Abstract
Numerous studies using a variety of imaging techniques have reported age-related differences in neural activity while subjects carry out cognitive tasks. Surprisingly little attention has been paid to the potential impact of age-associated changes in sensory acuity on these findings. Studies in the visual modality frequently report that their subjects had “normal or corrected- to-normal vision.” However, in most cases, there is no indication that visual acuity was actually measured, and it is likely that the investigators relied largely on self-reported visual status of subjects, which is often inaccurate. We investigated whether differences in visual acuity influence one of the most commonly observed findings in the event-related potentials literature on cognitive aging, a reduction in posterior P3b amplitude, which is an index of cognitive decision-making/updating. Well-matched young (n = 26) and old adults (n = 29) participated in a visual oddball task. Measured visual acuity with corrective lenses was worse in old than young adults. Results demonstrated that the robust age-related decline in P3b amplitude to visual targets disappeared after controlling for visual acuity, but was unaffected by accounting for auditory acuity. Path analysis confirmed that the relationship between age and diminished P3b to visual targets was mediated by visual acuity, suggesting that conveyance of suboptimal sensory data due to peripheral, rather than central, deficits may undermine subsequent neural processing. We conclude that until the relationship between age-associated differences in visual acuity and neural activity during experimental tasks is clearly established, investigators should exercise caution attributing results to differences in cognitive processing.
Keywords: aging, visual acuity, neural activity, ERPs, P3b
1. Introduction
Numerous studies have reported age-related differences in neural activity while subjects carry out cognitive tasks, using fMRI, PET, and ERPs as dependent variables (Cabeza et al., 2002; van Dinteren et al., 2014; Gazzaley et al., 2008; Madden et al., 1999; Madden et al., 2004; Madden et al., 2002; Miller et al., 2008; Reuter-Lorenz et al., 2000; Rossini et al., 2007; Rypma and D'Esposito, 2000; De Santi et al., 1995; Stern et al., 2005). Reports of task-related studies in the visual modality frequently indicate that their subjects had “normal or corrected-to-normal vision”. However, in most cases, there is no indication that visual acuity (VA) was actually measured, and it is likely that the investigators relied largely on subjects’ self-reported visual status. This approach is problematic for two major reasons: first, self-reports of VA are often inaccurate (Daffner et al., 2013; Friedman et al., 1999), and second, aging is associated with an increased likelihood of reduced VA, most frequently related to outdated prescriptions for corrective lenses (Skeel et al., 2003; Tielsch et al., 1990) or other peripheral, not central, problems (Kanthan et al., 2008; Klaver et al., 1998). These observations raise questions about the extent to which undetected differences in VA across age groups may contribute to the frequently reported age-related changes in neural activity during visual tasks, which are typically attributed to alterations in cognitive operations.
Here, we focused on one of the most commonly observed findings in the ERP literature on cognitive aging: reduction of the amplitude of the posterior P3b component in response to target visual stimuli, a result that has been published innumerable times over the last few decades, reflecting experiments conducted in either the visual or auditory modality (Alperin et al., 2014; Anderer et al., 1998; van Dinteren et al., 2014; Fabiani and Friedman, 1995; Fabiani et al., 1998; Kok, 2000; Li et al., 2013; Mullis et al., 1985; O'Connell et al., 2012; Polich, 1997). Age-associated decline in P3b amplitude has not been attributed to degraded sensory information that is delivered to neural systems involved in decision-making. Rather, based on well-established research regarding the cognitive and functional significance of the P3b component, its diminished size among older adults has been interpreted as an attenuation of the categorization process or the reduced ability to update working memory after a target has been categorized (Daffner et al., 2011; Donchin, 1981; Donchin and Coles, 1988). Within the framework of information processing theory, the age-associated decline in P3b amplitude reflects diminished transfer of information and greater remaining uncertainty about an event and its implications for the generation of expectations about future ones (Johnson, 1986; Picton, 1992). Recently, O'Connell and colleagues have suggested that the P3b1 may represent a theoretical decision variable involved in the accumulation and temporal integration of sensory evidence, that determines behavior once it crosses a threshold or boundary criterion (O'Connell et al., 2012). These investigators found that in young adults this component is very sensitive to systematic perturbation of physical evidence during decision formation, which suggested a tight, dynamic coupling between perceptual and decision processes in the human brain. In keeping with this perspective, even subtle age-associated reductions in sensory fidelity might undermine the decision process, be associated with greater residual uncertainty, and manifest as a smaller P3b.
There is a large body of research on the relationship between age-related impairments in sensory and cognitive processing (most often measured in terms of cognitive test performance), which has led to an ongoing debate between interpretations offered by the Common Cause (Baltes and Lindenberger, 1997; Christensen et al., 2001; Lindenberger and Baltes, 1994; Salthouse and Hancock, 1996) vs. the Sensory Deficit (Gilmore et al., 2006; Scialfa, 2002) hypotheses. Surprisingly, despite this literature, the issues at stake have received little attention in research investigating age-associated differences in underlying neural activity. To address this issue, the current study aimed to establish whether age-related deficits in VA mediate the decline in the P3b amplitude to target visual stimuli. To accomplish this objective, ERP data from a visual oddball task were analyzed before and after controlling for VA, as measured in young and old subjects. To test the modality specificity of the relationship between VA and the P3b to target visual stimuli, we determined whether the predicted age-related decline in P3b to visual targets would remain significant after controlling for measured auditory acuity in subjects. We chose to investigate young adults (late teens and early 20s) and older adults (mid 60s and 70s) to replicate the approach most typically found in the literature (Alperin et al., 2014; Anderer et al., 1998; van Dinteren et al., 2014; Fabiani and Friedman, 1995; Fabiani et al., 1998; Kok, 2000; Li et al., 2013; Mullis et al., 1985; O'Connell et al., 2012; Polich, 1997).
