays are significantly correlated with postural control during quiet stance and walking4.
Compensatory stepping responses are different than feet-in-place postural responses in that they may elicit a lateral weight shift (anticipatory postural adjustment; APA) prior to the step. In addition, the longer time necessary to generate a step than a feet-in-place postural response suggests that more central nervous system processing is involved5. Therefore, stepping responses may be “less automatic” and more easily modified by cortical processing and intention5. Compensatory stepping has been measured in individuals prone to falls, including individuals with Parkinson’s disease (PD) and older adults, showing slower and less effective stepping responses in these groups6–9. While delayed responses have been observed during feet-in-place perturbations in PwMS3, 4, compensatory stepping responses to recover equilibrium have not been characterized for this population. Responses to forward and backward surface perturbations have also not been directly compared in PwMS. Recent evidence suggests backward postural responses may rely more on startle responses in upper brain stem regions10 than forward perturbations. Given the high frequency of brainstem lesions in PwMS11, evaluating both forward and backward perturbations and both feet-in-place and stepping responses in PwMS is necessary to fully characterize postural response deficits.
A better understanding of postural control deficits in PwMS may improve the efficacy of balance rehabilitation aimed to reduce falls and improve quality of life. Therefore, we characterized feet-in-place and compensatory stepping postural responses after forward and backward surface perturbations in PwMS compared to control participants. We further determined whether postural response impairments correlated with clinical measures of MS disability.
METHODS
Participants
A convenience sample of 54 PwMS and 21 age-matched healthy controls were recruited (Table 1). PwMS were recruited through the Oregon Health & Science University (OHSU) MS clinic and the MS community. Healthy adults were recruited through fliers placed throughout the OHSU campus. All participants provided informed consent, and the research protocol was approved by the OHSU institutional review board. Inclusion criteria for MS participants were: diagnosis of MS by MS neurologist and no relapses within 60 days. Inclusion criteria for all participants included ability to walk 25ft without assistive device, and no other known biomechanical or neurological conditions affecting stepping or balance. PwMS and control participants were similar with respect to age, height, and weight. PwMS demonstrated longer timed 25 foot walk (T25FW) (Table 1).
Table 1.
Participant Characteristics- mean (SD)
| MS (n=52) | Control (n=21) | p-value | |
|---|---|---|---|
| Age (y) | 49.5 (9.8) | 49.9 (11.9) | 0.90 |
| Gender (%F) | 79.2 | 66.7 | -- |
| Height (in) | 65.4 (3.2) | 66.7 (3.4) | 0.13 |
| Weight (lbs) | 155.8 (32.5) | 156.6 (42.5) | 0.87 |
| 25ft walk (s) | 6.0 (1.9) | 4.4 (0.7) | <0.001 |
| srEDSS | 4.3 (0.9) | -- | -- |
| EDMUS | 3.3 (1.6) | -- | -- |
| Years with Disease | 12.7 (10.6) | -- | -- |
| MS diagnosis (RR/SP/PP) | 33 / 13 / 6 | -- | -- |
Legend: MS- Multiple Sclerosis, RR- relapsing remitting, SP- secondary progressive, PP- primary progressive, srEDSS- self-reported expanded disability status scale; EDMUS- European database of multiple sclerosis, disability score.
Data collection
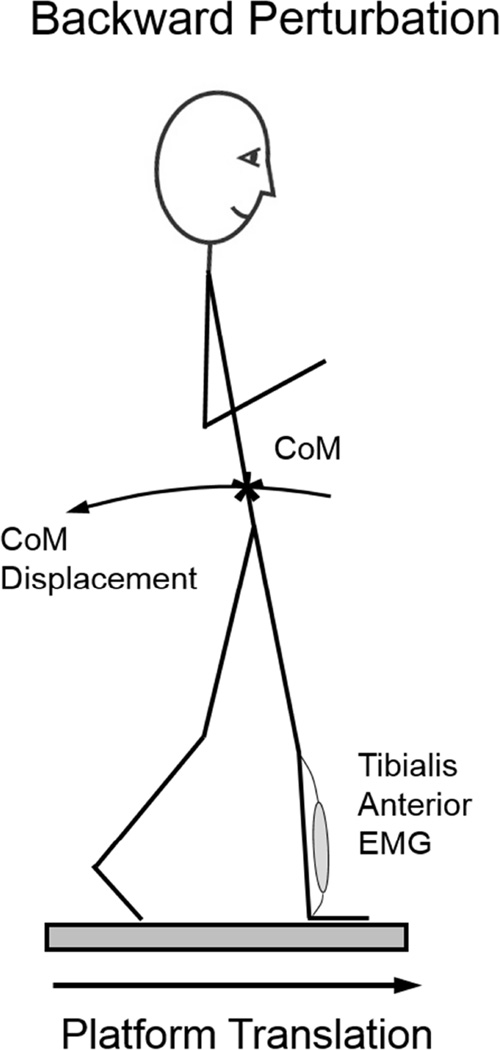
Participants stood on two computer/servo-controlled hydraulic platforms that translated forward or backward, resulting in backward and forward postural sway, respectively. Perturbation direction will refer to the direction of postural sway induced by the surface translation. Thus, a “backward” perturbation refers to forward movement of the support-surface inducing backward body sway whereas “Forward” perturbations refer to backward translation of the support-surface inducing forward body sway (Figure 1).
