Abstract
Objective
Although prior studies report a relationship between elevated lipoprotein-associated phospholipase A2 (Lp-PLA2) and incident cardiovascular disease, the prospective association of Lp-PLA2 with incident peripheral arterial disease (PAD) has not been studied. We investigated the association between Lp-PLA2 mass and activity and the risk of developing clinical PAD and low ankle-brachial index (ABI).
Approach and Results
Among Cardiovascular Health Study participants, a population-based cohort of 5888 adults aged 65 years or older enrolled in 1989–1990, Lp-PLA2 mass and activity were measured in 4537 individuals without baseline PAD. Clinical PAD, defined as leg artery revascularization or diagnosed claudication, was ascertained through 2011. Incident low ABI, defined as ABI <0.9 and decline of >=0.15, was assessed among 3537 individuals who had an ABI >0.9 at baseline and a second ABI measurement 3 or 6 years later. Analyses were adjusted for demographics, cholesterol, smoking, comorbidities, and C-reactive protein. Each standard deviation increment in Lp-PLA2 mass (117 ng/ml) was associated with a higher risk of developing clinical PAD (hazard ratio (HR) 1.28; 95% confidence interval (CI) 1.13, 1.45) and incident low ABI (odds ratio (OR) 1.16; 95% CI 1.00, 1.33). Results per standard deviation increment in Lp-PLA2 activity (13 nmol/min/ml) were similar for clinical PAD (HR 1.24; 95% CI 1.07, 1.44) and low ABI (OR 1.28; 95% CI 1.09, 1.50).
Conclusions
Higher Lp-PLA2 mass and activity were associated with development of both incident clinical PAD and low ABI. Future studies are needed to determine whether pharmacologic inhibition of Lp-PLA2 reduces the incidence of PAD.
Keywords: Inflammation, Epidemiology, Peripheral Artery Disease, Lipoprotein-associated Phospholipase A2
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is an enzyme synthesized primarily by inflammatory cells and is highly expressed by macrophages in atherosclerotic lesions, particularly within the necrotic core and fibrotic cap of rupture-prone plaques.1–5 Lp-PLA2 hydrolyzes oxidized phospholipids found on the LDL particle in plaques, resulting in proinflammatory and proatherogenic products.6, 7
The associations between elevated Lp-PLA2 concentration and both incident coronary heart disease and ischemic stroke are well established.8–13 The association of Lp-PLA2 with incident peripheral arterial disease (PAD) has not been evaluated, other than in cross-sectional studies.14–17
Over 20% of older men and women seen in primary care medical practices have a low ankle-brachial index (ABI) consistent with PAD.18 These individuals have higher mortality rates than persons without PAD.19 The need for increased research into the biology of atherosclerosis and thrombosis in PAD was recently highlighted in a report from the American Heart Association Vascular Disease Summit.20 Considering the prevalence of and mortality associated with PAD as well as the close association of Lp-PLA2 with incident atherosclerotic disease in other vascular territories, it is important to determine if high Lp-PLA2 levels are associated with the development of PAD as well. If observed, future studies could investigate if these individuals may benefit from more intensive measures to modify cardiovascular risk and, potentially, pharmacologic inhibition of Lp-PLA2.
The Cardiovascular Health Study offers a unique opportunity to examine the prospective relationship between Lp-PLA2 mass and activity levels and the development of PAD in an elderly population with long-term follow-up. We defined PAD using both clinical events and development of a low ABI. Since some but not all studies reported that Lp-PLA2 and C-reactive protein (CRP) were complementary in risk prediction for cardiovascular events, we also assessed for an interaction between markers of inflammation and Lp-PLA2.9, 11, 12, 21
Materials and Methods
Materials and Methods are available in the online-only Data Supplement
Results
There were 127 participants with clinical PAD and 715 participants low ABI at baseline. After adjusting for age, sex, race, clinic site, cigarette smoking, cardiovascular disease, diabetes, total cholesterol, hypertension, high-density lipoprotein-cholesterol, statin use, physical activity, CRP, and eGFR, Lp-PLA2 mass was associated with a low baseline ABI (OR: 1.20; 95% CI: 1.10, 1.30) but not clinical PAD (OR: 1.09; 95% CI: 0.91, 1.28). There were weak cross-sectional associations that were not statistically significant for Lp-PLA2 activity with low ABI (OR: 1.10; 95% CI: 0.99, 1.21) and with clinical PAD (OR: 1.13; 95% CI: 0.92, 1.37).
