Abstract
The bacterial repair enzyme MutT hydrolyzes the damaged nucleotide OdGTP (the 5′-triphosphate derivative of 8-oxo-2′-deoxyguanosine; OdG), which is a known mutagen and has been linked to antibacterial action. Previous work has indicated important roles for the C8-oxygen, N7-hydrogen, and C2-exocyclic amine during OdGTP recognition by MutT. In order to gain a more nuanced understanding of the contribution of these three sites to the overall activity of MutT, we determined the reaction parameters for dGTP, OdGTP, and nine of their analogues using steady state kinetics. Our results indicate that overall high reaction efficiencies can be achieved despite altering any one of these sites. However, altering two or more sites leads to a significant decrease in efficiency. The data also suggest that, similar to another bacterial OdG repair enzyme, MutM, a specific carbonyl in the enzyme can not only promote activity by forming an active site hydrogen bond with the N7-hydrogen of OdGTP, but can also hinder activity through electrostatic repulsion with the N7-lone pair of dGTP.
Graphical Abstract
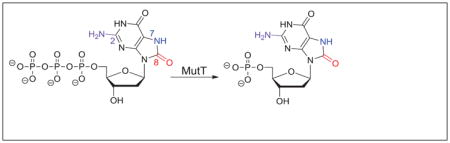
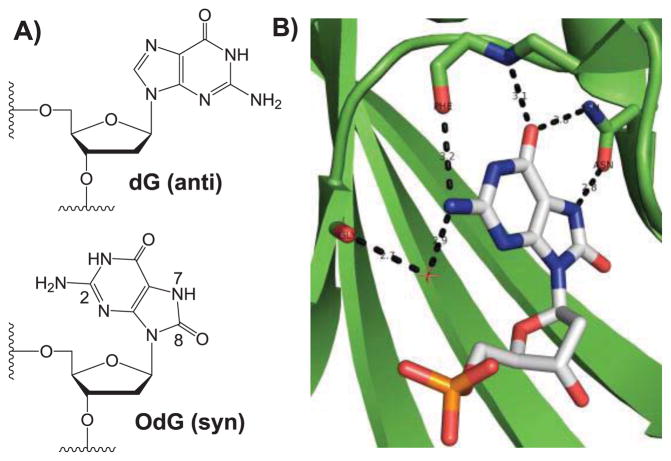
Spontaneous mutations, while necessary for evolution, can also be lethal to a cell. Among the damaged DNA bases, 8-oxo-2′-deoxyguanosine (OdG), which arises from the reaction between 2′-deoxyguanosine (dG) and reactive oxygen species, is well studied since it is both prominent in aerobic cells 1, 2 and is promutagenic due to its possible pairing with 2′-deoxyadenosine (dA). 3 OdG differs from dG only within the imidazole ring: dG contains a hydrogen bond accepting N7, where in OdG, the presence of the C8-oxygen leads to a hydrogen bond donating N7.4 Additionally, the added steric bulk off C8 in OdG reorients the glycosidic bond to the syn conformation from the more standard anti conformation (Fig. 1A).5 Cells have evolved several repair enzymes in efforts to limit the deleterious effects of OdG; in Escherichia coli, three enzymes (MutM, MutY, and MutT) are used, which together are referred to as the GO pathway.6, 7 MutM (also known as Fpg) removes the 8-oxoguanine base from OdG:dC base pairs,8 MutY removes the adenine base from OdG:dA base pairs,9 and MutT hydrolyzes the 5′-triphosphate derivative of OdG (OdGTP) to the corresponding monophosphate (OdGMP),10, 11 thereby preventing its insertion into DNA.