2. Material and methods
2.1 Participants
Subjects were recruited through community announcements in the Boston metropolitan area and underwent informed consent approved by the Partners Human Research committee. Participants were between 18 and 32 years in the young group or between 65 and 79 years, in the old group (See Alperin et al., 2014 for a more detailed account of the methods employed). Subjects underwent an initial telephone screen in which they were asked about vision, hearing, and medical history. To be included in this study subjects had to report that they had normal vision or corrected-to-normal vision with glasses or contact lenses. In addition, inclusion criteria required that subjects be English-speaking and have 12 or more years of education, a Mini Mental State Exam (MMSE) score (Folstein et al., 1973) ≥ 26, and an estimated Intelligence Quotient (IQ) on the American National Adult Reading Test (AMNART) (Ryan and Paolo, 1992) ≥ 100. Subjects were excluded if their mean performance on a battery of neuropsychological tests (Table 1) was < 33rd percentile (which was done to reduce the likelihood of including older subjects with mild cognitive impairment or early dementia), had a history of CNS diseases or major psychiatric disorders based on Diagnostic and statistical Manual of Mental Disorders, 4th ed. (DSM-IV) criteria (American Psychiatric Association, 1994), a history of clinically significant medical diseases, mean hearing loss (MHL) (see below) of > 40 dB, or > 20 dB difference between ears at any tested frequency, were unable to distinguish between the color red and blue, had a Beck Depression Inventory (Beck et al., 1988) (for young subjects) or a Geriatric Depression Scale (Yesavage et al., 1982) (for old subjects) score of ≥ 10, or had focal abnormalities on neurological examination consistent with a central nervous system lesion. Subjects were paid for their time.
Table 1.
Subjects Characteristics (Mean (SD))
| Variable | Young | Old |
|---|---|---|
| Number of subjects | 26 | 29 |
| Gender (male/female) | 12/14 | 14/15 |
| Age (years)a | 22.5 (2.2) | 72.8 (3.8) |
| Executive Capacity (%ile) | 67.3 (16.7) | 68.6 (7.54) |
| Years of Education | 15.1 (1.5) | 16.1 (3.1) |
| AMNART (estimated IQ) | 116.7 (6.6) | 118.3 (9.7) |
| MMSEb | 29.8 (.3) | 29.4 (.8) |
| Mean Hearing Lossc | −9.8 (4.9) | 13.5 (10.5) |
| Visual Acuityd | 1.02 (0.1)# | 0.74 (0.1)* |
| 20/12.5 | 1 | - |
| 20/16 | 7 | 1 |
| 20/20 | 16 | 2 |
| 20/25 | - | 11 |
| 20/30 | 1 | 12 |
| 20/35 | - | - |
| 20/40 | 1 | 3 |
p < .001 (young < old)
p = .01 (young > old)
p < 0.001
p < 0.001
≈ 20/20
≈ 20/25
Executive Capacity = Average (composite) percentile performance on the following tests: Digit Span Backward, Controlled Oral Word Association Test, Letter-Number Sequencing, Trail-Making Test Parts A and B, and Digit-Symbol Coding.
AMNART = American National Adult Reading Test
MMSE = Mini Mental State Exam
Binocular VA was measured in all subjects with a Snellen 10 feet model wall chart, and recorded as a decimal representation of 20/x, such that 20/20 = 1.0 and represents “normal” visual acuity. Worse than normal vision was represented with a visual acuity value of less than 1.0 (e.g., 20/40 = 0.5). Vision better than 20/20 was represented with a visual acuity value greater than 1.0.
Subjects also had a formal audiological examination in which hearing thresholds were tested at 6 frequencies: 250, 500, 1000, 2000, 4000, and 8000 Hz. Hearing acuity was determined by the decibel threshold for detecting sounds at each of the frequencies. Hearing loss was calculated as the difference between the measured dB level at threshold and 20 dB (Friedman et al., 1998). MHL was the average hearing loss across the 6 frequencies tested. Zero or negative values represents no hearing loss; positive values indicate some degree of hearing loss.
Two age groups were studied. The young subject group included 26 subjects with a mean age of 22.5 (2.2), and the old subject group included 29 subjects with a mean age of 72.8 (3.8). An additional 3 young and 5 old subjects completed the experiment, but were excluded due to excessively noisy ERP data. Table 1 provides a summary of information about subject characteristics.
2.2 Experimental Procedures
A visual oddball task was administered under low and high memory load. Under both loads, half the visual stimuli were presented in the color red and half in the color blue, in randomized order. The low load task required subjects to respond by button press on a computer mouse, to one specific target letter. To help minimize group differences in performance on the high load task, demands were made easier for old subjects. For the high load task, the number of target letters chosen for each age group was based on pilot data: young subjects responded to 5 target letters and older subjects responded to 4 target letters. This was done to allow us to draw inferences about age-related differences in neural activity and not performance-related differences (Daffner et al., 2011; Riis et al., 2008). Subjects were instructed to pay attention to letters appearing in the designated color while ignoring letters appearing in the other color, and respond by button press to target letters appearing in the designated color only. Subjects were asked to respond as quickly and as accurately as possible to target letters. Practice trials preceded each set of experimental runs. Task order and the hand used for target response were counterbalanced across subjects.
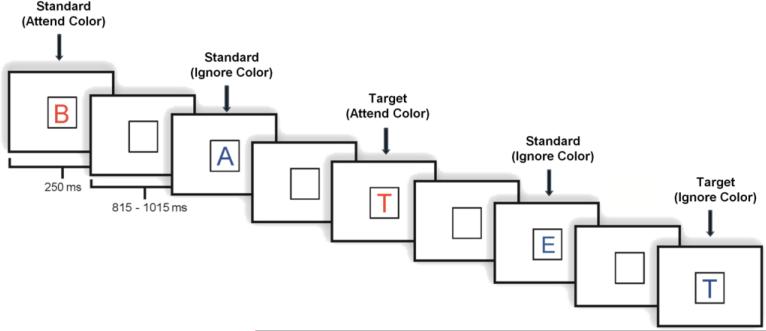
Each task included 800 stimulus trials divided into 8 blocks. In both the high load and low load tasks, stimuli appeared one at a time within a fixation box that remained on the screen at all times and subtended a visual angle of ~3.5° × 3.5° at the center of a high-resolution computer monitor. The distance between the participants’ eyes and the screen was approximately 5 feet. Thus, viewing of experimental stimuli depended on distance, not near, visual acuity. Target stimuli (7.5% in attend color; 7.5% in ignore color) were designated upper case letters and standard stimuli (70% overall; 35% in each color) were any non-target upper case letters. Novel stimuli, unusual/unfamiliar line drawings, such as impossible or fragmented objects (Kosslyn et al., 1994; Kroll and Potter,1984) (7.5% in each color), accounted for the remainder of stimuli presented. Visual stimuli subtended an angle of ~2.5° along their longest dimension and were presented for 250 ms. The inter-stimulus interval varied randomly between 815-1015 ms (mean ~ 915 ms) (Fig. 1). The current report focused on correct trials in response to target stimuli under the attend condition (target letters in the designated color) in the high memory load task2.
