Abstract
Objective
Symptomatic knee osteoarthritis (OA) is a condition commonly associated with increased pain, disability, and functional limitations. Given the poor correspondence between radiographic evidence and clinical pain, central sensitization has been implicated as a potential mechanism underlying pain facilitation in knee OA. Sex may be a moderator of centrally-mediated changes in knee OA pain; however, few studies have systematically assessed this. Therefore, the aim of this study was to examine differences in peripheral and central sensitization in men and women with symptomatic knee OA, as well as to determine whether these differences vary across age (middle-age vs. older-age).
Methods
Participants (N=288) between the ages of 45 and 85 completed a battery of quantitative sensory pain procedures assessing sensitivity to contact heat, cold pressor, mechanical pressure, and punctate stimuli. Differences in temporal summation (TS) were examined, as well as measures of clinical pain and functional performance.
Results
When compared to men, women exhibited greater sensitivity to multiple pain modalities (i.e., lower heat, cold, pressure thresholds/tolerances, greater TS of pain); however, there were no sex differences in clinical pain with the exception of greater widespread pain observed in women. Although there were select age-related differences in pain sensitivity, sex differences in pain varied minimally across age cohort.
Conclusion
Overall, these findings provide evidence for greater overall sensitivity to experimental pain in women with symptomatic knee osteoarthritis (OA), compared to men, suggesting that enhanced central sensitivity may be an important contributor to pain in this group.
Keywords: knee osteoarthritis, central sensitization, sex differences, experimental pain sensitivity, clinical pain
Osteoarthritis (OA) is the most common form of arthritis, with characteristic symptoms of joint pain, stiffness, and decreased functional mobility. OA drives significant healthcare utilization, psychosocial and physical impairment, and disability (1, 2). Age represents a significant risk factor for increased incidence and prevalence of OA (3), as older adults often present with greater pain complaints, more persistent pain, and greater pain-related disability than younger adults (4–6). Although OA can affect multiple joints in the body, the knee is one of the most commonly affected sites (7), with approximately 45% of older adults in the United States population estimated to be at risk for developing knee OA (8, 9).
Traditionally, knee OA has been characterized as a condition of peripheral pathology. However, given that radiological findings of joint damage do not impressively correlate with the degree of clinical symptoms, other centrally-mediated factors have been suggested to influence its pathology (10–12). While localized pain and hyperalgesia at the affected joint suggest a strong peripheral component to knee OA, more widespread pain and hyperalgesia indicate that central sensitization may contribute to pain-related symptomatology (12). Supporting this, persons with knee OA (relative to healthy individuals) exhibit greater sensitivity to mechanical pressure (13–15) and heat (14, 15) stimuli across multiple body sites, signifying potential disturbances in central pain processing.
Female sex appears to be a contributor to pain and disability in knee OA, regardless of radiographic severity of OA (16). In fact, women are at greater risk of developing knee OA, and they often experience greater pain, disability, and more advanced stages of the condition, relative to men (17, 18). Further, sex may be an important contributor to central changes in osteoarthritic pain. This is supported by several studies suggesting women have a higher propensity to develop chronic pain due to central sensitization (16, 19). Although less is known about sex differences in experimental pain processing in OA (20, 21), one study by Tonelli and colleagues (22) observed increased sensitivity to pressure and heat pain stimuli in women compared to men with knee OA. However, these authors examined a population with advanced knee OA immediately prior to joint replacement surgery; therefore, generalization may be restricted to individuals with more severe, late stage OA. Additionally, experimental pain assessment occurred at the operative knee site which limits assessment of both localized and widespread, somatosensory changes in pain.
The primary aim of the current study was to examine sex-related differences in pain responses in community-dwelling adults with symptomatic knee OA using quantitative sensory testing as a method to examine somatosensory dysfunction (23). We hypothesized that women would experience enhanced pain sensitivity (i.e., lower pain thresholds and tolerances) and temporal summation of pain, as well as greater clinical pain, disability, and decreased functional performance relative to men. Interestingly, the influence of sex on pain has also been shown to vary as a function of age (17, 24–27); therefore, age may mediate sex differences in pain. Notably, while sex-related differences have been well-established in younger adults, the degree to which these disparities exist across older populations has not been well characterized. Hence, a secondary aim of the study was to examine whether sex differences in experimental and clinical pain varied across participant age (middle-aged vs. older-aged adults).
Materials and Methods
Participants
Individuals (N=288) between the ages of 45 to 85 with symptomatic knee OA (based upon American College of Rheumatology criteria (28)) were recruited from the community through flyers and radio/newspaper advertisement. Participants self-identified as “black/African-American” or “white/Caucasian/European” and were recruited as part of a larger, multi-site (University of Florida; University of Alabama at Birmingham) study examining ethnic differences in knee osteoarthritis. Participants were excluded for: uncontrolled hypertension (>150/95); history of myocardial infarction; systemic rheumatic disorders; peripheral neuropathy; psychiatric hospitalization during previous year; cognitive impairment (MMSE score ≤ 22); excessive anxiety regarding study procedures; or daily opioid use.
Procedures
All procedures were fully approved by the University of Florida and University of Alabama-Birmingham Institutional Review Boards. Participants were initially evaluated for exclusion criteria via a phone screen. Those deemed eligible were asked to attend a health assessment session (HAS) during which a more thorough evaluation of eligibility criteria was conducted, health history and socio-demographic information was obtained, questionnaires were completed, and participants underwent a Short Physical Performance Battery (SPPB) test. All participants provided written informed consent prior to enrollment and were scheduled for their quantitative sensory testing (QST) session within 4 weeks of the HAS.
