Abstract
Bisphenol A (BPA), an endocrine disruptor used in a variety of consumer products, has been found to alter the number of neurons in multiple brain areas in rats following exposure in perinatal development. Both the number of neurons and glia also change in the medial prefrontal cortex (mPFC) during adolescence, and this process is known to be influenced by gonadal hormones which could be altered by BPA. In the current study, we examined Long-Evans male and female rats that were administered BPA (0, 4, 40, or 400 µg/kg/day) during adolescent development (postnatal days 27–46). In adulthood (postnatal day 150), the number of neurons and glia in the mPFC were stereologically assessed in methylene blue/azure II stained sections. There were no changes in the number of neurons, but there was a significant dose by sex interaction in number of glia in the mPFC. Pairwise comparisons between controls and each dose showed a significant increase in the number of glia between 0 and 40 µg/kg/day in females, and a significant decrease in the number of glia between 0 and 4 µg/kg/day in males. In order to determine the type of glial cells that were changing in these groups in response to adolescent BPA administration, adjacent sections were labelled with S100β (astrocytes) and IBA-1 (microglia) in the mPFC of the groups that differed. The number of microglia was significantly higher in females exposed to 40 µg/kg/day than controls and lower in males exposed to 4 µg/kg/day than controls. There were no significant effects of adolescent exposure to BPA on the number of astrocytes in male or females. Thus, adolescent exposure to BPA produced long-term alterations in the number of microglia in the mPFC of rats, the functional implications of which need to be explored.
Keywords: BPA, microglia, astrocytes, adolescence, medial prefrontal cortex
1. Introduction
Bisphenol A (BPA) is an environmental endocrine disruptor commonly found in polycarbonate plastics including dental sealants, medical devices, food storage containers, and water pipes. It is also a component of epoxy resins used to coat the inside of the metal cans used for foods (Vandenberg et al., 2007). It leaches from products and once inside the body, can bind to estrogen, androgen, and thyroid receptors (Gould et al., 1998; Kupier et al., 1998; Sohoni & Sumpter, 1998; Moriyama et al., 2002). Given this ubiquity, BPA has been detected in 92.6% of the US population (Calafat et al., 2008).
Previous research has shown effects of BPA on perinatal brain development in several areas. Exposure to BPA during gestation and lactation has been found to abolish sex differences in the number of corticotropin-releasing hormone neurons in the bed nucleus of the stria terminalis (Funabashi et al., 2004), in the number of neurons in the anteroventral periventricular nucleus and in the sexually dimorphic nucleus of the preoptic area (McCaffrey et al., 2013). Furthermore, it reverses the sex difference in the number of neurons in the locus coeruleus (Kubo et al., 2003). Few studies have investigated the effect of perinatal BPA exposure on areas of the brain associated with cognition, but Sadowski et al. (2014) found an increase in the number of neurons in the adult medial prefrontal cortex (mPFC) in male, but not female, rats.
In contrast to the plethora of perinatal studies, fewer studies have explored the effects of BPA on the developing adolescent brain. Adolescence is the period between childhood and adulthood that includes the restructuring of the brain in humans and rats (Spear, 2013; Juraska et al., 2013). Bowman et al (2015) introduced 40 µg/kg/day BPA or vehicle via subcutaneous injection to male and female rats from postnatal day (P) 42–49. After behavioral testing and a pre-sacrifice stress in adulthood, they calculated density of basal and apical spines in the CA1 area of the hippocampus and the mPFC. BPA caused a decrease in spine density on both basal and apical spines in the CA1 area of the hippocampus in males and females. However, there was no effect of BPA exposure on density of spines in the mPFC. The density of spines (number/length of dendrite) may not be an indicator of the total number of spines, which requires measurement of the total length of the dendritic tree. Thus, whether there are effects of adolescent exposure to BPA on the neuroanatomical structure of the mPFC, still remain unknown.
Adolescent humans show a decrease in the volume of frontal gray matter starting around age 12 that continues into adulthood (Lenroot & Geidd, 2006). Previous research from our laboratory has found a decrease in the number of neurons and glia in the rat mPFC between P35 (early adolescence) and P90 (adulthood) (Markham et al., 2007) with the most significant decrease occurring between P35 and 45 in females (Willing et al., 2015). This decline in humans and rats coincides with the average onset of puberty, suggesting a hormonal role in cortical restructuring. To support this hypothesis, Koss et al. (2015)) found that pre-pubertal gonadectomy did not have an effect in males, but prevented the neuron and glial loss in the mPFC in female rats, suggesting that ovarian hormones are involved in the loss of cortical neurons during adolescence. Given that gonadal hormones appear to be involved in the cortical restructuring that occurs during adolescence, it is plausible that endocrine disruptors, such as BPA, could alter normal development. Thus the adolescent exposure model adopted in the current study allows us to isolate the effects of BPA on a potentially vulnerable time period.
The current study explores the long-term effects of adolescent exposure to BPA on the number of neurons and glia in the mPFC in both males and females. After the initial analysis, glia were further examined at selected doses of BPA to quantify the number of IBA1-expressing microglia and S100β-expressing astrocytes.
2. Methods
2.1 Animals and Dosing Procedure
Twelve male and twelve female adult Long Evans rats from Harlan (Indianapolis, IN, USA) were used as breeders. All animals were housed in same sex pairs for at least one week before breeding when one male and one female rat were paired for nine nights and then housed individually. The day of birth was denoted as P0. The pups were weaned on P23 and housed in same sex, same treatment pairs. Four males and four females from each of the 12 litters were randomly selected and assigned into one of four treatment groups: 0, 4, 40 or 400 µg/kg/day BPA so that each dose group contained only one rat of each sex per litter. This allowed litter to be included as a variable.