Figure 1.
Backward Perturbations refer to a forward translation of the support-surface, with backward body sway and potential step. The tibialis anterior is the agonist muscle during backward perturbations.
Participants were instructed to rest arms naturally at their sides with eyes open and feet placed at a heel-to-heel distance of 10cm. Starting foot position was held constant across trials and participants. Participants were exposed to 14 support-surface perturbations. The first two perturbations (one forward and one backward at 4cm amplitude and 15cm/s velocity) were meant to familiarize participants with perturbations and habituate any initial startle responses. Then, participants underwent 6 small perturbations (3 forward followed by 3 backward; 4cm amplitude and 15cm/s velocity) to induce feet-in-place postural responses. Stepping occurred in <5% of these perturbations trials. Participants were then exposed to 6 larger perturbations (3 backward and 3 forward; 15cm amplitude and 56cm/s velocity) to induce stepping postural responses. All participants stepped in response to these perturbations. Participants were given the following instructions: "The surface is going to slide forward or backward underneath your feet. Do whatever is necessary to keep your balance. Be still but not rigid. Be relaxed, and try not to anticipate the timing or direction of perturbations”. The timing of perturbation onset was randomized between 2 and 10 seconds. Thirteen PwMS required physical support from the spotter for at least one large perturbation to prevent a fall. Data from two PwMS was excluded as they were unable to produce any steps in response to large perturbations.
Postural response outcomes
Step characterization and body CoM were derived from reflective markers placed on the lumbar spine and bilateral lateral malleoli. A marker was also placed on the platform to measure platform motion (Motion Analysis Co., Santa Rosa, CA). To measure postural response latencies, participants wore surface electromyography (EMG) units (Noraxon, Scottsdale, AZ) bilaterally on the medial gastrocnemius (MG) and tibialis anterior (TA).
Clinical outcomes
PwMS completed the self-reported Expanded Disability Status Scale (srEDSS)12 and the European Database of Multiple Sclerosis Scale of Disability (EDMUS). Patients also completed three, fast-as-possible, 25-foot walks to establish the T25FW13.
Data Processing
Reflective marker data for kinematic analyses were collected at 120 Hz and low-pass filtered at 50 Hz. Amplified EMG for latency of muscle onset were collected at 960 Hz, and bandpass filtered (15–480 Hz) via a 4th order butterworth filter14. EMG data were then rectified and low-pass filtered (50 Hz) to create a linear envelope15. Force-plate data for latency of foot lift were captured at 480 Hz. Center of pressure (COP) data (used for APA calculation) was calculated by averaging the position of force-vectors under the two independent force plates. All EMG, marker, and force-plate data were visually inspected.
Outcome Measures
Center of mass (CoM) displacement and stepping latency were identified as the two primary variables of interest. CoM displacement represents extent of disequilibrium induced by the perturbations, and serves as an overall measure of performance16, 17. Step latency was chosen because delayed feet-in-place postural responses are characteristic of prolonged somatosensory conduction delays in PwMS3, and similar delays in postural stepping responses may contribute to falls6.
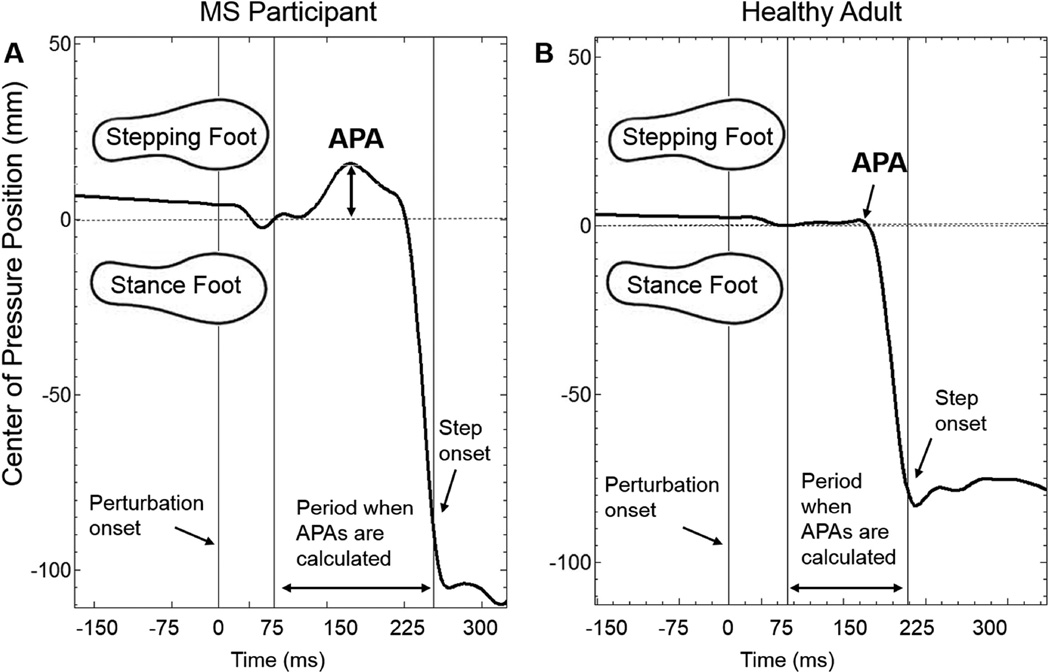
CoM displacement after a perturbation was estimated by calculating the maximum anterior/posterior displacement of the lumbar spine marker with respect to platform translation. Stepping latency was calculated as the time after perturbation that either the left or right force-plate was unloaded. Other stepping characteristics included: First step length- the distance between the left and right heel markers at the moment of first foot contact; Anticipatory postural adjustments (APAs)- calculated by measuring the medio-lateral displacement of center of pressure (CoP) data8, 9, 18 between 75ms after platform movement and step onset18; APA magnitude was defined as the maximum COP displacement towards the eventual stepping leg (Figure 2); and Muscle onset latency- the time between perturbation onset and an increase in agonist activity >2 standard deviations from baseline that was sustained for at least 50ms3, 4, 19. The agonist muscle for backward and forward perturbations was the TA and MG, respectively. Muscle onset latency was calculated as the median of the three trials collected in each direction.
