Abstract
An ion chromatographic method with conductivity detection for the precise and accurate analysis of lithium ions in phosphate-buffered saline, used as a cervicovaginal lavage (CVL) fluid, was developed and validated. The lithium ion dilution factor during the CVL is used to calculate the volume of cervicovaginal fluid (CVF) collected. Initial CVL Li+ concentrations of 1 mM and 10 mM were evaluated. The method is robust, practical, and afforded an accurate measurement (5% of the measurement, or better) at 24 μL of vaginal fluid simulant collected per mL of CVL fluid, as low as 5 μL mL−1 using 10 mM Li+ with a measurement accuracy of 6.7%. Ion chromatograms of real-world CVL samples collected in vivo from common animal models (sheep and pig-tailed macaque) and a human volunteer demonstrate that the analysis is interference-free. The method is readily transferrable and should enable the accurate measurement of CVF volume collected during CVLs benefitting a broad range of research disciplines, including pharmacokinetic, pharmacodynamic, metabolomic, and microbiome studies.
Keywords: Cervicovaginal fluid dilution, Cervicovaginal lavage, Ion chromatography, Lithium
1. Introduction
Cervicovaginal lavage (CVL) is a frequently used sampling method in preclinical and clinical studies. A lavage fluid –usually 2.5-10 mL in clinical trials [1, 2], but lower for small animal models (e.g., 1 mL for macaques)– is used to rinse the lower reproductive tract and collect the components of the mucosal surface, including small molecules (xenobiotics and metabolites), extracellular polymeric substances, proteins and peptides, microbial cells, and host cells. Sterile saline or phosphate-buffered saline (PBS) are the most commonly used lavage fluids, as water is hypotonic and can lyse cells, confounding measurements of extracellular and intracellular vaginal fluid components [1]. The CVL method provides the significant advantage of collecting a sample integrated over the entire lower female genital tract, rather than the local sample obtained with swabs, sponges, and tear test strips. Consequently, CVLs collect larger cervicovaginal fluid (CVF) volumes that do not need to be recovered from the sampling device at the time of analysis. CVL is a safe and simple procedure that can be carried out easily in the clinic and self-sampling by trial participants also has been reported. A new self-sampling device was evaluated by Rwandan women and found to be acceptable for CVL collection [3], increasing the potential for sample collection in longitudinal studies.
The advantages sample collection via CVL have been exploited in numerous, diverse studies aimed at measuring the CVF concentration of one or more target analytes. Vaginal proinflammatory cytokines and other markers of immune activation are routinely measured in CVL [4-9]. More recently, a multiplexed assay to analyze antimicrobial peptides in CVL has been reported [10]. Proteomic studies also have been conducted using CVL samples [11]. Molecular methods have been used to measure the abundance of viral pathogens in CVL samples, including: human papillomavirus (HPV) [12, 13], hepatitis C virus (HCV) [14], herpes simplex virus type 2 (HSV-2) [15-18], human immunodeficiency virus type 1 (HIV-1) [7, 15, 19], and cytomegalovirus (CMV) [20]. CVL samples from women volunteers have been analyzed to determine whether topical zinc deficiency is a risk factor in recurrent vulvovaginal candidiasis [21] and if dissolved nitric oxide gas is associated with bacterial vaginosis (BV) [22]. CVL samples even have been used to isolate bacterial DNA in culture-independent vaginal microbiome studies [23, 24] and metabolomics studies [25, 26]. The concentration of antiviral agents is measured in CVL samples as part of pharmacokinetic studies aimed at developing regimens for the prevention of vaginal HIV-1 [27-32] and HSV-2 [29, 33] infection. Recently, the analysis of CVL exosomes carrying microRNAs has been proposed as new biomarker for cervical cancer screening [34].
One fundamental drawback in current studies involving CVL sample analysis lies with the unknown amount of CVF collected, which can vary over more than one order of magnitude in women [2, 35]. This broad uncertainty can lead to large errors in the measurements, confounding the interpretation of results. The inclusion of an additive in the CVL fluid that is not endogenous to the vaginal mucosa could be used to calculate the dilution of CVFs. The additive should be selected according to the following criteria: does not interfere with downstream assays (e.g., ELISA) at the employed concentration; does not partition significantly into the vaginal mucosa during the lavage procedure; and can be analyzed with high measurement accuracy and precision. Both lithium chloride (LiCl) [2, 35] and gluconate [1] as inert CVL additives have been used to measure this dilution factor, but these methods suffer from insufficient precision. These significant limitations explain why the dilution factor in CVL samples is not measured routinely.
Here, a validated ion chromatography (IC) method for accurate and precise Li+ analysis in the determination of CVF dilution factors in CVL samples is described. The method is robust across lavage fluid volumes up to 10 mL and Li+ concentrations from 1-10 mM, and is applicable to a broad set of disciplines involved in the quantitative measurement of analytes in the vaginal mucosa.
2. Material and methods
2.1. Chemicals
Lithium chloride (molecular biology grade, L9650) and bovine serum albumin (BSA, ≥ 99%) were purchased from Sigma-Aldrich (St. Louis, MO) and methanesulfonic acid (MSA, 98+%) was purchased from Alfa Aesar (Haverhill, MA). Sodium chloride (USP-grade) and potassium hydroxide (USP-grade) were obtained from BDH through VWR International (Radnor, PA) and calcium hydroxide (98%, extra pure), D,L-lactic acid (85%), and glycerol (Reagent ACS, 99.6%) were obtained from Acros Organics through Thermo Fisher Scientific (Waltham, MA). D-Glucose, monohydrate (biotechnology grade) was obtained from Amresco (Solon, OH) and acetic acid (Certified ACS), urea (reagent grade), and phosphate-buffered saline (PBS, 10× solution, DNase-, RNase-, and protease-free) were purchased from Thermo Fisher Scientific. High purity water (HPW, > 18 MΩ-cm) was obtained from a Milli-Q UF Plus ultrapure water system (EMB Millipore, Billerica, MA). Vaginal fluid simulant (VFS) was prepared according to the recipe by Owen and Katz [36].