To limit environmental BPA exposure, all animals (breeders and offspring) were housed in polysulfone shoebox cages. Water bottles were glass with rubber stoppers, and the water was filtered through a reverse osmosis filtration system. The animals were fed a phytoestrogen-free diet (2020X Teklad Global Soy Protein-Free Extruded Rodent Diet). The animals were allowed food and water ad libitum. The light cycle was maintained at 12:12 (L:D). All animal procedures were approved by and conducted in accordance with the University of Illinois Urbana-Champaign IACUC and the National Research Council’s Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
BPA was orally administered on a voluntarily consumed cookie. This mode of administration mimics that most common route of exposure in humans and is not stressful for the animal subjects. The BPA doses of 0 (control), 4, 40 and 400 µg BPA/kg/day were made with concentrations of 0, .008, .08, and .8 mg of BPA (received from the Environmental Protection Agency (EPA); 99% purity) /ml tocopherol-stripped corn oil, respectively. The suspensions were stirred for five minutes before dosing procedures began each day. All solutions were coded so that the experimenters were blind to the treatments. To dose the animals, 0.5 µl/g of the BPA solution was pipetted onto a Newman’s Own Organic Vanilla Alphabet cookie. Each animal was placed into an individual cage and observed for the time it took to consume the cookie. Three animals were removed from the study because they did not reliably consume the entire cookie, resulting in a sample size of 10–11 animals per sex in each dose group. From P24–26, pups were given ¼ of a cookie with 0.5 µl/g of oil to habituate them to the dosing regimen. From P27–46, pups were given ¼ of a cookie with 0.5 µl/g of their respective BPA treatment solution. To mark puberty onset, female animals (n=5/dose group from 5 litters) were checked daily starting on P25 for vaginal opening (Castellano et al., 2011) and male animals (n=5/dose group from 5 litters) were checked daily starting on P35 for preputial separation (Korenbrot et al., 1977).
2.2 Histology
In adulthood (~P150), animals were anesthetized with 100 mg/kg i.p. injection of sodium pentobarbital. Females were not staged at sacrifice because the experimenters were analyzing total neuron and glial numbers, which do not change with the estrus cycle. Body weight and brain weight, length, and width were recorded at the time of perfusion. The brain was fixed through a transcardiac perfusion using .1M phosphate buffered saline (PBS) and 4% paraformaldehyde. Brains were removed immediately after the perfusion and were placed in 4% paraformaldehyde in .1M PBS for 24 hours, and then transferred to a 30% sucrose solution in .1 M PBS for 48 hours. 30 and 60 micron coronal sections were sliced using a freezing microtome. Slices were cut at 60µm for neuron and glia quantification, and in between these thicker sections, two 30µm sections were cut for optimal immunocytochemical staining. A punch in the left hemisphere in the lateral cortex was used to indicate left/right orientation. The 60µm slices at 240µm intervals were mounted on slides and stained with Methylene Blue-Azure II Nissl stain, (0 [n= 11 females; 11 males], 4 [n=11 females; 11 males], 40 [n=10 females; 10 males], 400 µg BPA/kg/day [n=11 females; 10 males]). The slides were allowed to dry for 24 hours prior to coverslipping. Adjacent 30 micron sections were stored in a storage solution (30% ethylene glycol, 30% glycerol, 30% distilled water, 10% .1M PBS) in a −20 C freezer until immunocytochemical staining.