Figure 1.
a) 2′-deoxyguanosine (dG) and 8-oxo-2′-deoxyguanosine (OdG) when in the anti and syn conformations, respectively. b) Active site of a binary MutT:OdGMP complex (PDB 3A6T).12
Due to its capacity to promote mutations, both the replication and repair of OdG have been extensively studied when it is within an oligonucleotide. Recently, there has been much additional interest in the free nucleotide, OdGTP, due to its ability to cause mutation13, 14 and the role it might play during antibiotic initiated bacterial cell death. Though controversial,15–18 it has been postulated that some bactericidal agents, like β-lactams and quinolones, initiate cell death through double-strand DNA breaks caused by incomplete repair of closely spaced OdG lesions. Through knockout and overexpression studies, it was shown that the incorporation of OdGTP plays a major role in this lethal pathway.16 Thus, OdGTP may be important not only to genetic mutation, but also to the potency of some antibiotics. For these reasons, studies into the ability of MutT to remove OdGTP from the nucleotide pool are of even greater interest.
MutT belongs to the Nudix hydrolase family, which have a 23 residue highly conserved signature sequence, GX5EX7REUXEEXGU, where U is a bulky hydrophobic residue and X is any amino acid.19, 20 Previous studies have shown that while OdGTP is a significantly better substrate for MutT than dGTP,10 other modified nucleotides, like 8-Bromo-dGTP (BrdGTP) can also have significant activity.21 Consistent with these results, crystal structure studies of a binary MutT complex containing OdGMP (Fig. 1B) indicated i) the presence of a hydrogen bond between Oδ of Asn119 and the N7-hydrogen of OdG, an interaction not possible with dGTP, and ii) that the 8-oxoguanine base is held in the syn conformation, which is preferred when large atoms, like oxygen and bromine, are present off C8.5 The structural studies also revealed interactions between the enzyme and the C6-oxygen and C2-exocyclic amine, 12 which are both standard to all dGTP based substrates.
Though the structural studies provide much insight into MutT substrate recognition, they are not able to discern the relative importance of each distinct interaction or property. In general, there has been surprisingly little research on MutT substrate flexibility and there has been no systematic study on the degree to which individual substrate properties contribute to MutT activity. Previously, we have used OdG analogues to establish structure-activity relationships of OdG with repair enzymes and various polymerases.22–24 In this study, nine OdGTP analogues were used to investigate the contribution of three sites to MutT activity. Those sites include i) C8, where a large atom can promote the syn N-glycosidic bond conformation5, 25 that is observed in the active site of the binary MutT:OdGMP structure, ii) N7, where the presence of a hydrogen can lead to a stabilizing interaction with Asn119, and iii) C2, where the exocyclic amine can form both a direct hydrogen bond with the main chain carbonyl of Phe35 and a water-mediated hydrogen bond with the main chain carbonyl of Gly37 (Fig. 1B).12
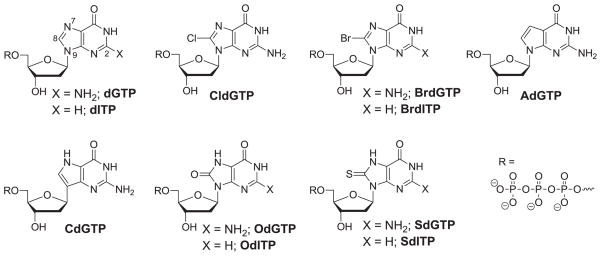
The nine analogues used in this study, illustrated in Figure 2, include i) 8-chloro-dGTP (CldGTP) and BrdGTP, which both mimic dGTP, but with the addition of a large atom off C8, ii) 7-deaza-dGTP (AdGTP), which contains a carbon at position 7, removing any hydrogen bonding ability at that site, iii) 9-deaza-dGTP (CdGTP) and 8-thio-dGTP (SdGTP), which both contain an N7-hydrogen, but vary from OdGTP at the C8 position, and iv) the 5′-triphosphate derivative of 2′-deoxyinosine (dITP), as well as 8-Bromo-dITP (BrdITP), 8-oxo-dITP (OdITP), and 8-thio-dITP (SdITP), which all lack the C2-exocyclic amine. By testing the activity of each of these analogues, it is possible to gain a more nuanced understanding of MutT substrate recognition at the three sites (C8, N7, and C2).
Figure 2.
5′-Triphosphate derivatives of dG, OdG, and nine of their analogues.