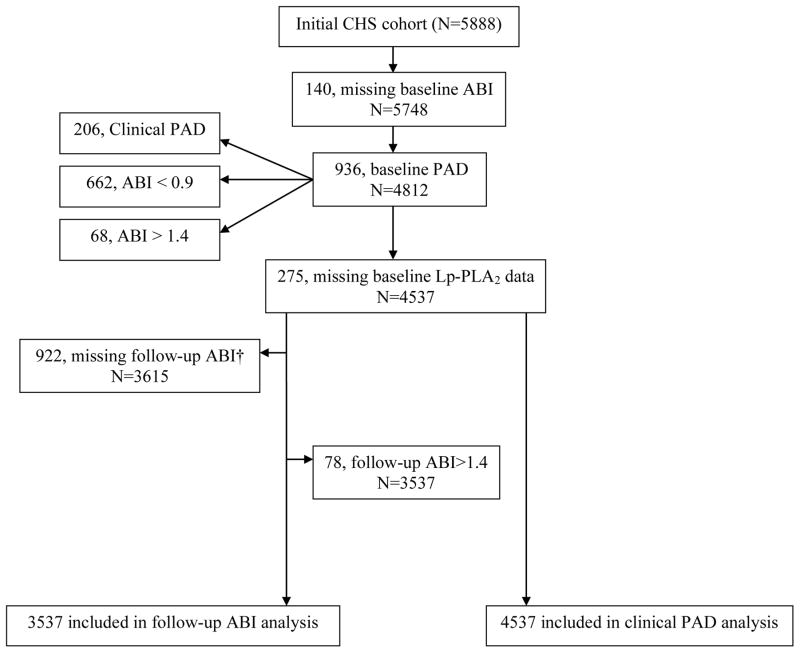
Figure 1 shows the flow of participants that were included in the prospective analysis for the clinical PAD and incident low ABI outcome. There were 205 incident cases of clinical PAD among the 4537 participants followed for the development of this outcome over a median duration of 14.2 years. In Table 1, compared to participants who did not develop clinical PAD, participants who developed clinical PAD were more likely to smoke, have diabetes, have hypertension, and have clinical cardiovascular disease at baseline. Triglyceride, CRP, and fibrinogen were higher while baseline ABI and high-density lipoprotein-cholesterol were lower in those who developed clinical PAD.
Figure 1.
Flowchart of participants included in the analysis for developing clinical PAD and incident low ABI*
*ABI=ankle-brachial index; PAD=peripheral arterial disease
† 261 deceased prior to 2nd ABI exam
Table 1.
Baseline characteristics of Cardiovascular Health Study participants according to presence of absence of incident clinical PAD*
| Characteristic | Cases (n=205) | Non-cases (n=4332) | p-value† |
|---|---|---|---|
| Age | 72.0 (4.6) | 72.2 (5.2) | 0.49 |
| Female, % | 114 (55.6) | 2580 (59.6) | 0.26 |
| Black, % | 33 (16.1) | 620 (14.3) | 0.48 |
| Smoking status, % | <0.001 | ||
| Never | 79 (38.5) | 2083 (48.1) | |
| Former | 86 (42.0) | 1807 (41.7) | |
| Current | 40 (19.5) | 442 (10.2) | |
| Pack-years if ever smoked | 40 (22–56) | 26 (11–47) | <0.001 |
| Diabetes, % | 53 (25.9) | 586 (13.5) | <0.001 |
| Hypertension, % | 133 (64.9) | 2372 (54.8) | 0.004 |
| Prevalent Cardiovascular Disease‡, % | 66 (32.2) | 853 (19.7) | <0.001 |
| ABI | 1.06 (1.01–1.13) | 1.10 (1.04–1.18) | <0.001 |
| Body mass index, kg/m2 | 27.3 (5.1) | 26.7 (4.7) | 0.11 |
| Physical activity, kcal/week | 1120 (406–2582) | 1131 (418–2430) | 0.85 |
| Total cholesterol, mg/dL | 214 (40) | 211 (38) | 0.20 |
| LDL cholesterol, mg/dL | 133 (37) | 129 (35) | 0.10 |
| HDL cholesterol, mg/dL | 51.2 (16.0) | 55.0 (15.6) | 0.001 |
| TG, mg/dL | 161 (92) | 137 (75) | <0.001 |
| eGFR from creatinine | 67.4 (18.6) | 69.9 (18.2) | 0.06 |
| Lp-PLA2 activity, nmol/min/ml | 42.5 (15.2) | 39.1 (12.9) | 0.002 |
| Lp-PLA2 mass, ng/ml | 372 (130) | 340 (116) | <0.001 |
| Interleukin-6, mg/dL | 1.8 (1.3–2.4) | 1.6 (1.1–2.5) | 0.08 |
| C-reactive protein, mg/L | 2.4 (1.3–4.7) | 1.8 (0.9–3.2) | <0.001 |
| Fibrinogen, mg/dL | 334 (76) | 319 (64) | 0.007 |
| Statin use, % | 3 (1.5) | 97 (2.2) | 0.55 |
Continuous variables are expressed as mean (SD) or median (interquartile range).
Categorical variables are N (percent).
Comparisons were made between cases and noncases.
Cardiovascular disease was defined as having one of the following at baseline: history of myocardial infarction, angina, stroke, transient ischemic attack, or coronary artery bypass surgery or angioplasty.
