Abstract
Background
Urine uric acid (UUA) has been implicated in the pathogenesis of diabetic nephropathy via its effect on tubular cells. We hypothesized that UUA would be greater in adolescents with type 1 diabetes (T1D) compared to those without. Second, we hypothesized that UUA and fractional uric acid excretion (FeUA) would be greater in adolescents with T1D and hyperfiltration (estimated glomerular filtration rate (eGFR) ≥141 mL/min/1.73m2) compared to those without hyperfiltration
Methods
Adolescents with (n=239) and without T1D (n=75) had UUA measured and FeUA calculated. Serum creatinine and cystatin C were used to calculate eGFR by the Zappitelli equation.
Results
Adolescents with T1D had higher eGFR (mean±standard deviation (SD): 120±22 vs. 112±16 mL/min/1.73m2, p=0.0006), lower urine pH (6.2±0.8 vs. 6.5±1.0, p=0.01), higher UUA (37.7±18.6 vs. 32.8±18.1 mg/dL, p=0.049) and FeUA (median, IQR: 6.2 [4.3–8.7] vs. 5.2 [3.6–7.0]%, p=0.02) compared to non-diabetic adolescents. Among adolescents with T1D, those with hyperfiltration had higher FeUA (8.6 [5.2–9.9] vs. 6.0 [4.2–8.3]%, p=0.02) compared to those without hyperfiltration.
Conclusions
Adolescents with T1D had higher eGFR, higher UUA and more acidic urine than non-diabetic controls, which may increase their risk of UUA crystallization. Adolescents with T1D and hyperfiltration had higher FeUA than those without hyperfiltration. These hypothesis-generating observations may suggest a potential pathophysiologic association between uricosuria and hyperfiltration.
Keywords: glomerular filtration rate (GFR), hyperfiltration, urine uric acid, fractionated excretion of uric acid, type 1 diabetes, children
Introduction
Diabetic nephropathy (DN) is the single most important cause of end-stage renal disease (ESRD) in the Western world [1–3]. Early manifestations of DN, including microalbuminuria and hyperfiltration, are present in adolescents and young adults with type 1 diabetes (T1D). Hyperfiltration is an early phenotype of DN, thought to precede microalbuminuria, and is associated with cardiovascular disease (CVD) and progression of DN [4]. The mechanisms responsible for hyperfiltration in diabetes are not completely understood, but are likely due to a combination of glomerular hemodynamic, vasoactive and metabolic factors that together increase intraglomerular pressure and flow, leading to structural changes including mesangial expansion and glomerular basement membrane thickening [5, 6]. Previous studies have for the most part supported a role for hyperfiltration in the initiation and progression of DN in T1D [7], but how hyperfiltration is associated with DN progression in T1D remains poorly understood.
Serum uric acid (SUA) is a recognized risk factor for DN in T1D [8], but emerging mechanistic data also support urine uric acid (UUA) as an important mediator of DN via its harmful effects on tubular cells [9, 10]. Under acidic conditions UUA precipitates and causes crystallization which can lead to tubular injury. Furthermore, there is a report that elevated concentrations of soluble UUA may also cause epithelial mesenchymal changes in tubular cells [11]. In diabetes, glycosuria leads to elevated UUA through the uricosuric effect, i.e. glucose increases excretion of uric acid in urine, but it is unclear whether hyperfiltration could increase fractional uric acid excretion (FeUA). Individuals with diabetes are also thought to be relatively volume depleted from glycosuria [12], which could lead to increased proximal tubular absorption of sodium and bicarbonate and consequent acidic urine. It is however unknown whether adolescents with T1D have more acidic urine than their non-diabetic counterparts in the absence of ketoacidosis. Accordingly, in this study we examined urine pH, UUA and FeUA in adolescents with and without T1D. We hypothesized that adolescents with T1D would have more acidic urine and higher UUA than their non-diabetic counterparts. Second, we examined uricosuria in adolescents with T1D with and without hyperfiltration. We hypothesized that hyperfiltration would be associated with elevated FeUA in adolescents with T1D.
Materials and methods
The Determinants of Macrovascular Disease in Adolescents with Type 1 Diabetes study was initiated to investigate atherosclerotic disease risk in youth with T1D [13, 14]. The study enrolled 300 subjects 12–19 years old from 2008 to 2010, with type 1 diabetes and 100 non-diabetic controls of similar age [13]. Study participants with T1D were diagnosed by islet cell antibody or by provider clinical diagnosis, had diabetes duration >5 years at entry into the study, and received care at the Barbara Davis Center for Childhood Diabetes. Control subjects were recruited from friends of the study subjects as well as from campus and community advertisements. No siblings or first-degree relatives of patients with T1D were included. Subjects were excluded for diabetes of any other type, or for a history of abnormal cardiac anatomy or arrhythmia that would preclude the subject from vascular function measurements. Participants with (n=239) and without (n=75) T1D and data on UUA were included in our analyses. The study was approved by the Colorado Multiple Institution Review Board, and informed consent and assent (for subjects <18 years) were obtained from all subjects.