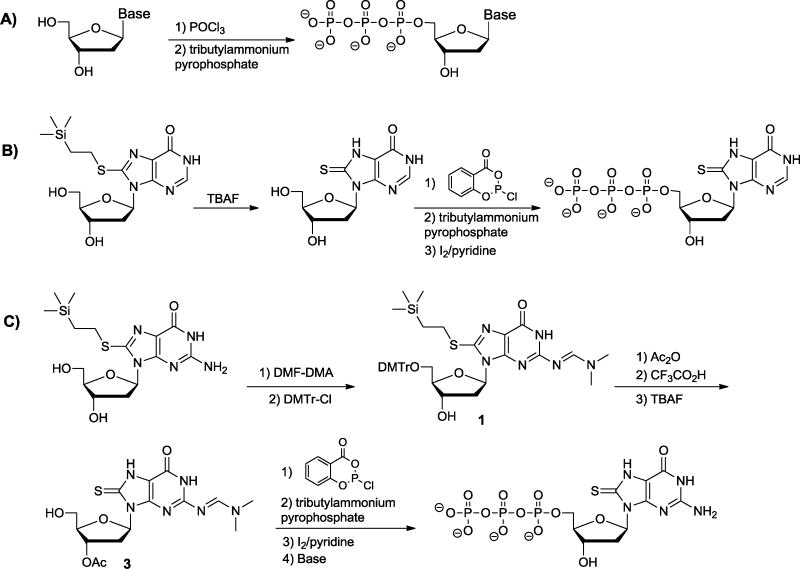
dGTP, BrdGTP, OdGTP, AdGTP, and dITP are all commercially available, OdITP has been reported previously,26 and the mononucleosides of three of the other analogues (CldGTP, CdGTP, BrdITP) have been reported previously by our lab or others.25, 27, 28 We used standard reaction and purification methods to convert the CldG, CdG, and BrdI monomers to their corresponding 5′-triphosphate derivatives (Fig. 3A). Though we have previously incorporated 8-thio-2′-deoxyinosine (SdI) into oligonucleotides using phosphoramidite chemistry, this study necessitated the mononucleoside, which we had not previously synthesized. Therefore, we first obtained SdI monomer from our previously characterized 8-thio protected SdI monomer23 through deprotection using tetrabutylammonium fluoride (TBAF); we then synthesized SdITP using salicyl chlorophosphite (2-chloro-1,3,2-benzodioxaphosphorin-4-one) and tributylammonium pyrophosphate (Fig. 3B). 29 Synthesis to SdGTP required protection of the 3′-hydroxyl and C2-exocyclic amine. As shown in Figure 3C, using the 8-thio protected SdG monomer,30 we protected the C2-exocylic amine, 5′-hydroxyl, and 3′-hydroxyl with dimethylformamide (DMF), dimethoxytrityl (DMTr), and acetyl (Ac) groups, respectively, using standard chemistry. The 5′-hydroxy and 8-thio protecting groups were then removed with trifluoroacetic acid and TBAF, respectively, before salicyl chlorophosphite and tributylammonium pyrophosphate were once again used to add the 5′-triphosphate.
Figure 3.
General methods for the syntheses of a) CldGTP, CdGTP, BrdITP, and OdITP, b) SdITP, and c) SdGTP. TBAF = tetrabutyl ammonium fluoride, DMF-DMA = N, N-dimethyl formamide dimethyl acetal, DMTr = dimethoxytrityl, Ac = acetyl.