2.3 Immunocytochemistry
30 micron slices were removed from the storage solution and rinsed three times in trisbuffered saline (TBS; pH=7.6). The slices were then blocked in TBS containing1% hydrogen peroxide, 20% normal goat serum (NGS) and 1% bovine serum albumin (BSA) for 30 minutes, and incubated in primary antibody (Anti IBA1 Rabbit, Wako Industries [microglia]; or Anti S- 100β Rabbit, Millipore [astrocytes]) for 48 hours. The primary antibody concentration was 1:5000 for both Anti IBA1 and Anti S-100β, and made in TBS containing 2% NGS and 0.3% Triton-X-100 (TTG). After primary incubation, the slices were rinsed three times in TTG, and incubated for 90 minutes in secondary antibody (biotinylated goat, anti-rabbit IgG). They were then rinsed twice in TTG, twice in TBS, incubated for one hour in ABC solution (Vectastain Elite ABC Kit, Vector Laboratories, Burlingame, CA, USA), rinsed three times in TBS, and then immersed in DAB solution (Sigma Aldrich fast tabs, St. Louis, MO, USA) for two minutes followed by several rinses in TBS. The slices were then mounted on charged slides and allowed to dry for 24 hours prior to coverslipping.
2.4 Cell Number Quantification
2.4.1 Nissl Stain
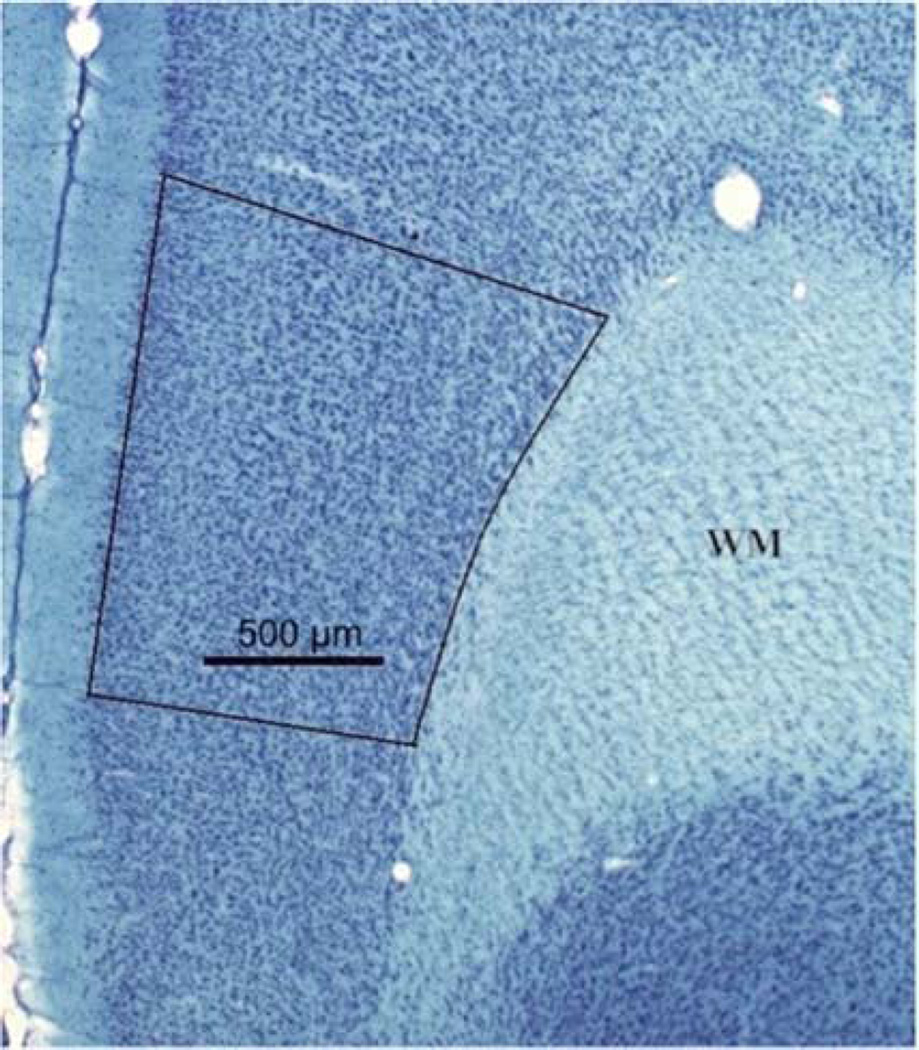
The Nissl stained slices were used to quantify the total number of neurons and glia within the mPFC. A Zeiss light microscope with camera lucida was used to parcellate the prelimbic (PL) area of the mPFC using distinguishing neuroanatomical features as described previously (Markham et al., 2007). Layer I is nearly devoid of cell bodies and can be easily distinguished from Layer II. Layer VI is bound by white matter on the lateral side. The dorsal border of PL is distinguished by a denser band of cells and a widening of layer V. The ventral border of PL is distinguished by a loss of clear lamination of the layers (Fig. 1). Each parcellation was scanned into a computer and the area calculated using Image J (NIH). The thickness of each slice was calculated via the StereoInvestigator software (Microbrightfield, Williston, VT, USA). The area of PL was multiplied by the thickness of each slice and these volumes were summed to obtain a volume for each animal. The experimenter was blind to the treatment and sex of the animal in all histological evaluations.
Figure 1.
The prelimbic area of the rat mPFC in a coronal section. Scale bar = 500 µm.
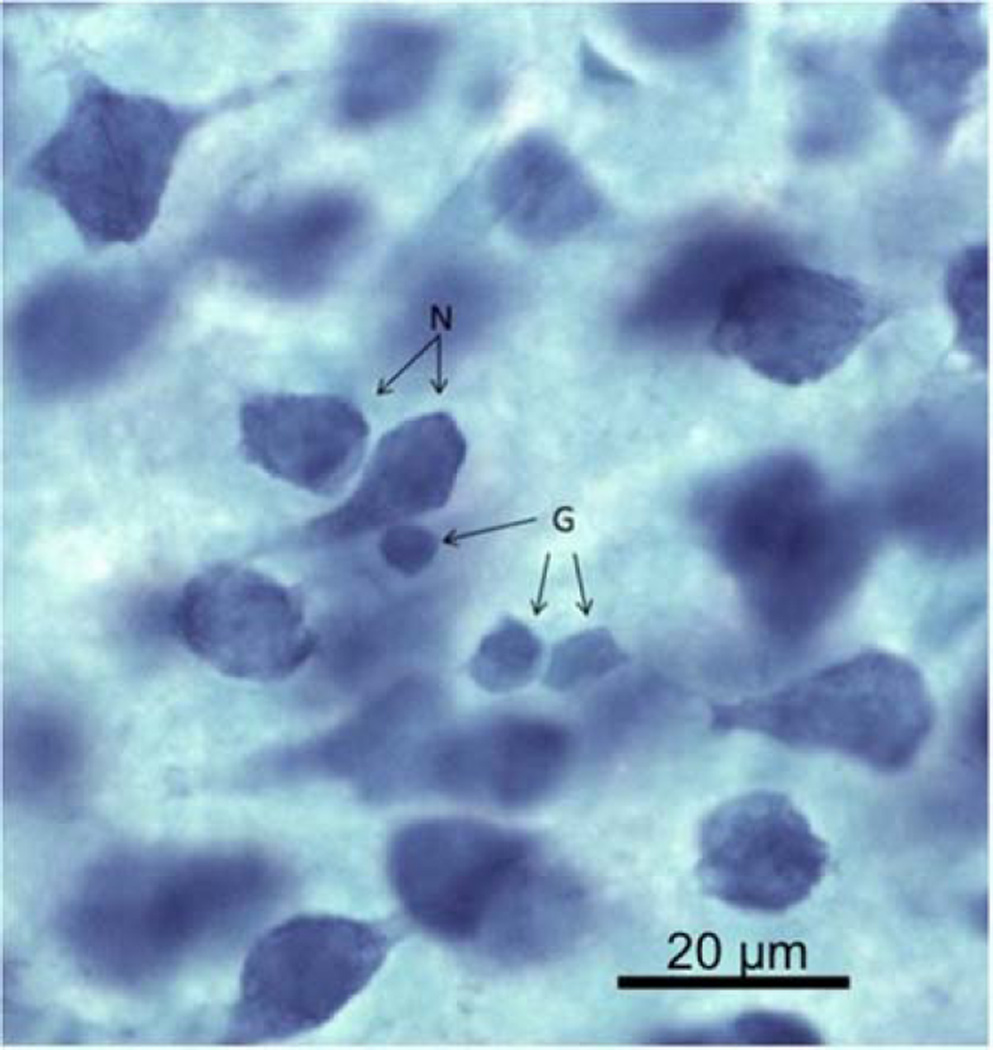
The optical disector with StereoInvestigator software (Microbrightfield, Williston, VT, USA) was used to quantify the number of neurons and glia in the PL area of the mPFC using techniques outlined in Gundersen et al (1988). Counting was performed with a 35 µm × 35 µm box with ‘exclusion’ and ‘inclusion’ lines (Fig. 2). Any cell within or touching the inclusion lines was counted, and any cell touching the exclusion lines was not counted. The optical disector also had a depth in the z-axis (14 µm) with 1 µm guard zones on the top and bottom of each frame. The bottom of each cell had to be in focus within the 14 µm z-axis depth in order to be counted. This protected against over counting the number of larger cells in relation to smaller cells. The software randomly selected areas to be counted within a defined region of interest.
Figure 2.