During the QST session, participants were given a brief overview of study procedures and health status was reviewed to confirm eligibility requirements. The session included the following sensory testing procedures: heat pain, mechanical and punctate pressure pain, cold pressor pain. To control for potential order and carry-over effects, heat and pressure pain procedures were counterbalanced across participants and mandatory rest breaks were provided prior to cold pressor tasks. Standardized instructions were delivered via a recording prior to the commencement of each procedure. Sensory testing occurred on the participant’s index (most affected) knee and sites ipsilateral to the index knee. The index knee was chosen based upon self-report of pain in the designated knee. If participants reported bilateral knee pain, they were asked to identify their most painful knee (selected as the “index” site for testing).
Questionnaires
Pain Intensity/Unpleasantness Rating Scales
Participants verbally rated pain intensity and unpleasantness using two separate 0–100 numerical rating scales (NRS) where 0 indicated “no pain/unpleasantness” and 100 indicated the “most intense pain/unpleasantness imaginable.”
Western Ontario and McMaster Universities Index of Osteoarthritis (WOMAC)
The WOMAC (29) is a 24-item scale assessing symptoms of knee OA over the past 48 hours. Items are scored on a 5-point scale ranging from 0–4 with higher scores indicating greater symptom severity. Three subscales comprise the WOMAC: 1) pain during activities (5 items); 2) daytime stiffness (2 items); and 3) impairments in physical function (17 items). The WOMAC demonstrates good construct validity and reliability (30).
Graded Chronic Pain Scale (GCPS)
The GCPS (31) is a 7-item scale asking about pain severity and disability over the past 6 months. Using a 0–10 scale, participants rated the intensity of their current knee pain and the worst and average pain during the past 6 months (Characteristic Pain Intensity score). Participants also rated the degree to which their knee pain interfered with daily activities during the past 6 months (Disability score). All items were averaged and multiplied by 10 to generate index scores for pain intensity and disability, with higher scores indicating greater symptomatology. High internal consistency and reliability has been reported for the GCPS (32).
Pain Sites
The number of pain sites was assessed by having participants indicate the number of regions in which they experienced pain on “more days than not over the past three months.” From a list of twelve body areas (i.e., hands, arms, shoulders, neck, head or face, chest, stomach, upper back, lower back, knees, legs, feet and ankles), participants reported areas in which they currently have pain (reported bilaterally). Items were summed to create a total score ranging from 0–24.
Short Physical Performance Battery (SPPB)
The SPPB (33) assesses limitation in functional movement, including standing balance, 4-meter gait speed, and chair rising tasks. A single summary performance score is calculated, ranging from 0–12, with lower scores indicating greater functional limitation.
Experimental Pain Outcomes
Mechanical Pain
Pressure pain threshold (PPTh) was assessed through three trials of pressure pain stimuli delivered to the medial and lateral joint line of the index knee, and the ipsilateral trapezius muscle, dorsal forearm, and quadriceps muscle (order counterbalanced). Stimuli were delivered at a constant rate of 30 kilopascals (kPa) per second to each site, with the participant indicating their pain threshold by pressing a button. PPTh (in kPa) was calculated by averaging the three trials for each site.
Punctate mechanical stimuli were delivered to the patella and the dorsal surface of the hand (order counterbalanced) using a 300g nylon monofilament. First, a single stimulus was delivered and the participant provided a rating of pain intensity. Then, 10 consecutive stimuli (1 second inter-trial interval) were delivered and the participant provided a rating of the peak pain intensity experienced during the series of 10 stimuli (i.e., TS of punctate pressure). Pain ratings for the two trials of single stimuli and for the series of 10 contacts were averaged for each site.
Heat Pain
Thermal stimuli were delivered using a computer-controlled device (Medoc Pathway Thermal Sensory Analyzer; Ramat Yishai, Israel). Thermal stimuli were delivered to the ventral forearm and the medial aspect of the index knee. Using an ascending method of limits and starting at 32 °C, heat temperature was increased at a rate of 0.5 °C/s (max 52 °C) until participants first felt warmth (WTh), first felt pain (HPTh), or could no longer tolerate the pain (HPTo). Participants provided their response by pressing a computer mouse button. Three trials each of WTh, HPTh, and HPTo were collected at each area (forearm, knee), with the average of these responses being recorded. Pain ratings (0–100) were obtained after each HPTh and HPTo trial.
For temporal summation of heat pain, participants verbally rated the intensity of pain evoked by each of 5 brief, repetitive suprathreshold heat pulses on the 0 to 100 scale. Three target temperatures (44 °C, 46 °C, and 48 °C) were delivered by a Contact Heat-Evoked Potential Stimulator thermode for less than 1 second, with a 2.5-second inter-pulse interval during which the temperature of the contactor returned to baseline (32 °C).
Cold Pain
A modified cold pressor procedure was conducted during which participants were asked to immerse their right hand up to the wrist in 1-minute water immersions (Neslab refrigeration unit; Portsmouth, NH), with temperatures set at 12 °C and 8 °C (in descending order). The time until participants first reported pain (CPTh) and withdrew their hand (CPTo) was recorded. Participants also provided pain intensity and pain unpleasantness ratings at 60 seconds after immersion (or upon hand withdrawal). A 5-minute rest break was provided between each cold pressor trial.