After subjects had been laying supine for a minimum of 5 minutes, blood pressure measurements were obtained using a Dynapulse 5200A (Pulse Metric, San Diego, California), and 3 measurements were averaged. Height was measured to the nearest 0.1 cm with shoes removed using a wall-mounted stadiometer and weight was measured to the nearest 0.1 kg using a Detecto scale (Detecto, Webb City, Missouri).
All subjects fasted overnight (≥8 hours) prior to blood and urine collection. Urine samples were centrifuged at 3900 rpm for 10 minutes at 4° C and the urine pH was measured from supernatant by Accumet basic AB 15 plus pH meter (Fisher Scientific, New Hampshire, USA). UUA and SUA were evaluated using a QuantiChrom TM Uric Acid kit assay (DIUA-250) with quantitative colorimetric uric acid determination at 590 nm (BioAssay System – California, USA). UUA concentrations were measured once the urine pH was buffered to 7.0 or greater with sodium bicarbonate. FeUA was calculated as 100*[(UUA*serum creatinine)/(SUA*urine creatinine)]. Urine creatinine and albumin were also measured (RIA, Diagnostic Products), and urine albumin to creatinine (ACR) ratio calculated. Serum creatinine was measured at the University of Colorado Hospital clinical lab using commercially available assays [15]. Cystatin C was measured using the commercially available Dade-Behring assay following package insert instructions on a BNII instrument. Estimated glomerular filtration rate (eGFR) (mL/min/1.73m2) was determined using the Zappitelli equation (eGFR = (507.76 * e(0.003 * height)) / (serum cystatin C0.635 * serum creatinine0.547))[16–18]. Hyperfiltration is typically defined as 2 standard deviations above the mean GFR in healthy participants, and we defined hyperfiltration a priori as eGFR ≥141 mL/min/1.73m2, which represents eGFR at the 95th percentile for the non-diabetic controls in our cohort, and 99th percentile for healthy adolescents in the National Health and Nutrition Examination Survey (NHANES) [7, 18]. As a sensitivity analysis we ran similar models with eGFR calculated by the Bouvet equation (eGFR = 63.2 *[1.2/cystatin C]0.56 * [(96/88.4)/serum creatinine]0.35 * [weight/45]0.30 * [age/14]0.40) [16]. HbA1c was measured on the DCA Advantage by Siemens (Princeton, New Jersey) at the Children's Hospital Colorado main clinical lab.
Statistical analysis
Analyses were performed in SAS (version 9.4 or higher; SAS Institute, Cary, NC). Demographic and clinical characteristics among adolescents with and without T1D and among T1D adolescents with and without hyperfiltration were compared using Student’s t-test for normally distributed continuous variables, Wilcoxon’s test for non-normally continuous variables (e.g. ACR and FeUA) and χ2 test for categorical variables. Generalized linear regression models were employed to evaluate the associations between glucose, HbA1c, eGFR and FeUA, in addition to differences in FeUA between participants with and without hyperfiltration adjusted for ACR and HbA1c. In the generalized linear regression model FeUA was natural log-transformed due to positively skewed distribution. Significance was based on an α-level of 0.05.
Results
Adolescents with T1D have greater FeUA and more acidic urine compared to controls
Adolescents with T1D had lower SUA compared to their non-diabetic peers (Table 1). However, adolescents with T1D had higher eGFR, more acidic urine, greater UUA and FeUA compared to their non-diabetic peers (Table 1). UUA concentration is measured with a buffered urine pH of 7.0 or greater, as UUA solubility depends on urine pH. As a sensitivity analysis, we also measured UUA concentration in the native urine pH and adjusted for the urine pH in a generalized linear regression model. The pH-adjusted UUA remained significantly greater in adolescents with T1D compared to their non-diabetic peers (Least square means, 95% confidence interval: 25.2 [95% CI 23.5–27.0] vs. 20.0 [16.9–23.2] mg/dL, p=0.005).
Table 1.