Examples of neurons (N) and glia (G) with the methylene blue/azureII stain. Scale bar
To distinguish between cell types, cell morphology, color, and size of neurons and glia were considered. Neuronal cell bodies have a distinct nucleus, stain a dark blue, and are larger in size than glia. Glial cell bodies have an amorphous shape, stain a lighter teal blue, and are small in size (Fig. 2). At least 400 neurons and 300 glia were counted from each brain. The total number of counted cells was divided by the number of counting sites to find a density of cells per disector volume. The density of cells per disector volume was then multiplied by the total volume of the mPFC to calculate the total number of neurons and glia in the mPFC.
2.4.2 Astrocyte and microglia quantification
After finding significant differences in the total number of glia cells between control and 40 µg/kg/day BPA in females as well as control and 4 µg/kg/day BPA in the males, the adjacent sections from these groups only were stained for particular types of glia. Since the other treatment groups were not significantly different from control, the glial subtypes of those groups were not analyzed. The same stereological method described in 2.4.1. above was used to quantify the number of IBA-1 expressing microglia (n = 9 animals for control females; n = 8 animals for 40 µg/kg/day BPA females; n = 7 animals for control males; n = 8 animals for 4 µg/kg/day BPA males) and S100β expressing astrocytes (n = 11 animals for control females; n = 9 animals for 40 µg/kg/day BPA females; n = 11 animals for control males; n = 10 animals for 4 µg/kg/day BPA males) in the mPFC. The mPFC was not intact in some tissue slices, so that these samples were not used for staining and quantification, resulting in a lower number of animals in some groups. In order to account for thinner tissue and a lower number of cells, the optical disector dimensions were changed to 75 µm × 75 µm (L×W) and 12 µm depth on the z axis with 1 µm guard zones on the top and bottom. Every microglia cell body within the inclusion zone was considered regardless of morphological state. Again, the density of cells was multiplied by the volume of the region to give the total number of each types of glia.
2.5 Statistics
A 2 (sex) × 4 (treatment) ANOVA with litter as a random factor was used to analyze all measures except the glial subtypes. The assumptions of ANOVA were met. The data were normal, independent and identically distributed.. A critical p-value of p<.05 was used to define a significant difference between groups. Following the significant interaction, post hoc comparisons (Dunnett’s) between controls and treatment groups in males and females were run. For the analysis of astrocytes and microglia, a one way ANOVA that enabled the inclusion of litter as a random factor was used to analyze each sex and subtype separately. Standardized effect size (Cohen’s d, Cohen, 1992) was calculated (treatment group compared to control) for each significant finding in cell number quantification.