Figure 1.
Illustration of an experimental run.
2.3 Behavioral Data
Mean target accuracy and mean reaction time (RT) were measured. A response was considered a hit if it occurred between 200–1000 ms after stimulus presentation. Target stimuli correctly responded to (target hits) and stimuli incorrectly identified as targets (false alarms) were measured in order to determine an overall accuracy score (% target hits - % false alarms).
2.4 ERP recordings
An ActiveTwo electrode cap (Behavioral Brain Sciences Center, Birmingham, UK) was used to hold to a full array of 128 Ag-AgCl BioSemi (Amsterdam, The Netherlands) “active” electrodes to the scalp, at locations determined by a pre-configured montage. Electrodes were arranged in equidistant concentric circles from the International 10-20 system position Cz. In addition to the 128 electrodes on the scalp, 6 mini bio-potential electrodes were placed, over the left and right mastoid, beneath each eye, and next to the outer canthi of the eyes to capture eye blinks and vertical and horizontal eye movements. EEG activity was digitized at a sampling rate of 512 Hz.
2.5 Data analysis
EEG data were analyzed using ERPLAB (www.erpinfo.org/erplab) (Lopez-Calderon and Luck, 2014) and EEGLAB (http://sccn.ucsd.edu/eeglab) toolboxes that operate within the MATLAB framework (Delorme and Makeig, 2004). Raw EEG data were resampled to 256 Hz and referenced off-line to the algebraic average of the right and left mastoids. EEG signals were filtered using an IIR filter with a bandwidth of 0.03–40 Hz (12 dB/octave roll-off). Eye artifacts were removed through an independent component analysis. Individual bad channels were corrected with the EEGLAB interpolation function. Epochs were discarded from the analyses if they contained baseline drift or movement artifacts greater than ±90 μV.
2.6 Averaged ERP data
For the average waveform ERPs, the analysis focused on P3b amplitude in response to target visual stimuli under the attend condition, high memory load task. The amplitude of the target P3b was measured as the mean value at Pz of the 100 ms interval around local peak latency between 400 and 700 ms.
2.7 Principal Component Analyses (PCA)
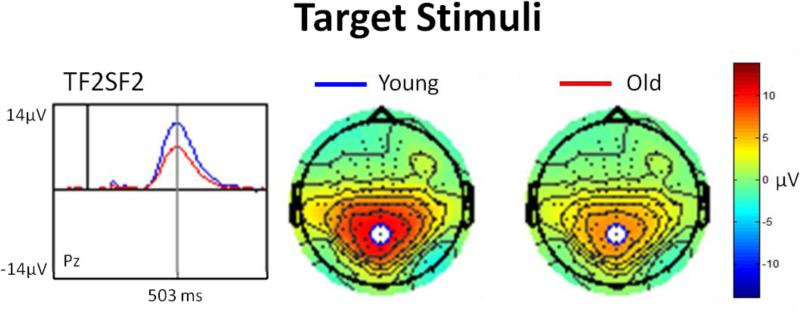
PCA was used to confirm the results of the averaged waveform analysis. PCA is a data-driven method that decomposes ERP waveforms into their underlying components and is particularly useful in parsing spatially and temporally overlapping components. Following the recommendations of Dien et al. (Dien et al., 2007), a two-step temporospatial procedure (Promax rotations) was conducted on all subjects’ individual ERP averages, at all 134 electrode sites, using the ERP PCA toolkit 2.39 (Dien, 2010). A parallel test was used to restrict the number of factors generated for each PCA (Dien et al., 2007). Examination of the latency and topography of the PCA output led to the identification of factor corresponding to the P3b for the visual task (Fig. 3). Factor scores (amplitudes) were submitted to statistical analysis.
Figure 3.
PCA-derived waveform and topographical distribution of the visual P3b component in response to target stimuli.
2.8 Statistical analysis
An ANOVA was conducted to assess whether there were differences between age groups (young vs. old) in size of the average amplitude at the electrode Pz and the PCA-derived P3b factor. Differences between groups (young vs. old) were reanalyzed after controlling for VA or MHL, using ANCOVA. Path analysis was used to confirm the hypothesis about the relationships between variables of interest.
3. Results
3.1 Visual Acuity
VA (with corrective lenses), as measured by a Snellen 10 feet model wall chart, ranged from 20/16 to 20/40 in both groups, with a mean of 1.02 (0.1) ≈ 20/20 in the young group and 0.74 (0.1) ≈ 20/25 in the old group (Table 1). Old subjects had worse VA than young subjects F(1,53) = 42.7, p < 0.004, partial η2 = 0.44. Age inversely correlated with VA: the older the subject, the worse the visual acuity (r = −0.67, p < 0.001).
3.2 Auditory Acuity (mean hearing loss)
Hearing thresholds were determined by a formal audiological evaluation. Mean hearing loss (MHL) across 6 tested frequencies was calculated as the average difference between measured dB level at threshold and 20dB (Friedman et al, 1998), with positive values representing diminished hearing acuity. In the young group, MHL ranged from −15.4 to 0.8, with a mean of −9.8 (4.9); in the old group MHL ranged from −8.7 to 31.1, with a mean of 13.5 (10.5) (Table 1). Old subjects had greater MHL than young subjects F(1,53) = 108.3, p < 0.001, partial η2 = 0.67. Age directly correlated with MHL: the older the subject, the greater the mean hearing loss (r = 0.83, p < 0.001).
3.3 Behavioral data
Comparison of target hits, false alarms and overall accuracy rates did not reveal differences between groups; however, the reaction time of the young group (610.7 (52) ms) was shorter than that of the old group (644.1 (59) ms), F(1,53) = 4.72, p = 0.03, partial η2 = 0.08 (Supplementary Table 1).