Data Analysis
Statistical analyses were conducted using 2 (Sex) × 2 (Age) ANCOVAs with SPSS 22.0 (SPSS Inc., Chicago, IL) software. An age cut-point of 56 years was employed to discriminate between the middle-aged (45–56 years) and older-aged (57–85 years) groups, and is consistent with previous research (34–37). Since race and BMI have been associated with clinical and experimental pain in prior research, these variables were included as covariates in all analyses. Due to differences amongst sex and age groups in education, marital status, and employment, these variables were also included as covariates. MANCOVA models were used when families of dependents were highly correlated (r>.50), while repeated measures ANCOVAs were conducted when time/trial was involved (i.e., TS of heat and punctate stimuli). If sphericity was violated, Greenhouse-Geisser corrections were used. To obtain effect size estimates associated with F-tests, partial eta-squared (ηp2) was calculated from GLM analyses (small=.01, medium=.06, large=.14). Significance was set at p≤0.05 (two-tailed).
Results
Participants
Table 1 presents demographic and clinical data for the sample. Women had higher educational attainment and were more likely to be employed. The older age group had a higher proportion of whites, were more highly educated and married, less likely to be employed, and had a lower BMI and chronic pain grade.
Table 1.
Demographic and clinical characteristics of participants across sex and age
| Women N=183 |
Men N=105 |
Group Comparison |
Middle-Age N=161 |
Older-Age N=127 |
Group Comparison |
|||
|---|---|---|---|---|---|---|---|---|
|
M or % (SD) |
M or % (SD) |
p | d |
M or % (SD) |
M or % (SD) |
p | d | |
| Age | 56.8(7.8) | 56.5(7.2) | .69 | .05 | 51.1(3.3) | 63.9(5.2) | <.001 | 2.89 |
| Sex | .82 | |||||||
| Female | 63.5 | 64.0 | 62.7 | |||||
| Male | 36.5 | 36.0 | 37.3 | |||||
| Race | 1.00 | <.001 | ||||||
| White/Caucasian | 42.9 | 42.9 | 29.2 | 60.6 | ||||
| Black/African-American | 57.1 | 57.1 | 70.8 | 39.4 | ||||
| Income ($) | .20 | .27 | ||||||
| ≤20,000 | 29.2 | 47.1 | 39.9 | 30.4 | ||||
| 20,000–39,999 | 34.8 | 18.3 | 25.3 | 33.6 | ||||
| 40,000–59,999 | 15.2 | 12.5 | 13.3 | 15.2 | ||||
| ≥60,000 | 20.8 | 22.1 | 21.5 | 20.8 | ||||
| Education | .02 | .02 | ||||||
| ≤High School degree | 41.2 | 57.2 | 52.2 | 40.9 | ||||
| 2-year college degree | 24.2 | 19.0 | 20.5 | 24.4 | ||||
| 4-year college degree | 19.8 | 14.3 | 19.3 | 15.7 | ||||
| Graduate degree | 14.8 | 9.5 | 8.0 | 19.0 | ||||
| BMI | 32.7(8.1) | 30.9(6.8) | .06 | .24 | 32.7(8.4) | 31.3(6.4) | .13 | .18 |
| Marital Status | .80 | .01 | ||||||
| Married | 37.4 | 48.6 | 39.2 | 44.9 | ||||
| Not Married | 60.4 | 50.4 | 58.9 | 53.5 | ||||
| Other | 2.2 | 1.0 | 1.9 | 1.6 | ||||
| Employment | .01 | <.001 | ||||||
| Employed | 55.3 | 32.2 | 56.3 | 35.4 | ||||
| Other | 2.8 | 4.8 | 1.9 | 5.5 | ||||
| GCPS | .72 | <.001 | ||||||
| Grade 0 | 0.0 | 2.0 | 0.6 | 0.8 | ||||
| Grade 1 | 38.9 | 41.1 | 29.9 | 52.4 | ||||
| Grade 2 | 27.8 | 16.7 | 24.9 | 22.2 | ||||
| Grade 3 | 17.2 | 19.6 | 22.9 | 11.9 | ||||
| Grade 4 | 16.1 | 20.6 | 21.7 | 12.7 | ||||
Note. BMI=Body Mass Index; GCPS=Graded Chronic Pain Scale.
Experimental Pain Outcomes
Mechanical and Punctate Pressure
The main effect of sex was significant for all mechanical pressure pain outcomes (Table 2), signifying that relative to men, thresholds were lower for women at all sites (p’s<.001). The main effect of age was significant for pressure pain thresholds at the quadriceps and epicondyle, with older adults exhibiting lower thresholds at both sites (p’s<.05). The Sex × Age interaction for all mechanical pressure pain outcomes was non-significant (p’s>.05).
Table 2.
Descriptive and inferential statistics for measures of mechanical pressure across sex and age
| Women | Men | Sex (S) | Middle-Age | Older-Age | Age (A) | SxA | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pain Outcome | Mean (SD) | Mean (SD) | F | ηp2 | Mean (SD) | Mean (SD) | F | ηp2 | F | ηp2 |
| Mechanical Pressure (kPa) | ||||||||||
| Medial joint line (knee) | 243.8(145.4) | 357.0(167.8) | *37.10 | .12 | 304.5(162.5) | 296.3(165.0) | .17 | .00 | 2.32 | .01 |
| Lateral joint line (knee) | 254.5(153.9) | 386.3(167.8) | *47.97 | .15 | 322.8(165.8) | 318.1(176.3) | .05 | .00 | 1.98 | .01 |
| Quadriceps | 373.2(200.5) | 490.5(243.9) | *18.39 | .06 | 460.9(237.5) | 402.8(203.5) | *3.93 | .01 | .01 | .00 |
| Trapezius | 226.0(142.8) | 331.0(200.5) | *25.30 | .09 | 298.8(186.4) | 258.2(152.2) | 3.30 | .01 | .74 | .00 |
| Epicondyle | 202.2(114.0) | 309.7(212.5) | *29.40 | .10 | 277.0(177.9) | 234.8(145.0) | *3.95 | .01 | .05 | .00 |
Note. kPa=kilopascals.
p ≤ .05.