2.2. Optimized IC Method for Li+ Analysis
The analytical procedure was based on published methods [37, 38]. The ion chromatography (IC) system consisted of a Model G1329A autosampler (Agilent Technologies, Santa Clara, CA) using an injection volume of 10 μL, a Model AS50 chromatography compartment (Dionex, Sunnyvale, CA), a Model GP50 gradient pump (Dionex) operating at a flow rate of 1.0 mL min−1, a Model ED40 electrochemical detector (Dionex) in conductivity mode with the conductivity cell contained in a DS30 temperature stabilization compartment. The Dionex IC components and data acquisition was controlled by Chromeleon software version 6.80 (Dionex). The autosampler was controlled by Chemstation software version B.01.03 (Agilent Technologies) that was synchronized with the Dionex components through external relay contacts on the GP50. Separation of lithium ions (Li+) was achieved using a Dionex IonPac CS12A, 4 × 250 mm column and IonPac CG12A 4 × 50 mm guard column maintained at 30°C, under isocratic elution conditions with 10 mM MSA as the mobile phase. Ions were detected by conductivity using cation suppression with a Dionex CSRS 300 self-regenerating suppressor operated at 50 mA. Runs were started after 1 h of equilibration at 1 mL min−1 flow, the run time was 12.5 min, and the Li+ peak had a retention time of 5.4 min. The column was regenerated as needed to achieve good Li+ peak shapes by ramping the eluant at 1 mL min−1 from 10 mM to 100 mM MSA over 8 min, holding at 100 mM for 20 min, and ramping from 100 mM to 10 mM over 8 min. The column was then equilibrated for 23 min with 10 mM MSA prior to injecting the next set of samples.
Measurement stability was evaluated by carrying out sixty consecutive measurements of a PBS sample containing either 1 mM or 10 mM LiCl without column regeneration.
2.3. Optimization of Parameters for Determination of CVL Li+ Dilution in by CVF
The coefficients of variation (CV) of consecutive Li+ measurements as a function of the number of replicates (N = 3-12) per sample at 1 mM and 10 mM LiCl in CVL fluid was determined. Three concentrations of VFS in the CVL were used: 5 μL mL−1; 50 μL mL−1; and 250 μL mL−1. The samples (0.1 mL) were diluted with HPW (1 mL) prior to analysis using high quality, locked, mechanical pipettors.
The measurement variation introduced by the above dilution step was investigated by comparing six successive analyses of a single CVL sample, diluted with HPW (0.1 mL with 1 mL) with single analyses of six samples prepared from the same CVL stock, but diluted independently with HPW (0.1 mL CVL diluted with 1.0 mL HPW); i.e., six separate dilutions, one for each measurement.
2.4. Calculation of CVF Volume Collected in the CVL
The calculation of CVF volume collected in the CVL is based on the method by Belec et al. [35], with some important differences. Rather than using a Li+ concentration measurement derived from the instrument signal and a suitable calibration curve, Li+ peak area is used directly in equation 1.
| (1) |
where fv (μL mL−1) is the volume of CVF (μL) collected per mL of lavage fluid, A1 is the Li+ peak area in the naïve CVL fluid, and A2 is the Li+ peak area in the collected CVL sample.
In the optimized method, each fv measurement is made up of four replicate sets of paired A1-A2 analyses (eight analyses total); i.e., two samples –the CVL stock solution prepared for the study (naïve reference) and the CVL collected in the study (diluted sample)– are measured sequentially four times.
2.5. Measurement Accuracy
Eight synthetic standards spanning the fv = 5-404 μL mL−1 range were prepared using PBS and VFS and analyzed as described above. A plot of the measured simulated CVF volume (Vmeas) versus actual simulated CVF volume (Vact) collected in 1 mL of CVL fluid was used to analyze the linearity of the response and the deviation from ideality. Measurement accuracy at fv = 5.0 and 24.1 μL mL−1 was determined with and without calibration of the calculated fv values (eq. 1) using the slope and intercept from a plot of Vmeas versus Vact, with the corresponding samples (i.e., either 5.0 or 24.1 μL mL−1) omitted.
2.6. Analysis of CVL Samples Collected in Vivo
Real-world, Li-containing CVL samples were collected in vivo for IC analysis. The sheep sample (1 mM Li+, 10 mL) was collected at Colorado State University (Fort Collins, CO) according to NIH guidelines in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) using established procedures [27, 29, 31]. The protocols were approved by Institutional Animal Care and Use Committees at Colorado State University (Animal Welfare Assurance Number: A3572-01). Details on the pig-tailed macaque pharmacokinetic study that included the collection of Li-containing CVL samples (1 mM, 5 mL) have been presented elsewhere [32]. The human CVL sample (10 mM, 2.5 mL) was collected under approval by the Institutional Review Board of the University of Texas Medical Branch at Galveston (IRB 14-0479).