Participant characteristics stratified by type 1 diabetes (T1D) status
| Variables | Adolescents | P-value | |
|---|---|---|---|
| T1D N=239 |
Non-diabetic N=75 |
||
| Age (years) | 15±2 | 15±2 | 0.88 |
| Sex (female) | 49% | 53% | 0.52 |
| Duration (years) | 8.8±3.0 | -- | -- |
| Weight (kg) | 63±14 | 60±15 | 0.11 |
| BMI (kg/m2) | 22.8±3.8 | 21.8±4.2 | 0.04 |
| BMI z-score | 0.61±0.76 | 0.29±1.02 | 0.004 |
| HbA1c (%) | 9.0±1.6 | 5.3±0.3 | <0.0001 |
| Glucose (mg/dL) | 185±84 | 83±7 | <0.0001 |
| eGFR (mL/min/1.73m2) [Zappitelli] | 120±22 | 112±16 | 0.0006 |
| ACRa (mg/g) | 7.3 (4.3–12.7) | 7.9 (4.2–19.9) | 0.18 |
| SUA (mg/dL) | 4.6±0.8 | 5.0±1.0 | <0.0001 |
| Urine pH | 6.2±0.8 | 6.5±1.0 | 0.01 |
| UUA (mg/dL) | 37.7±18.6 | 32.8±18.1 | 0.049 |
| FeUAa (%) | 6.2 (4.3–8.7) | 5.2 (3.6–7.0) | 0.02 |
| SBP (mm Hg) | 113±9 | 109±9 | <0.0001 |
| SBP (percentile) | 57±23 | 43±23 | <0.0001 |
| DBP (mm Hg) | 68±7 | 64±6 | <0.0001 |
| DBP (percentile) | 59±21 | 45±20 | <0.0001 |
All data presented as mean ± standard deviation (SD) unless otherwise specified.
Median and interquartile range (IQR).
BMI = body mass index, HbA1c = hemoglobin A1c, eGFR = estimated glomerular filtration rate, ACR = albumin-to-creatinine ratio, SUA = serum uric acid, UUA = urine uric acid, FeUA = fractional excretion of uric acid, SBP = systolic blood pressure, DBP = diastolic blood pressure
Adolescents with T1D and hyperfiltration have greater uricosuria compared to adolescents with T1D without hyperfiltration
Almost 13 % of adolescents with T1D had renal hyperfiltration. Compared to adolescents with T1D without hyperfiltration (n=203), those with hyperfiltration (n=29) had higher HbA1c and ACR (Table 2). Furthermore, adolescents with hyperfiltration had lower SUA and greater FeUA compared to those without hyperfiltration (Table 2). Adjusting for ACR and HbA1c did not attenuate the difference in FeUA (least square means, 95% confidence interval: 7.6 [6.2–9.3] vs. 5.9 [5.5–6.3], p=0.02).
Table 2.
Type 1 diabetes (T1D) participant characteristics stratified by renal hyperfiltration status
| Variables | Adolescents with T1D Renal hyperfiltration |
P-value | |
|---|---|---|---|
| No N=203 |
Yes N=29 |
||
| Age (years) | 15±2 | 15±2 | 0.27 |
| Sex (female) | 47% | 67% | 0.06 |
| Duration (years) | 8.8±3.0 | 7.9±2.6 | 0.17 |
| Weight (kg) | 63.7±14.1 | 57.8±12.9 | 0.03 |
| BMI (kg/m2) | 22.8±3.3 | 22.1±3.9 | 0.30 |
| BMI z-score | 0.63±0.74 | 0.48±0.90 | 0.31 |
| HbA1c (%) | 8.7±1.4 | 10.5±2.0 | <0.0001 |
| Glucose (mg/dL) | 179±77 | 205±86 | 0.09 |
| eGFR (mL/min/1.73m2) | 113±15 | 160±14 | <0.0001 |
| ACRa (mg/g) | 7 (4–12) | 10 (6–22) | 0.006 |
| SUA (mg/dL) | 4.6±0.8 | 4.2±0.7 | 0.004 |
| Urine pH | 6.2±0.8 | 6.1±0.9 | 0.91 |
| FeUAa (%) | 6.0 (4.2–8.3) | 8.6 (5.2–9.9) | 0.02 |
| UUA (mg/dL) | 37.7±18.4 | 37.0±20.0 | 0.85 |
| SBP (mm Hg) | 113±9 | 112±8 | 0.49 |
| SBP (percentile) | 57±23 | 61±24 | 0.39 |
| DBP (mm Hg) | 68±6 | 71±8 | 0.02 |
| DBP (percentile) | 58±21 | 71±22 | 0.002 |
232 of the 239 participants with T1D had data on eGFR by the Zappitelli equation and were included in this table. All data presented as mean ± standard deviation (SD) unless otherwise specified.
Median and interquartile range (IQR).
