Abstract
Background
There are conflicting reports on the role of fibrates in CVD-risk. Several studies indicate beneficial effects of fibrates on CVD risk in type-2 diabetic patients. We tested how fenofibrate changes lipoprotein subfractions and glucose homeostasis in type-2 diabetic patients.
Study design
Selected markers of lipid and glucose homeostasis and inflammation were measured in 204 diabetic patients who participated in the Diabetes Atherosclerosis Intervention Study (DAIS) and were randomly assigned to 200 mg fenofibrate or placebo for a minimum of 3 years.
Results
Triglyceride and remnant-like particle cholesterol (RLP-C) levels decreased significantly, as well as LpPLA2 activity on fenofibrate compared to placebo. HDL-C and apoA-I levels increased on fenofibrate. In contrast to other lipid-modifying drugs that increase HDL-C and the large (α-1) HDL particles; on fenofibrate, the medium and small (α-3 and α-4) HDL particles increased. Although, preβ-1 HDL particle levels decreased significantly from baseline on fenofibrate, they remained elevated compared to normal level (28.6 mg/dl vs. <15 mg/dl). The concentrations of total LDL-C and small dense LDL-C did not change on fenofibrate compared to placebo. On fenofibrate, glycoalbumin levels increased moderately, while insulin and adiponectin levels did not change.
Conclusion
On fenofibrate, lipid homeostasis improved and Lp-PLA2 activity decreased while there was no improvement in glucose homeostasis. Despite of increasing HDL-C and apoA-I levels fenofibrate failed to change the HDL subpopulation profile beneficially.
Keywords: fenofibrate, lipoproteins, sdLDL-C, HDL particles, CVD risk
INTRODUCTION
The major cause of death in patients with type-2 diabetes is cardiovascular disease (CVD) (1, 2). Diabetic patients, if they are not receiving insulin, often have decreased high-density lipoprotein cholesterol (HDL-C) and elevated triglyceride (TG) levels (3). In the Helsinki Heart Study (HHS) and the Veterans Affairs HDL Intervention Trial (VA-HIT), administration of gemfibrozil, a PPAR-α agonist, caused a concomitant decrease in CVD risk by increasing HDL-C and decreasing TG levels (4, 5). However, in a post-hoc analysis of VA-HIT, Robins et al. concluded that the CVD risk-lowering effects of gemfibrozil could not be entirely explained by the modest increase in HDL-C observed in the treatment arm (6). The reduction in CVD was greatest in those individuals who had at least some of the characteristics of the metabolic syndrome both in the HHS and the VA-HIT (4, 5). In the latter, Rubins et al. found that the beneficial effects of gemfibrozil on CVD events were greater in patients with either type-2 diabetes or pre-diabetes (7). Moreover, measurement of HDL subpopulations in VA-HIT participants indicated that gemfibrozil decreased the levels of the large, anti-atherogenic α-1 HDL particles; though baseline levels of α-1 HDL were inversely associated with future CVD events (8, 9).
In previous lipid-lowering intervention trials, only post-hoc subgroup analyses on people with diabetes have been presented. The Diabetes Atherosclerosis Intervention Study (DAIS) was the first study specifically designed to investigate whether correcting dyslipidemia in type-2 diabetes mellitus with fenofibrate would reduce coronary artery disease (CAD) as determined by angiography (10). We present an analysis on the effects of fenofibrate on LDL and HDL subpopulations and other emerging CVD risk factors on a subset of the DAIS study.