Table 2 compares participants with a follow-up ABI to those participants who were alive but without an ABI at the time of follow-up. Compared to participants with a follow-up ABI, participants without a follow-up ABI were older and more likely to be black, to smoke, have diabetes, have hypertension, and have clinical cardiovascular disease at baseline. Body mass index, CRP, interleukin-6 (IL-6) and fibrinogen were higher while baseline ABI and physical activity were lower in those without a follow-up ABI. Lp-PLA2 mass and activity levels did not differ between the two groups.
Table 2.
Baseline characteristics of Cardiovascular Health Study participants alive at follow-up with and without an ABI measure.
| Characteristic | ABI at follow-up (n=3615) | ABI at follow-up missing (n=661) | p-value† |
|---|---|---|---|
| Age | 71.8 (4.9) | 73.3 (5.7) | <0.001 |
| Female, % | 2163 (59.8) | 418 (63.2) | 0.10 |
| Black, % | 424 (11.7) | 155 (23.5) | <0.001 |
| Smoking status, % | 0.002 | ||
| Never | 1722 (47.6) | 324 (49.0) | |
| Former | 1533 (42.4) | 245 (37.1) | |
| Current | 360 (10.0) | 92 (13.9) | |
| Pack-years if ever smoked | 26 (11–47) | 26 (9–46) | .70 |
| Diabetes, % | 445 (12.3) | 122 (18.5) | <0.001 |
| Hypertension, % | 1939 (53.6) | 408 (61.7) | <0.001 |
| Prevalent Cardiovascular Disease‡, % | 660 (18.3) | 159 (24.1) | <0.001 |
| ABI | 1.11 (1.04–1.18) | 1.09 (1.03–1.16) | <0.001 |
| Body mass index, kg/m2 | 26.6 (4.5) | 27.7 (5.5) | <0.001 |
| Physical activity, kcal/week | 1226 (478–2582) | 853 (267–1762) | <0.001 |
| Total cholesterol, mg/dL | 211 (38) | 211 (41) | 0.97 |
| LDL cholesterol, mg/dL | 130 (34) | 129 (38) | 0.89 |
| HDL cholesterol, mg/dL | 55.0 (15.5) | 54.4 (15.5) | 0.37 |
| TG, mg/dL | 137 (71) | 142 (88) | 0.17 |
| eGFR from creatinine | 70.0 (17.6) | 69.8 (19.3) | 0.77 |
| Lp-PLA2 activity, nmol/min/ml | 39.3 (12.9) | 38.9 (13.3) | 0.49 |
| Lp-PLA2 mass, ng/ml | 341 (116) | 339 (118) | 0.73 |
| Interleukin-6, mg/dL | 1.5 (1.1–2.3) | 1.7 (1.2–2.6) | <0.001 |
| C-reactive protein, mg/L | 1.7 (0.88–3.0) | 2.1 (1.1–4.4) | <0.001 |
| Fibrinogen, mg/dL | 316 (62) | 333 (70) | <0.001 |
| Statin use, % | 82 (2.3) | 15 (2.3) | 0.99 |
Continuous variables are expressed as mean (SD) or median (interquartile range).
Categorical variables are N (percent).
Comparisons were made between participants with ABI at follow-up vs. participants missing ABI at follow-up.
Cardiovascular disease was defined as having one of the following at baseline: history of myocardial infarction, angina, stroke, transient ischemic attack, or coronary artery bypass surgery or angioplasty.
Associations for Lp-PLA2 mass and Lp-PLA2 activity with incident clinical PAD
The additive model plots supported the use of linear models for the association of Lp-PLA2 mass and activity with incident PAD. Table 3 shows the hazard ratios for developing clinical PAD for both Lp-PLA2 mass and Lp-PLA2 activity. Adjusting for the same variables as those reported in the cross-sectional analysis above, each higher standard deviation of Lp-PLA2 mass (117 ng/ml) increased risk of PAD by 28% (p<0.001) and each higher standard deviation of Lp-PLA2 activity (13 nmol/min/ml) increased risk of PAD by 24% (p<0.001). Results were similar after additional adjustment for baseline ABI, with hazard ratios of 1.27 (95% CI: 1.11, 1.44) and 1.23 (95% CI: 1.07, 1.43) per SD increment of Lp-PLA2 mass and activity respectively. Results were also similar after adjustment for inflammatory markers instead of CRP; the hazard ratios per SD increment of Lp-PLA2 mass were 1.29 (95% CI: 1.14, 1.47) and 1.30 (95% CI: 1.14, 1.48) for the addition of fibrinogen and IL-6 respectively. The hazard ratios per SD increment of Lp-PLA2 activity were 1.26 (95% CI: 1.09, 1.46) and 1.26 (95% CI: 1.09, 1.46) for the addition of fibrinogen and IL-6 respectively.
Table 3.