Figure 2.
Anticipatory postural response (APA) calculation. APAs were calculated as the motion of the center of pressure towards the stepping foot after perturbation onset, but before stepping onset. Representative data shown for MS (A), and control (B) participants.
Statistics
Stepping data was non-normally distributed, and transformations did not uniformly improve non-normality of data across variables. Thus, non-parametric statistical assessments were utilized to assess group, direction, and group by direction interactions. Group effects (collapsed across direction) were assessed via Mann Whitney U tests. Direction effects (collapsed across groups) were assessed via Wilcoxon-Signed Rank tests. To assess interaction effects between direction and group (i.e. whether the difference between performance on forward and backward perturbations were similar across groups), we first calculated the difference between forward and backward perturbations separately for each group. This difference score (forward minus backward) was compared across groups via Mann Whitney U tests. Significance levels were set at alpha<0.05, and trend levels at alpha<0.1. If significance or trends toward group by perturbation direction interaction were observed, post-hoc across-group Mann Whitney U tests were run for each direction to determine whether PwMS and control groups performed differently on forward perturbations or backward perturbations.
Spearman’s Rho correlation statistic (r[s]) was used to identify whether primary outcome measures (CoM motion and step latency) were related to 1) clinical measures: the EDMUS and T25FW time, or 2) other measures of postural responses: number of steps, step length, APA magnitude, and muscle onset latency. Spearman correlation analyses were also run to determine whether muscle onset latencies for feet-in-place and stepping responses were related. The effect size of correlation coefficients are categorized as small (0.1<r[s]<0.3), medium (0.1<r[s]<0.3), and large (r[s]>0.5)20.
RESULTS
Means and standard deviations for feet-in-place and stepping postural response kinematics, as well as statistical analyses are shown in Table 2.
Table 2.
Postural response kinematics and statistical results (p-values) of control participants and PwMS during feet-in-place and stepping response postural responses.
| Direction of lean |
Control | PwMS |
#group effect |
‡direction effect |
#group by direction interaction |
#post
hoc direction effect (across group, each direction) |
||
|---|---|---|---|---|---|---|---|---|
| Feet-in-place Response | ||||||||
| CoM (m) | Forward | 0.035 (0.004) | 0.036 (0.004) | 0.028 | <0.001 | 0.012 | NS | |
| Backward | 0.046 (0.006) | 0.053 (0.014) | 0.020 | |||||
| Stepping Response | ||||||||
| CoM (m) | Forward | 0.21 (0.06) | 0.24 (0.17) | 0.003 | <0.001 | 0.001 | NS | |
| Backward | 0.29 (0.70) | 0.47 (0.23) | <0.001 | |||||
| Step Latency (ms) | Forward | 369 (152) | 367 (117) | 0.028 | <0.001 | 0.054 | NS | |
| Backward | 254 (37) | 318 (114) | 0.004 | |||||
| Number of Steps | Forward | 1.08 (0.14) | 1.58 (0.62) | <0.001 | <0.001 | 0.032 | 0.002 | |
| Backward | 1.37 (0.50) | 2.52 (1.27) | <0.001 | |||||
| Step Length (m) | Forward | 0.32 (0.10) | 0.28 (0.12) | 0.069 | 0.002 | NS | -- | |
| Backward | 0.27 (0.09) | 0.23 (0.09) | -- | |||||
| APA (mm) | Forward | 11.3 (10.0) | 8.7 (6.7) | NS | NS | 0.036 | NS | |
| Backward | 3.8 (4.1) | 9.0 (9.3) | 0.040 | |||||
Legend: CoM- center of mass, APA- anticipatory postural response, PwMS- people with multiple sclerosis
P-values >0.1 are noted as “NS”.