METHODS
Study design and population
DAIS took place in 11 clinical centers in Canada, Finland, France, and Sweden between 1996 and 1999, as described (10). Eligible participants were patients with dyslipidemia and type-2 diabetes aged 40–65 years, with or without previous coronary intervention. The lipid and diabetes eligibility characteristics were assessed during an 8-week baseline period during which participant were not receiving lipid-lowering medications of any kind but were following an American Heart Association/National Cholesterol Education Program Step 1 diet. The same diet was maintained throughout the treatment period. Lipid entry criteria were: total cholesterol to HDL-C ratio ≥ 4:1, plus either an LDL-C concentration of 3.5–4.5 mmol/L and TG concentration of ≤ 5.2 mmol/L, or a TG concentration of 1.7–5.2 mmol/L and ≤LDL-C 4.5 mmol/L. Diabetes entry criteria were: 1) type-2 diabetes as indicated by a fasting plasma glucose concentration without treatment of more than 7.8 mmol/L, or a plasma glucose concentration of 11.0 mmol/L or more 2 h after a 75 g oral glucose load, or on treatment with glucose-lowering drugs; 2) diagnosis after age 35 years; 3) no history of ketoacidosis; and 4) adequate glycemic control (hemoglobin A1c <170% of laboratory’s upper normal limit). DAIS was not a trial of the effects of glycemic control; as such participants’ physicians were allowed to adjust the glucose-lowering drug regimen to optimize control in individual patients. Eligible patients were assigned to fenofibrate or placebo with stratification by sex, previous coronary intervention, and clinic center using a permuted blocks randomization procedure. The treatment period was at least 3 years. The protocol was reviewed and approved by each institution’s ethics committee, and all participants gave informed consent to take part.
The DAIS analyzed 207 subjects in the fenofibrate and 211 in the placebo arm. Adequate plasma samples for further lipid measurements, including LDL and HDL subpopulation profile determination, were available for 108 subjects (51.2%) in the fenofibrate arm and 96 (45.5%) subjects in the placebo arm. Tufts University IRB committee approval was obtained for measurements and analyses performed at Tufts University.
Laboratory measurements
Fasting plasma samples stored at −80°C were used. Automated chemistries were measured on a Hitachi 911 analyzer. Total cholesterol, TG, and HDL-C were measured using kits from Roche. ApoA-I and highly-sensitive C-reactive protein (hsCRP) were measured using immunoturbidimetric assay kits from Wako Diagnostics (Richmond, VA). Small dense LDLC (sdLDL-C) and LDL-C were measured using kits from Denka-Seiken (Japan). Remnant-like particle cholesterol (RLP-C) was measured using kits from Kyowa-Medex (Japan). Insulin was measured with kits from Kamiya Biomedical (Seattle, WA), glycated albumin was measured with kits from Asahi Kasei Pharma (Japan), adiponectin was measured with kits from Otsuka Pharmaceutical (Japan). Lipoprotein-associated phospholipase A2 (Lp-PLA2) concentration and activity were measured at DiaDexus (San Francisco, CA).
ApoA-I-containing HDL particles were determined by 2-dimensional, non-denaturing gel electrophoresis followed by immunodetection and image analysis as described earlier (11,12). Briefly: in the first dimension, HDL was separated from 4 μl plasma on 0.7% agarose gel by charge into preβ-, α-, and preα-mobility particles. In the second dimension, each sample was further separated according to size by non-denaturing polyacrylamide gel electrophoresis (on 3-35% concave gradient gels). Gels were electro-transferred to nitrocellulose membranes. ApoA-I was immunolocalized by incubation with monospecific goat human apoA-I antibody for 6 h. After the unbound first antibody was washed off with PBST, membranes were incubated with 125I-labeled secondary antibody. Signals were quantitatively determined by image analysis using a FluoroImager (Molecular Dynamics, Sunnyvale, CA). Ten apoA-I-containing HDL subpopulations were delineated; signals were measured in each area and used for calculating the percent distribution. Concentration of each subpopulations were calculated by multiplying percentiles by total plasma apoA-I concentration.
Data and Statistical Analysis
Percent changes from baseline (visit 4) until a minimum of 3 years on therapy (visit 11) were calculated for all study parameters. Assays which yielded data outside of the measureable level were imputed as the lower or upper limit of detection as appropriate. Missing data secondary to plasma volume insufficiency was imputed using multiple imputation by chained equations (MICE) utilizing all lipid parameters in the MICE model. A burn in of 10 iterations was used to reach converge to produce each of 20 multiple imputations for the final analytical data set. Therefore, all 204 participants were included in the analysis. The normality of percent differences were assessed via a Shapiro-Wilk test, and means and standard deviations were calculated. Intra- and intergroup differences from baseline were analyzed with univariate and bivariate linear regression, respectively. The median and interquartile range of percent changes were reported for parameters which violated the normality assumption, and intra- and inter-group differences were analyzed with median quantile regression. All p values and confidence intervals are reported unadjusted, but the false discovery rate method was employed (13). All analyses were performed using STATA version 12 (StataCorp, TX, USA).