Associations between Lp-PLA2 and incident clinical peripheral arterial disease*
| Incident Cases of PAD | Incidence rate† (95% CI) | Model 1‡ HR (95% CI) |
Model 2 § HR (95% CI) |
|
|---|---|---|---|---|
| Lp-PLA2 Mass, ng/ml (n=4537) | ||||
| 1st quartile (57.5–255.1) | 35 | 2.10 (1.51, 2.93) | 1.00 (Referent) | 1.00 (Referent) |
| 2nd quartile (255.1–325.2) | 46 | 2.92 (2.19, 3.90) | 1.45 (0.93, 2.25) | 1.47 (0.94, 2.29) |
| 3rd quartile (325.3–402.3) | 55 | 3.52 (2.70, 4.58) | 1.73 (1.13, 2.66) | 1.74 (1.12, 2.70) |
| 4th quartile (402.3–944.3) | 69 | 4.73 (3.73, 5.99) | 2.36 (1.56, 3.59) | 2.15 (1.39, 3.30) |
|
| ||||
| Per SD (117.4) | 1.34 (1.18, 1.51) | 1.28 (1.13, 1.45) | ||
| p-value(linear) | <0.001 | <0.001 | ||
|
| ||||
| Lp-PLA2 Activity, nmol/min/ml (n=4537) | ||||
| 1st quartile (8.6–30.2) | 39 | 2.44 (1.78, 3.34) | 1.00 (Referent) | 1.00 (Referent) |
| 2nd quartile (30.2–37.4) | 35 | 2.17 (1.56, 3.02) | 0.90 (0.57, 1.43) | 0.92 (0.57, 1.47) |
| 3rd quartile (37.4–46.3) | 72 | 4.68 (3.71, 5.89) | 1.95 (1.31, 2.91) | 1.80 (1.17, 2.77) |
| 4th quartile (46.3–146.7) | 59 | 3.91 (3.03, 5.05) | 1.64 (1.07, 2.51) | 1.36 (0.84, 2.21) |
|
| ||||
| Per SD (13.0) | 1.28 (1.14, 1.45) | 1.24 (1.07, 1.44) | ||
| p-value (linear) | <0.001 | 0.004 | ||
Results of multivariable proportional hazards regression models are shown for both Lp-PLA2 mass and activity by quartiles of the distribution, with each Lp-PLA2 quartile compared with the 1st quartile (referent quartile), and per standard deviation increment
Rates are per 1000 person years
Model 1 adjusted for age (years), male sex, race, clinic site
Model 2 adjusted for Model 1 + cigarette smoking, cardiovascular disease, diabetes, total cholesterol, hypertension, HDL-c, statin use, physical activity, CRP, and eGFR
Table 3 also shows incidence rates and adjusted hazard ratios for developing clinical PAD according to quartiles of both Lp-PLA2 mass and Lp-PLA2 activity. Compared to the 1st quartile of Lp-PLA2 mass, participants in the 3rd and 4th quartile had a 74% increased risk of developing clinical PAD (95% CI: 1.12, 2.70) and participants in the 4th quartile had a 2.15-fold increased risk of developing clinical PAD (95% CI: 1.39, 3.30). Compared to the 1st quartile of Lp-PLA2 activity, only participants in the 3rd quartile had a significantly increased risk of developing clinical PAD (HR 1.80; 95% CI: 1.17, 2.77).
Associations for Lp-PLA2 mass and Lp-PLA2 activity with incident low ABI
There were 212 incident cases of a low ABI among the 3537 participants followed for this outcome. Only 30 of these 212 cases also had incident clinical PAD. Table 4 shows the adjusted odds ratios (Model 2) for incident low ABI for both Lp-PLA2 mass and Lp-PLA2 activity. Each higher standard deviation of Lp-PLA2 mass and Lp-PLA2 activity were associated with a 16% (p=0.046) and 28% (p=0.002) higher risk of incident low ABI respectively. Results were similar after additional adjustment for baseline ABI. The odds ratios were 1.14 (95% CI: 0.99, 1.32) and 1.25 (95% CI: 1.06, 1.46) per SD increment of Lp-PLA2 mass and activity respectively. Results from the inverse probability weighted analysis were slightly attenuated for both Lp-PLA2 mass (OR 1.15; 95% CI: 0.99, 1.33) and Lp-PLA2 activity (OR 1.16; 95% CI: 0.98, 1.33). No significant interquartile associations existed for developing a low ABI in the quartile-specific analyses for either Lp-PLA2 mass and Lp-PLA2 activity in adjusted analyses.
Table 4.