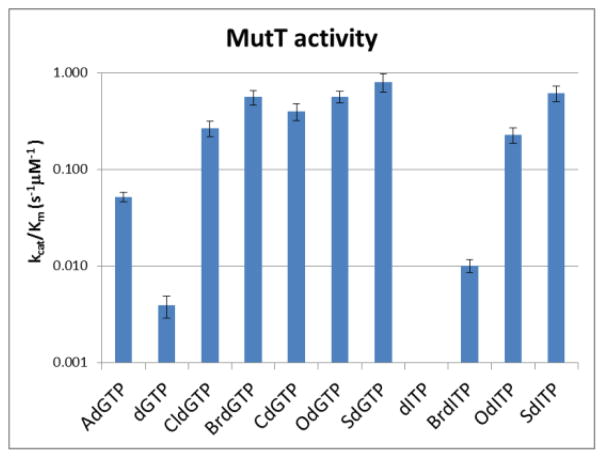
dGTP, OdGTP, and the nine analogues were tested for their overall activity with MutT using steady-state Michaelis-Menten conditions. Reverse phase HPLC was used to separate the triphosphate substrates from the monophosphate products. Similar to previous results with MutT, 31 reactions with OdGTP were roughly 150-fold more efficient than with dGTP (Fig. 4). All of the analogues had quantifiable activity except dITP, which showed little (< 5%) reaction even after incubation for up to an hour with ten times the normal enzyme concentration (data not shown). BrdITP and AdGTP were 2.6- and 13-fold more efficient substrates than dGTP, respectively, while the rest of the analogues had activities that were all within 0.5 to 1.5-fold of OdGTP. Interestingly, the six analogues with high activity varied in the size of the atom off C8, the presence of the N7-hydrogen, or the presence of the C2-exocyclic amine. Thus, while the crystal structure suggests an important role for each of these sites during substrate recognition, none of them are absolutely required for significant steady-state activity with MutT.
Figure 4.
Efficiencies of steady-state reactions with MutT. kcat/Km ± standard deviations were calculated from Michaelis-Menten plots using at least three independent experiments. The individual parameters for kcat and Km, as well as Michaelis-Menten curves for each reaction can be found in the Supporting Information.
Our data instead suggests that, at minimum, two of the three factors are required. For example, CldGTP and BrdGTP both contain a large atom off C8 and the C2-exocyclic amine, but no N7-hydrogen; CdGTP contains both the N7-hydrogen and the C2-exocyclic amine, but no large atom off C8; OdITP and SdITP contain both a large atom off C8 and the N7-hydrogen, but no C2-exocyclic amine. When only one of these three properties is present, as in dGTP (only the C2-exocyclic amine) or BrdITP (only a large atom off C8), a roughly 100-fold drop in activity is observed. If none of the three properties is present, as in dITP, then the reaction is so poor as to not allow for accurate quantification. Analyzing the data for AdGTP provides even greater insight into the N7 position. While the presence of the N7-hydrogen in OdGTP allows for a stabilizing hydrogen bond to Oδ of Asn119, the N7-lone pair of dGTP would have a repulsive interaction with the same atom. AdGTP contains neither a stabilizing N7-hydrogen nor a repulsive N7-lone pair (though it does have the C2-exocyclic amine) and has intermediate activity in our assays. Thus, it is possible Asn119 is used not only to promote hydrolysis of the damaged nucleotide OdGTP, but also discourage hydrolysis of the undamaged nucleotide dGTP. Additionally, AdGTP is a better steric mimic of OdGTP than dGTP at the N7 position, which may also contribute to its intermediate activity.