The proposal of this work was reviewed and approved by Tufts University Health Science Campus Institutional Review Board.
RESULTS
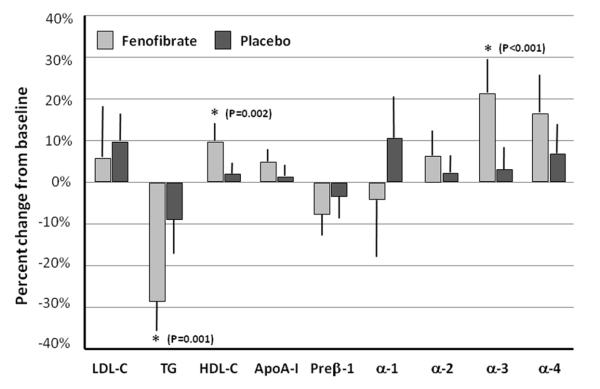
Table 1 shows plasma lipid, inflammatory and metabolic parameters in the fenofibrate and placebo groups. LDL-C increased 10.1% (p=0.01) in the placebo and 5.5% (p=0.43) in the fenofibrate group resulting in no significant difference between the two treatment groups (p=0.57). Concentration of sdLDL-C slightly increased in the placebo group (3.5% p=0.48) and slightly decreased (−11.8% p=0.07) in the fenofibrate group, but the difference between the two groups was not significant (p=0.60). TG decreased more in the fenofibrate (−29.1% p<0.001) than in the placebo group (−9.4% p=0.04) resulting in a significant treatment difference (p<0.001). Concomitantly, RLP-C decreased more in the fenofibrate (−31.9% p<0.001) than in the placebo (−7.2% p=0.11) group with a treatment difference of p<0.001. Fenofibrate increased HDL-C more (9.9% p<0.001) than placebo treatment (2.0% p=0.13) resulting in significant difference between the two groups (p=0.002). ApoA-I increased slightly more in the fenofibrate than in the placebo group (5.1% p=0.002 vs. 1.2% p=0.39), but the difference between the two treatments was not significant (p=0.07). Glycated albumin (GA), a marker of diabetes, increased significantly in both the placebo and the fenofibrate arms (5.3% p=0.01 and 10.3% p<0.001, respectively) with no significant difference between groups (p=0.07). Insulin and adiponectin levels did not change significantly in either group. While concentrations of hsCRP and Lp-PLA2 did not change significantly, the activity of Lp-PLA2 decreased significantly in the fenofibrate group (−13.4% p<0.001).
Table 1.