BMI = body mass index, HbA1c = hemoglobin A1c, eGFR = estimated glomerular filtration rate, ACR = albumin-to-creatinine ratio, SUA = serum uric acid, UUA = urine uric acid, FeUA = fractional excretion of uric acid, SBP = systolic blood pressure, DBP = diastolic blood pressure
Glucose and eGFR correlates with FeUA in adolescents with T1D
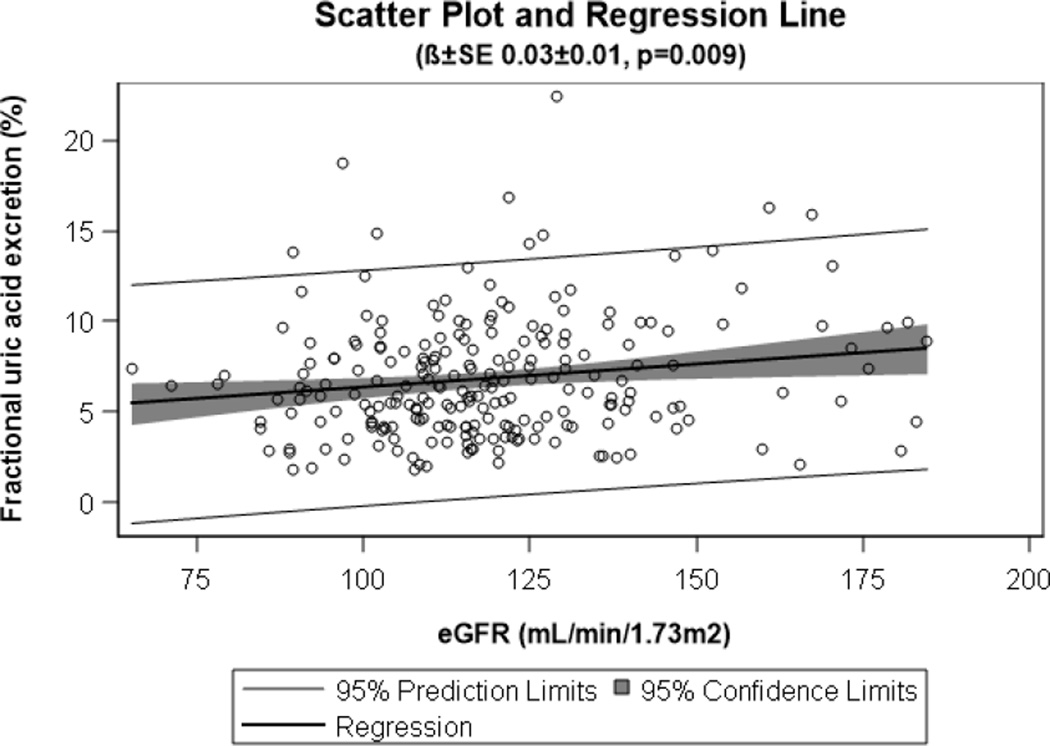
In adolescents with T1D, concurrent fasting glucose (β±SE: 0.01±0.00, p=0.01) but not HbA1c (β±SE: 0.02±0.15, p=0.91) was associated with FeUA. Furthermore, eGFR was positively associated with FeUA (Figure 1).
Figure 1. Relationship between eGFR and FeUA in adolescents with T1D.
Scatter plot and regression line demonstrating the relationship between eGFR and FeUA in adolescents with T1D (n=232).
eGFR = estimated glomerular filtration rate, FeUA = fractional excretion of uric acid, T1D = type 1 diabetes
Discussion
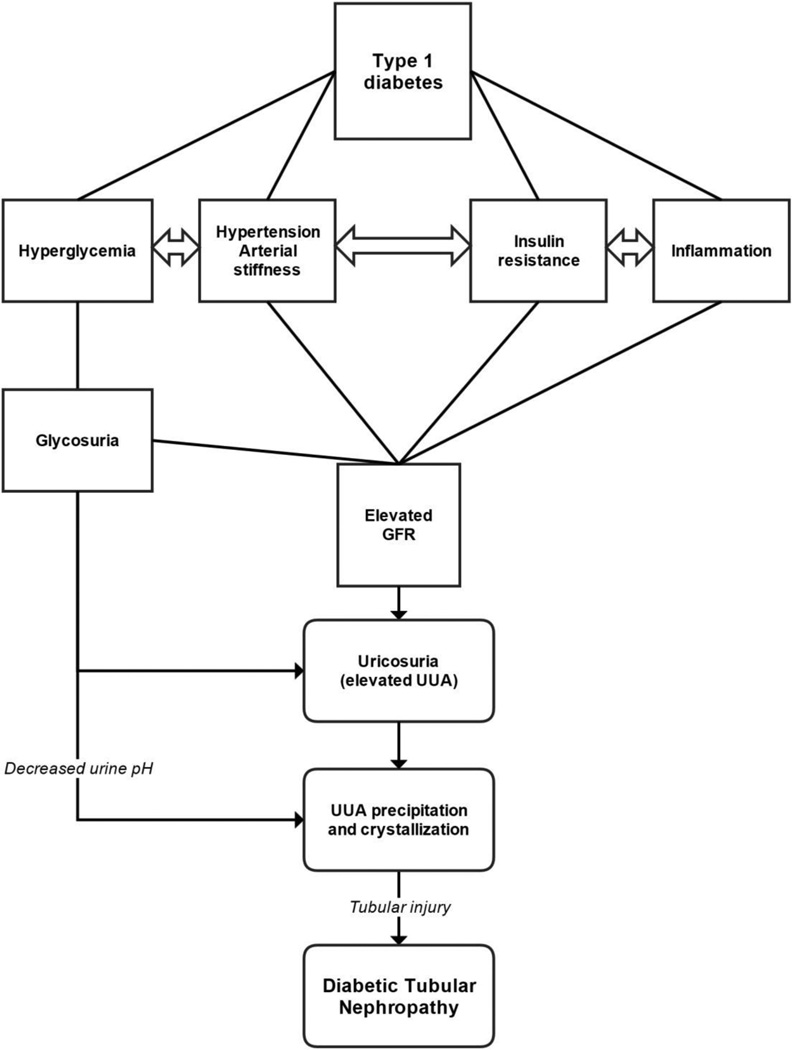
Adolescents with T1D had higher eGFR, more acidic urine and higher FeUA than their non-diabetic counterparts. Furthermore, adolescents with T1D and hyperfiltration had higher FeUA compared to their peers without hyperfiltration. While our observations are cross-sectional and cannot imply causality, they support our hypothesis that adolescents with T1D and renal hyperfiltration may be at increased risk of UUA precipitation and crystallization due to relative uricosuria in the setting of acidic urine (Figure 2).