3.4 ERP data
3.4.1 P3b to target visual stimuli before and after controlling for visual acuity
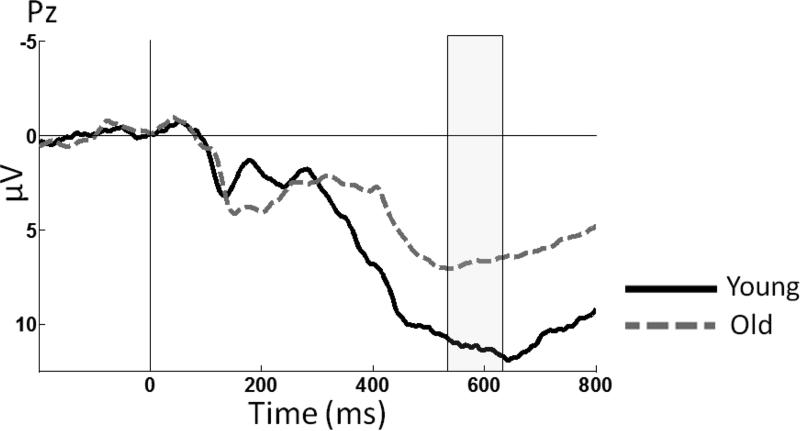
Figure 2 illustrates the grand average waveforms of young and old subjects in response to target visual stimuli at the midline posterior electrode Pz, where the P3b component is most commonly measured. An ANOVA demonstrated an effect of age group, F(1,53) = 8.95, p = 0.004, partial η2 = 0.14, which was present because young subjects generated a larger mean target P3b amplitude than old subjects. An ANCOVA revealed that after controlling for VA, there were no age-related differences in the amplitude of the target P3b, F(1,52) = 1.11, p = 0.29, partial η2 = 0.02.
Figure 2.
Grand-average ERP waveforms at midline electrode site Pz in response to target stimuli. The shaded area represents the approximate temporal interval in which the average amplitutude was measured for the P3b.
PCA revealed a factor (TF2SF2) whose temporal interval and spatial distribution was consistent with the P3b component. It had positive polarity, peaked at 503 ms and accounted for 9.22% of the total variance. Figure 3 illustrates the topography and amplitude (factor scores) of the P3b factor. An ANOVA demonstrated an effect of age (young > old), F(1,53) = 5.44, p = 0.02, partial η2 = 0.09. An ANCOVA revealed that after controlling for VA, age-related differences in P3b amplitude did not survive, F(1,52) = 0.24, p = 0.62, partial η2 = 0.0053.
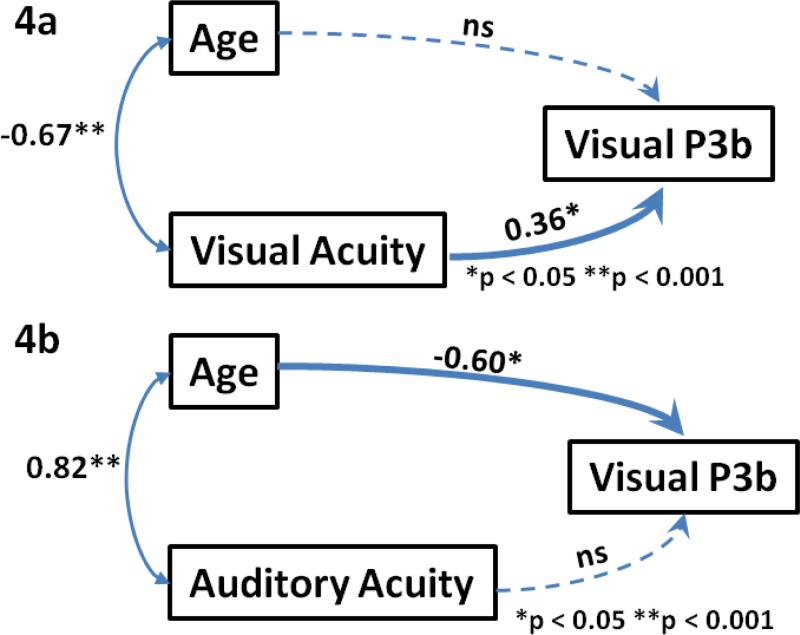
To further address the major question of this study, a path analysis was conducted examining the relationships between age (as a continuous variable), VA, and the PCA-derived target P3b amplitude (Figure 4a). It revealed a strong inverse correlation between age and VA, with a correlation coefficient of −.67 (p < .01). There was also a correlation between VA and P3b amplitude that remained significant after controlling for the impact of age (with a path coefficient = .36, p < .05). In contrast, after controlling for the impact of VA, there was no association between age and P3b amplitude (correlation coefficient = −.03, p > 0.8), suggesting that VA mediates the relationship between age the P3b amplitude. The same pattern of results was found when using age group as a categorical rather than age as a continuous variable. A path analysis was also conducted for the averaged ERP data at Pz and yielded results that were similar to the PCA-derived data (supplementary data Fig. 1a).
Figure 4.
Path analysis examining the relationships between a) PCA-derived P3b factor, age and visual acuity and b) PCA-derived P3b factor, age and auditory acuity (mean hearing loss).
* p < 0.05, ** p < 0.01, ns: non-significant.
3.4.2 P3b to target visual stimuli before and after controlling for mean hearing loss
Given the tight correlation between visual decline and aging, the causal link between VA and age-related decline in the visual target P3b remains ambiguous. As the Common Cause hypothesis suggests, a latent common factor may be responsible for age-associated deterioration in both non-cognitive (sensory) and cognitive processes (Baltes and Lindenberger, 1997; Christensen et al., 2001; Lindenberger and Baltes, 1994; Salthouse and Hancock, 1996). To address the issue of whether specific sensory input is a critical factor that mediates later neural processing of visual events, the original visual P3b data were re-analyzed after controlling for both measures of auditory and visual acuity. Of note, MHL (auditory acuity) also strongly correlated with age, but theoretically should not directly influence visual processing and the amplitude of the P3b to visual targets. Finding that age-related differences in visual target P3b survive controlling for MHL, but not for VA, would be inconsistent with the Common Cause hypothesis.
Analysis of covariance indicated that after controlling for MHL, the difference between the groups (young > old) remained significant: for averaged P3b amplitude at Pz, F(1,52) = 7.44, p = 0.009, eta2 = 0.12, and for the PCA P3b factor, F(1,52) = 7.02, p = 0.01, partial η2 = 0.11.
To further validate these findings, a path analysis was used to examine the relationships between age (as a continuous variable), MHL, and the PCA-derived visual target P3b amplitude (Fig. 4b). It revealed a strong correlation between age and MHL, with a correlation coefficient of .83 (p < .001). There was also a correlation between age and P3b amplitude that remained significant after controlling for the impact of MHL (with a path coefficient = −0.6, p < .01). However, there was no reliable relationship between MHL and P3b amplitude (p = .1). Thus, in contrast to VA, auditory acuity did not impact the relationship between age and P3b to visual targets.