Major lipid and metabolic parameters at baseline, on-treatment, and changes (Δ%) during the follow up
| Placebo (n=96) | Fenofibrate (n=108) | Group Differences | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Lipid parameters | Baseline | On-treatment | Δ% (min, max) | p value | Baseline | On-treatment | Δ% (min, max) | p value | Δ (95% CI) | p value |
| Total-C (mg/dl) | 245 (45) | 239 (41) | −1.0 (−4.7, 2.7) | 0.60 | 239 (37) | 222 (41) | −6.3 (−9.4, −3.2) | <0.001 | −5.3 (−10.0, −0.6) | 0.03 |
| LDL-C (mg/dl) | 134 (47) | 139 (38) | 10.1 (2.5, 17.7) | 0.01 | 130 (42) | 133 (33) | 5.5 (−8.4, 19.4) | 0.43 | −4.6 (−20.5, 11.3) | 0.57 |
| sdLDL-C (mg/dl) | 31.5 (22.4) | 37.1 (22.9) | 3.5 (−6.4, 13.5) | 0.48 | 31.8 (15.6) | 30.8 (21.4) | −11.8 (−24.6, 0.9) | 0.07 | −15.3 (−31.2, 0.5) | 0.60 |
| Triglycerides† (mg/dl) | 220 (135) | 198 (120) | −9.4 (−18.3, −0.5) | 0.04 | 219 (141) | 144 (91) | −29.1 (−36.5, −21.5) | <0.001 | −19.7 (−30.8, −8.5) | 0.001* |
| RLP-C (mg/dl) | 21.5 (8.9) | 18.6 (8.8) | −7.2 (−16.1, 1.6) | 0.11 | 21.7 (11.1) | 13.4 (9.1) | −31.9 (−40.0, −23.7) | <0.001 | −24.6 (−36.6, −12.6) | <0.001* |
| HDL-C (mg/dl) | 39.5 (7.5) | 40.2 (9.1) | 2.0 (−0.6, 4.7) | 0.13 | 39.2 (8.5) | 42.7 (11.3) | 9.9 (5.9, 13.9) | <0.001 | 7.9 (3.0, 12.8) | 0.002* |
| ApoA-I (mg/dl) | 123 (18) | 124 (20) | 1.2 (−1.5, 3.9) | 0.39 | 124 (19) | 130 (27) | 5.1 (1.9, 8.3) | 0.002 | 3.9 (−0.3, 8.2) | 0.07 |
|
| ||||||||||
| Insulin† (μIU/mL) | 8.2 (8.4) | 7.9 (8.8) | 1.1 (−12.3, 14.4) | 0.87 | 7.4 (9.8) | 7.6 (9.0) | −1.7 (−14.7, 11.2) | 0.79 | −2.8 (−18.5, 12.9) | 0.73 |
| Glycoalbumin (%) | 19.8 (4.3) | 20.4 (4.3) | 5.3 (1.3, 9.3) | 0.01 | 19.2 (4.1) | 21.0 (4.8) | 10.3 (6.6, 13.9) | <0.001 | 5.0 (0.4, 10.4) | 0.07 |
| Adiponectin (μg/mL) | 7.2 (3.0) | 7.2 (2.9) | 2.5 (−2.4, 7.4) | 0.32 | 7.4 (3.4) | 7.0 (3.1) | −3.0 (−7.7, 1.7) | 0.21 | −5.5 (−12.2, 1.3) | 0.11 |
| hsCRP† (mg/L) | 2.2 (4.4) | 2.9 (3.6) | 10.1 (−7.8, 28.1) | 0.26 | 1.8 (3.7) | 2.4 (3.5) | 7.1 (−13.2, 25.7) | 0.49 | −3.0 (−28.6, 22.6) | 0.82 |
| Lp-PLA2, (ng/mL) | 258 (66) | 247 (54) | −1.6 (−7.2, 4.1) | 0.58 | 259 (61) | 244 (59) | −3.6 (−8.0, 0.7) | 0.10 | −2.0 (−9.1, 5.0) | 0.56 |
| Lp-PLA2 activity (nmol/mL/min) |
153 (34) | 151 (58) | −0.6 (−8.2, 7.0) | 0.87 | 150 (27) | 132 (30) | −13.4 (−16.6, −10.2) | <0.001 | −12.8 (−20.7.1, −4.9) | 0.002* |
Data shown as mean (SD) and univariate or multivariate regression of intra- and intergroup differences of percent change.
Data shown as median (IQR) and median quantile regression of intra- and intergroup differences of percent change.
All data shown are after multiple imputation.
Significant using FDR after adjusting for all intergroup comparisons.
Table 2 shows data on apoA-I-containing HDL particles at baseline and on treatment. Concentration of the small preβ-1 HDL particles decreased more on fenofibrate than on placebo (7.8% p=0.004 vs. 3.7% p=0.15), but there was no significant difference between the two treatments (p=0.27). Concentration of the large α-1 particles increased on placebo treatment by 11.5% (p=0.03) while decreased on fenofibrate by −2.0% (p=0.80), but no group significant difference between them (p=0.12). The medium-sized α-3 HDL particles increased more in the fenofibrate (21.4% p<0.001) than in the placebo (3.1% p=0.33) group and this difference was significant (p<0.001). The small-sized α-4 HDL particles also increased more in the fenofibrate than in the placebo group (17.3% p<0.001 vs. 7.5% p=0.04) but the difference was not significant (p=0.08).