Associations between Lp-PLA2 and incident low ankle-brachial index*
| Incident Cases of Low ABI | Model 1† | Model 2‡ | |
|---|---|---|---|
|
| |||
| OR (95% CI) | OR (95% CI) | ||
| Lp-PLA2 Mass, ng/ml (n=3537) | |||
| 1st quartile (57.5–255.1) | 51 | 1.0 (Referent) | 1.0 (Referent) |
| 2nd quartile (255.1–325.2) | 41 | 0.94 (0.61, 1.46) | 0.91 (0.58, 1.44) |
| 3rd quartile (325.3–402.3) | 53 | 1.42 (0.93, 2.17) | 1.34 (0.86, 2.08) |
| 4th quartile (402.3–944.3) | 67 | 1.69 (1.12, 2.56) | 1.49 (0.96, 2.30) |
|
| |||
| Per SD (117.4) | 1.22 (1.07, 1.40) | 1.16 (1.00, 1.33) | |
| p-value (linear) | .004 | .046 | |
|
| |||
| Lp-PLA2 Activity, nmol/min/ml (n=3537) | |||
| 1st quartile (8.6–30.2) | 57 | 1.0 (Referent) | 1.0 (Referent) |
| 2nd quartile (30.2–37.4) | 37 | 0.75 (0.48, 1.17) | 0.67 (0.42, 1.07) |
| 3rd quartile (37.4–46.3) | 49 | 1.15 (0.76, 1.76) | 0.96 (0.61, 1.53) |
| 4th quartile (46.3–146.7) | 69 | 1.79 (1.18, 2.71) | 1.45 (0.89, 2.35) |
|
| |||
| Per SD (13.0) | 1.32 (1.16, 1.52) | 1.28 (1.09, 1.50) | |
| p-value (linear) | <.001 | .002 | |
Results of multivariable logistic regression models are shown for both Lp-PLA2 mass and activity by quartiles of the distribution, with each Lp-PLA2 quartile compared with the 1st quartile (referent quartile), and per standard deviation increment
Model 1 adjusted for age (years), male sex, black, clinic site and time between ABI measures
Model 2 adjusted for Model 1 + cigarette smoking, cardiovascular disease, diabetes, total cholesterol, HDL-c, hypertension, statin use, physical activity, CRP, and eGFR
Lp-PLA2 mass and Lp-PLA2 activity levels were lower in African American participants compared to the rest of the study population (285.8 ng/ml vs. 350.6 ng/ml, p<0.01 and 32.2 nmol/min/ml vs. 40.4 nmol/min/ml, p<0.01 for Lp-PLA2 mass and Lp-PLA2 activity respectively). A significant p-value for interaction between Lp-PLA2 mass and African American race for the outcome of incident PAD (p=0.03) was noted in adjusted analysis and the association for Lp-PLA2 mass per standard deviation increment with incident PAD was not significant for this subgroup (HR 0.81; 95% CI: 0.53, 1.26). This finding, however, was not replicated for Lp-PLA2 activity with incident clinical PAD or for either Lp-PLA2 measure with incident low ABI.
Interaction between Lp-PLA2 mass and Lp-PLA2 activity with other Inflammatory Markers
Lp-PLA2 mass and activity correlated moderately with each other (r=0.54, p<0.001); however, there were no substantial correlations between either Lp-PLA2 mass or activity with CRP, fibrinogen, or IL-6 (correlation coefficients all <0.07). There were no significant additive or multiplicative interactions of Lp-PLA2 mass or activity with inflammatory markers (see supplemental tables I & II).
Discussion
In a cohort of community-dwelling older adults, higher baseline Lp-PLA2 mass and activity were associated with an increased risk of future clinical PAD and low ABI after adjustment for traditional risk factors and other markers of inflammation. The inflammatory markers CRP, fibrinogen, or IL-6 in combination with Lp-PLA2 mass did not modify the effect of Lp-PLA2 alone in predicting incident PAD. The interquartile associations for Lp-PLA2 mass and activity with both incident clinical PAD and low ABI were not as uniformly significant compared to associations of the continuous variables for the biomarkers, perhaps due to loss of power by categorization of the data. Further, we observed a much wider range of values for 4th quartiles of both Lp-PLA2 mass and activity compared to the 2nd and 3rd quartiles. Consequently, by reducing a continuous measure to 4 groups, this quantification did not adequately capture risk of PAD associated with the highest values when included in such a broadly defined interval.
To our knowledge, no previous studies reported associations of either Lp-PLA2 mass or Lp-PLA2 activity with incident PAD. The relationships of Lp-PLA2 biomarkers with coronary heart disease and ischemic stroke have been established; however, PAD is a distinct form of atherosclerosis. In the Reduction of Atherothrombosis for Continued Health registry, 40% of people with PAD had no concomitant coronary or cerebrovascular disease.22 Although traditional cardiovascular risk factors are common to all types of atherosclerotic disease, significant differences in the strength of these associations have been demonstrated depending on the form of disease. For example, cigarette smoking and diabetes have particularly strong associations with the development of PAD, carrying an over 3-fold increased risk each, compared to hypertension and dyslipidemia, which have more modest effects.23 Cigarette smoking is 2 to 3 times more likely to cause PAD compared to coronary artery disease.24 Considering these factors, the relationship of novel risk factors with PAD may differ from their relationships to other cardiovascular diseases. Prior evaluation into the role of inflammation biomarkers in risk of PAD has found a consistently increased risk associated with certain biomarkers such as CRP and fibrinogen but not others, namely IL-6 or adhesion molecules.25–27 Thus, assessing the relationship between Lp-PLA2 and incident PAD is important.