Figure 1 and Supplementary Figure 1 present results for punctate pressure outcomes. For punctate pain at the hand, the main effects of sex [F(1, 276)=2.81, p=.10, ηp2=.01] and age [F(1, 276)=2.34, p=.13, ηp2=.01] were both non-significant for the single stimulus. Additionally, there were no differences in TS of punctate pressure pain at the hand across sex [F(1, 275)=.24, p=.63, ηp2=.00] or age [F(1, 275)=.53, p=.47, ηp2=.00]. For punctate pain at the knee, there was a significant main effect of sex [F(1, 276)=3.81, p=.05, ηp2=.01] for the single stimulus pain rating; however, no significant effects occurred for age [F(1, 276)=3.31, p=.07, ηp2=.01]. Specifically, women (d=.24) provided higher pain ratings for the single stimulus compared to men. Women also exhibited greater TS of punctate pressure at the knee, relative to men [F(1, 275)=12.61, p<.001, ηp2=.04, d=.35], whereas there were no significant differences across age groups for TS [F(1, 275)=.15, p=.70, ηp2=.00]. The Sex × Age interaction for all punctate pressure outcomes was non-significant (all p’s>.05).
Figure 1.
Adjusted means and standard errors for punctate pressure across sex. For punctate pressure at the hand, the main effect of sex was non-significant for the single stimulus and temporal summation (TS). For punctate pressure at the knee, women had higher pain ratings for the single stimulus compared to men, and had greater TS of punctate pressure pain.
Heat Pain
The main effect of sex was significant for all heat pain outcomes (Table 3) indicating that women exhibited lower thresholds and tolerances at both the arm and knee (p’s<.05). Further, intensity ratings at the arm and knee for tolerance were lower in women (p’s<.01). The main effect of age and the Sex × Age interaction for all heat pain outcomes were non-significant (all p’s>.05).
Table 3.
Descriptive and inferential statistics for measures of thermal heat and cold pain across sex and age
| Women | Men | Sex (S) | Middle-Age | Older-Age | Age (A) | SxA | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pain Outcome | Mean (SD) | Mean (SD) | F | ηp2 | Mean (SD) | Mean (SD) | F | ηp2 | F | ηp2 |
| Heat Trials (Knee) | ||||||||||
| Warmth Thr (°C) | 37.0(2.7) | 37.9(3.0) | *5.41 | .02 | 37.1(2.7) | 37.8(2.9) | 2.89 | .01 | .28 | .00 |
| Pain Thr (°C) | 41.6(3.2) | 42.5(3.4) | *4.93 | .02 | 42.2(3.3) | 41.8(3.3) | .79 | .00 | .42 | .00 |
| Pain Tol (°C) | 45.1(2.7) | 46.7(2.7) | *25.65 | .09 | 46.0(2.9) | 45.9(2.8) | .05 | .00 | .66 | .00 |
| Heat Trials (Arm) | ||||||||||
| Warmth Thr (°C) | 35.3(2.1) | 36.2(2.9) | *9.78 | .03 | 36.0(2.7) | 35.5(2.2) | 1.81 | .01 | .40 | .00 |
| Pain Thr (°C) | 41.2(3.2) | 42.3(3.3) | *7.34 | .03 | 42.0(3.3) | 41.6(3.3) | .62 | .00 | .59 | .00 |
| Pain Tol (°C) | 45.2(2.5) | 46.7(2.7) | *25.88 | .09 | 46.1(2.6) | 45.8(2.7) | .49 | .00 | 2.38 | .01 |
| Heat Pain Ratings (Knee) | ||||||||||
| Pain Thr (0–100) | 24.0(21.5) | 28.8(22.5) | 2.63 | .01 | 26.0(22.2) | 27.8(21.9) | .06 | .00 | .65 | .00 |
| Pain Tol (0–100) | 55.0(27.7) | 66.4(24.9) | *9.79 | .04 | 62.4(27.5) | 59.0(27.1) | .73 | .00 | .20 | .00 |
| Heat Pain Ratings (Arm) | ||||||||||
| Pain Thr (0–100) | 22.7(19.9) | 27.0(22.1) | 2.39 | .01 | 25.7(21.8) | 23.9(19.8) | .36 | .00 | .43 | .00 |
| Pain Tol (0–100) | 52.7(25.5) | 62.0(25.0) | *7.28 | .03 | 59.0(26.1) | 55.8(25.2) | .71 | .00 | .26 | .00 |
| Cold Pressor (12 °C) | ||||||||||
| Pain Thr (in sec) | 18.2(15.1) | 21.1(15.8) | 2.19 | .01 | 21.3(15.4) | 18.0(15.4) | 2.24 | .01 | 1.99 | .01 |
| Withdrawal (in sec) | 51.4(14.3) | 54.3(12.8) | 2.95 | .01 | 53.1(13.4) | 52.5(14.4) | .12 | .00 | .76 | .00 |
| Intensity @ 60-sec | 62.2(31.0) | 56.2(34.6) | 2.08 | .01 | 55.8(32.9) | 62.5(31.8) | 2.16 | .01 | 2.44 | .01 |
| Unpl. @ 60-sec | 65.8(31.0) | 60.4(32.8) | 1.77 | .01 | 62.9(32.1) | 63.3(31.3) | .93 | .00 | 2.12 | .01 |
| Cold Pressor (8°C) | ||||||||||
| Pain Thr (in sec) | 11.1(10.9) | 13.8(11.7) | 3.46 | .01 | 13.8(11.9) | 11.1(10.4) | 2.81 | .01 | *6.67 | .03 |
| Withdrawal (in sec) | 43.3(19.3) | 48.3(18.1) | *4.79 | .02 | 45.7(18.9) | 46.0(19.1) | .01 | .00 | .94 | .00 |
| Intensity @ 60-sec | 76.5(27.1) | 68.0(31.2) | *5.37 | .02 | 71.2(29.9) | 73.3(27.6) | .27 | .00 | *5.54 | .02 |
| Unpl. @ 60-sec | 78.2(26.4) | 73.2(29.5) | 1.98 | .01 | 75.7(27.5) | 75.6(27.6) | .00 | .00 | *3.85 | .02 |
Note. Thr=Threshold; Tol=Tolerance.