2.7. Statistical Analyses
Data were analyzed using GraphPad Prism (version 6.05; GraphPad Software, Inc., La Jolla, CA). Comparisons between groups were carried out using an unpaired Student t-test with statistical significance defined as a two-tailed P value of < 0.05.
3. Results
The optimized IC method for Li+ determination in PBS requires precise sample dilution –0.1 mL CVL diluted with 1 mL of HPW was used here– to avoid sodium (from the PBS) buildup on the column that degrades the Li+ peak shape and quantitation accuracy. Controlling the column temperature at 30°C to improve chromatographic reproducibility and replacing the autosampler that formed part of the original IC system (Model AS50, Dionex) with a Model G1329A unit (Agilent Technologies) to increase injection volume accuracy and precision significantly improved the method reliability.
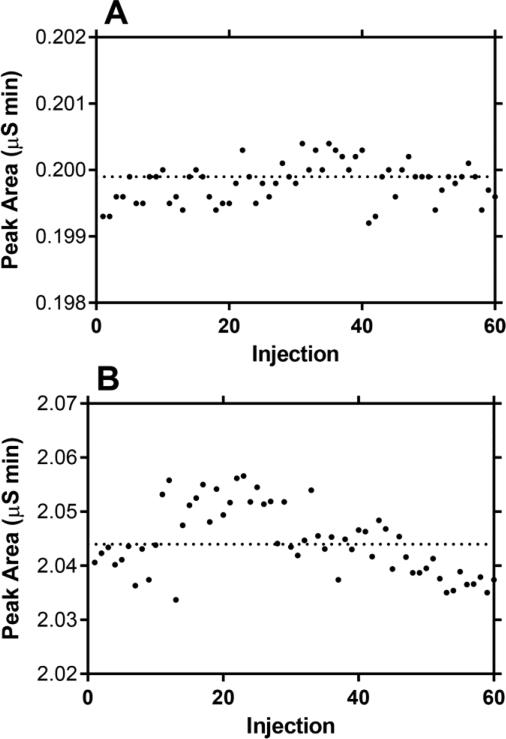
The stability of sixty, sequential, replicate measurements is illustrated by Figure 1 using PBS containing LiCl (Fig. 1A, 1 mM; Fig. 1B, 10 mM) diluted 0.1 mL into 1 mL of HPW. No column washes or regeneration cycles were carried out between sample injections. The CV for the six consecutive deciles were 0.08-0.16% (1 mM Li+) and 0.10-0.31% (10 mM Li+), while the CV for the complete sets of sixty measurements were 0.15% (1 mM Li+) and 0.31% (10 mM Li+).
Fig. 1.
Lithium ion peak areas measured in sixty sequential injections of PBS spiked with (A) 1 mM LiCl and (B) 10 mM LiCl after dilution with HPW (0.1 mL with 1.0 mL).
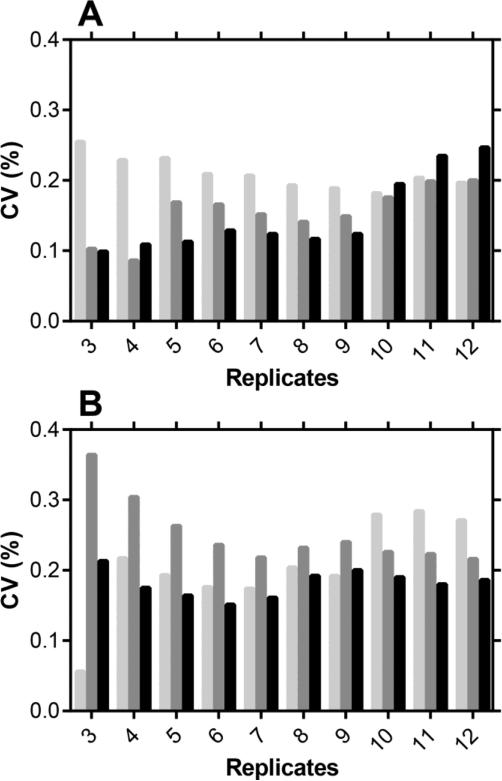
The high level of precision –defined as the “closeness of agreement between indications or measured quantity values obtained by replicate measurements on the same or similar objects under specified conditions” [39], and characterized by a low CV– observed in Figure 1 by repeated injections of the same sample led to the next phase of method development aimed at determining the minimum number of measurement replicates needed to maintain a low CV. Figure 2 shows the CV of repeated measurements (simulated fv = 5, 50, and 250 μL mL−1) as a function of the number of replicates (N = 3-12). Using six or more replicate measurements did not increase the analytical precision. The results show that a high precision (CV < 0.3%) is obtained routinely using four replicate measurements of the sample, with CVs in the 0.1-0.2% range observed in four out of six experiments. While it appears feasible to obtain acceptable precision with three repeated measurements of the same sample, four replicates represent the most useful compromise between precision and practicality.
Fig. 2.
Lithium ion measurement precision (CV) as a function of the number of replicates per sample at (A) 1 mM LiCl and (B) 10 mM LiCl in the CVL fluid. Pale grey bars, fv = 5 μL mL−1 VFS; dark grey bars, fv = 50 μL mL−1 VFS; black bars, fv = 250 μL mL−1 VFS.