Mann- Whitney U test,
Wilcoxon Signed-Rank Test. See “Statistics” section for more details.
CoM Displacement
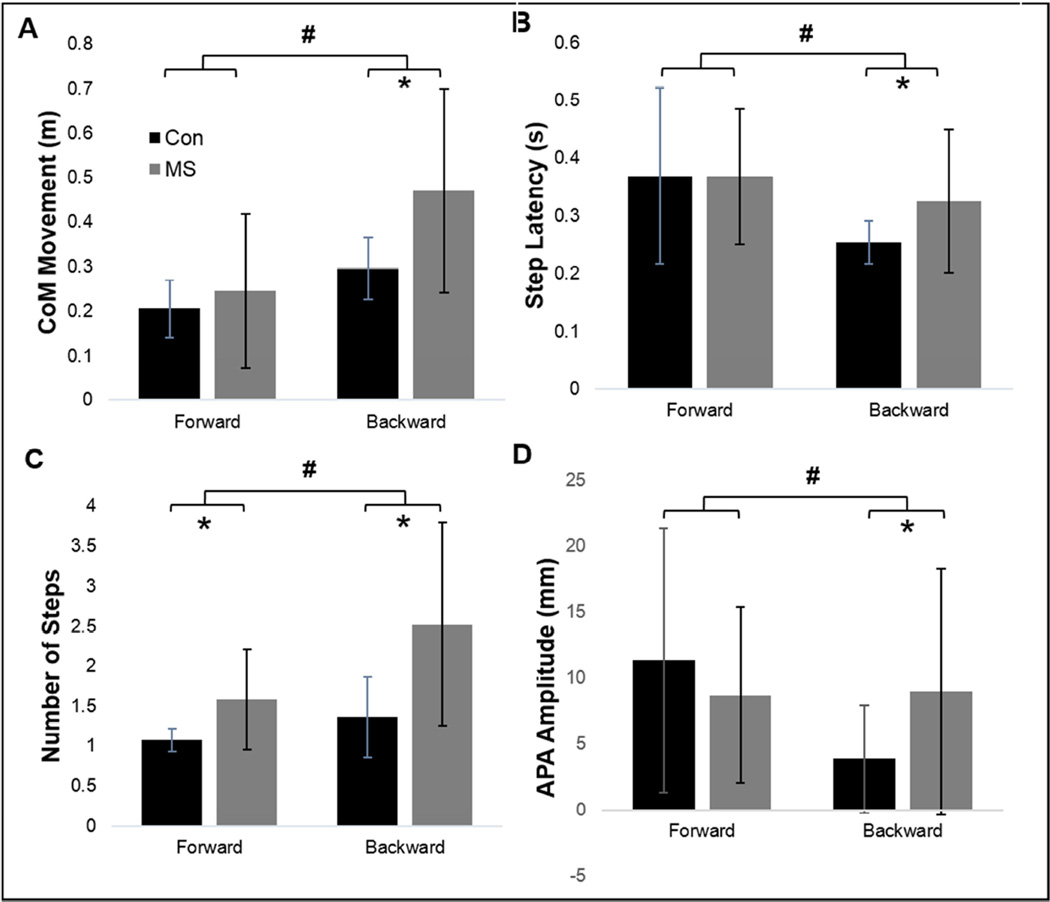
CoM displacements in response to feet-in-place perturbations were significantly larger in MS compared to control participants. Backward perturbations resulted in significantly larger CoM displacements than forward perturbations. There was also a group by direction interaction, which was due to significantly larger CoM displacements for MS than control participants for backward stepping, but not forward stepping.
Similar to feet-in-place responses, compensatory stepping responses resulted in significantly larger CoM displacements in MS compared to control participants. Backward perturbations resulted in significantly larger CoM displacements than forward perturbations (Figure 3a). A significant interaction between group and direction was due to larger CoM displacements for PwMS than control participants for backward stepping, but not for forward stepping.
Figure 3.
Kinematics after forward and backward stepping. A) Center of mass (CoM) movement after perturbations, B) step latency, and C) number of steps, D) Anticipatory postural response (APA) amplitude. Backward perturbations resulted in more pronounced across-group differences than forward perturbations. *Significant (p<0.05) difference between MS and control participants. #Significant (p<0.05) difference between forward and backward perturbations.
Step Latency
Step latency was significantly longer in PwMS compared to control participants, and was shorter during backward stepping with respect to forward. A trend (p=0.054) toward a significant interaction between group and direction was due to significantly shorter step latencies in control participants compared to PwMS for backward stepping, but not forward stepping (Figure 3b).