3. Results
3.1 Growth and development indices
The expected significant sex differences were found in body weight (F(1,11)=707.81; p<.001), brain width (F(1,11)=19.24; p<.001), brain length (F(1,11)=49.12; p<.001), and brain weight (F(1,11)=118.40; p<.001) (Table 1). There was no effect of treatment on any of these measures. Similarly, there was a significant difference in the day of puberty onset between males and females (F(1,4)=58.93; p<.002), but no effect of treatment.
Table 1.
Growth and Development
| Male | Female | ||
|---|---|---|---|
| Body Weight (g)* | |||
| 0 | 418.55 ± 9.46 | 251.72 ± 6.18 | |
| 4 | 406.45 ± 9.95 | 259.64 ± 7.04 | |
| 40 | 419.1 ± 6.29 | 245.6 ± 4.27 | |
| 400 | 421.09 ± 9.69 | 261.27 ± 4.80 | |
| Brain Length (mm)* | |||
| 0 | 1.60 ± .01 | 1.52 ± .02 | |
| 4 | 1.59 ± .01 | 1.52 ± .01 | |
| 40 | 1.57 ± .01 | 1.54 ± .01 | |
| 400 | 1.59 ± .01 | 1.54 ± .02 | |
| Brain Width (mm)* | |||
| 0 | 1.59 ± .01 | 1.54 ± .02 | |
| 4 | 1.60 ± .01 | 1.54 ± .02 | |
| 40 | 1.59 ± .01 | 1.54 ± .01 | |
| 400 | 1.60 ± .01 | 1.55 ± .01 | |
| Brain Weight (g)* | |||
| 0 | 2.03 ± .02 | 1.83 ± .02 | |
| 4 | 2.02 ± .02 | 1.86 ± .02 | |
| 40 | 2.02 ± .02 | 1.87 ± .01 | |
| 400 | 2.03 ± .03 | 1.90 ± .02 | |
| Puberty Onset (days)* | |||
| 0 | 43.6 ± 1.17 | 35.6 ± .93 | |
| 4 | 45.5 ± 1.73 | 35.8 ± .39 | |
| 40 | 45.4 ± 1.03 | 34.8 ± .49 | |
| 400 | 44.8 ± .94 | 35.8 ± .58 | |
Data reported as mean ± SEM
significant sex difference
3.2 Neurons and Glia
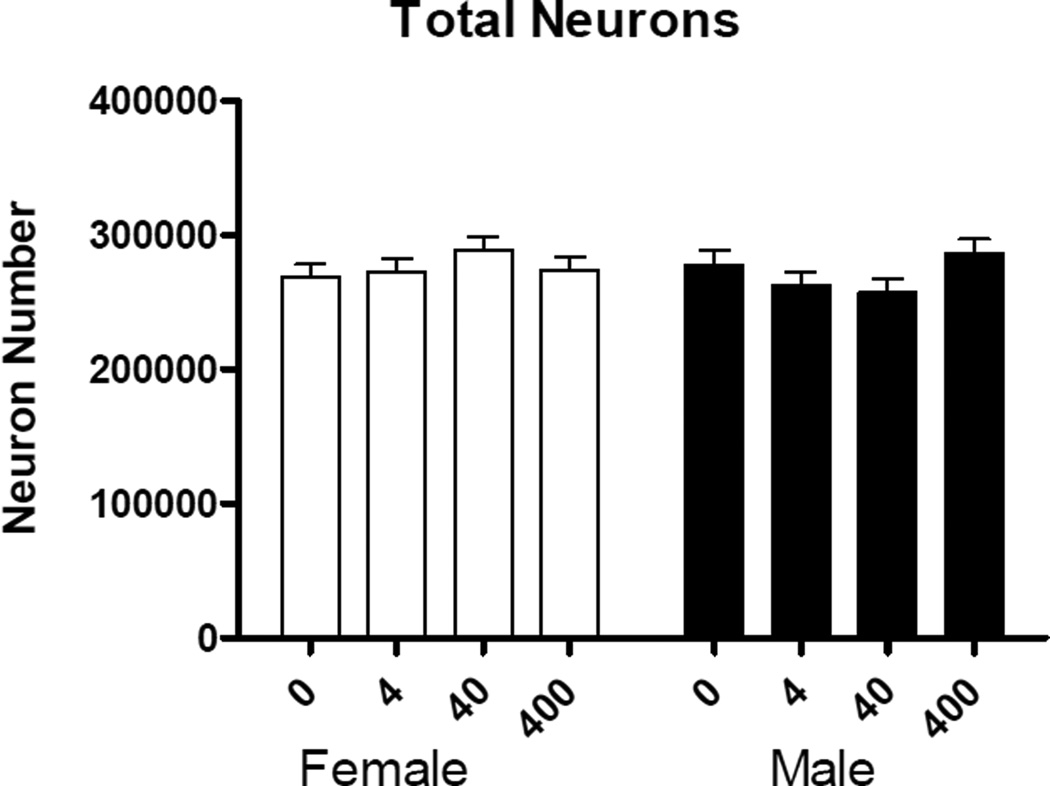
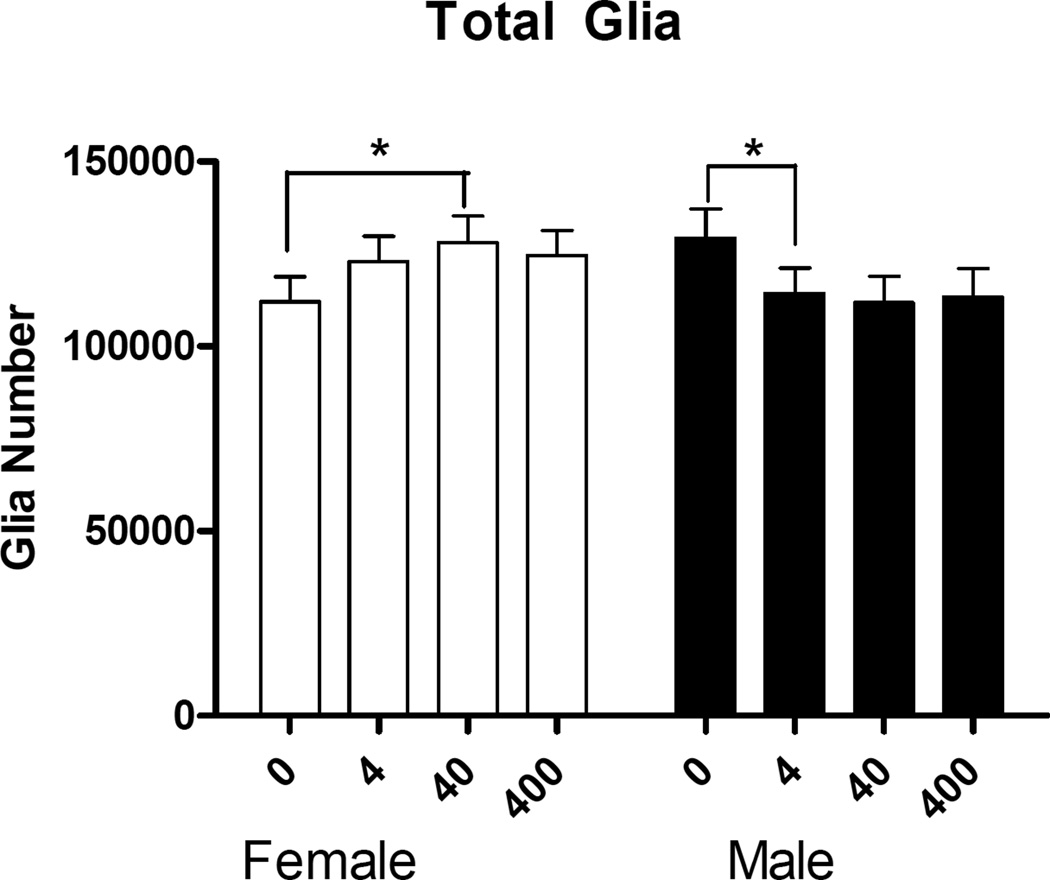
There were no main effects of sex or treatment in the number of neurons in the mPFC (Fig. 3). There was no main effect of sex or treatment in the number of glia. However, there was a significant treatment × sex interaction (F(3,27)=2.93, p<.05) (Fig. 4). Post hoc comparisons revealed significant increase in the number of glia between control and 40 µg/kg/day BPA in females (p<.04, d=0.71), and a significant decrease in the number of glia between control and 4 µg/kg/day BPA in males (p<.05, d=0.95) (Fig. 4).
Figure 3.
The number of neurons (mean + SEM) in the mPFC between males and females in the BPA treatment groups (µg/kg/day). There were no significant effects of treatment.
Figure 4.
The number of glia (mean + SEM) in the mPFC between males and females in the BPA treatment groups (µg/kg/day). There was a significant treatment × sex interaction (p<.05). Post hoc comparisons showed a significant increase in the number of glia between control and 40 µg/kg/day BPA in females (p<.05) and a significant decrease in the number of glia between control and 4 µg/kg/day BPA in males (p<.05). *p<.05
3.3 Astrocytes
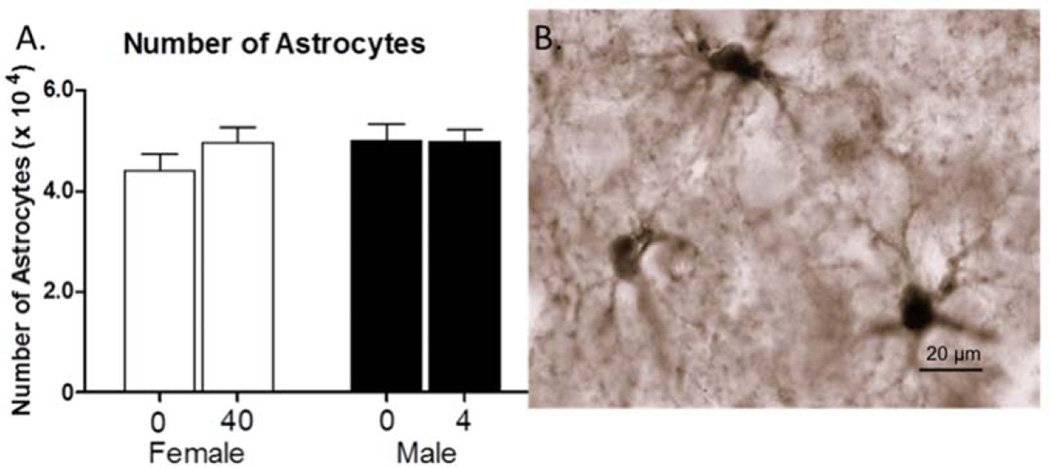
A one-way analysis of the number of astrocytes between controls and 40 µg/kg/day BPA in females were not significant (Fig. 5). Likewise, a one way analysis of the number of astrocytes between controls and 4 µg/kg/day BPA in males was also not significant (Fig. 5).