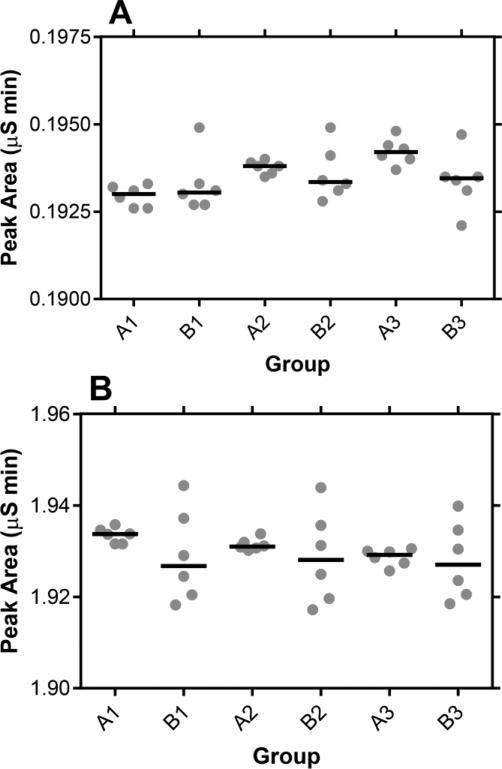
Dilution of the CVL samples is required to lower the sodium content of the fluid, as discussed above, enabling at least sixty successive samples to be analyzed without regenerating the column. While high quality, locked, mechanical pipettors were used to obtain the dilutions, it was important to quantify the variability introduced by this procedure. Figure 3 compares measurements from three paired experiments. All six measurements in group A were performed on the same diluted sample while all six samples in group B were prepared by separate dilutions. A Student t test showed that there was no statistically significant difference between groups A and B in these tests (Fig. 3A, 1 mM Li+, P = 0.050-0.617; Fig. 3B, 10 mM Li+, P = 0.299-0.832), while the variability in the measurements consistently was greater in group B. The reduction in measurement precision introduced by the dilution step needs to be taken into account when defining quality control parameters, as discussed below.
Fig. 3.
Scatter plots of three paired sets of Li+ measurements in CVL fluid containing (A) 1 mM and (B) 10 mM LiCl, with the horizontal line representing the median. Measurements A1-A3 were obtained by measuring six successive times a single CVL sample, diluted with HPW (0.1 mL with 1.0 mL), while measurements B1-B3 were obtained by measuring six samples prepared from the same CVL stock, but diluted independently with HPW (0.1 mL with 1.0 mL); i.e., six separate dilutions, one for each measurement.
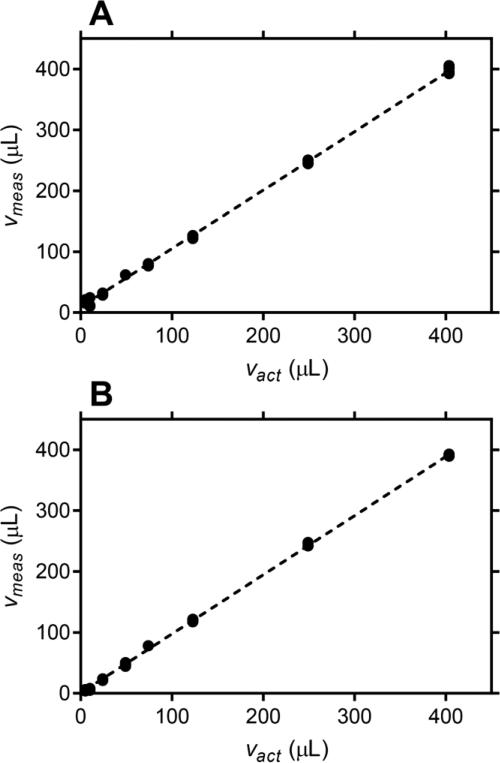
The accuracy of the measurements was determined by preparing a set of samples simulating fv values in the 5-400 μL mL−1 range, representative of the range of CVF volumes expected to be collected in vivo [2, 35]. Accuracy is defined as the “closeness of agreement between a measured quantity value and a true quantity value of a measurand” [39], where the measurand is the true value that would be obtained with a perfect measurement. Figure 4 shows excellent agreement between actual and measured simulated CVF volumes, with less than 4% and 3% deviation from ideality for 1mM and 10 mM Li+, respectively. Measurement accuracy was determined for samples of simulated CVF in 1 mL CVL using the calibration data from Fig. 4. For 1 mM Li+, measurement accuracy of 2.2% was obtained for fv = 24.1 μL mL−1 CVF in CVL, and for 10 mM Li+, measurement accuracies of 3.1% and 1.4% were obtained for fv = 5.0 and 24.1 μL mL−1 samples, respectively. For the accuracy determinations, data corresponding to 5.0 μL or 24.1 μL of simulated CVF were omitted from the linear fit. Calibration of the calculated fv values was not required at 10 mM Li+. Measurement accuracies of 6.7 and 4.3% were obtained for fv = 5.0 and 24.1 μL mL−1, respectively, without calibration.
Fig. 4.
Plot of measured simulated CVF volume (Vmeas) versus actual simulated CVF volume (Vact) collected in 1 mL of CVL fluid. Four individual replicates are shown for each measurement and the broken line represents a linear fit to all data. (A) 1 mM Li+, slope = 0.9610 ± 0.0063, y-intercept = 8.909 ± 1.117 μL, R2 = 0.9987; and (B) 10 mM Li+, slope = 0.9706 ± 0.00372, y-intercept = 0.4794 ± 0.6568 μL, R2 = 0.9996.