5.1 Discussion
The purpose of this study was to investigate whether unrecognized differences in VA across age groups contribute to the frequently reported age-related changes in neural activity during visual tasks. We replicated a very well-established observation in the ERP literature on cognitive aging (Alperin et al., 2014; Anderer et al., 1998; Fabiani and Friedman, 1995; Fabiani et al., 1998; Kok, 2000; Li et al., 2013; Mullis et al., 1985; O'Connell et al., 2012; Polich, 1997) by finding a robust age-associated decline in P3b amplitude to target visual stimuli. Consistent with numerous publications, all participants reported having normal or corrected-to-normal vision. However, actual measurements of VA revealed that, on average, young adults had better than, and old adults, worse than 20/20 vision, which was associated with a reliable difference in VA between groups. After controlling for VA, the age-related reduction in P3b amplitude to target visual stimuli disappeared. Path analysis confirmed that the relationship between age and diminished P3b to target visual stimuli was mediated by VA. These results are particularly striking since the age-associated impairments in VA were subtle (with an average acuity for old subjects of ~20/27, and no subject having worse than 20/40 vision), and the presented visual stimuli relatively large (subtending an angle of ~2.5°). Of note, even the relatively infrequent studies that have measured VA have not excluded subjects with very mild visual impairment, presumably reflecting the assumption that such deficits would not have an impact on the results (Alperin et al., 2014; Logan et al., 2002; Nielson et al., 2002; Park et al., 2012).
The study also demonstrated that the relationship between sensory input and the neural responses indexed by the P3b component to visual targets may be modality-specific. Controlling for differences in auditory acuity (MHL) did not eliminate the age-related decline in the P3b to targets in the visual modality, which was confirmed by path analysis. These results suggest that accounting for differences in visual acuity (even relatively subtle ones) may influence how one interprets observed differences between young and old adults in neural activity elicited by experimental tasks in the visual modality, and provide a challenge to the status quo approach to investigating this subject.
Given the existence of a separate, large body of research highlighting the strong link between age-associated changes in sensory and cognitive processing (Baltes and Lindenberger, 1997; Gilmore et al., 2006; Lindenberger and Baltes, 1994; Salthouse and Hancock, 1996; Scialfa, 2002), it is curious that a discussion of this issue has not commonly been included in the literature addressing age-related changes in neural activity underlying cognition. The classic version of the Common Cause hypothesis (Baltes and Lindenberger, 1997; Christensen et al., 2001; Lindenberger and Baltes, 1994; Salthouse and Hancock, 1996) suggests that a common, biologically-based factor is responsible for age-related deterioration at all levels of functioning, including ones that mediate peripheral sensory operations. Within this framework, deficits in sensory fidelity among older adults would be viewed as another marker of the aging process, and not as a causally meaningful, intervening variable. In contrast, the Sensory Deficit hypothesis suggests that impaired sensory fidelity can have a direct effect on subsequent cognitive processing (Gilmore et al., 2006; Scialfa, 2002) by reducing the capacity to rapidly extract critical information upon which decisions depend. The data upon which these theories have been developed have largely involved paper and pencil tests of cognitive performance and not indices of neural activity. The results of our path analysis and the apparent modality specificity of sensory acuity on visual P3b amplitude are more consistent with the Sensory Deficit than the Common Cause hypothesis.
According to Mesulam, many aspects of cognition reflect the extensive associative elaboration and attentional modulation of sensory information (Mesulam, 1998). Downstream transmodal areas serve as critical gateways for transforming perception into recognition, and depend on the integrity of the products of earlier sensory processing. As suggested by sequential sampling models, decisions dependent on perceptual processing are mediated by higher-level brain regions like the lateral intraparietal area (LIP) that integrate output from lower-level sensory regions until a criterion amount of evidence is accumulated (Heekeren et al., 2008; Smith and Ratcliff , 2004) Consistent with these perceptual decision-making models, the conveyance of suboptimal sensory data due to diminished VA would undermine the accumulation of sensory evidence. This would disrupt subsequent categorization/decision-making processes, resulting in the transfer of less information (i.e., greater residual uncertainty), which can be indexed by a reduction in the size of the P3b (O'Connell et al., 2012). Viewed from this perspective, the age-related decline in posterior P3b need not be attributed to impairment in cognitive systems mediating decision-making or memory updating, but may be the consequence of the delivery of degraded visual information.
Several models of cognitive aging and adaptation have been developed to explain differences in the recruitment of neural resources across age groups, including the “posterior-anterior shift in aging” or PASA, the “compensation-related utilization of neural circuits hypothesis” or CRUNCH, and the “scaffolding theory of aging and cognition” or STAC (Cabeza et al, 2002; van Dinteren et al., 2014; Gazzaley et al., 2008; Madden et al., 1999; Madden et al., 2004; Madden et al., 2002; Miller et al., 2008; Reuter-Lorenz et al., 2000; Rossini et al., 2007; Rypma and D'Esposito, 2000; De Santi et al., 1995; Stern et al., 2005). These models converge in terms of suggesting that age-related increases in anterior neural activity may be a compensatory response in older adults to inefficiencies of early stages of sensory-perceptual processing that are mediated by posterior cortical regions. Since most of these studies did not seem to measure, and none controlled for differences in VA, the extent to which apparent age-associated dysfunction of early cortical sensory processing may be due to undocumented differences in VA remains an open question. In a previous study, we reported that accounting for VA had a substantial impact on whether older and younger adults differed in the amplitude and latency of ERP indices of early visual processing, the P1 and N1 components (Daffner et al., 2013). These results challenge existing hypotheses that have attributed such age-related changes in the P1 and N1 components to degradation of posterior brain areas, alteration in parvocellular and magnocellular pathways, decline in the integrity of white matter tracts between different nodes of the visual system, or a disruption of the cortical processing indexed by these components (Daffner et al., 2013).