Figure 5.
A. The number of astrocytes (mean + SEM) in the mPFC seen in males and females in the BPA treatment groups. B. Image of S-100β-expressing astrocytes labeled with DAB under oil. S-100β primarily labels cell bodies of astrocytes. Scale bar = 20 µm.
3.4 Microglia
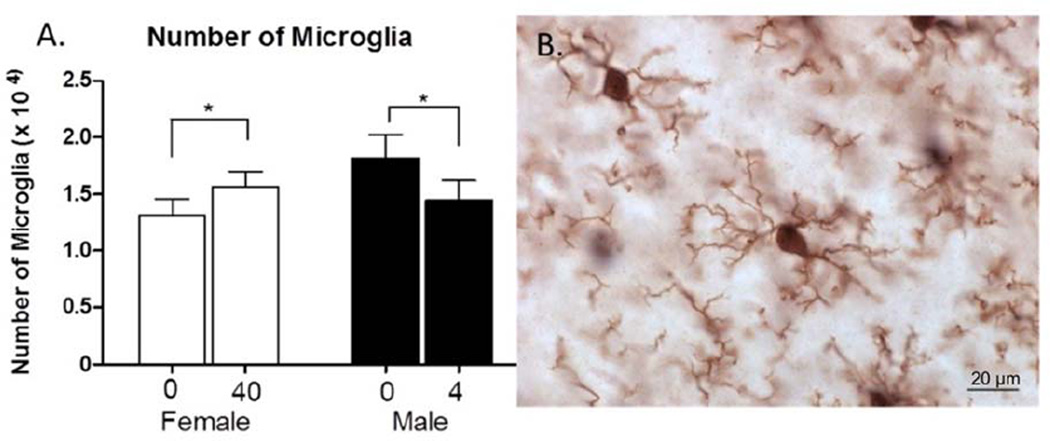
One way analysis of the number of microglia between 0 and 40 µg/kg/day BPA in females resulted in a significant increase in the number of microglia with BPA exposure (F(1,4)=7.541, p=.05, d=0.68) (Fig. 6). Also, a one way analysis of the number of microglia between 0 and 4 µg/kg/day BPA in males showed a significant decrease with BPA exposure (F(1,5)=10.377, p=.02, d=0.76) (Fig. 6).
Figure 6.
A. The number of microglia (mean + SEM) observed in the mPFC in the BPA treatment groups. There was a significant increase the number of microglia between control and 40 µg/kg/day BPA in females and a significant decrease in the number of microglia between control and 4 µg/kg/day BPA in males. B. Image of IBA1-expressing microglia labeled with DAB under oil. Scale bar = 20 µm.
4. Discussion
The current experiment showed that exposure to BPA during adolescence has differential long-term effects between males and females as well as between cell types in the mPFC. We did not observed a significant effect of BPA exposure on the number of neurons. This contrasts with to the findings of Sadowski et al. (2014), in which perinatal BPA exposure resulted in an increase in the number of neurons in adult male rats. This indicates a difference in vulnerability during the perinatal versus adolescent period of development. This is not surprising given the diverse effects of hormones on the number of neurons in another part of the cortex, the visual cortex, between the perinatal and adolescent period (Nuñez et al., 2000; 2002).
The present study found a significant effect of exposure to BPA on the number of glia, resulting in an increase in females with 40 µg/kg/day BPA and a decrease in males with 4 µg/kg/day BPA compared to controls. These effects were in the moderate (females) to large range (males) according to the magnitude of the effect size (Cohen’s d; Cohen, 1992). Interestingly, the same pattern was found with the other doses of BPA, although they did not reach significance. Further classification of two subtypes of glia revealed that the number of microglia significantly increased with BPA exposure in females, while it significantly decreased in males, again in the moderate effect size range for both sexes. Unlike microglia, effects were not detected in the number of astrocytes.
The doses used in the current study (4, 40 and 400 µg/kg/day) include two doses below the EPA’s safe reference dose of 50 µg/kg/day and a relatively high dose (400 µg/kg/day) that still may be relevant for human exposure levels. Taylor et al. (2011) examined the pharmacokinetics of BPA exposure level in rodents and non-human primates, and the 400 µg/kg/day resulted in a serum concentration that is similar to previously reported human levels. Interestingly, the doses used in the present study resulted in a non-monotonic dose response curve with the doses below the EPA safe dose having effects. BPA and other endocrine disrupting chemicals have been shown to commonly produce a dose response that is not a linear association between dose and biological effect (Vandenberg et al., 2012). Therefore, it is not without precedent to observe an increase in effect at a low dose and then a reduction in the effect at a higher dose, such as was demonstrated in the number of glia in females in this study.