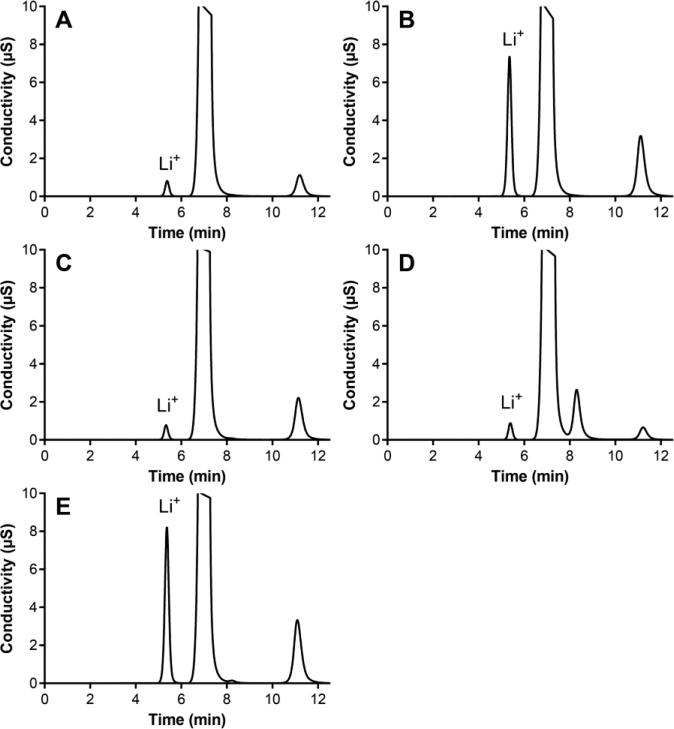
Figure 5 overlays chromatograms from simulated and in vivo CVL samples containing different Li+ levels. These results confirm the interference-free measurement of Li+ in real-world samples and illustrate the excellent Li+ peak shape.
Fig. 5.
Ion chromatograms of CVL (PBS containing LiCl) samples: (A) synthetic, fv = 100 μL mL−1 VFS, 1 mM Li+; (B) synthetic, fv = 100 μL mL−1 VFS, 10 mM Li+; (C) sheep, fv = 141 μL mL−1, 1 mM Li+; (D) pig-tailed macaque, fv = 40 μL mL−1, 1 mM Li+; and (E) human subject, fv = 107 μL mL−1, 10 mM Li+. All CVL samples were diluted 0.1 mL into 1 mL of HPW. The large peak at 7.2 min corresponds to Na+ and the peak at 11.1 min to K+. The peak at 8.3 min likely corresponds to NH +4.
4. Discussion
The measurement of CVF volume collected during a CVL represents a unique analytical challenge. There are no known naïve components of CVF that could act as internal markers of dilution, so a suitable additive needs to be included with the CVL fluid. The CVL volume used in human studies generally falls within the 2.5-10 mL range. The CVF volume collected in a CVL can vary over more than one order of magnitude across individuals with median values in the 0.3-0.5 mL range depending on the study and the phase of menstrual cycle [2, 35]. On average, the dilution of the CVL fluid component (2.5-10 mL) by the collected CVF (0.4 mL) therefore will be in the 4-14% range, depending on the CVL volume. The measurement of CVF volume based on dilution of a marker analyte in the CVL therefore will involve accurate analysis of small changes on a relatively large signal; the largest signal will be obtained in the naïve, undiluted lavage fluid.
The measurement precision and accuracy of the assay will dictate the lowest achievable fv value. For example, Belec et al. reported a measurement precision of 8.5% for 10 mM Li+ over 15 replicates and a CV of 2.5% for the Li+ measurement, although it is not clear whether this referred to the accuracy at full-scale (3 meq L−1) or at the measured Li+ concentration [35]. This modest precision and accuracy will be limiting at low fv values. Here, a measurement accuracy of 5%, or better, was achieved at fv = 5.0 μL mL−1 (10 mM Li+), sufficient to satisfy the analytical needs of most common applications. The accuracy of the method is achieved based on high measurement precision (CV < 0.3%), the analytical strategy based on the mean of four peak area measurement pairs per sample, and the linearity of the instrument response.
The measurement precision and accuracy requirements of CVF volume determination in a CVL have led to the development of a set of practical recommendations:
When planning the study, use 10 mM Li+ in the lavage fluid and minimize the CVL volume for sampling. For human and sheep studies a CVL volume of 2.5 mL is recommended and a volume of 1 mL is recommended for nonhuman primate studies. Higher lavage volumes would reduce the lowest CVF volume quantifiable based on a 5.0 μL mL−1 (10 mM Li+) limit,
Always store a refrigerated, representative stock of naïve CVL fluid from each batch used in animal or human studies and use this stock as the reference sample in the corresponding analyses,
CVL reference (A1, see 2.4.) and in vivo CVL sample (A2) are analyzed sequentially as an A1-A2 pair, with four A1-A2 replicate measurements per sample (Fig. 2),
The peak area CV for each set of four replicate measurements is calculated and CVs greater than 0.5% are an indicator of suboptimal precision and may need to be reanalyzed,
Peak area, not the Li+ concentration, are used in eq. 1 to calculate fv, there-by avoiding errors introduced by calibration of the instrument response. This approach is possible because the strict requirement of linearity in instrument response to Li+ concentration is met over the target analytical range (Fig. 4),
A calibration curve may not be required to remove measurement biases, but is recommended as a quality control.
5. Conclusions
An IC method for the interference-free, accurate, and precise analysis of Li+ in PBS has been developed to measure CVF dilution during a CVL. The simplicity of the method and the broad applicability of the analytical technique is expected to enable wide-scale adoption by researchers measuring the concentration of vaginal analytes in CVL samples. Accurate compensation for dilution of the CVF collected in the CVL will significantly improve the rigor of associated analyses in diverse fields, including: pharmacokinetic, pharmacodynamic, metabolomic, and microbiome studies.