Figure 2. Proposed hyperfiltration-uricosuric pathway.
GFR = glomerular filtration rate, UUA = urine uric acid
T1D in adolescents and young adults is commonly accompanied by hyperfiltration [19]. Hyperfiltration is associated with increased intraglomerular pressure, and may promote the development of diabetic nephropathy by unclear mechanisms [20, 21]. While hyperfiltration is associated with progression of DN in some patients with T1D [4], the risk of nephropathy is also affected by a number of other important factors including blood pressure, arterial stiffness, inflammation and glycemic control [7]. In this report we demonstrated an association between hyperfiltration and elevated FeUA in adolescents with T1D. Elevated UUA has been associated with tubular nephropathy, especially in the setting of acidic urine which promotes UUA precipitation and crystallization [22]. Urine pH in the normal population tends to average 6.5 (normal range between 5.0 to 8.5) and urine pH has been shown to be very stable over time if stored at −20°C or lower [23]. Solubility of UUA depends on urine pH, and for that reason urine pH is typically increased to 7.0 or above prior to measuring the concentration of UUA. We also observed lower levels of urinary pH in adolescents with T1D. The cause of the urinary acidification is unclear, but it may be related to a relative volume depletion in diabetes in the setting of glycosuria with consequent proximal absorption of sodium and bicarbonate.
Diabetic tubular nephropathy is associated with basement membrane thickening, tubular hypertrophy, epithelial-mesenchymal transition, glycogen accumulation and interstitial inflammation [24]. Although glomerular changes have received significantly more attention from researchers and clinicians than tubulointerstitial changes in diabetes, tubular injury is known to be more strongly associated with renal function than glomerular injury [9, 25]. In fact, tubular damage may be induced earlier than glomerular injury in the course of diabetic nephropathy [10]. Animal studies have demonstrated that blocking uric acid production protects the kidney from tubulointerstitial injury, which suggests a causal role for uric acid in the development of diabetic tubular injury [26]. Furthermore, allopurinol has been shown to attenuate the diabetic tubular injury associated with the KK-A(y)/Ta mouse model [27], and the PERL study is investigating the effect of allopurinol on uric acid lowering to slow decline in GFR in adults with T1D [28]. Moreover, it was recently shown that UUA promoted apoptosis in human proximal tubular cells by oxidative stress and activation of NADPH oxidase NOX4 [29].
Glucosuria is a recognized cause of elevated urate load in T1D [12]. Herein, we report for the first time that hyperfiltration may also be associated with elevated FeUA in adolescents with T1D. The increased urate load in T1D may lead to increased intratubular uric acid concentrations that form crystals in the setting of urinary acidification (Figure 2). Crystalline and non-crystalline UUA and monosodium urate activate tubular cells via both crystalline and non-crystalline effects, resulting in kidney injury [11, 30]. Crystalline uric acid likely stimulates inflammasome activation, toll-like receptors and chemotactic factors [31, 32]. Urate crystals have been reported to cause proximal tubular injury [30]. The observation that people with URAT1 mutations develop exercise-induced AKI with marked uricosuria suggests a crystal-dependent mechanism, since these individuals have significantly reduced ability to reabsorb soluble uric acid and have an increased risk for uric acid kidney stones [33]. Potential non-crystalline effects may also be involved, including induction of oxidative stress, inhibition of cell proliferation, epithelial-mesenchymal cell transformation and stimulation of inflammatory pathways [11, 34–36]. Taken together, these findings suggest that UUA might directly act on the proximal tubular epithelial cells to cause inflammation and apoptosis in the diabetic kidney.