p ≤ .05.
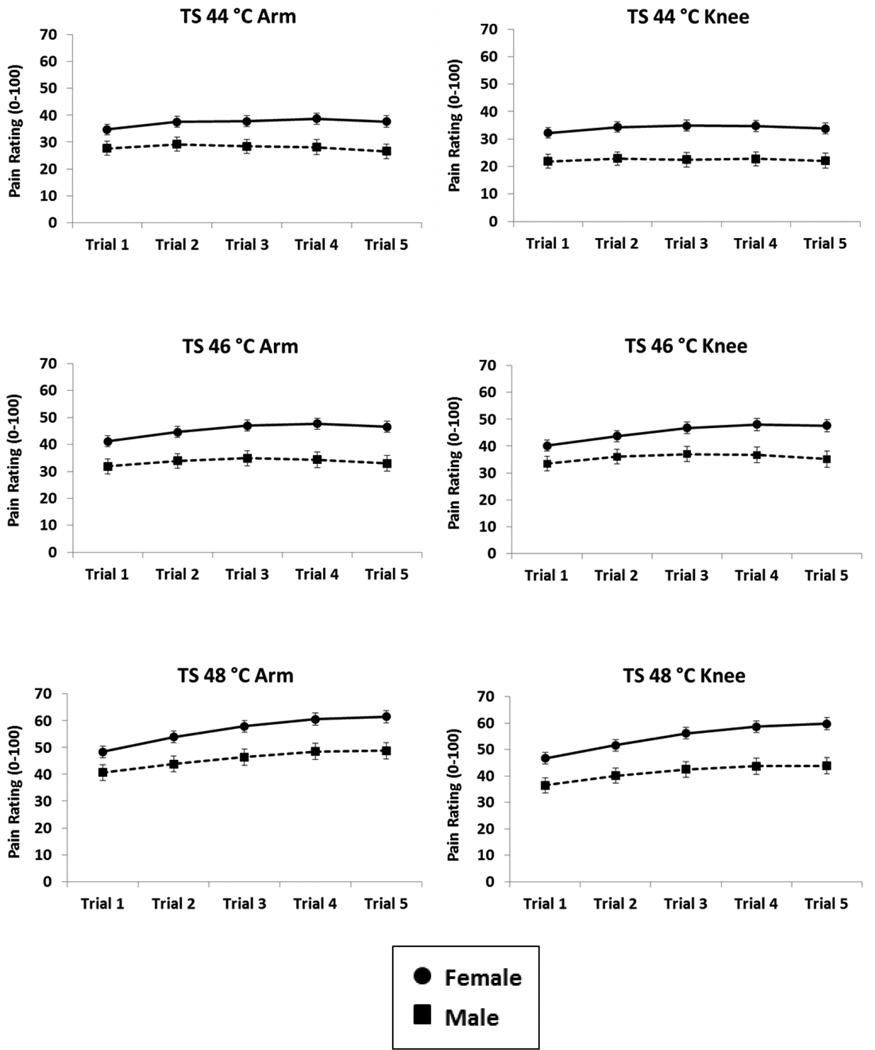
For TS of heat pain, the main effect of sex was significant for all temperatures and sites, indicating that women produced overall higher heat pain ratings than men (44 °C Arm: F(1,260)=8.03, p=.005, ηp2=.03; 46 °C Arm: F(1,265)=12.43, p<.001, ηp2=.05; 48 °C Arm: F(1,269)=8.91, p=.003, ηp2=.03; 44 °C Knee: F(1,255)=13.19, p<.001, ηp2=.05; 46 °C Knee: F(1,266)=7.70, p=.006, ηp2=.03; 48 °C Knee: F(1,270)=13.32, p<.001, ηp2=.05). There was also a significant Sex × Trial interaction for TS at the arm for 48 °C (F(1.65,443.11)=3.21, p=.05, ηp2=.01), as well as at the knee for 46 °C (F(1.53,407.65)=4.49, p=.02, ηp2=.02) and 48 °C (F(1.47,397.73)=3.95, p=.04, ηp2=.01), indicating that the sex difference (women>men) for TS was greater across subsequent trials. All other main effects and interactions for sex (Figure 2), age (Supplementary Figure 2), and trial were non-significant (all p’s>.05).