The cross-sectional associations of Lp-PLA2 with PAD observed here were consistent with existing literature, which only includes three cross-sectional studies that reported no or weak associations. One cross-sectional study of 247 patients found that Lp-PLA2 mass had a borderline-significant association with an ABI<0.9 (p=0.05) after adjustment for conventional risk factors, CRP and statin use.15 In the Rotterdam study those in the highest tertile of Lp-PLA2 activity had significantly higher odds of having an ABI<0.9 when compared to participants in the lowest tertile, however, this was not independent of cholesterol level.17 The Framingham Offspring Study reported no association between Lp-PLA2 mass or Lp-PLA2 activity with prevalent PAD.16
Our prospective findings, therefore, differ considerably from cross-sectional associations of Lp-PLA2 and PAD. It may be that markers of inflammation identify and correlate better with unstable, rupture-prone plaques rather than simply reflect atherosclerotic disease burden. Although CRP is associated with incident PAD, it is not strongly correlated with extent of noncoronary atherosclerotic disease as measured by carotid artery duplex or ABI.15, 28, 29 Prior work in the Cardiovascular Health Study found that CRP was higher in participants with ultrasound characteristics of vulnerable plaque in the carotid, and there was an interaction of CRP and presence of carotid atherosclerosis in predicting cardiovascular events.30 Similar results have been demonstrated with associations of Lp-PLA2 and coronary artery disease. In a study of over 500 patients undergoing coronary angiography, higher Lp-PLA2 mass did not independently correlate with extent of angiographic disease but was associated with a higher incidence of cardiovascular events in the same study.31
Our results on PAD are consistent with prospective data on Lp-PLA2 concentration and incident disease in other atherosclerotic beds. A recent meta-analysis reported that the risk ratios for Lp-PLA2 and incident cardiovascular disease were significantly elevated whether mass or activity was measured.8 Lp-PLA2 mass and Lp-PLA2 activity were only moderately correlated with each other in prior studies and our results were consistent with these.32–34 Future studies will be needed to confirm whether use of either measure have similar discriminatory ability in identifying increased risk of incident PAD.
Our study has limitations. The Cardiovascular Health Study participants were all aged 65 years and older at baseline and results may not be generalizable to younger aged cohorts. PAD progression may have been inadequately assessed, particularly in diabetics, due to exclusion of participants with an ABI >1.4 at baseline. While follow up for incident clinical PAD was complete, the significant missing data for the follow-up ABI may have introduced bias. Participants alive at the time of follow-up but without a follow-up ABI were more likely to have comorbidities compared to those with follow-up data. Reassuringly, baseline Lp-PLA2 mass and activity levels did not significantly differ by whether follow-up ABI was measured, and results were only mildly attenuated after accounting for the missing data. Moreover, our results on low ABI were strikingly consonant with those for clinical PAD, in which outcomes were available on nearly all participants. Finally, ABI measurements did not include a post-exercise value and, therefore, may not have detected PAD in a small number of individuals.
In conclusion, these results demonstrate that, in an elderly population, higher Lp-PLA2 mass and activity were significantly associated with an increased risk for incident clinical PAD and development of a low ABI. Prospective studies of associations between Lp-PLA2 and PAD appear to be superior to cross-sectional ones but further studies are needed in other observational cohorts to corroborate these findings. Although recent large scale randomized trials have found no significant reduction in cardiovascular events with pharmacologic Lp-PLA2 inhibition, PAD is a distinct form of atherosclerosis and it may be important to determine, if our results are confirmed, whether individuals at higher risk for incident PAD based on Lp-PLA2 levels may benefit from pharmacologic inhibition of Lp-PLA2 or more intensive cardiovascular risk modification.35, 36
Supplementary Material
Significance.
Peripheral artery disease (PAD) is an increasingly prevalent disease associated with higher mortality rates compared to individuals without PAD. While the associations between elevated Lp-PLA2 concentration and both incident coronary heart disease and ischemic stroke are well established, we report here, for the first time, an independent association between both Lp-PLA2 activity and mass with incident PAD, using either clinical endpoints or the ankle-brachial index. Our results suggest that elevated Lp-PLA2 levels may help identify individuals at increased risk for the development of PAD. Future study is needed to determine whether these individuals deemed to be at higher risk for incident PAD based on Lp-PLA2 levels may benefit from pharmacologic inhibition of Lp-PLA2 or more intensive cardiovascular risk modification.
Acknowledgments
Sources of Funding: This research was supported by NHLBI contracts N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133 and NHLBI grants HL080295 and K12-HL083790, with additional contribution from the NINDS. Other support was provided by an investigator-initiated grant from GlaxoSmithKline (GSK). A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.
Footnotes
Disclosures: Dr. Koro is an employee at GSK. Dr. Cushman and Dr. Jenny have had funding from diaDexus. The funding sources did not have a role in the design, analysis or approval of the manuscript.