Number of steps
Number of steps was significantly larger in PwMS compared to control participants. Backward perturbations resulted in significantly more steps than forward perturbations (Figure 3c). There was also a significant interaction between group and direction with backward stepping having a more pronounced effect on PwMS step counts than forward perturbations.
Step Length
Step length was not significantly different between the MS and control groups. Backward perturbations resulted in significantly smaller steps than forward perturbations. No group by direction interaction was observed.
APA magnitude
A significant group by direction interaction was due to larger APAs in PwMS in response to backward, but not forward, perturbations (Figure 3d).
Muscle onset latency
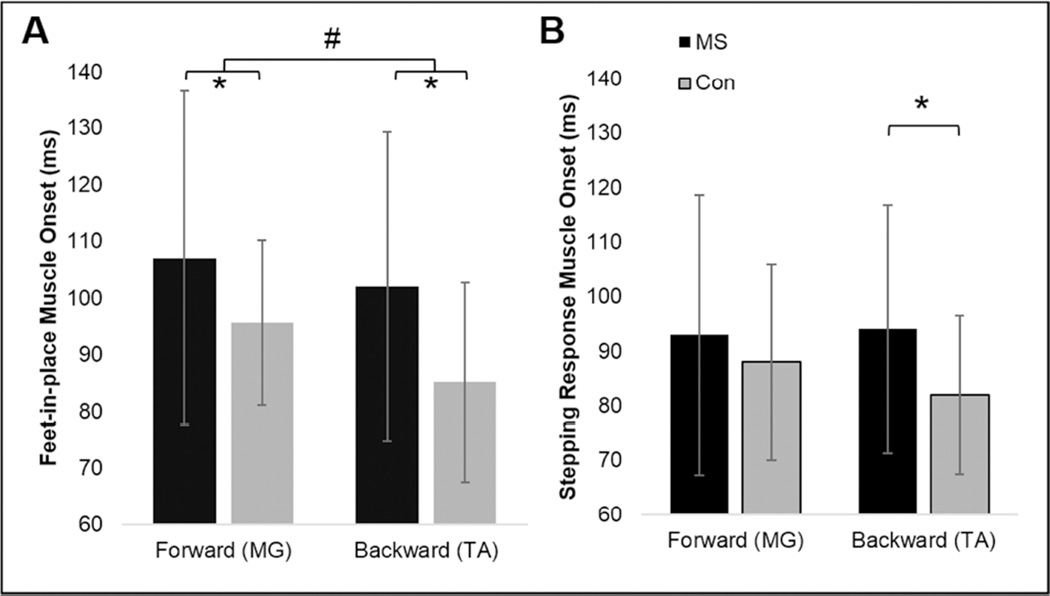
Feet-in-place muscle onset latencies were significantly longer in PwMS compared to control participants (Figure 4, Table 3). Postural response latencies to backward perturbations were significantly shorter than to forward perturbations. There was no interaction between group and direction for feet-in-place response latencies.
Figure 4.
muscle onset during A) feet-in-place and B) stepping perturbations. *Significant (p<0.05) difference between MS and control participants. #Significant (p<0.05) difference between forward and backward perturbations.
Table 3.
Muscle onset latency in milliseconds (ms) and statistical results (p-values) for control participants and people with multiple sclerosis (PwMS) during feet-in-place and stepping response perturbations.
| Direction of lean |
Control | PwMS |
#group effect |
‡direction effect |
#group by direction interaction |
#post
hoc direction effect (across group, each direction) |
|
|---|---|---|---|---|---|---|---|
| Feet-in-place Response | Forward | 95 (14) | 107 (29) | 0.044 | 0.008 | 0.186 | -- |
| Backward | 85 (17) | 101 (27) | -- | ||||
| Stepping Response | Forward | 88 (17) | 93 (25) | NS | NS | 0.027 | NS |
| Backward | 82 (14) | 94 (23) | 0.048 | ||||
P-values >0.1 are noted as “NS”.
Mann-Whitney U test,
Wilcoxon Signed-Rank Test. See “Statistics” section for more details.
Stepping response muscle onset latencies were not different between groups or directions. However, there was a group by direction interaction due to significantly longer stepping latencies for PwMS compared to control participants in response to backward perturbations, but not forward perturbations (Table 3).
Correlation analyses
During stepping perturbations, forward and backward CoM displacements had significant positive correlations with number of steps taken and muscle onset latency (Table 4). Step latency had a significant positive correlation to amplitude of APAs, muscle latency, and number of steps (Table 4). T25FW and EDMUS had significant, positive correlations to CoM displacement and step latency in response to backward stepping response perturbations (Figure 5), but not forward (Table 4). Feet-in-place muscle latencies were significantly correlated to stepping response muscle latencies for both forward (r[s]=0.72, p< 0.001) and backward (r[s]=0.82; p<0.001) perturbations.
Table 4.