Figure 2.
Adjusted means and standard errors for temporal summation (TS) across sex. Women had overall higher heat pain ratings than men. The Sex × Trial interaction for 46 °C (knee) and 48 °C (arm, knee) was significant indicating that women had greater TS across the 5 repeated trials.
Cold Pain
The main effect of sex was significant for cold pain outcomes (Table 3) at 8 °C, with women demonstrating lower tolerances and greater pain intensity ratings than men (p’s<.05). The main effect of age was non-significant for all outcomes (p’s>.05). There were also significant Sex × Age interactions for CPTh, CP intensity ratings, and CP unpleasantness ratings at 8 °C, suggesting that women exhibited lower pain thresholds, as well as higher pain intensity and unpleasantness ratings than males, but only in the older-aged group (p’s<.05).
Clinical Pain
Table 4 presents descriptive and inferential statistics, as well as internal consistency estimates (range .83 to .97) for clinical pain outcomes. The main effect of sex was non-significant for all outcomes, with the exception of women having a greater number of pain sites relative to men (p=.001). The main effect of age was significant for clinical pain intensity and disability on the GCPS, with middle-aged adults reporting greater pain intensity and disability (p’s<.05). The Sex × Age interaction for all clinical pain outcomes was non-significant (p’s>.05).
Table 4.
Descriptive and inferential statistics for measures of clinical pain across sex and age
| Women | Men | Sex (S) | Middle-Age | Older-Age | Age (A) | SxA | Cronbach’s Alpha | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pain Outcome | Mean (SD) | Mean (SD) | F | ηp2 | Mean (SD) | Mean (SD) | F | ηp2 | F | ηp2 | α |
| WOMAC | |||||||||||
| Total Score (0–96) | 34.5(20.5) | 34.1(20.7) | .03 | .00 | 35.2(21.1) | 33.3(19.5) | .53 | .00 | .00 | .00 | |
| WOMAC Subscales | |||||||||||
| Pain (0–20) | 7.3(4.2) | 7.1(4.6) | .13 | .00 | 7.4(4.5) | 7.0(4.2) | .54 | .00 | .46 | .00 | .88 |
| Stiffness (0–8) | 3.3(2.0) | 3.5(1.9) | .88 | .00 | 3.5(2.0) | 3.4(2.0) | .13 | .00 | .92 | .00 | .91 |
| Physical Function (0–68) | 23.7(15.1) | 23.3(14.9) | .07 | .00 | 24.2(15.5) | 22.8(14.2) | .52 | .00 | .01 | .00 | .97 |
| GCPS | |||||||||||
| Pain Intensity (0–100) | 50.8(22.4) | 49.8(23.6) | .16 | .00 | 53.9(22.4) | 46.7(22.0) | *7.00 | .03 | .18 | .00 | .83 |
| Disability (0–100) | 43.2(29.1) | 44.3(29.5) | .10 | .00 | 48.1(28.5) | 39.4(29.0) | *5.47 | .02 | .00 | .00 | .92 |
| Number of Pain Sites | 6.0(4.7) | 4.3(3.2) | *11.42 | .04 | 5.4(4.6) | 4.9(3.8) | .99 | .00 | 1.37 | .01 | |
| SPPB | |||||||||||
| Total Score (0–12) | 9.6(1.9) | 9.9(1.7) | 1.13 | .00 | 10.0(1.8) | 9.5(2.0) | 3.15 | .01 | 1.16 | .00 | |
Note. WOMAC=Western Ontario and McMaster Universities Index of Osteoarthritis; GCPS=Graded Chronic Pain Scale; SPPB=Short Physical Performance Battery.
p ≤ .05.
Discussion
The aim of the current study was to examine sex and age differences in experimental and clinical pain, in addition to functional and physical performance in adults with symptomatic knee OA. Several notable findings were observed: 1) clear sex differences existed in experimental pain, with women exhibiting greater pain sensitivity to multiple pain modalities, compared to men; 2) clinical pain did not differ across sex; and 3) sex differences in experimental and clinical pain varied minimally across age cohorts (middle-age vs. older-age).
As expected, women with knee OA, compared to their male counterparts, exhibited greater sensitivity to a number of stimuli, including lower pain thresholds and tolerances to heat, cold, and pressure stimuli, greater mechanical pressure pain, and higher mechanical and heat temporal summation of pain. In contrast to the consistent sex differences found in responses to experimental noxious stimuli (20, 21, 26), men and women did not differ on measures of clinical pain, with the exception of greater widespread pain reported by women. Although it is unclear why women did not exhibit enhanced clinical pain as has been observed in other studies (20, 21), it is possible that sex differences in pain processing in older adults with knee OA are more easily detected using evoked pain measures rather than retrospective report of clinical symptoms.
It is well-known that sex differences in pain exist in healthy adults, yet little research has examined these effects among older adults, particularly among clinical samples. To our knowledge, only one study has examined sex differences in both experimental and clinical pain sensitivity in individuals with knee OA. Specifically, in 208 older adults with late-stage OA, Tonelli and colleagues (22) found that women, relative to men, exhibited greater sensitivity to heat and mechanical pressure stimuli at the operative knee, as well as lower functional performance and greater clinical pain. However, Tonelli and colleagues recruited a sample of individuals with severe OA requiring total joint replacement; therefore, sample characteristics may have led to variability across studies. Our study, which included a community-dwelling sample with less severe knee OA, extends these findings by also revealing that women demonstrate enhanced hyperalgesia (i.e., greater experimental and widespread pain, greater temporal summation) both locally and distally relative to the affected joint.