Limitations to the present study include the observational and cross-sectional design which prevents determination of causality and direction, and whether the association holds true longitudinally. Furthermore, our study included more participants with than without T1D to optimize power for the study’s main hypotheses, and only a small number met our criteria for hyperfiltration. For these reasons, the data should be viewed strictly as hypothesis generating. Also, we used an estimate of GFR to assess renal function. However, Zappitelli’s combined creatinine and cystatin C equation [17, 18, 37, 38] is considered state-of-the-art and has been shown to have high accuracy in estimating GFR in children and adolescents with GFR >90 mL/min/1.73m2 [17, 18, 38], and imprecision would bias our results towards the null. As a sensitivity analysis we ran similar models with GFR estimated by the Bouvet equation and obtained similar results.
In summary, adolescents with T1D have higher eGFR and more acidic urine than their non-diabetic peers, which may increase their UUA concentration and decrease the urine solubility of uric acid, thereby predisposing to UUA precipitation and crystallization. Furthermore, adolescents with T1D and hyperfiltration had the highest FeUA. Uricosuria is an emerging risk factor for DN in animal models, and could also explain how hyperfiltration contributes to progression of DN. We submit this as a hypothesis to be proven or disproven. We also recognize that the hyperfiltration-uricosuria hypothesis does not exclude other important mechanisms contributing to the progression of DN in the setting of hyperfiltration. Understanding how hyperfiltration contributes to progression of DN may direct development of therapies to prevent or delay GFR loss. Further studies are needed to examine the longitudinal relationships between hyperfiltration, UUA and progression of DN, and to determine if the relationships between hyperfiltration, UUA and DN hold true longitudinally.
Acknowledgements
Support for this study was provided by NIDDK grants (T32DK06387, DK075360), JDRF (11-2007-694) and CTSI UL-1 RR025780. The study was performed at the Barbara Davis Center for Childhood Diabetes, Aurora, CO. Dr. Maahs was supported by a grant from NIDDK (DK075360), Dr. Snell-Bergeon by an American Diabetes Association Junior Faculty Award (7-13-CD-10) and Dr. Wadwa by an early career award from the Juvenile Diabetes Research Foundation (11-2007-694).
Footnotes
Author Contributions
PB researched, wrote, contributed to discussion, and reviewed/edited the manuscript; LP researched, performed the statistical analyses, contributed to the discussion, reviewed/edited the manuscript; CR researched, contributed to the discussion, reviewed/edited the manuscript; MGL researched, contributed to the discussion, reviewed/edited the manuscript; JKSB researched, contributed to the discussion, reviewed/edited the manuscript; RJJ researched, contributed to the discussion, reviewed/edited the manuscript; RPW researched, contributed to discussion, and reviewed/edited the manuscript; DMM researched, contributed to discussion, and reviewed/edited the manuscript.
Conflict of interest disclosure
Drs. Bjornstad, Roncal, Pyle Lanaspa, Snell-Bergeon, Wadwa and Maahs and Ms. Harra and Bishop have no conflict of interest to disclose. Dr Johnson holds a patent related to lowering uric acid in the treatment of diabetic nephropathy and has shares with XORT therapeutics.
Duality of interest
Drs. Bjornstad, Pyle and Maahs are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Compliance with ethical standards
The study was approved by the Colorado Multiple Institution Review Board, and informed consent and assent (for subjects <18 years) were obtained from all subjects.
References
- 1.Maahs DM, Rewers M. Editorial: Mortality and renal disease in type 1 diabetes mellitus--progress made, more to be done. J Clin Endocrinol Metab. 2006;91:3757–3759. doi: 10.1210/jc.2006-1730. [DOI] [PubMed] [Google Scholar]