References
- 1.Stafforini DM, Elstad MR, McIntyre TM, Zimmerman GA, Prescott SM. Human macrophages secret platelet-activating factor acetylhydrolase. J Biol Chem. 1990;265:9682–9687. [PubMed] [Google Scholar]
- 2.Asano K, Okamoto S, Fukunaga K, Shiomi T, Mori T, Iwata M, Ikeda Y, Yamaguchi K. Cellular source(s) of platelet-activating-factor acetylhydrolase activity in plasma. Biochem Biophys Res Commun. 1999;261:511–514. doi: 10.1006/bbrc.1999.1066. [DOI] [PubMed] [Google Scholar]
- 3.Tarbet EB, Stafforini DM, Elstad MR, Zimmerman GA, McIntyre TM, Prescott SM. Liver cells secrete the plasma form of platelet-activating factor acetylhydrolase. J Biol Chem. 1991;266:16667–16673. [PubMed] [Google Scholar]
- 4.Kolodgie FD, Burke AP, Skorija KS, Ladich E, Kutys R, Makuria AT, Virmani R. Lipoprotein-associated phospholipase A2 protein expression in the natural progression of human coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:2523–2529. doi: 10.1161/01.ATV.0000244681.72738.bc. [DOI] [PubMed] [Google Scholar]
- 5.Hakkinen T, Luoma JS, Hiltunen MO, Macphee CH, Milliner KJ, Patel L, Rice SQ, Tew DG, Karkola K, Yla-Herttuala S. Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase, is expressed by macrophages in human and rabbit atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 1999;19:2909–2917. doi: 10.1161/01.atv.19.12.2909. [DOI] [PubMed] [Google Scholar]
- 6.Macphee C, Benson GM, Shi Y, Zalewski A. Lipoprotein-associated phospholipase A2: A novel marker of cardiovascular risk and potential therapeutic target. Expert Opin Investig Drugs. 2005;14:671–679. doi: 10.1517/13543784.14.6.671. [DOI] [PubMed] [Google Scholar]
- 7.MacPhee CH, Moores KE, Boyd HF, Dhanak D, Ife RJ, Leach CA, Leake DS, Milliner KJ, Patterson RA, Suckling KE, Tew DG, Hickey DM. Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase, generates two bioactive products during the oxidation of low-density lipoprotein: Use of a novel inhibitor. Biochem J. 1999;338:479–487. [PMC free article] [PubMed] [Google Scholar]
- 8.The Lp-PLA2 Studies Collaboration. Lipoprotein-associated phospholipase A2 and risk of coronary disease, stroke, and mortality: Collaborative analysis of 32 prospective studies. Lancet. 2010;375:1536–1544. doi: 10.1016/S0140-6736(10)60319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Heiss G, Sharrett AR. Lipoprotein-Associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2004;109:837–842. doi: 10.1161/01.CIR.0000116763.91992.F1. [DOI] [PubMed] [Google Scholar]
- 10.Packard CJ, O’Reilly DS, Caslake MJ, McMahon AD, Ford I, Cooney J, Macphee CH, Suckling KE, Krishna M, Wilkinson FE, Rumley A, Lowe GD. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease: West of Scotland Coronary Prevention Study Group. N Engl J Med. 2000;343:1148–1155. doi: 10.1056/NEJM200010193431603. [DOI] [PubMed] [Google Scholar]
- 11.Koenig W, Khuseyinova N, Lowel H, Trischler G, Meisinger C. Lipoprotein-associated phospholipase A2 adds to risk prediction of incident coronary events by C-reactive protein in apparently healthy middle-aged men from the general population: results from the 14-year follow-up of a large cohort from southern germany. Circulation. 2004;110:1903–1908. doi: 10.1161/01.CIR.0000143377.53389.C8. [DOI] [PubMed] [Google Scholar]
- 12.Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Chambless LE, Myerson M, Wu KK, Sharrett AR, Boerwinkle E. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident ischemic stroke in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Arch Intern Med. 2005;165:2479–2484. doi: 10.1001/archinte.165.21.2479. [DOI] [PubMed] [Google Scholar]
- 13.Jenny NS, Solomon C, Cushman M, Tracy RP, Nelson JJ, Psaty BM, Furberg CD. Lipoprotein-associated phospholipase A2 and risk of cardiovascular disease in older adults: results from the Cardiovascular Health Study. Atherosclerosis. 2010;209:528–32. doi: 10.1016/j.atherosclerosis.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allison MA, Denenberg JO, Nelson JJ, Natarajan L, Criqui MH. The association between lipoprotein-associated phospholipase A2 and cardiovascular disease and total mortality in vascular medicine patients. J Vasc Surg. 2007;46:500–506. doi: 10.1016/j.jvs.2007.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santos S, Rooke TW, Bailey KR, McConnell JP, Kullo IJ. Relation of markers of inflammation (C-reactive protein, white blood cell count, and lipoprotein-associated phospholipase A2) to the ankle-brachial index. Vasc Med. 2004;9:171–176. doi: 10.1191/1358863x04vm543oa. [DOI] [PubMed] [Google Scholar]
- 16.Murabito JM, Keyes MJ, Guo CY, Keaney JF, Jr, Vasan RS, D’Agostino RB, Sr, Benjamin EJ. Cross-sectional relations of multiple inflammatory biomarkers to peripheral arterial disease: The Framingham Offspring Study. Atherosclerosis. 2009;203:509–514. doi: 10.1016/j.atherosclerosis.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kardys I, Oei HH, van der Meer IM, Hofman A, Breteler MM, Witteman JC. Lipoprotein-associated phospholipase A2 and measures of extracoronary atherosclerosis: The Rotterdam Study. Arterioscler Thromb Vasc Biol. 2006;26:631–636. doi: 10.1161/01.ATV.0000201289.83256.cf. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 19.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 20.Creager MA, Beckman JA, Conte MS. A Vascular Disease Thought Leaders Summit Report 2015: Improving Vascular Disease Prevention, Detection, and Treatement. [Accessed December 5, 2015];Scientificsessionsorg. 2015 Available at: http://scientificsessions.org/VascularDiseaseReport.