Regarding age differences, older participants, compared to the middle-aged group, displayed lower mechanical pain thresholds in the quadriceps and epicondyle. These results are somewhat surprising, since previous evidence suggests that older adults show increased pain thresholds relative to their younger counterparts (38). However, most previous comparisons have examined healthy samples, and the presence of OA pain could alter the pattern of age-related differences in pain perception. Also, age-related effects in pain sensitivity may be dependent upon pain modality (36), such that older adults may exhibit dysregulation in the processing of sustained C-fiber mediated pressure pain, and in particular, among sites distal to the affected joint. Interestingly, the middle-aged group reported greater clinical pain intensity and disability relative to older-aged adults. While factors such as pain-related beliefs (i.e., believing pain is a normal part of aging) and stoicism among older adults may have influenced results, differences among groups in physical activity could also account for findings. That is, older adults often decrease their physical activity (39), which could lead to lower pain and disability since pain may be more commonly evoked upon movement in knee OA, while decreased physical activity is also associated with increased pain sensitivity (40). Further, it is important to note that a higher proportion of our middle-aged sample reported being employed, and with the increase in physical activity that often coincides with employment, this could potentially augment pain and subsequent pain-related disability. Hence, middle-aged adults may represent a cohort at greater risk for adverse pain-related functional outcomes (4). These findings are speculative, however, and warrant further exploration.
We found little evidence that sex differences vary with age, as the only effects observed were in cold pressor pain. In particular, women exhibited lower cold pain thresholds, as well as higher pain intensity and unpleasantness ratings relative to men, but only in older-aged adults. Notably, there have been few studies examining the interactive roles of sex and age in relation to pain sensitivity. Riley and Gilbert (27) reported that among adults between the ages of 45–64, females with orofacial pain reported greater clinical pain than males; however, no sex differences emerged in the older age group (ages 65+). Further, Pickering and colleagues (41) found that although healthy females, compared to men, showed lower thermal pain threshold levels, these differences were attenuated in the older adult group (ages 75+), relative to the younger-aged cohort (ages 18–25). Although we did not employ a younger-aged sample to examine whether similar effects would be found in younger adults, our results are in accordance with others suggesting that sex differences in pain may vary minimally in older adults (27, 41) and more specifically, in older individuals with knee osteoarthritis.
There are some limitations of the current study that warrant acknowledgement. First, this sample was restricted to middle-aged and older adults from the community who generally had mild to moderate knee OA symptoms; therefore, results may not generalize to clinic-based samples with more severe symptoms. Second, participants had to be willing to undergo two laboratory sessions, as well as an extensive battery of experimental pain testing. Thus, our sample may have been more resilient (e.g., less functionally limited; greater functional abilities) than other samples with symptomatic knee OA. Third, although there were clear sex differences observed in experimental pain, we did not detect any differences across men and women in measures of clinical pain, a finding that differs from those of many previous studies. It is unclear whether these findings are due to our study methodology (e.g., type of clinical measures) or to our sample, or perhaps reflective of some other unmeasured factor.
Despite these limitations, there are several strengths that are worth noting. First, whereas most studies have focused mainly on investigating sex differences in younger individuals, we attempted to expand previous research by examining these differences in a large sample of middle-aged and older adults with knee OA. Second, we utilized a multi-modal battery of quantitative sensory and clinical measures to examine pain sensitivity in our sample. And third, limited research has examined sex differences in both clinical and experimental pain among persons with symptomatic knee OA. Therefore, our study highlights the role that sex differences may have on pain sensitivity in individuals with knee OA.
Conclusions and Future Directions
Overall, study findings suggest that central sensitization may contribute to pain in women with knee OA, as observed from the pattern of findings in this group (i.e., greater temporal summation, heightened sensitivity to multiple pain modalities, greater number of pain sites, pain in areas distal to the knee joint). This may have important treatment implications as centrally-acting therapies (e.g., tricyclics, SSRI’s) targeting central nervous system hyperexcitability may be more effective in women with knee OA rather than peripherally-targeted treatments (e.g., topicals, NSAID’s). To a large extent, these differences did not vary significantly across age groups. Given the limited research examining pain sensitivity in older adults, further investigation is warranted to clarify whether sex-related differences in pain exist in other aging clinical populations. Continued research is also needed to elucidate the underlying biological and psychosocial mechanisms that not only increase vulnerability for enhanced pain sensitivity in women with knee OA, but that also identify why men may be particularly resilient toward augmented pain sensitivity in this population. Identification of these factors may facilitate the development of mechanistically-based therapeutic strategies that could be individualized based upon patient sex. Further, methods by which to effectively evaluate and diagnose centrally-mediated pain (e.g., developing diagnostic criteria for central sensitization) (12), both in the research and clinical setting, merits attention as this could optimize pain treatment and ultimately reduce pain management disparities existing among men and women.
Supplementary Material
Significance and Innovations.
In community-dwelling older adults with symptomatic knee osteoarthritis, women exhibit hyperalgesia to multiple experimental pain modalities and greater widespread pain, relative to men.
The enhanced pain sensitivity observed in women with knee osteoarthritis may have a strong central component to its pathology.
Select age-related differences were detected in pain sensitivity; however, sex differences in pain varied minimally across middle- and older-aged adults.