This is the first study to show long-term effects of adolescent BPA exposure on glial cells in the mPFC. The adolescent period is a crucial time of rewiring in the prefrontal cortex, a process that is heavily dependent upon glia for pruning and synaptogenesis (Theodosis et al., 2008; Eroglu & Barres, 2010). Glia have been shown to express hormone receptors, including ERβ, the estrogen receptor to which BPA has the highest affinity (Azcoitia et al., 1999; Arvanitis et al., 2004; Ishihara et al., 2015; Kupier et al., 1998). The exposure to endocrine disruptors during adolescence could impact normal development through their effects on microglia. Microglia recently have been implicated in synaptic pruning and structural remodeling in addition to the often-studied immune responses (Schafer et al., 2013; Bilimoria & Stevens, 2015). Parkhurst et al. (2013) examined the effects of microglia deletion on synaptic remodeling in the motor cortex of a transgenic male mouse model and showed a significant deficit in both spine formation and deletion, as well as a decrease in the number of learning-dependent spines formed. These results suggest that alterations in the number of microglia could have a dramatic impact on the maintenance and function of dendritic spines. Previous work on BPA and synaptic remodeling has shown BPA to block the synaptic response to estradiol in adult nonhuman primates (Leranth et al., 2008a) and to testosterone in adult male rats (Leranth et al., 2008b). Thus, both the increase in females and decrease in males in the number of microglia in the mPFC could lead to physiological effects on synaptic number and function. This does contrast with Bowman et al (2015) who found no effect of BPA during adolescence on spine density in the mPFC. However, dendritic length was not taken into account to calculate total number of spines. Also, Bowman et al (2015) examined a different age range, dosing from P42–49, while the exposure in the present study was P27–46. Previous work on the changes in the dendritic tree across adolescence indicate growth early in adolescence followed by pruning later (Koss et al., 2014). Thus the effects of BPA may depend on the cellular changes occurring during exposure. Further research is necessary to explicitly examine this idea and to elucidate the implications of the loss or gain of microglia within the mPFC.
Beyond the impact of adolescent exposure to BPA on microglia, other glial cell types could be affected. The total number of microglia and astrocytes counted in the mPFC do not sum to the total number of glia cells counted in the initial Nissl stained tissue. The remaining glia cell types, oligodendrocytes and NG2 cells, were not examined in this study. Oligodendrocytes are responsible for myelination of the neural axons within the cortex. Sexual dimorphisms of oligodendrocyte cell number, proliferation, and death have been described in previous research (Cerghet et al., 2006). The sexual dimorphism in overall number of glia evidenced in the current study could be influenced by an alteration in the rate of cell death and turnover of oligodendrocytes due to BPA exposure. NG2 cells are progenitor cells for new oligodendrocytes, although several other functions have been suggested (Hill & Nishiyama, 2014). It is possible that the remaining glia cell types are also affected by adolescent BPA exposure, which awaits further research.
Another intriguing finding in this study is the differential reactions between male and female animals. Adolescent BPA exposure in female animals resulted in an increase, while exposure in male animals resulted in a decrease, in the number of total glia and microglia in the mPFC. Previous research from our laboratory suggests the number of glia in the mPFC are also susceptible to changes in gonadal hormones. Pre-pubertal gonadectomy at P20 resulted in a higher number of glia, but only in females (Koss et al., 2015). The differential response of males and females to gonadectomy indicates that ovarian hormones are playing a role in the alteration of glia number. Glia have estrogen and other hormone receptors and can be influenced by changes in hormone levels (Azcoitia et al., 1999; Arvanitis et al., 2004; Ishihara et al., 2015). Since BPA binds to estrogen receptors (Kupier et al., 1998), a similar mechanism could be responsible for the increase in number of glia in females. The results of this study mimic the effect of pre-pubertal gonadectomy in female animals, suggesting that BPA is acting as an estrogen antagonist in the mPFC of females and could be indicative of masculinization of the mPFC. In male rats, in contrast, adolescent exposure to BPA caused a decrease in the number of glia and microglia in the mPFC in male rats, perhaps indicating that it acted as an agonist. It has been well established that BPA can act as a selective estrogen response modulator and can respond differently between receptor and tissue types (Welshons et al., 2006), partially explaining the opposite effect of BPA exposure between males and females. In addition, whether BPA acts as an agonist or antagonist could depend on the hormonal milieu.
5. Conclusion
In conclusion, adolescent exposure to BPA did not affect the number of neurons in the male or female mPFC. However, there was a significant increase in the number of glia between controls and 40µg/kg/day BPA in females and a significant decrease in the number of glia between controls and 4 µg/kg/day BPA in males. The changes in the number of glia in the mPFC were mirrored by the increase in the number of microglia in females and the decrease in the number of microglia in males, but no significant differences were detected in the number of astrocytes in either sex. These changes in microglia may indicate synaptic and functional changes in the mPFC that differ between the sexes. Further research should examine the behavioral effects of adolescent exposure to BPA in order to understand the broader implications of these effects.
Highlights.