Table 2.
Concentration and changes of apoA-I-containing HDL particles
| Placebo (n=96) | Fenofibrate (n=108) | Group Differences | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Baseline | On-treatment | Δ% (min, max) | p value | Baseline | On-treatment | Δ% (min, max) | p value | Δ (95% CI) | p value | |
| Pre-β1 | 32.0 (10.2) | 30.2 (9.3) | −3.7 (−8.6, 1.3) | 0.15 | 31.6 (10.7) | 28.6 (9.9) | −7.8 (−13.0, −2.7) | 0.004 | −4.2(−11.6, 3.2) | 0.27 |
| Pre-β2 | 2.3 (1.4) | 2.3 (1.2) | 11.1 (−0.1, 22.2) | 0.05 | 2.3 (1.4) | 2.3 (1.3) | 12.2 (−2.2, 26.6) | 0.09 | 1.2 (−17.2, 19.5) | 0.90 |
| α1† | 6.8 (5.2) | 7.5 (5.4) | 11.5 (1.5, 21.4) | 0.03 | 6.9 (5.9) | 7.2 (6.3) | −2.0 (−18.2, 14.2) | 0.80 | −13.5 (−30.5, 3.6) | 0.12 |
| α2 | 32.9 (7.8) | 33.4 (9.3) | 2.6 (−1.9, 7.0) | 0.26 | 32.7 (8.5) | 34.4 (11.4) | 6.6 (0.2, 13.0) | 0.04 | 4.1 (−4.1, 12.2) | 0.33 |
| α3 | 24.2 (6.2) | 24.2 (6.5) | 3.1 (−3.1, 9.2) | 0.33 | 24.5 (7.0) | 28.7 (7.8) | 21.4 (13.4, 29.3) | <0.001 | 18.3 (8.4, 28.2) | <0.001* |
| α4 | 13.7 (3.7) | 14.2 (4.2) | 7.5 (0.4, 14.5) | 0.04 | 13.6 (3.9) | 15.5 (5.7) | 17.3 (8.6, 26.0) | <0.001 | 9.9 (−1.3, 21.0) | 0.08 |
| Pre-α1† | 1.5 (1.6) | 1.8 (1.8) | 16.6 (−3.0, 36.3) | 0.10 | 1.7 (2.0) | 1.5 (1.8) | −10.9 (−29.7, 7.9) | 0.25 | −27.5 (−58.8, −1.2) | 0.04 |
| Pre-α2 | 3.8 (1.5) | 4.2 (1.6) | 18.6 (8.6, 28.6) | <0.001 | 4.0 (1.5) | 4.3 (1.9) | 13.1 (3.2, 22.9) | 0.01 | −5.5 (−19.6, 8.5) | 0.44 |
| Pre-α3 | 2.8 (1.1) | 2.9 (1.2) | 8.9 (−0.1, 17.9) | 0.05 | 2.9 (1.2) | 3.4 (1.4) | 23.4 (14.0, 32.9) | <0.001 | 14.5 (1.4, 27.7) | 0.03 |
| Pre-α4 | 1.7 (0.7) | 1.7 (0.8) | 8.1 (−2.3, 18.5) | 0.12 | 1.8 (0.9) | 1.9 (0.9) | 13.8 (2.6, 24.9) | 0.02 | 5.7 (−10.0, 21.3) | 0.47 |
Data shown as mean (mg/dl) (SD) and univariate or multivariate regression of intra- and intergroup differences of percent change.
Data shown as median (IQR) and median quantile regression of intra- and intergroup differences of percent change.
All data shown are after multiple imputation.
Significant using FDR after adjusting for all intergroup comparisons.