- 21.Blake GJ, Dada N, Fox JC, Manson JE, Ridker PM. A prospective evaluation of lipoprotein-associated phospholipase A2 levels and the risk of future cardiovascular events in women. J Am Coll Cardiol. 2001;38:1302–1306. doi: 10.1016/s0735-1097(01)01554-6. [DOI] [PubMed] [Google Scholar]
- 22.Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, Goto S, Liau CS, Richard AJ, Rother J, Wilson PW REACH Registry Investigators. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–189. doi: 10.1001/jama.295.2.180. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch AT, Haskel ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the Management of Patients With Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic) Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 24.Price JF, Mowbray PI, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Relationship between smoking and cardiovascular risk factors in the development of peripheral arterial disease and coronary artery disease: Edinburgh Artery Study. Eur Heart J. 1999;20:344–353. doi: 10.1053/euhj.1998.1194. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97:425–428. doi: 10.1161/01.cir.97.5.425. [DOI] [PubMed] [Google Scholar]
- 26.Pradhan AD, Rifai N, Ridker PM. Soluble intercellular adhesion molecule-1, soluble vascular adhesion molecule-1, and the development of symptomatic peripheral arterial disease in men. Circulation. 2002;106:820–825. doi: 10.1161/01.cir.0000025636.03561.ee. [DOI] [PubMed] [Google Scholar]
- 27.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Inflammatory, haemostatic, and rheological markers for incident peripheral arterial disease: Edinburgh Artery Study. Eur Heart J. 2007;28:354–362. doi: 10.1093/eurheartj/ehl441. [DOI] [PubMed] [Google Scholar]
- 28.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of c-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol as predictors of peripheral arterial disease. JAMA. 2001;285:2481–5. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- 29.Folsom AR, Pankow JS, Tracy RP, Arnett DK, Peacock JM, Hong Y, Djousse L, Eckfeldt JH. Association of c-reactive protein with markers of prevalent of atheorsclerotic disease. Am J Cardiol. 2001;88:112–7. doi: 10.1016/s0002-9149(01)01603-4. [DOI] [PubMed] [Google Scholar]
- 30.Cao JJ, Arnold AM, Manolio TA, Polak JF, Psaty BM, Hirsch CH, Kuller LH, Cushman M. Association of carotid artery intima-media thickness, plaques, and c-reactive protein with future cardiovascular disease and all-cause mortality: The Cardiovascular Health Study. Circulation. 2007;116:32–8. doi: 10.1161/CIRCULATIONAHA.106.645606. [DOI] [PubMed] [Google Scholar]
- 31.Brilakis ES, McConnll JP, Lennon RJ, Elesber AA, Meyer JG, Berger P. Association of lipoprotein-associated phospholipase A2 levels with coronary artery disease risk factors, angiographic coronary artery disease, and major adverse events at follow-up. Eur Heart J. 2005;26:137–44. doi: 10.1093/eurheartj/ehi010. [DOI] [PubMed] [Google Scholar]
- 32.Persson M, Hedblad B, Nelson JJ, Berglund G. Elevated Lp-PLA2 levels add prognostic information to the metabolic syndrome on incidence of cardiovascular events among middle-aged nondiabetic subjects. Arterioscler Thromb Vasc Biol. 2007;27:1411–1416. doi: 10.1161/ATVBAHA.107.142679. [DOI] [PubMed] [Google Scholar]
- 33.Iribarren C, Gross MD, Darbinian JA, Jacobs DR, Sidney S, Loria CM. Association of lipoprotein-associated phospholipase A2 mass and activity with calcified coronary plaque in young adults. Arterioscler Thromb Vasc Biol. 2005;25:216–21. doi: 10.1161/01.ATV.0000148322.89911.44. [DOI] [PubMed] [Google Scholar]
- 34.Caslake MJ, Packard CJ, Suckling KE, Holmes SD, Chamberlain P, Macphee CH. Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase: a potential new risk factor for coronary artery disease. Atherosclerosis. 2000;150:413–9. doi: 10.1016/s0021-9150(99)00406-2. [DOI] [PubMed] [Google Scholar]
- 35.STABILITY Investigators. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med. 2014;370:1702–11. doi: 10.1056/NEJMoa1315878. [DOI] [PubMed] [Google Scholar]
- 36.O’Donoghue ML, Braunwald E, White HD, et al. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. JAMA. 2014;312:1006–15. doi: 10.1001/jama.2014.11061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.