5.2 Limitations
Our study has several limitations. First, no formal ophthalmologic examination was performed to evaluate abnormalities of the lens, or to determine if cataracts or retinal pathology were present, which are relatively common in older individuals (Friedman et al., 1999; Kanthan et al., 2008; Klaver et al., 1998; Skeel et al., 2003; Tielsch et al., 1990). The most benign explanation for reduced VA is that the subjects had inadequate refraction and needed an updated prescription for corrective lenses, which is a common occurrence (Skeel et al., 2003; Tielsch et al., 1990). Most often, age-related decreases in VA are due to peripheral impairment. However, in rare cases, more central causes of visual impairment may have contributed. Second, although the number of subjects in the current study was as large (n = 55) as most reported in the P3 literature upon which inferences about age-related changes in cognitive operations have been made, the findings need to be replicated with a larger sample size, ideally one that includes subjects with a wider range of VA. The current study did not address whether the tight link between sensory fidelity and P3b amplitude would be found in modalities other than vision. Future research is needed to determine whether analogous results occur in experimental tasks involving the auditory, tactile, or olfactory modalities. A strategy for more directly determining if there is a causal link between visual impairment and age-related changes in neural activity would be to experimentally degrade visual acuity or stimulus signal in young adults and examine the impact on P3b amplitude. There is preliminary evidence that employing such an approach has been associated with a reduction of the size of early visual evoked potentials (Millodot, 1970) as well as the P3b to visual targets (Heinrich et al., 2010; Marhöfer et al., 2015). Moreover, some studies have suggested that degrading visual stimulus quality in young adults to simulate the contrast sensitivity loss of older adults can diminish or eliminate age and disease effects on a variety of cognitive tests (Cronin-Golomb et al., 2007; Gilmore et al., 2006; Toner et al., 2012).
6. Conclusion
In summary, the most critical message of this report is that until the relationship between age-associated differences in visual acuity and late markers of neural processing is clearly established, investigators need to be very careful when interpreting their findings. This cautionary tale applies to studies not only using ERPs, but also other functional imaging techniques. It may not make sense to utilize high-tech methods to precisely measure neural activity, while not collecting readily available, low-tech information about factors such as visual acuity. Our results suggest that it is not sufficient to simply ask subjects if they have normal or corrected-to-normal vision (Daffner et al., 2013; Friedman et al., 1999; Skeel et al., 2003). Objective tests of VA are necessary. Investigators may need to account for differences between age groups not only in typically assessed variables like socioeconomic status, education, sex, and estimated IQ, but also sensory acuity. In addition, research on neural changes associated with normal cognitive aging may be enhanced by collecting information not only on disorders that may impact neuropsychological functioning, such as dementia, cerebrovascular disease, diabetes, and depression, but also on conditions that can impair vision, such as cataracts, glaucoma, and macular degeneration. The issues raised by this report will become increasingly relevant as more studies focus on normal cognitive aging in adults over the age of 80, which is the fastest growing sector of our population, and for whom primary sensory deficits are extremely common. There is a strong need for additional research to help develop guidelines about the most appropriate ways to account for age-related differences in sensory fidelity in the study of neural markers of normal cognitive aging.
Supplementary Material
Highlights.
Age-related differences in task-related neural activity are very commonly reported
Impact of group differences in sensory acuity on these findings has been neglected
Here, we replicate a classical ERP finding: a decline in P3b amplitude with aging
Age-associated differences in P3b disappeared after controlling for visual acuity
Researchers should be cautious ascribing results to cognitive, not sensory processing
Acknowledgements
The Laboratory of Healthy Cognitive Aging has been sustained by NIA Grant R01 AG017935 and ongoing support from the Wimberly family, the Muss family, and the Mortimer/Grubman family. The first author (FHGP) received a scholarship from the program “Science without Borders” (Coordination for the Improvement of Higher Education Personnel / Brazil), number 99999.003029/2014-00, which supports advanced training in the USA. Also, the authors would like to thank Sarah Fackler for her excellent administrative assistance.
List of abbreviations
- AMNART
American National Adult Reading Test
- DSM-IV
Diagnostic and statistical Manual of Mental Disorders, 4th ed
- ERPs
Event-related potentials
- LIP
Lateral intraparietal area
- MHL
Mean hearing loss
- MMSE
Mini Mental State Exam
- PCA
Principal Component Analyses
- RT
Reaction time
- VA
Visual acuity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests: The authors report no conflict of interest or financial disclosur
A component the investigators labeled the centro-parietal positivity, which they suggested share all of the characteristics of the P3b.
An analysis (not included here) of the low memory load task revealed the same pattern of results as was observed in the high memory load task.
ANCOVA was repeated using LogMAR (MAR = minimal angle of resolution) values for VA, which unlike Snellen values reflect a linear scale. The same pattern emerged: after controlling for VA using LogMAR values, age-related differences in P3b amplitude did not survive, F(1,52) = 0.28, p = 0.59, partial η2 = 0.005.
References
- Alperin BR, Mott KK, Rentz DM, Holcomb PJ, Daffner KR. Investigating the age-related “anterior shift” in the scalp distribution of the P3b component using principal component analysis. Psychophysiology. 2014;51:620–633. doi: 10.1111/psyp.12206. doi:10.1111/psyp.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Anderer P, Pascual-Marqui RD, Semlitsch HV, Saletu B. Electrical sources of P300 event-related brain potentials revealed by low resolution electromagnetic tomography. 1. Effects of normal aging. Neuropsychobiology. 1998;37:20–27. doi: 10.1159/000026472. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychol Aging. 1997;12:12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Christensen H, Mackinnon AJ, Korten A, Jorm AF. The “common cause hypothesis” of cognitive aging: evidence for not only a common factor but also specific associations of age with vision and grip strength in a cross-sectional analysis. Psychol Aging. 2001;16:588–599. doi: 10.1037//0882-7974.16.4.588. [DOI] [PubMed] [Google Scholar]