Spearman’s rho correlation coefficients between primary outcome measures (center of mass movement and step latency) and stepping characteristics (step length, APA amplitude), muscle onset latency, and clinical assessments of MS (25 foot walk time and EDMUS). Panel A shows correlations after backward perturbations (i.e. backward stepping). Panel B shows correlations after forward perturbations (i.e. forward stepping).
| A) Backward Perturbations |
Number of steps |
Step Latency |
Step Length |
APA magnitude |
Muscle onset latency (TA) |
T25FW | EDMUS |
|---|---|---|---|---|---|---|---|
| CoM Movement | .850** | .193 | −.207 | −.037 | .290* | .473** | .590** |
| Step Latency | .095 | --- | .117 | .630** | .777** | .364** | .440** |
| B) Forward Perturbations |
Number of steps |
Step Latency |
Step Length |
APA magnitude |
Muscle onset latency (MG) |
T25FW | EDMUS |
|---|---|---|---|---|---|---|---|
| CoM Movement | .535** | .519** | .396** | .441** | .419** | .196 | .193 |
| Step Latency | .342* | --- | .218 | .459** | .615** | .291* | .274 |
Legend: CoM: Center of mass; TA: Tibialis anterior; MG: Medial gastrocnemius; T25FW: Timed 25 Foot Walk; EDMUS: European database of multiple sclerosis scale of disability
Correlation is significant at the 0.01 level.
Correlation is significant at the 0.05 level.
Figure 5.
Correlations (Spearman’s Rho; r[s]) between A) Center of mass (CoM) motion and muscle onset latency, B) anticipatory postural adjustment (APA) and step latency, and C) CoM motion and 25 foot walk time (T25FW). All relationships represent data from backward perturbations.
DISCUSSION
PwMS demonstrate both abnormal compensatory stepping responses and abnormal feet-in-place postural responses in reaction to surface perturbations. In addition, CoM displacement after perturbations was significantly correlated to delayed postural responses, such as muscle onset latency. Finally, PwMS demonstrate more pronounced dysfunction during backward stepping than during forward stepping, and backward stepping performance was significantly correlated to clinical tests including the T25FW and the EDMUS.
PwMS exhibited larger CoM displacements (falling farther before recovering equilibrium), prolonged step latency, and required more steps to recover balance compared to healthy control participants of similar age. These findings are consistent with previous work from this laboratory that demonstrated feet-in-place postural response latencies are significantly slowed in PwMS, and are related to slowed spinal, somatosensory conduction3, 4. Results from the current study also show that muscle onset latency in PwMS are associated with large CoM displacements, i.e. more disequilibrium during stepping. This association suggests that the long latencies of postural responses in PwMS are contribute to disequilibrium in response to large perturbations. However, although statistically significant, the correlation effect sizes were “small”20 between CoM displacement and muscle onset latency (r[s]=0.290), suggesting other untested variables also contribute to reduced postural response performance in PwMS.
Differences between the MS and control groups were more pronounced for backward stepping responses than for forward stepping responses. Although the underlying cause of these differences is unknown, one possibility is that backward stepping may involve more startle-pathways, located in the upper brainstem, than forward stepping10. Recent evidence suggests the effect of startling stimuli is diminished in PwMS, possibly due to poor preparation and storage of prepotent motions that are released with startling stimuli21. This is consistent with the fact that brainstem pathophysiology is often observed in PwMS11. Alternatively, more pronounced impairments of backward than forward stepping could be due to reduced visual information for backward step placement. PwMS rely heavily on visual information for balance22, so the absence of vision to direct foot placement during backward stepping may affect PwMS more than control subjects. Finally, the TA muscle is responsible for the shortest latencies and largest torque response to backward disequilibrium. This muscle is much smaller than the gastrocnemius/soleus complex, which is primarily responsible for forward postural recovery14. The smaller tibialis motor neuron pools extend over a much smaller extent of the spinal cord than the tripartite gastrocnemius/soleus23. Because they encompass a smaller area of the spinal cord, any white matter pathways associated with the tibialis motor neuron could be more vulnerable to damage from localized MS plaques. Regardless of the cause, these results suggest that backward stepping responses are more affected in PwMS than forward stepping responses, and therefore more sensitive at identifying deficiencies in postural control in PwMS.
Backward stepping performance was also shown to have ‘large’ or ‘medium’ correlation effect sizes8 with a commonly used clinical measure, the T25FW. This illustrates the importance of postural response characteristics for capturing functional mobility. Previous work in healthy older adults and individuals with PD has shown that interventions that improved compensatory stepping also improved performance in other balance tasks and may reduce falls24, 25 (For review, see26). This co-variance of the quality of postural stepping responses and gait speed lends further support to the importance of postural stability for functional mobility.