DISCUSSION
The DAIS investigators reported that fenofibrate reduced the angiographic progression of CAD and that the beneficial effects of fenofibrate, at least partially, were due to the correction of lipoprotein abnormalities (10). In a follow-up paper, they reported that changes in LDL size and plasma lipid levels account for part of the antiatherogenic effects of fenofibrate in type-2 diabetes (14). In this sample set, we did not measure LDL size, but measured sdLDL-C concentration, the most atherogenic part of LDL (15, 16). We have seen a slight decrease in sdLDL-C on fenofibrate treatment, however, it was not significantly different from the changes on placebo treatment (group difference p=0.60). Therefore, we believe that the effects of fenofibrate on sdLDL-C do not impact CVD risk significantly.
Our findings in this study are similar to our former findings in VA-HIT. In that trial, gemfibrozil increased HDL-C and that was associated with increased levels of medium-sized (α-3) HDL particles but not with large-sized (α-1) HDL particles (8,9). In the present study, fenofibrate increased HDL-C and apoA-I significantly and that was associated with increased levels of the small and medium (α-4 and α-3) not the large (α-1) HDL particles. Although, fenofibrate significantly decreased concentrations of preβ-1 level from baseline, after treatment for three years it was still markedly higher (28.6 mg/dl) than our historic normal level (<15 mg/dl).
In contrast fibrates, statin- and/or niacin-mediated increase in HDL-C and decrease in TG levels are usually accompanied by significant decrease in small-sized preβ-1 and significant increase in large-sized α-1 HDL particles (17-21). Statins decrease TG-rich lipoproteins (TRL) in plasma through decreasing cholesterol synthesis, thereby reducing VLDL production/secretion by the liver. As a result, the concentrations of both major apoB-containing lipoprotein classes, VLDL and LDL, are reduced in plasma. Decreased level of apoB-containing lipoproteins causes lower cholesterol ester transfer protein (CETP) activity. In turn, decreased CETP activity results in increased α-1 and decreased preβ-1 HDL particle levels due to decreased fractional catabolic rate of the large α-1 particles.
In contrast to statins, which decrease synthesis/secretion of TRL from the liver, fibrates reduce plasma TG levels by increasing TRL clearance from plasma (22) by decreasing apoA-V and apoC-III levels [both are lipoprotein lipase (LPL) inhibitors] resulting in increased LPL activity (23-25). In patients with high TG level, LPL increases VLDL catabolism and as a result VLDL to LDL turn over (26). In this case, neither the number of apoB-containing particles nor CETP-mediated exchange of TG for cholesterol ester (CE) between TRL and α-1 HDL change. TG-enriched α-1 HDL particles are good substrates for hepatic lipase, which increases α-1 catabolism into smaller HDL particles (27).
In conclusion, we have verified that, similar to gemfibrozil, fenofibrate significantly increases HDL-C and significantly decreases plasma TG and RLP-C levels, but in our study, had only moderate effects on total LDL-C and sdLDL-C levels. Also, similar to gemfibrozil, fenofibrate-mediated improvements in TG and HDL-C levels were not accompanied with improvement in the HDL subpopulation profile. We believe that fenofibrate decreases CVD risk by ameliorating high plasma TG level by increasing LPL activity and by decreasing inflammation, marked by decreased Lp-PLA2 activity, but not by increasing HDL-C and improving the HDL subpopulation profile. Despite of fenofibrate treatment for three years, DAIS patients did not have normal HDL remodeling, which was indicated by the very low level of the large and the very high level of the small HDL particles.
Highlights.
Compared to placebo, fenofibrate significantly decreased TG, but not LDL-C.
Compared to placebo, fenofibrate significantly increased HDL-C, but not apoA-I.
Despite of decreasing TG and increasing HDL-C, there was no beneficial change in the HDL profile.
Fenofibrate’s effect was more pronounced on the small HDL particles than on the large ones.