- Cronin-Golomb A, Gilmore GC, Neargarder S, Morrison SR, Laudate TM. Enhanced stimulus strength improves visual cognition in aging and Alzheimer's disease. Cortex. 2007;43:952–966. doi: 10.1016/s0010-9452(08)70693-2. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Chong H, Sun X, Tarbi EC, Riis JL, McGinnis SM, Holcomb PJ. Mechanisms underlying age- and performance-related differences in working memory. J Cogn Neurosci. 2011;23:1298–1314. doi: 10.1162/jocn.2010.21540. doi:10.1162/jocn.2010.21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffner KR, Haring AE, Alperin BR, Zhuravleva TY, Mott KK, Holcomb PJ. The impact of visual acuity on age-related differences in neural markers of early visual processing. NeuroImage. 2013;67:127–136. doi: 10.1016/j.neuroimage.2012.10.089. doi:10.1016/j.neuroimage.2012.10.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. doi:10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santi S, de Leon MJ, Convit A, Tarshish C, Rusinek H, Tsui WH, Sinaiko E, Wang GJ, Bartlet E, Volkow N. Age-related changes in brain: II. Positron emission tomography of frontal and temporal lobe glucose metabolism in normal subjects. Psychiatr Q. 1995;66:357–370. doi: 10.1007/BF02238755. doi:10.1007/BF02238755. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dien J. The ERP PCA Toolkit: An open source program for advanced statistical analysis of event-related potential data. J Neurosci Methods. 2010;187:138–145. doi: 10.1016/j.jneumeth.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Dien J. Applying Principal Components Analysis to Event-Related Potentials: A Tutorial. Dev Neuropsychol. 2012;37:497–517. doi: 10.1080/87565641.2012.697503. doi:10.1080/87565641.2012.697503. [DOI] [PubMed] [Google Scholar]
- Dien J, Khoe W, Mangun GR. Evaluation of PCA and ICA of simulated ERPs: Promax vs. Infomax rotations. Hum Brain Mapp. 2007;28:742–763. doi: 10.1002/hbm.20304. doi:10.1002/hbm.20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin E. Presidential address, 1980. Surprise!...Surprise? Psychophysiology. 1981;18:493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behav Brain Sci. 1988;11:357–374. doi:10.1017/S0140525X00058027. [Google Scholar]
- Fabiani M, Friedman D. Changes in brain activity patterns in aging: the novelty oddball. Psychophysiology. 1995;32:579–594. doi: 10.1111/j.1469-8986.1995.tb01234.x. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Friedman D, Cheng JC. Individual differences in P3 scalp distribution in older adults, and their relationship to frontal lobe function. Psychophysiology. 1998;35:698–708. [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1973;12:189–198. doi: 10.1016/0022-3956(75)90026-6. doi:10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friedman D, Kazmerski V, Cycowicz Y. Effects of aging on the novelty P3 during attend and ignore oddball tasks. Psychophysiology. 1998;35:508–520. doi: 10.1017/s0048577298970664. doi:10.1017/S0048577298970664. [DOI] [PubMed] [Google Scholar]
- Friedman SM, Munoz B, Rubin GS, West SK, Bandeen-Roche K, Fried LP. Characteristics of discrepancies between self-reported visual function and measured reading speed. Salisbury Eye Evaluation Project Team. Invest Ophthalmol Vis Sci. 1999;40:858–864. [PubMed] [Google Scholar]
- Gazzaley A, Clapp W, Kelley J, McEvoy K, Knight RT, D'Esposito M. Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proc Natl Acad Sci U S A. 2008;105:13122–13126. doi: 10.1073/pnas.0806074105. doi:10.1073/pnas.0806074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore GC, Spinks RA, Thomas CW. Age effects in coding tasks: Componential analysis and test of the sensory deficit hypothesis. Psychol Aging. 2006;21:7–18. doi: 10.1037/0882-7974.21.1.7. doi:10.1037/0882-7974.21.1.7. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Marrett S, Ungerleider LG. The neural systems that mediate human perceptual decision making. Nat Rev Neurosci. 2008;9:467–479. doi: 10.1038/nrn2374. [DOI] [PubMed] [Google Scholar]
- Heinrich SP, Marhöfer D, Bach M. “Cognitive” visual acuity estimation based on the event-related potential P300 component. Clin Neurophysiol. 2010;121:1464–1472. doi: 10.1016/j.clinph.2010.03.030. doi:10.1016/j.clinph.2010.03.030. [DOI] [PubMed] [Google Scholar]
- Johnson R., Jr. A triarchic model of P300 amplitude. Psychophysiology. 1986;23:367–384. doi: 10.1111/j.1469-8986.1986.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Kanthan GL, Wang JJ, Rochtchina E, Tan AG, Lee A, Chia EM, Mitchell P. Ten-year incidence of age-related cataract and cataract surgery in an older Australian population. The Blue Mountains Eye Study. Ophthalmology. 2008;115:808–814. e1. doi: 10.1016/j.ophtha.2007.07.008. doi:10.1016/j.ophtha.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Klaver CC, Wolfs RC, Vingerling JR, Hofman A, de Jong PT. Age-specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam Study. Arch Ophthalmol. 1998;116:653–658. doi: 10.1001/archopht.116.5.653. [DOI] [PubMed] [Google Scholar]
- Kok A. Age-related changes in involuntary and voluntary attention as reflected in components of the event-related potential (ERP). Biol Psychol. 2000;54:107–143. doi: 10.1016/s0301-0511(00)00054-5. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Alpert NM, Thompson WL, Chabris CF, Rauch SL, Anderson AK. Identifying objects seen from different viewpoints. A PET investigation. Brain. 1994;117:1055–1071. doi: 10.1093/brain/117.5.1055. [DOI] [PubMed] [Google Scholar]
- Kroll JF, Potter MC. Recognizing words, pictures, and concepts: A comparison of lexical, object, and reality decisions. Journal of Verbal Learning and Verbal Behavior. 1984;23:39–66. [Google Scholar]