The delayed stepping responses in PwMS may have been due to increased size of their APAs. An APA prior to stepping unweights the stepping leg prior to movement, enabling a large and controlled step. However, APAs also delay stepping onset9. Thus, during compensatory steps, where speed of stepping is critical, healthy adults exhibit reduced or absent APAs27. In the current study, we observed larger APA magnitudes during backward perturbations in PwMS than control subjects, and larger APAs were positively correlated to delays in step onset. Previous work from our laboratory suggested that PwMS increased the magnitude and anticipatory scaling of their feet-in-place postural responses to compensate for their delayed latencies3. It is possible that exaggerated APAs in PwMS are a strategy to compensate for slow step initiation since larger weight shifts to the stance leg provides more time for the stepping leg to be lifted off the ground without disequilibrium. Alternatively, large APAs may represent exaggerated preparatory postural forces under the feet due to somatosensory loss, or are related to cerebellar hypermetria/ataxia. Regardless of the cause, if large APAs contribute to slower step initiation it is possible that reducing the size of APAs could reduce step latency.
Study Limitations
Several limitations should be noted. First, participants knew the direction of the upcoming support-surface perturbation. Anticipation of an upcoming postural perturbation could be associated with anticipatory leaning. However, previous research suggests that this preleaning likely does not contribute substantially to post-perturbation performance17. Further, preleaning was minimized by coaching to maintain consistent initial COP position. Second, perturbations via movement of the support-surface do not perfectly mimic slips or trips in the community, possibly limiting external validity. We chose this perturbation for consistent perturbation size; however, future work should determine the ecological validity of this perturbation. Finally, it should be noted that postural control is a multi-factorial task that also relies on central sensory integration and muscle strength, which were not directly assessed in this investigation.
Conclusions
PwMS exhibit significant impairments in postural stepping responses to external displacements. These differences were most frequently observed during backward falling, suggesting that this task is a more sensitive approach to identify and monitor postural performance in PwMS. Abnormal postural responses during compensatory stepping appear clinically relevant as they relate with clinical disability. Future studies could investigate the ability of neurorehabilitation therapies to improve postural stepping responses and prevent falls in the MS population.
Acknowledgments
We would like to acknowledge the assistance of Geetanjali Gera, Jessica Nguyen, and Austen Yeager for their help with data collection. This work was supported by grants from the National Multiple Sclerosis Society (RG 4914A1/2; PI: JH), the NIH National Center for Advancing Translational Science (1KL2TR000119; PI: JH), the United States Department of Veteran’s Affairs Rehabilitation Research and Development Service (Career Development Award-1: #I01BX007080; PI: DP) & VA Merit Award (E1075-R; PI: FH), the National Institutes of Health (R01 AG006457 29 PI: FH), and the Medical Research Foundation of Oregon (Early Investigator Award; PI: DP). The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
ABBREVIATIONS
- PwMS
people with multiple sclerosis
- APA
anticipatory postural response
- CoP
center of pressure
- CoM
center of mass
- MG
medial gastrocnemius
- TA
tibialis anterior
- srEDSS
self-reported expanded disability status scale
- EDMUS
European database of multiple sclerosis scale of disability
- T25FW
Timed 25 Foot Walk
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures / Conflict of interest
Dr. Horak and OHSU have an equity/interest in APDM, a company that may have a commercial interest in the results of the study. This potential conflict of interest has been reviewed and managed by the Research & Development Committee at the Portland VA Medical Center and OHSU. No other authors declare any conflict of interest.
References
- 1.Peterson EW, Ben Ari E, Asano M, Finlayson ML. Fall attributions among middle-aged and older adults with multiple sclerosis. Arch Phys Med Rehabil. 2013;94(5):890–895. doi: 10.1016/j.apmr.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 2.Cowan JM, Rothwell JC, Dick JP, Thompson PD, Day BL, Marsden CD. Abnormalities in central motor pathway conduction in multiple sclerosis. Lancet. 1984;2(8398):304–307. doi: 10.1016/s0140-6736(84)92683-7. [DOI] [PubMed] [Google Scholar]
- 3.Cameron MH, Horak FB, Herndon RR, Bourdette D. Imbalance in multiple sclerosis: a result of slowed spinal somatosensory conduction. Somatosensory & motor research. 2008;25(2):113–122. doi: 10.1080/08990220802131127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huisinga JM, St George RJ, Spain R, Overs S, Horak FB. Postural response latencies are related to balance control during standing and walking in patients with multiple sclerosis. Arch Phys Med Rehabil. 2014;95(7):1390–1397. doi: 10.1016/j.apmr.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm. 2007;114(10):1339–1348. doi: 10.1007/s00702-007-0657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maki BE, McIlroy WE. Control of rapid limb movements for balance recovery: age-related changes and implications for fall prevention. Age Ageing. 2006;35(Suppl 2):ii12–ii8. doi: 10.1093/ageing/afl078. [DOI] [PubMed] [Google Scholar]