-
-
After BPA exposure in adolescence, the adult medial prefrontal cortex was examined
-
-
Female rats had a significant increase in the number of microglia
-
-
Male rats had a significant decrease in the number of microglia
-
-
There were no effects on the number of neurons or astrocytes
Acknowledgments
The authors would like to thank the University of Illinois Microscopy Suite for their assistance with the stereology workstation. This work was supported by NIEHS P20 ES018163, USEPA RD 83459301-Project 4; NIEHS P01 ES002848, USEPA 83543401-Project 3.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arvanitis DN, Wang H, Bagshaw RD, Callahan JW, Boggs JM. Membrane-associated estrogen receptor and caveolin-1 are present in central nervous system myelin and oligodendrocyte plasma membranes. J Neurosci Res. 2004;75(5):603–613. doi: 10.1002/jnr.20017. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Garcia-Segura LM. Localization of estrogen receptor β-immunoreactivity in astrocytes of the adult rat brain. Glia. 1999;26(3):260–267. [PubMed] [Google Scholar]
- Bilimoria PM, Stevens B. Microglia function during brain development: New insights from animal models. Brain Res. 2015;1617:7–17. doi: 10.1016/j.brainres.2014.11.032. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Luine V, Diaz Weinstein S, Khandaker H, DeWolf S, Frankfurt M. Bisphenol-A exposure during adolescence leads to enduring alterations in cognition and dendritic spine density in adult male and female rats. Horm Behav. 2015;69:89–97. doi: 10.1016/j.yhbeh.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat A, Ye X, Wong L-Y, Reidy J, Needham L. Exposure of the U.S. Population to Bisphenol A and 4-tertiary-Octylphenol: 2003–2004. Environ Health Perspect. 2008;116(1):39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Bentsen AH, Sanchez-Garrido MA, Ruiz-Pino F, Romero M, Garcia-Galiano D, Tena-Sempere M. Early metabolic programming of puberty onset: Impact of changes in postnatal feeding and rearing conditionins on the timing of puberty and development of the hypothalamic kisspeptin system. Endocrinology. 2011;152(9):3396–3408. doi: 10.1210/en.2010-1415. [DOI] [PubMed] [Google Scholar]
- Cerghet M, Skoff RP, Bessert D, Zhang Z, Mullins C, Ghandour MS. Proliferation and death of oligodendrocytes and myelin proteins are differentially regulated in male and female rodents. J Neurosci. 2006;26(5):1439–47. doi: 10.1523/JNEUROSCI.2219-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature. 2010;468(7321):223–231. doi: 10.1038/nature09612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabashi T, Kawaguchi M, Furuta M, Fukushima A, Kimura F. Exposure to bisphenol A during gestation and lactation causes loss of sex difference in corticotropin-releasing hormone-immunoreactive neurons in the bed nucleus of the stria terminalis of rats. Psychoneuroendocrinology. 2004;29(4):475–85. doi: 10.1016/s0306-4530(03)00055-6. [DOI] [PubMed] [Google Scholar]
- Gould JC, Leonard LS, Maness SC, Wagner BL, Conner K, Zacharewski T, et al. Bisphenol A interacts with the estrogen receptor alpha in a distinct manner from estradiol. Mol Cell Endocrinol. 1998;142(1–2):203–14. doi: 10.1016/s0303-7207(98)00084-7. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, West MJ. The new stereological tools: Disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. AMPIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Hill RA, Nishiyama A. NG2 cells (polydendrocytes): Listeners to the neural network with diverse properties. Glia. 2014;62(8):1195–1210. doi: 10.1002/glia.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara Y, Itoh K, Ishida A, Yamazaki T. Selective estrogen-receptor modulators suppress microglial activation and neuronal cell death via an estrogen receptor-dependent pathway. J Steroid Biochem Mol Biol. 2015;145:85–93. doi: 10.1016/j.jsbmb.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Juraska JM, Sisk CL, DonCarlos LL. Sexual differentiation of the adolescent rodent brain: hormonal influences and developmental mechanisms. Horm Behav. 2013;64(2):203–210. doi: 10.1016/j.yhbeh.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biology of Reproduction. 1977;17(2):298–303. doi: 10.1095/biolreprod17.2.298. [DOI] [PubMed] [Google Scholar]
- Koss WA, Belden CE, Hristov AD, Juraska JM. Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse. 2014;68:61–72. doi: 10.1002/syn.21716. [DOI] [PubMed] [Google Scholar]
- Koss WA, Lloyd MM, Sadowski RN, Wise LM, Juraska JM. Gonadectomy before puberty increases the number of neurons and glia in the medial prefrontal cortex of female, but not male, rats. Dev Psychobiol. 2015;57(3):305–12. doi: 10.1002/dev.21290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo K, Arai O, Omura M, Watanabe R, Ogata R, Aou S. Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neurosci Res. 2003;45(3):345–356. doi: 10.1016/s0168-0102(02)00251-1. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, Saag PT, van der, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, Szigeti-Buck K, Bober J, MacLusky NJ. Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proc Natl Acad Sci. 2008a;105(37):14187–14191. doi: 10.1073/pnas.0806139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Szigeti-Buck K, MacLusky NJ, Hajszan T. Bisphenol A prevents the synaptogenic response to testosterone in the brain of adult male rats. Endocrinology. 2008b;149(3):988–994. doi: 10.1210/en.2007-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham J, Morris J, Juraska J. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144(3):961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- McCaffrey KA, Jones B, Mabrey N, Weiss B, Swan SH, Patisaul HB. Sex specific impact of perinatal bisphenol A (BPA) exposure over a range of orally administered doses on rat hypothalamic sexual differentiation. Neurotoxicology. 2013;36:55–62. doi: 10.1016/j.neuro.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, et al. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab. 2002;87(11):5185–5190. doi: 10.1210/jc.2002-020209. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. 8th. (US): National Academies Press; 2011. [PubMed] [Google Scholar]
- Nunez JL, Jurgens HA, Juraska JM. Androgens reduce cell death in the developing rat visual cortex. Dev Brain Res. 2000;125:83–88. doi: 10.1016/s0165-3806(00)00126-7. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Sodhi J, Juraska JM. Ovarian hormones after postnatal day 20 reduce neuron number in the rat primary visual cortex. J Neurobiol. 2002;52:312–21. doi: 10.1002/neu.10092. [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, Lafaille JJ, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155(7):1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski RN, Wise LM, Park PY, Schantz SL, Juraska JM. Early exposure to bisphenol A alters neuron and glia number in the rat prefrontal cortex of adult males, but not females. Neuroscience. 2014;279:122–131. doi: 10.1016/j.neuroscience.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D, Lehrman E, Stevens B. The “quad-partite” synapse: Microglia-synapse interactions in the developing and mature CNS. Glia. 2013;61(1):24–36. doi: 10.1002/glia.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohoni P, Sumpter JP. Several environmental oestrogens are also anti-androgens. J Endocrinol. 1998;158(3):327–339. doi: 10.1677/joe.0.1580327. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent neurodevelopment. J Adolesc Health. 2013;52:7–13. doi: 10.1016/j.jadohealth.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, vom Saal FS, Welshons WV, Drury B, Rottinghaus G, Hunt PA, VandeVoort CA. Similarity of Bisphenol A pharmacokinetics in Rhesus monkeys and mice: Relevance for human exposure. Environ Health Perspect. 2011;119:422–430. doi: 10.1289/ehp.1002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA, Oliet SH. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev. 2008;88(3):983–1008. doi: 10.1152/physrev.00036.2007. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24(2):139–77. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee D-H, Myers JP. Hormones and endocrine disrupting chemicals: Low-dose effects and nonmonotonic dose responses. 2012;33(3):378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing J, Juraska JM. The timing of neuronal loss across adolescence in the medial prefrontal cortex of male and female rats. Neuroscience. 2015;301:268–275. doi: 10.1016/j.neuroscience.2015.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147:56–69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]