Acknowledgement
This work was supported by grants from ABBOTT Laboratories and the NIH (HL117933) PI: Asztalos. We thank DiaDexus for measuring LpPLA2 in DAIS samples at no cost.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vaccaro O, Stamler J, Neaton JD. Sixteen-year coronary mortality in black and white men with diabetes screened for the Multiple Risk Factor Intervention Trial (MRFIT) Int J Epidemiol. 1998;27(4):636–41. doi: 10.1093/ije/27.4.636. [DOI] [PubMed] [Google Scholar]
- 2.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 3.Steiner G. The dislipoproteinaemias of diabetes. Atherosclerosis. 1994;110:S27–S33. doi: 10.1016/0021-9150(94)05373-q. [DOI] [PubMed] [Google Scholar]
- 4.Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, Huttunen JK, Kaitaniemi P, Koskinen P, Manninen V, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317(20):1237–45. doi: 10.1056/NEJM198711123172001. [DOI] [PubMed] [Google Scholar]
- 5.Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, Wilt TJ, Wittes J. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341(6):410–8. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 6.Robins SJ, Collins D, Wittes JT, Papademetriou V, Deedwania PC, Schaefer EJ, McNamara JR, Kashyap ML, Hershman JM, Wexler LF, Rubins HB, VA-HIT Study Group Veterans Affairs High-Density Lipoprotein Intervention Trial. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. JAMA. 2001;285(12):1585–91. doi: 10.1001/jama.285.12.1585. [DOI] [PubMed] [Google Scholar]
- 7.Rubins HB, Robins SJ, Collins D, Nelson DB, Elam MB, Schaefer EJ, Faas FH, Anderson JW. Diabetes, plasma insulin, and cardiovascular disease: subgroup analysis from the Department of Veterans Affairs high-density lipoprotein intervention trial (VA-HIT) Arch Intern Med. 2002;162(22):2597–604. doi: 10.1001/archinte.162.22.2597. [DOI] [PubMed] [Google Scholar]
- 8.Asztalos BF, Collins D, Cupples LA, Demissie S, Horvath KV, Bloomfield HE, Robins SJ, Schaefer EJ. Value of high-density lipoprotein (HDL) subpopulations in predicting recurrent cardiovascular events in the Veterans Affairs HDL Intervention Trial. Arterioscler Thromb Vasc Biol. 2005;25(10):2185–91. doi: 10.1161/01.ATV.0000183727.90611.4f. [DOI] [PubMed] [Google Scholar]
- 9.Asztalos BF, Collins D, Horvath KV, Bloomfield HE, Robins SJ, Schaefer EJ. Relation of gemfibrozil treatment and high-density lipoprotein subpopulation profile with cardiovascular events in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Metabolism. 2008;57(1):77–83. doi: 10.1016/j.metabol.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diabetes Atherosclerosis Intervention Study investigators Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomised study. Lancet. 2001;357(9260):905–10. [PubMed] [Google Scholar]
- 11.Asztalos BF, Roheim PS, Milani RL, Lefevre M, McNamara JR, Horvath KV, Schaefer EJ. Distribution of ApoA-I-containing HDL subpopulations in patients with coronary heart disease. Arterioscler Thromb Vasc Biol. 2000;20(12):2670–6. doi: 10.1161/01.atv.20.12.2670. [DOI] [PubMed] [Google Scholar]
- 12.Asztalos BF, Sloop CH, Wong L, Roheim PS. Two-dimensional electrophoresis of plasma lipoproteins: Recognition of new apo A-I-containing subpopulations. Biochim Biophys Acta - Lipids Lipid Metab. 1993;1169:291–300. doi: 10.1016/0005-2760(93)90253-6. [DOI] [PubMed] [Google Scholar]
- 13.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statistic Soc B. 1995;57(1):289–300. [Google Scholar]
- 14.Vakkilainen J, Steiner G, Ansquer JC, Aubin F, Rattier S, Foucher C, Hamsten A, Taskinen MR, DAIS Group Relationships between low-density lipoprotein particle size, plasma lipoproteins, and progression of coronary artery disease: the Diabetes Atherosclerosis Intervention Study (DAIS) Circulation. 2003;107(13):1733–7. doi: 10.1161/01.CIR.0000057982.50167.6E. [DOI] [PubMed] [Google Scholar]