Interestingly, the OdG repair enzyme MutM appears to use a similar discriminatory system at N7. While also holding the 8-oxoguanine base in the syn-conformation, MutM uses the main chain carbonyl of Ser200 to form a stabilizing hydrogen bond to the N7-hydrogen of 8-oxoguanine when excising it from OdG:dC base pairs.32 That same carbonyl can also clash with the N7-lone pair in dG, rendering dG:dC base pairs inactive.33 Similar to the biochemical results with AdGTP reported herein, AdG:dC base pairs are only 10-fold less active with MutM than OdG:dC base pairs.22 Expanding to the other two sites, MutM, like MutT, is tolerant to the loss of the C2-exocyclic amine from OdG; previous experiments have shown that the 8-oxohypoxanthine base is removed from OdI:dC pairs with higher efficiency as compared to 8-oxoguanine removal from OdG:dC pairs.34 Finally, MutM appears to be more sensitive than MutT at the C8 position; dissimilar to the results herein, SdG:dC pairs were significantly less active with MutM than OdG:dC pairs.22
In conclusion, we have used nine analogues of dGTP and OdGTP to investigate the importance of the C8, N7, and C2 positions to the overall reaction efficiency of the MutT repair enzyme. We have found that no one site is critically important to activity, but rather it is the combination of at least two of the interactions and/or properties that affords significant activity. It may be useful to study the activities of the analogues with MutT using pre-steady state (single-turnover) kinetic experiments in which the individual steps of nucleotide hydrolysis can be analyzed. Detailed kinetic studies have shown that while the chemistry step is partially rate limiting with poor substrates, such as dGTP, in highly active substrates, such as OdGTP, product release and/or an enzyme conformational change are likely rate limiting.31
Supplementary Material
Acknowledgments
This work was supported by an NSF-RUI (CHE-1305548) to M.L.H., an NIH-AREA grant (GM066715-01) to S.F.O., the Beckman Foundation, the University of Richmond, and the Rochester Institute of Technology. M.L.H. is a Henry Dreyfus Teacher-Scholar. The authors thank J. Pollock for critical reading of the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kunkel T. Trends Genet. 1999;15:93. doi: 10.1016/s0168-9525(98)01664-3. [DOI] [PubMed] [Google Scholar]
- 2.Park EM, Shigenaga MK, Degan P, Korn TS, Kitzler JW, Wehr CM, Kolachana P, Ames BN. Proc Natl Acad Sci USA. 1992;89:3375. doi: 10.1073/pnas.89.8.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gannett P, Sura T. Chem Res Toxicol. 1993;6:690. doi: 10.1021/tx00035a015. [DOI] [PubMed] [Google Scholar]
- 4.Cho B, Kadlubar F, Culp S, Evans F. Chem Res Toxicol. 1990;3:445. doi: 10.1021/tx00017a010. [DOI] [PubMed] [Google Scholar]
- 5.Uesugi S, Ikehara M. J Am Chem Soc. 1977;99:3250. doi: 10.1021/ja00452a008. [DOI] [PubMed] [Google Scholar]
- 6.Michaels ML, Miller JH. J Bacteriol. 1992;174:6321. doi: 10.1128/jb.174.20.6321-6325.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tajiri T, Maki H, Sekiguchi Mut Res. 1995;336:257. doi: 10.1016/0921-8777(94)00062-b. [DOI] [PubMed] [Google Scholar]
- 8.Karakaya A, Jaruga P, Bohr V, Grollman A, Dizdaroglu M. Nucleic Acids Res. 1997;25:474. doi: 10.1093/nar/25.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu AL, Yuen DS, Cillo J. J Biol Chem. 1996;271:24138. doi: 10.1074/jbc.271.39.24138. [DOI] [PubMed] [Google Scholar]
- 10.Maki H, Sekiguchi M. Nature. 1992;355:273. doi: 10.1038/355273a0. [DOI] [PubMed] [Google Scholar]