- 2.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2010;53:2312–2319. doi: 10.1007/s00125-010-1860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjornstad P, Cherney D, Maahs DM. Early diabetic nephropathy in type 1 diabetes: new insights. Curr Opin Endocrinol Diabetes Obes. 2014;21:279–286. doi: 10.1097/MED.0000000000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjornstad P, Cherney DZ, Snell-Bergeon JK, Pyle L, Rewers M, Johnson RJ, Maahs DM. Rapid GFR decline is associated with renal hyperfiltration and impaired GFR in adults with Type 1 diabetes. Nephrol Dial Transplant. 2015;30:1706–1711. doi: 10.1093/ndt/gfv121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest. 2006;116:288–296. doi: 10.1172/JCI27699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Premaratne E, Verma S, Ekinci EI, Theverkalam G, Jerums G, MacIsaac RJ. The impact of hyperfiltration on the diabetic kidney. Diabetes Metab. 2014;41:5–17. doi: 10.1016/j.diabet.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia. 2009;52:691–697. doi: 10.1007/s00125-009-1268-0. [DOI] [PubMed] [Google Scholar]
- 8.Bjornstad P, Maahs DM, Rivard CJ, Pyle L, Rewers M, Johnson RJ, Snell-Bergeon JK. Serum uric acid predicts vascular complications in adults with type 1 diabetes: the coronary artery calcification in type 1 diabetes study. Acta Diabetol. 2014;51:783–791. doi: 10.1007/s00592-014-0611-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int. 1999;56:1627–1637. doi: 10.1046/j.1523-1755.1999.00721.x. [DOI] [PubMed] [Google Scholar]
- 10.Ginevri F, Piccotti E, Alinovi R, DeToni T, Biagini C, Chiggeri GM, Gusmano R. Reversible tubular proteinuria precedes microalbuminuria and correlates with the metabolic status in diabetic children. Pediatr Nephrol. 1993;7:23–26. doi: 10.1007/BF00861555. [DOI] [PubMed] [Google Scholar]
- 11.Ryu ES, Kim MJ, Shin HS, Jang YH, Choi HS, Jo I, Johnson RJ, Kang DH. Uric acid-induced phenotypic transition of renal tubular cells as a novel mechanism of chronic kidney disease. Am J Physiol Renal Physiol. 2013;304:F471–F480. doi: 10.1152/ajprenal.00560.2012. [DOI] [PubMed] [Google Scholar]
- 12.Lytvyn Y, Škrtić M, Yang GK, Yip PM, Perkins BA, Cherney DZ. Glycosuria-mediated urinary uric acid excretion in patients with uncomplicated type 1 diabetes mellitus. Am J Physiol Renal Physiol. 2015;308:F77–F83. doi: 10.1152/ajprenal.00555.2014. [DOI] [PubMed] [Google Scholar]
- 13.Bjornstad P, Pyle L, Nguyen N, Snell-Bergeon JK, Bishop FK, Wadwa RP, Maahs DM. Achieving International Society for Pediatric and Adolescent Diabetes and American Diabetes Association clinical guidelines offers cardiorenal protection for youth with type 1 diabetes. Pediatr Diabetes. 2015;16:22–30. doi: 10.1111/pedi.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Specht BJ, Wadwa RP, Snell-Bergeon JK, Nadeau KJ, Bishop FK, Maahs DM. Estimated insulin sensitivity and cardiovascular disease risk factors in adolescents with and without type 1 diabetes. J Pediatr. 2013;162:297–301. doi: 10.1016/j.jpeds.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maahs DM, Prentice N, McFann K, Snell-Bergeon JK, Jalal D, Bishop FK, Aragon B, Wadwa RP. Age and sex influence cystatin C in adolescents with and without type 1 diabetes. Diabetes Care. 2011;34:2360–2362. doi: 10.2337/dc11-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bacchetta J, Cochat P, Rognant N, Ranchin B, Hadj-Aissa A, Dubourg L. Which creatinine and cystatin C equations can be reliably used in children? Clin J Am Soc Nephrol. 2011;6:552–560. doi: 10.2215/CJN.04180510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fadrowski JJ, Neu AM, Schwartz GJ, Furth SL. Pediatric GFR estimating equations applied to adolescents in the general population. Clin J Am Soc Nephrol. 2011;6:1427–1435. doi: 10.2215/CJN.06460710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiarelli F, Cipollone F, Romano F, Tumini S, Costantini F, di Ricco L, Pomilio M, Pierdomenico SD, Marini M, Cuccurullo F, Mezzetti A. Increased circulating nitric oxide in young patients with type 1 diabetes and persistent microalbuminuria: relation to glomerular hyperfiltration. Diabetes. 2000;49:1258–1263. doi: 10.2337/diabetes.49.7.1258. [DOI] [PubMed] [Google Scholar]
- 20.Hostetter TH, Rennke HG, Brenner BM. Compensatory renal hemodynamic injury: a final common pathway of residual nephron destruction. Am J Kidney Dis. 1982;1:310–314. doi: 10.1016/s0272-6386(82)80032-2. [DOI] [PubMed] [Google Scholar]
- 21.Hostetter TH, Rennke HG, Brenner BM. The case for intrarenal hypertension in the initiation and progression of diabetic and other glomerulopathies. Am J Med. 1982;72:375–380. doi: 10.1016/0002-9343(82)90490-9. [DOI] [PubMed] [Google Scholar]
- 22.Roncal-Jimenez C, Garcia-Trabanino R, Barregard L, Lanaspa MA, Wesseling C, Harra T, Aragόn A, Grases F, Jarquin ER, González MA, Weiss I, Glaser J, Sánchez-Lozada LG, Johnson RJ. Heat stress nephropathy from exercise-induced uric acid crystalluria: a perspective on mesoamerican nephropathy. Am J Kidney Dis. 2015 doi: 10.1053/j.ajkd.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 23.Cook JD, Strauss KA, Caplan YH, Lodico CP, Bush DM. Urine pH: the effects of time and temperature after collection. J Anal Toxicol. 2007;31:486–496. doi: 10.1093/jat/31.8.486. [DOI] [PubMed] [Google Scholar]