- 7.de Kam D, Nonnekes J, Oude Nijhuis LB, Geurts AC, Bloem BR, Weerdesteyn V. Dopaminergic medication does not improve stepping responses following backward and forward balance perturbations in patients with Parkinson's disease. J Neurol. 2014 doi: 10.1007/s00415-014-7496-3. [DOI] [PubMed] [Google Scholar]
- 8.King LA, Horak FB. Lateral stepping for postural correction in Parkinson's disease. Arch Phys Med Rehabil. 2008;89(3):492–499. doi: 10.1016/j.apmr.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King LA, St George RJ, Carlson-Kuhta P, Nutt JG, Horak FB. Preparation for compensatory forward stepping in Parkinson's disease. Arch Phys Med Rehabil. 2010;91(9):1332–1338. doi: 10.1016/j.apmr.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nonnekes J, Scotti A, Oude Nijhuis LB, Smulders K, Queralt A, Geurts AC, et al. Are postural responses to backward and forward perturbations processed by different neural circuits? Neuroscience. 2013;245:109–120. doi: 10.1016/j.neuroscience.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 11.Lu Z, Zhang B, Qiu W, Kang Z, Shen L, Long Y, et al. Comparative brain stem lesions on MRI of acute disseminated encephalomyelitis, neuromyelitis optica, and multiple sclerosis. PLoS One. 2011;6(8):e22766. doi: 10.1371/journal.pone.0022766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowen J, Gibbons L, Gianas A, Kraft GH. Self-administered Expanded Disability Status Scale with functional system scores correlates well with a physician-administered test. Multiple sclerosis. 2001;7(3):201–206. doi: 10.1177/135245850100700311. [DOI] [PubMed] [Google Scholar]
- 13.Fischer JS, Rudick RA, Cutter GR, Reingold SC. The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Multiple sclerosis. 1999;5(4):244–250. doi: 10.1177/135245859900500409. [DOI] [PubMed] [Google Scholar]
- 14.Dimitrova D, Horak FB, Nutt JG. Postural muscle responses to multidirectional translations in patients with Parkinson's disease. J Neurophysiol. 2004;91(1):489–501. doi: 10.1152/jn.00094.2003. [DOI] [PubMed] [Google Scholar]
- 15.Kamen G, Gabriel D. Essentials of Electromyography. Human Kinetics. 2010 [Google Scholar]
- 16.Lee PY, Gadareh K, Naushahi MJ, Gresty M, Bronstein AM. Protective stepping response in Parkinsonian patients and the effect of vibrotactile feedback. Mov Disord. 2013;28(4):482–489. doi: 10.1002/mds.25227. [DOI] [PubMed] [Google Scholar]
- 17.Welch TD, Ting LH. Mechanisms of motor adaptation in reactive balance control. PLoS One. 2014;9(5):e96440. doi: 10.1371/journal.pone.0096440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs JV, Nutt JG, Carlson-Kuhta P, Stephens M, Horak FB. Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Exp Neurol. 2009;215(2):334–341. doi: 10.1016/j.expneurol.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horak FB, Diener HC, Nashner LM. Influence of central set on human postural responses. J Neurophysiol. 1989;62(4):841–853. doi: 10.1152/jn.1989.62.4.841. [DOI] [PubMed] [Google Scholar]
- 20.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. New Jersey: 1988. [Google Scholar]
- 21.Cabib C, Llufriu S, Casanova-Molla J, Saiz A, Valls-Sole J. Defective sensorimotor integration in preparation for reaction time tasks in patients with multiple sclerosis. J Neurophysiol. 2015;113(5):1462–1469. doi: 10.1152/jn.00591.2014. [DOI] [PubMed] [Google Scholar]
- 22.Van Emmerik RE, Remelius JG, Johnson MB, Chung LH, Kent-Braun JA. Postural control in women with multiple sclerosis: effects of task, vision and symptomatic fatigue. Gait Posture. 2010;32(4):608–614. doi: 10.1016/j.gaitpost.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Enoka R. Neuromechanics of Human Movement. 5th ed. Human Kinetics; 2015. [Google Scholar]
- 24.Mansfield A, Peters AL, Liu BA, Maki BE. Effect of a perturbation-based balance training program on compensatory stepping and grasping reactions in older adults: a randomized controlled trial. Phys Ther. 2010;90(4):476–491. doi: 10.2522/ptj.20090070. [DOI] [PubMed] [Google Scholar]
- 25.Jobges M, Heuschkel G, Pretzel C, Illhardt C, Renner C, Hummelsheim H. Repetitive training of compensatory steps: a therapeutic approach for postural instability in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2004;75(12):1682–1687. doi: 10.1136/jnnp.2003.016550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mansfield A, Wong JS, Bryce J, Knorr S, Patterson KK. Does perturbation-based balance training prevent falls? Systematic review and meta-analysis of preliminary randomized controlled trials. Phys Ther. 2015;95(5):700–709. doi: 10.2522/ptj.20140090. [DOI] [PubMed] [Google Scholar]
- 27.McIlroy WE, Maki BE. Do anticipatory postural adjustments precede compensatory stepping reactions evoked by perturbation? Neurosci Lett. 1993;164(1–2):199–202. doi: 10.1016/0304-3940(93)90891-n. [DOI] [PubMed] [Google Scholar]