- 11.Saraswat V, Massiah MA, Lopez G, Amzel LM, Mildvan AS. Biochemistry. 2002;41:15566. doi: 10.1021/bi020552p. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura T, Meshitsuka S, Kitagawa S, Abe N, Yamada J, Ishino T, Nakano H, Tsuzuki T, Doi T, Kobayashi Y, Fujii S, Sekiguchi M, Yamagata Y. J Biol Chem. 2010;285:444. doi: 10.1074/jbc.M109.066373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng KC, Cahill DS, Kasai H, Nishimura S, Loeb LA. J Biol Chem. 1992;267:166. [PubMed] [Google Scholar]
- 14.Katafuchi A, Nohmi T. Mutat Res. 2010;703:24. doi: 10.1016/j.mrgentox.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Dwyer DJ, Belenky PA, Yang JH, MacDonald IC, Martell JD, Takahashi N, Chan CTY, Lobritz MA, Braff D, Schwarz EG, Ye JD, Pati M, Vercruysse M, Ralifo PS, Allison KR, Khalil AS, Ting AY, Walker GC, Collins JJ. Proc Natl Acad Sci USA. 2014;111:E2100. doi: 10.1073/pnas.1401876111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foti JJ, Devadoss B, Winkler JA, Collins JJ, Walker GC. Science. 2012;336:315. doi: 10.1126/science.1219192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keren I, Wu YX, Inocencio J, Mulcahy LR, Lewis K. Science. 2013;339:1213. doi: 10.1126/science.1232688. [DOI] [PubMed] [Google Scholar]
- 18.Liu YY, Imlay JA. Science. 2013;339:1210. doi: 10.1126/science.1232751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLennan AG. Cell Mol Life Sci. 2006;63:123. doi: 10.1007/s00018-005-5386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bessman MJ, Frick DN, Ohandley SF. J Biol Chem. 1996;271:25059. doi: 10.1074/jbc.271.41.25059. [DOI] [PubMed] [Google Scholar]
- 21.Bhatnagar SK, Bullions LC, Bessman MJ. J Biol Chem. 1991;266:9050. [PubMed] [Google Scholar]
- 22.Hamm ML, Gill TJ, Nicolson S, Summers MR. J Am Chem Soc. 2007;129:7724. doi: 10.1021/ja0716453. [DOI] [PubMed] [Google Scholar]
- 23.Hamm ML, Crowley KA, Ghio M, Del Giorno L, Gustafson MA, Kindler KE, Ligon CW, Lindell MAM, McFadden EJ, Siekavizza-Robles C, Summers MR. Biochemistry. 2011;50:10713. doi: 10.1021/bi201383c. [DOI] [PubMed] [Google Scholar]
- 24.Hamm ML, Crowley KA, Ghio M, Lindell MAM, McFadden EJ, Silberg JSL, Weaver AM. Chem Res Toxicol. 2012;25:2577. doi: 10.1021/tx300365g. [DOI] [PubMed] [Google Scholar]
- 25.Hamm ML, Rajguru S, Downs AM, Cholera R. J Am Chem Soc. 2005;127:12220. doi: 10.1021/ja052578k. [DOI] [PubMed] [Google Scholar]
- 26.Kamiya H, Yakushiji H, Dugue L, Tanimoto M, Pochet S, Nakabeppu Y, Harashima H. J Mol Biol. 2004;336:843. doi: 10.1016/j.jmb.2003.12.060. [DOI] [PubMed] [Google Scholar]
- 27.Gibson ES, Lesiak K, Watanabe KA, Gudas LJ, Pankiewicz KW. Nucleosides Nucleotides. 1999;18:363. doi: 10.1080/15257779908043082. [DOI] [PubMed] [Google Scholar]
- 28.Russo M, Jimenez LB, Mulazzani QG, D’Angelantonio M, Guerra M, Miranda MA, Chatgilialoglu C. Chem Eur J. 2006;12:7684. doi: 10.1002/chem.200600040. [DOI] [PubMed] [Google Scholar]
- 29.Ludwig JEF. J Org Chem. 1989;54:631. [Google Scholar]
- 30.Hamm M, Cholera R, Hoey C, Gill T. Org Lett. 2004;6:3817. doi: 10.1021/ol0484097. [DOI] [PubMed] [Google Scholar]
- 31.Xia ZY, Azurmendi HF, Mildvan AS. Biochemistry. 2005;44:15334. doi: 10.1021/bi0513599. [DOI] [PubMed] [Google Scholar]
- 32.Fromme J, Verdine G. J Biol Chem. 2003;278:51543. doi: 10.1074/jbc.M307768200. [DOI] [PubMed] [Google Scholar]
- 33.Tchou J, Kasai H, Shibutani S, Chung MH, Laval J, Grollman A, Nishimura S. Proc Natl Acad Sci USA. 1991;88:4690. doi: 10.1073/pnas.88.11.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tchou J, Bodepudi V, Shibutani S, Antoshechkin I, Miller J, Grollman AP, Johnson F. J Biol Chem. 1994;269:15318. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.