- 15.Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, Couper D, Virani SS, Kathiresan S, Boerwinkle E, Ballantyne CM. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2014 May;34(5):1069–77. doi: 10.1161/ATVBAHA.114.303284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai MY, Steffen BT, Guan W, McClelland RL, Warnick R, McConnell J, Hoefner DM, Remaley AT. New automated assay of small dense low-density lipoprotein cholesterol identifies risk of coronary heart disease: the Multi-ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2014 Jan;34(1):196–201. doi: 10.1161/ATVBAHA.113.302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asztalos BF, Horvath KV, McNamara JR, Roheim PS, Rubinstein JJ, Schaefer EJ. Effects of atorvastatin on the HDL subpopulation profile of coronary heart disease patients. J Lipid Res. 2002;43(10):1701–7. doi: 10.1194/jlr.m200037-jlr200. [DOI] [PubMed] [Google Scholar]
- 18.Asztalos BF, Horvath KV, McNamara JR, Roheim PS, Rubinstein JJ, Schaefer EJ. Comparing the effects of five different statins on the HDL subpopulation profiles of coronary heart disease patients. Atherosclerosis. 2002;164:361–369. doi: 10.1016/s0021-9150(02)00149-1. [DOI] [PubMed] [Google Scholar]
- 19.Asztalos BF, Le Maulf F, Dallal GE, et al. Comparison of the Effects of High Doses of Rosuvastatin Versus Atorvastatin on the Subpopulations of High-Density Lipoproteins. Am J Cardiol. 2007;99:681–685. doi: 10.1016/j.amjcard.2006.09.117. [DOI] [PubMed] [Google Scholar]
- 20.Asztalos BF, Batista M, Horvath KV, Cox CE, Dallal GE, Morse JS, Brown GB, Schaefer EJ. Change in alpha1 HDL concentration predicts progression in coronary artery stenosis. Arterioscler Thromb Vasc Biol. 2003;23(5):847–52. doi: 10.1161/01.ATV.0000066133.32063.BB. [DOI] [PubMed] [Google Scholar]
- 21.Asztalos BF, Cupples LA, Demissie S, et al. High-density lipoprotein subpopulation profile and coronary heart disease prevalence in male participants of the Framingham Offspring Study. Arterioscler Thromb Vasc Biol. 2004;24:2181–2187. doi: 10.1161/01.ATV.0000146325.93749.a8. [DOI] [PubMed] [Google Scholar]
- 22.Grundy SM, Vega GL. Fibric acids: effects on lipids and lipoprotein metabolism. Am J Med. 1987;83(5B):9–20. doi: 10.1016/0002-9343(87)90866-7. [DOI] [PubMed] [Google Scholar]
- 23.Barter PJ, Rye KA. Is there a role for fibrates in the management of dyslipidemia in the metabolic syndrome? Arterioscler Thromb Vasc Biol. 2008;28(1):39–46. doi: 10.1161/ATVBAHA.107.148817. [DOI] [PubMed] [Google Scholar]
- 24.Steiner G. Fenofibrate for cardiovascular disease prevention in metabolic syndrome and type 2 diabetes mellitus. Am J Cardiol. 2008;102(12A):28L–33L. doi: 10.1016/j.amjcard.2008.09.072. [DOI] [PubMed] [Google Scholar]
- 25.Vu-Dac N, Gervois P, Jakel H, Nowak M, Bauge E, Dehondt H, Staels B, Pennacchio LA, Rubin EM, Fruchart-Najib J, Fruchart JC. Apolipoprotein A5, a crucial determinant of plasma triglyceride levels, is highly responsive to peroxisome proliferator-activated receptor alpha activators. J Biol Chem. 2003;278(20):17982–5. doi: 10.1074/jbc.M212191200. [DOI] [PubMed] [Google Scholar]
- 26.Ginsberg HN. Changes in lipoprotein kinetics during therapy with fenofibrate and other fibric acid derivatives. Am J Med. 1987;83(5B):66–70. doi: 10.1016/0002-9343(87)90873-4. [DOI] [PubMed] [Google Scholar]
- 27.Asztalos BF, Schaefer EJ, Horvath KV, Yamashita S, Miller M, Franceschini G, Calabresi L. Role of LCAT in HDL remodeling: investigation of LCAT deficiency states. J Lipid Res. 2007;48(3):592–9. doi: 10.1194/jlr.M600403-JLR200. [DOI] [PubMed] [Google Scholar]