- Li L, Gratton C, Fabiani M, Knight RT. Age-related frontoparietal changes during the control of bottom-up and top-down attention: an ERP study. Neurobiol Aging. 2013;34:477–488. doi: 10.1016/j.neurobiolaging.2012.02.025. doi:10.1016/j.neurobiolaging.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: a strong connection. Psychol Aging. 1994;9:339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Lopez-Calderon J, Luck SJ. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front Hum Neurosci. 2014;8:213. doi: 10.3389/fnhum.2014.00213. doi:10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, Denny LL, Hawk TC, Gottlob LR, Coleman RE. Adult age differences in the functional neuroanatomy of verbal recognition memory. Hum Brain Mapp. 1999;7:115–135. doi: 10.1002/(SICI)1097-0193(1999)7:2<115::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, Denny LL, Langley LK, Hawk TC, Coleman RE. Aging and attentional guidance during visual search: functional neuroanatomy by positron emission tomography. Psychol Aging. 2002;17:24–43. doi: 10.1037//0882-7974.17.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Provenzale JM, Huettel SA. Age-related Changes in Neural Activity during Visual Target Detection Measured by fMRI. Cereb Cortex. 2004;14:143–155. doi: 10.1093/cercor/bhg113. doi:10.1093/cercor/bhg113. [DOI] [PubMed] [Google Scholar]
- Marhöfer DJ, Bach M, Heinrich SP. Objective measurement of visual resolution using the P300 to self-facial images. Doc Ophthalmol. 2015;131:137–148. doi: 10.1007/s10633-015-9502-1. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamäki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci U S A. 2008;105:2181–2186. doi: 10.1073/pnas.0706818105. doi:10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millodot M, Riggs LA. Refraction Determined Electrophysiologically. Arch Ophthalmol. 1970;84:272–278. doi: 10.1001/archopht.1970.00990040274003. doi:10.1001/archopht.1970.00990040274003. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Johnson W. Neural reorganization and compensation in aging. J Cogn Neurosci. 2015;27:1275–1285. doi: 10.1162/jocn_a_00783. doi:10.1162/jocn_a_00783. [DOI] [PubMed] [Google Scholar]
- Mullis RJ, Holcomb PJ, Diner BC, Dykman RA. The effects of aging on the P3 component of the visual event-related potential. Electroencephalogr Clin Neurophysiol. 1985;62:141–149. doi: 10.1016/0168-5597(85)90026-7. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Langenecker SA, Garavan H. Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychol Aging. 2002;17:56–71. doi: 10.1037//0882-7974.17.1.56. [DOI] [PubMed] [Google Scholar]
- O'Connell RG, Balsters JH, Kilcullen SM, Campbell W, Bokde AW, Lai R, Upton N, Robertson IH. A simultaneous ERP/fMRI investigation of the P300 aging effect. Neurobiol Aging. 2012;33:2448–2461. doi: 10.1016/j.neurobiolaging.2011.12.021. doi:10.1016/j.neurobiolaging.2011.12.021. [DOI] [PubMed] [Google Scholar]
- O'Connell RG, Dockree PM, Kelly SP. A supramodal accumulation-to-bound signal that determines perceptual decisions in humans. Nat Neurosci. 2012;15:1729–1735. doi: 10.1038/nn.3248. doi:10.1038/nn.3248. [DOI] [PubMed] [Google Scholar]
- Owsley C. Aging and vision. Vision Res. 2006;51:1610–1622. doi: 10.1016/j.visres.2010.10.020. doi: 10.1016/j.visres.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Carp J, Kennedy KM, Rodrigue KM, Bischof GN, Huang CM, Rieck JR, Polk TA, Park DC. Neural broadening or neural attenuation? Investigating age-related dedifferentiation in the face network in a large lifespan sample. J Neurosci. 2012;32:2154–2158. doi: 10.1523/JNEUROSCI.4494-11.2012. doi:10.1523/JNEUROSCI.4494-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips LH, Andrés P. The cognitive neuroscience of aging: New findings on compensation and connectivity. Cortex. 2010;46:421–424. doi: 10.1016/j.cortex.2010.01.005. doi:10.1016/j.cortex.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Picton TW. The P300 wave of the human event-related potential. J Clin Neurophysiol. 1992;9:456–749. doi: 10.1097/00004691-199210000-00002. doi:10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- Polich J. EEG and ERP assessment of normal aging. Electroencephalogr Clin Neurophysiol. 1997;104:244–156. doi: 10.1016/s0168-5597(97)96139-6. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci. 2008;17:177–182. [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Riis JL, Chong H, Ryan KK, Wolk DA, Rentz DM, Holcomb PJ, Daffner KR. Compensatory neural activity distinguishes different patterns of normal cognitive aging. NeuroImage. 2008;39:441–454. doi: 10.1016/j.neuroimage.2007.08.034. doi:10.1016/j.neuroimage.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Rossi S, Babiloni C, Polich J. Clinical neurophysiology of aging brain: From normal aging to neurodegeneration. Prog Neurobiol. 2007;83:375–400. doi: 10.1016/j.pneurobio.2007.07.010. doi:10.1016/j.pneurobio.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Ryan JJ, Paolo AM. A screening procedure for estimating premorbid intelligence in the elderly. Clin Neuropsychol. 1992;6:53–62. doi:10.1080/13854049208404117. [PubMed] [Google Scholar]
- Rypma B, D'Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci. 2000;3:509–515. doi: 10.1038/74889. doi:10.1038/74889. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Hancock HE, Meinz EJ, Hambrick DZ. Interrelations of age, visual acuity, and cognitive functioning. J Gerontol B Psychol Sci Soc Sci. 1996;51:317–30. doi: 10.1093/geronb/51b.6.p317. doi:10.1093/geronb/51B.6.P317. [DOI] [PubMed] [Google Scholar]
- Scialfa CT. The role of sensory factors in cognitive aging research. Can J Exp Psychol. 2002;56:153–163. doi: 10.1037/h0087393. doi:10.1037/h0087393. [DOI] [PubMed] [Google Scholar]
- Skeel RL, Nagra A, VanVoorst W, Olson E. The relationship between performance-based visual acuity screening, self-reported visual acuity, and neuropsychological performance. Clin Neuropsychol. 2003;17:129–136. doi: 10.1076/clin.17.2.129.16509. doi:10.1076/clin.17.2.129.16509. [DOI] [PubMed] [Google Scholar]
- Smith PL, Ratcliff R. Psychology and neurobiology of simple decisions. Trends Neurosci. 2004;27:161–168. doi: 10.1016/j.tins.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Stern Y, Habeck C, Moeller J, Scarmeas N, Anderson KE, Hilton JH, Flynn J, Sackeim H, van Heertum R. Brain networks associated with cognitive reserve in healthy young and old adults. Cereb Cortex. 2005;15:394–402. doi: 10.1093/cercor/bhh142. doi:10.1093/cercor/bhh142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tielsch JM, Sommer A, Witt K, Katz J, Royall RM. Blindness and visual impairment in an American urban population. The Baltimore Eye Survey. Arch Ophthalmol. 1990;108:286–290. doi: 10.1001/archopht.1990.01070040138048. [DOI] [PubMed] [Google Scholar]
- Toner CK, Reese BE, Neargarder S, Riedel TM, Gilmore GC, Cronin-Golomb A. Vision-fair neuropsychological assessment in normal aging, Parkinson's disease and Alzheimer's disease. Psychol Aging. 2012;27:785–790. doi: 10.1037/a0026368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dinteren R, Arns M, Jongsma ML, Kessels RP. P300 Development across the Lifespan: A Systematic Review and Meta-Analysis. PLoS ONE. 2014;9:e87347. doi: 10.1371/journal.pone.0087347. doi:10.1371/journal.pone.0087347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.