- 24.Drummond K, Mauer M. The early natural history of nephropathy in type 1 diabetes: II. Early renal structural changes in type 1 diabetes. Diabetes. 2002;51:1580–1587. doi: 10.2337/diabetes.51.5.1580. [DOI] [PubMed] [Google Scholar]
- 25.Bjornstad P, Lanaspa MA, Ishimoto T, Kosugi T, Kume S, Jalal D, Maahs DM, Snell-Bergeon JK, Johnson RJ, Nakagawa T. Fructose and uric acid in diabetic nephropathy. Diabetologia. 2015;58:1993–2002. doi: 10.1007/s00125-015-3650-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanaspa MA, Ishimoto T, Cicerchi C, Tamura Y, Roncal-Jimenez CA, Chen W, Tanabe K, Andres-Hernando A, Orlicky DJ, Finol E, Inaba S, Li N, Rivard CJ, Kosugi T, Sanchez-Lozada LG, Petrash JM, Sautin YY, Ejaz AA, Kitagawa W, Garcia GE, Bonthron DT, Asipu A, Diggle CP, Rodriguez-Iturbe B, Nakagawa T, Johnson RJ. Endogenous fructose production and fructokinase activation mediate renal injury in diabetic nephropathy. J Am Soc Nephrol. 2014;25:2526–2538. doi: 10.1681/ASN.2013080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SM, Choi YW, Seok HY, Jeong KH, Lee SH, Lee TW, Ihm CG, Lim SJ, Moon JY. Reducing serum uric acid attenuates TGF-beta1-induced profibrogenic progression in type 2 diabetic nephropathy. Nephron Exp Nephrol. 2012;121:e109–e121. doi: 10.1159/000343567. [DOI] [PubMed] [Google Scholar]
- 28.Maahs DM, Caramori L, Cherney DZ, Galecki AT, Gao C, Jalal D, Perkins BA, Pop-Busui R, Rossing P, Mauer M, Doria A PERL Consortium. Uric acid lowering to prevent kidney function loss in diabetes: the preventing early renal function loss (PERL) allopurinol study. Curr Diab Rep. 2013;13:550–559. doi: 10.1007/s11892-013-0381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verzola D, Ratto E, Villaggio B, Parodi EL, Pontremoli R, Garibotto G, Viazzi F. Uric acid promotes apoptosis in human proximal tubule cells by oxidative stress and the activation of NADPH oxidase NOX 4. PLoS One. 2014;9:e115210. doi: 10.1371/journal.pone.0115210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schepers MS, van Ballegooijen ES, Bangma CH, Verkoelen CF. Crystals cause acute necrotic cell death in renal proximal tubule cells, but not in collecting tubule cells. Kidney Int. 2005;68:1543–1553. doi: 10.1111/j.1523-1755.2005.00566.x. [DOI] [PubMed] [Google Scholar]
- 31.Kim YG, Huang XR, Suga S, Mazzali M, Tang D, Metz C, Bucala R, Kivlighn S, Johnson RJ, Lan HY. Involvement of macrophage migration inhibitory factor (MIF) in experimental uric acid nephropathy. Mol Med. 2000;6:837–848. [PMC free article] [PubMed] [Google Scholar]
- 32.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 33.Ichida K, Hosoyamada M, Hisatome I, Enomoto A, Hikita M, Endou H, Hosoya T. Clinical and molecular analysis of patients with renal hypouricemia in Japan-influence of URAT1 gene on urinary urate excretion. J Am Soc Nephrol. 2004;15:164–173. doi: 10.1097/01.asn.0000105320.04395.d0. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Y, Fang L, Jiang L, Wen P, Cao H, He W, Dai C, Yang J. Uric acid induces renal inflammation via activating tubular NF-kappaB signaling pathway. PLoS One. 2012;7:e39738. doi: 10.1371/journal.pone.0039738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han HJ, Lim MJ, Lee YJ, Lee JH, Yang IS, Taub M. Uric acid inhibits renal proximal tubule cell proliferation via at least two signaling pathways involving PKC, MAPK, cPLA2, and NF-kappaB. Am J Physiol Renal Physiol. 2007;292:F373–F381. doi: 10.1152/ajprenal.00104.2006. [DOI] [PubMed] [Google Scholar]
- 36.Cirillo P, Gersch MS, Mu W, Scherer PM, Kim KM, Gesualdo L, Henderson GN, Johnson RJ, Sautin YY. Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J Am Soc Nephrol. 2009;20:545–553. doi: 10.1681/ASN.2008060576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouvet Y, Bouissou F, Coulais Y, Séronie-Vivien S, Tafani M, Decramer S, Chatelut E. GFR is better estimated by considering both serum cystatin C and creatinine levels. Pediatr Nephrol. 2006;21:1299–1306. doi: 10.1007/s00467-006-0145-z. [DOI] [PubMed] [Google Scholar]
- 38.Sharma AP, Yasin A, Garg AX, Filler G. Diagnostic accuracy of cystatin C-based eGFR equations at different GFR levels in children. Clin J Am Soc Nephrol. 2011;6:1599–1608. doi: 10.2215/CJN.10161110. [DOI] [PubMed] [Google Scholar]