Highlights.
Uncertainty in collected vaginal fluid volume is key drawback of lavage technique
Validated IC method for accurate and precise measurement lithium, marker of dilution
Chromatograms of samples collected in vivo underscore method utility
Acknowledgements
Research reported in this publication was supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under Award Numbers U19AI113048 and 5R33AI079791. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Priya Srinivasan and James M. Smith (Centers for Disease Control and Prevention), Jeremiah Easley (Colorado State University), and Richard B. Pyles and Kathleen L. Vincent (University of Texas Medical Branch at Galveston) for providing in vivo cervicovaginal lavage samples for analysis.
Abbreviations
- BSA
bovine serum albumin
- BV
bacterial vaginosis
- CVF
cervicovaginal fluid
- CVL
cervicovaginal lavage
- HPW
high purity water
- MSA
methanesulfonic acid
- VFS
vaginal fluid simulant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Dezzutti CS, Hendrix CW, Marrazzo JM, Pan ZY, Wang L, Louissaint N, Kalyoussef S, Torres NM, Hladik F, Parikh U, Mellors J, Hillier SL, Herold BC. Performance of Swabs, Lavage, and Diluents to Quantify Biomarkers of Female Genital Tract Soluble Mucosal Mediators. PLoS One. 2011;6:e23136. doi: 10.1371/journal.pone.0023136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell C, Paul K, Agnew K, Gaussman R, Coombs RW, Hitti J. Estimating Volume of Cervicovaginal Secretions in Cervicovaginal Lavage Fluid Collected for Measurement of Genital HIV-1 RNA Levels in Women. J. Clin. Microbiol. 2011;49:735–736. doi: 10.1128/JCM.00991-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ndayisaba G, Verwijs MC, van Eeckhoudt S, Gasarabwe A, Hardy L, Borgdorff H, Kestelyn E, Jespers VA, van de Wijgert J. Feasibility and Acceptability of a Novel Cervicovaginal Lavage Self-Sampling Device Among Women in Kigali, Rwanda. Sex. Transm. Dis. 2013;40:552–555. doi: 10.1097/OLQ.0b013e31828e5aa5. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell CM, Balkus J, Agnew KJ, Cohn S, Luque A, Lawler R, Coombs RW, Hitti JE. Bacterial Vaginosis, not HIV, is Primarily Responsible for Increased Vaginal Concentrations of Proinflammatory Cytokines. AIDS Res. Hum. Retroviruses. 2008;24:667–671. doi: 10.1089/aid.2007.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott ME, Wilson SS, Cosentino LA, Richardson BA, Moscicki AB, Hillier SL, Herold BC. Interlaboratory Reproducibility of Female Genital Tract Cytokine Measurements by Luminex: Implications for Microbicide Safety Studies. Cytokine. 2011;56:430–434. doi: 10.1016/j.cyto.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyongo JK, Jespers V, Goovaerts O, Michiels J, Menten J, Fichorova RN, Crucitti T, Vanham G, Arien KK. Searching for Lower Female Genital Tract Soluble and Cellular Biomarkers: Defining Levels and Predictors in a Cohort of Healthy Caucasian Women. PLoS One. 2012;7:e43951. doi: 10.1371/journal.pone.0043951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mukura LR, Ghosh M, Fahey JV, Cu-Uvin S, Wira CR. Genital Tract Viral Load in HIV Type 1-Positive Women Correlates with Specific Cytokine Levels in Cervicalvaginal Secretions but Is not a Determinant of Infectious Virus or Anti-HIV Activity. AIDS Res. Hum. Retroviruses. 2012;28:1533–1539. doi: 10.1089/aid.2011.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masson L, Mlisana K, Little F, Werner L, Mkhize NN, Ronacher K, Gamieldien H, Williamson C, McKinnon LR, Walzl G, Karim QA, Karim SSA, Passmore JAS. Defining Genital Tract Cytokine Signatures of Sexually Transmitted Infections and Bacterial Vaginosis in Women at High Risk of HIV Infection: a Cross-sectional Study. Sex. Transm. Infect. 2014;90:580–U521. doi: 10.1136/sextrans-2014-051601. [DOI] [PubMed] [Google Scholar]
- 9.Archary D, Liebenberg LJ, Werner L, Tulsi S, Majola N, Naicker N, Dlamini S, Hope TJ, Samsunder N, Karim SSA, Morris L, Passmore JAS, Garrett NJ. Randomized Cross-Sectional Study to Compare HIV-1 Specific Antibody and Cytokine Concentrations in Female Genital Secretions Obtained by Menstrual Cup and Cervicovaginal Lavage. PLoS One. 2015;10:e0131906. doi: 10.1371/journal.pone.0131906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boesch AW, Zhao YF, Landman AS, Garcia MR, Fahey JV, Wira CR, Ackerman ME. A Multiplexed Assay to Detect Antimicrobial Peptides in Biological Fluids and Cell Secretions. J. Immunol. Methods. 2013;397:71–76. doi: 10.1016/j.jim.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birse KM, Burgener A, Westmacott GR, McCorrister S, Novak RM, Ball TB. Unbiased Proteomics Analysis Demonstrates Significant Variability in Mucosal Immune Factor Expression Depending on the Site and Method of Collection. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nobbenhuis MAE, Helmerhorst TJM, van den Brule AJC, Rozendaal L, Jaspars LH, Voorhorst FJ, Verheijen RHM, Meijer CJ. Primary Screening for High Risk HPV by Home Obtained Cervicovaginal Lavage is an Alternative Screening Tool for Unscreened Women. J. Clin. Pathol. 2002;55:435–439. doi: 10.1136/jcp.55.6.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gok M, Heideman DAM, van Kemenade FJ, Berkhof J, Rozendaal L, Spruyt JWM, Voorhorst F, Belien JAM, Babovic M, Snijders PJF, Meijer C. HPV Testing on Self Collected Cervicovaginal Lavage Specimens as Screening Method for Women Who Do not Attend Cervical Screening: Cohort Study. Brit. Med. J. 2010;340 doi: 10.1136/bmj.c1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belec L, Legoff J, Si-Mohamed A, Bloch F, Keou FXM, Becquart P, Matta M, Prazuck T, Petite JP, Gutmann L, Payan C. Mucosal Humoral Immune Response to Hepatitis C Virus E1/E2 Surface Glycoproteins and HCV Shedding in Saliva and Cervicovaginal Fluids from Chronically HCV-infected Patients. J. Hepatol. 2003;38:833–842. doi: 10.1016/s0168-8278(03)00119-3. [DOI] [PubMed] [Google Scholar]
- 15.Delany S, Rosas R, Mlaba N, Clayton T, Akpomiemie G, LeGoff J, Capovilla A, Belec L, Slevens W, Mayaud P. Comparison of Cervicovaginal Lavage, Cervicovaginal Lavage Enriched with Cervical Swab, and Vaginal Tampon for the Detection of HIV-1 RNA and HSV-2 DNA in Genital Secretions. J. Acquir. Immune Defic. Syndr. 2008;49:406–409. doi: 10.1097/qai.0b013e31818c7f75. [DOI] [PubMed] [Google Scholar]
- 16.Aumakhan B, Hardick A, Quinn TC, Laeyendecker O, Gange SJ, Beyrer C, Cox C, Anastos K, Cohen M, Greenblatt RM, Merenstein DJ, Minkoff H, Nowicki M, Gaydos CA. Genital Herpes Evaluation by Quantitative TaqMan PCR: Correlating Single Detection and Quantity of HSV-2 DNA in Cervicovaginal Lavage Fluids with Cross-sectional and Longitudinal Clinical Data. Virol. J. 2010;7 doi: 10.1186/1743-422X-7-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watson-Jones D, Wald A, Celum C, Lingappa J, Weiss HA, Changalucha J, Baisley K, Tanton C, Hayes RJ, Marshak JO, Gladden RG, Koelle DM. Use of Acyclovir for Suppression of Human Immunodeficiency Virus Infection Is Not Associated with Genotypic Evidence of Herpes Simplex Virus Type 2 Resistance to Acyclovir: Analysis of Specimens from Three Phase III Trials. J. Clin. Microbiol. 2010;48:3496–3503. doi: 10.1128/JCM.01263-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aumakhan B, Gange SJ, Beyrer C, Gaydos CA, Minkoff H, Merenstein DJ, Cohen MH, Anastos K, Greenblatt R, Nowicki MJ, Quinn TC. Quantitative and Qualitative Correlates of Cervicovaginal Herpes Simplex Virus Type 2 Shedding Among HIV-infected Women in the Women's Interagency HIV Study. Int. J. STD AIDS. 2011;22:273–277. doi: 10.1258/ijsa.2009.009296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Homans J, Christensen S, Stiller T, Wang CH, Mack W, Anastos K, Minkoff H, Young M, Greenblatt R, Cohen M, Strickler H, Karim R, Spencer LY, Operskalski E, Frederick T, Kovacs A. Permissive and Protective Factors Associated With Presence, Level, and Longitudinal Pattern of Cervicovaginal HIV Shedding. J. Acquir. Immune Defic. Syndr. 2012;60:99–110. doi: 10.1097/QAI.0b013e31824aeaaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoenfisch AL, Dollard SC, Amin M, Gardner LI, Klein RS, Mayer K, Rompalo A, Sober JD, Cannon MJ. Cytomegalovirus (CMV) Shedding is Highly Correlated with Markers of Immunosuppression in CMV-seropositive Women. J. Med. Microbiol. 2011;60:768–774. doi: 10.1099/jmm.0.027771-0. [DOI] [PubMed] [Google Scholar]
- 21.Bohler K, Meisinger V, Klade H, Reinthaller A. Zinc Levels of Serum and Cervicovaginal Secretion in Recurrent Vulvo-vaginal Candidiasis. Genitourin. Med. 1994;70:308–310. doi: 10.1136/sti.70.5.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genc MR, Delaney ML, Onderdonk AB, Witkin SS, Grp MAPS. Vaginal Nitric Oxide in Pregnant Women with Bacterial Vaginosis. Am. J. Reprod. Immunol. 2006;56:86–90. doi: 10.1111/j.1600-0897.2006.00388.x. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell C, Balkus JE, Fredricks D, Liu CZ, McKernan-Mullin J, Frenkel LM, Mwachari C, Luque A, Cohn SE, Cohen CR, Coombs R, Hitti J. Interaction Between Lactobacilli, Bacterial Vaginosis-associated Bacteria, and HIV Type 1 RNA and DNA Genital Shedding in U.S. and Kenyan Women. AIDS Res. Hum. Retroviruses. 2013;29:13–19. doi: 10.1089/aid.2012.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ursell LK, Gunawardana M, Chang S, Mullen M, Moss JA, Herold BC, Keller MJ, McDonald D, González Peña A, Knight R, Baum MM. Comparison of the Vaginal Microbial Communities in HSV-2 Seropositive Women Receiving Medicated Intravaginal Rings. Antiviral Res. 2014;102:87–94. doi: 10.1016/j.antiviral.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srinivasan S, Morgan MT, Fiedler TL, Djukovic D, Hoffman NG, Raftery D, Marrazzo JM, Fredricks DN. Metabolic Signatures of Bacterial Vaginosis. Mbio. 2015;6 doi: 10.1128/mBio.00204-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vitali B, Cruciani F, Picone G, Parolin C, Donders G, Laghi L. Vaginal Microbiome and Metabolome Highlight Specific Signatures of Bacterial Vaginosis. Eur. J. Clin. Microbiol. Infect. Dis. 2015 doi: 10.1007/s10096-015-2490-y. DOI. Epub ahead of print Sep. 18. [DOI] [PubMed] [Google Scholar]
- 27.Moss JA, Baum MM, Malone AM, Kennedy S, Kopin E, Nguyen C, Gilman J, Butkyavichene I, Willis R, Vincent KL, Motamedi M, Smith TJ. Tenofovir and Tenofovir Disoproxil Pharmacokinetics from Intravaginal Rings. Aids. 2012;26:707–710. doi: 10.1097/QAD.0b013e3283509abb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moss JA, Malone AM, Smith TJ, Butkyavichene I, Cortez C, Gilman J, Kennedy S, Kopin E, Nguyen C, Sinha P, Hendry RM, Guenthner P, Holder A, Martin A, McNicholl J, Mitchell J, Pau C-P, Srinivasan P, Smith JM, Baum MM. Safety and Pharmacokinetics of Intravaginal Rings Delivering Tenofovir in Pig-tailed Macaques. Antimicrob. Agents Chemother. 2012;56:5952–5960. doi: 10.1128/AAC.01198-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moss JA, Malone AM, Smith TJ, Kennedy S, Kopin E, Nguyen C, Gilman J, Butkyavichene I, Vincent KL, Motamedi M, Friend DR, Clark MR, Baum MM. Simultaneous Delivery of Tenofovir and Acyclovir via an Intravaginal Ring. Antimicrob. Agents Chemother. 2012;56:875–882. doi: 10.1128/AAC.05662-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hendrix CW, Chen BA, Guddera V, Hoesley C, Justman J, Nakabiito C, Salata R, Soto-Torres L, Patterson K, Minnis AM, Gandham S, Gomez K, Richardson BA, Bumpus NN. MTN-001: Randomized Pharmacokinetic Cross-over Study Comparing Tenofovir Vaginal Gel and Oral Tablets in Vaginal Tissue and Other Compartments. PLoS One. 2013;8:e55013. doi: 10.1371/journal.pone.0055013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moss JA, Malone AM, Smith TJ, Kennedy S, Nguyen C, Vincent KL, Motamedi M, Baum MM. Pharmacokinetics of a Multipurpose Pod-intravaginal Ring Simultaneously Delivering Five Drugs in the Ovine Model. Antimicrob. Agents Chemother. 2013;57:3994–3997. doi: 10.1128/AAC.00547-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moss JA, Srinivasan P, Smith TJ, Butkyavichene I, Lopez G, Brooks AA, Martin A, Dinh CT, Smith JM, Baum MM. Pharmacokinetics and Preliminary Safety Study of Pod-Intravaginal Rings Delivering Antiretroviral Combinations for HIV Prophylaxis in a Macaque Model. Antimicrob. Agents Chemother. 2014;58:5125–5135. doi: 10.1128/AAC.02871-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keller MJ, Malone AM, Carpenter CA, Lo Y, Huang M, Corey L, Willis R, Nguyen C, Kennedy S, Gunawardana M, Guerrero D, Moss JA, Baum MM, Smith TJ, Herold BC. Safety and Pharmacokinetics of Acyclovir in Women Following Release From a Silicone Elastomer Vaginal Ring. J. Antimicrob. Chemother. 2012;67:2005–2012. doi: 10.1093/jac/dks151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Sun H, Wang XL, Yu Q, Li SH, Yu XY, Gong WW. Increased Exosomal MicroRNA-21 and MicroRNA-146a Levels in the Cervicovaginal Lavage Specimens of Patients with Cervical Cancer. Int. J. Mol. Sci. 2014;15:758–773. doi: 10.3390/ijms15010758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belec L, Meillet D, Levy M, Georges A, Tevibenissan C, Pillot J. Dilution Assessment of Cervicovaginal Secretions Obtained by Vaginal Washing for Immunological Assays. Clin. Diagn. Lab. Immunol. 1995;2:57–61. doi: 10.1128/cdli.2.1.57-61.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Owen DH, Katz DF. A Vaginal Fluid Simulant. Contraception. 1999;59:91–95. doi: 10.1016/s0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, De Borba B, Rohrer J. IC Assay for Lithium, Sodium, and Calcium in Lithium Carbonate, Application Note 1090. Thermo Fisher Scientific; Sunnyvale, CA: 2014. [Google Scholar]
- 38.Patil S, Rohrer J. Ion Chromatography Assay for Lithium in Lithium Citrate, Application Note 1121. Thermo Fisher Scientific; Sunnyvale, CA: 2015. [Google Scholar]
- 39.International Vocabulary of Metrology – Basic and General Concepts and Associated Terms (VIM) Joint Committee for Guides in Metrology; Sèvres, France: 2012. p. 108. [Google Scholar]