Acknowledgments
This work was supported by NIH Grant AG033906 and CTSA Grant TR000064. Dr. Emily J. Bartley was supported by NINDS training grant T32NS045551 to the University of Florida Pain Research and Intervention Center of Excellence. The authors would like to thank Terry Weber, Ralisa Pop, Rebekah Bush, and all of our research volunteers for help with data collection.
Disclosures: Supported by National Institutes of Health/National Institute on Aging Grant (R01AG033906); National Center for Advancing Translational Science Clinical and Translational Science Award granted to the University of Florida (UL1TR000064); National Center for Advancing Translational Science and National Center for Research Resources Clinical and Translational Science Award granted to the University of Alabama-Birmingham (UL1TR000165). Dr. Fillingim has received consulting fees, speaking fees, and/or honoraria from WebMD and Algynomics (less than $10,000 each) and owns stock or stock options in Algynomics. Dr. Bradley has received royalty payments (less than $10,000) from UpToDate Rheumatology.
References
- 1.Wright EA, Katz JN, Cisternas MG, Kessler CL, Wagenseller A, Losina E. Impact of knee osteoarthritis on health care resource utilization in a US population-based national sample. Medical Care. 2010;48(9):785–791. doi: 10.1097/MLR.0b013e3181e419b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. British Medical Bulletin. 2013;105(1):185–199. doi: 10.1093/bmb/lds038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brochet B, Michel P, Barberger-Gateau P, Dartigues JF. Population-based study of pain in elderly people: A descriptive survey. Age and Ageing. 1998;27(3):279–284. [Google Scholar]
- 4.Rustøen T, Wahl AK, Hanestad BR, Lerdal A, Paul S, Miaskowski C. Age and the experience of chronic pain: Differences in health and quality of life among younger, middle-aged, and older adults. The Clinical Journal of Pain. 2005;21(6):513–523. doi: 10.1097/01.ajp.0000146217.31780.ef. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis and Rheumatism. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: Cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP) PAIN. 2004;110(1–2):361–368. doi: 10.1016/j.pain.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: New Insights. Part 1: The Disease and Its Risk Factors. Annals of Internal Medicine. 2000;133(8):635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 8.Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis and Cartilage. 2013;21(9):1145–1153. doi: 10.1016/j.joca.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Care & Research. 2008;59(9):1207–1213. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finan PH, Buenaver LF, Bounds SC, Hussain S, Park RJ, Haque UJ, et al. Discordance between pain and radiographic severity in knee osteoarthritis: Findings from quantitative sensory testing of central sensitization. Arthritis Rheum. 2013;65(2):363–372. doi: 10.1002/art.34646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Practice & Research Clinical Rheumatology. 2011;25(2):141–154. doi: 10.1016/j.berh.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, et al. Sensitization in patients with painful knee osteoarthritis. PAIN. 2010;149(3):573–581. doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 14.King CD, Sibille KT, Goodin BR, Cruz-Almeida Y, Glover TL, Bartley E, et al. Experimental pain sensitivity differs as a function of clinical pain severity in symptomatic knee osteoarthritis. Osteoarthritis and Cartilage. 2013;21(9):1243–1252. doi: 10.1016/j.joca.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YC, Lu B, Bathon JM, Haythornthwaite JA, Smith MT, Page GG, et al. Pain sensitivity and pain reactivity in osteoarthritis. Arthritis Care & Research. 2011;63(3):320–327. doi: 10.1002/acr.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glass N, Segal NA, Sluka KA, Torner JC, Nevitt MC, Felson DT, et al. Examining sex differences in knee pain: The Multicenter Osteoarthritis Study. Osteoarthritis and Cartilage. 2014;22(8):1100–1106. doi: 10.1016/j.joca.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis and Cartilage. 2005;13(9):769–781. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Felson D, Zhang Y, Hannan M, Naimark A, Weissman B, Aliabadi P, et al. The incidence and natural history of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1995;38:1500–1505. doi: 10.1002/art.1780381017. [DOI] [PubMed] [Google Scholar]
- 19.Sluka KA, Berkley KJ, O’Connor MI, Nicolella DP, Enoka RM, Boyan BD, et al. Neural and psychosocial contributions to sex differences in knee osteoarthritic pain. Biology of Sex Differences. 2012;3:26. doi: 10.1186/2042-6410-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., III Sex, gender, and pain: A review of recent clinical and experimental findings. The Journal of Pain. 2009;10(5):447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartley EJ, Fillingim RB. Sex differences in pain: A brief review of clinical and experimental findings. British Journal of Anaesthesia. 2013;111(1):52–58. doi: 10.1093/bja/aet127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tonelli S, Rakel B, Cooper N, Angstom W, Sluka K. Women with knee osteoarthritis have more pain and poorer function than men, but similar physical activity prior to total knee replacement. Biology of Sex Differences. 2011;2(1):12. doi: 10.1186/2042-6410-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cruz-Almeida Y, Fillingim RB. Can quantitative sensory testing move us closer to mechanism-based pain management? Pain Med. 2014;15(1):61–72. doi: 10.1111/pme.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leveille SG, Zhang Y, McMullen W, Kelly-Hayes M, Felson DT. Sex Differences in musculoskeletal pain in older adults. Pain. 2005;116(3):332–338. doi: 10.1016/j.pain.2005.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scudds RJ, Robertson JM. Pain factors associated with physical disability in a sample of community-dwelling senior citizens. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2000;55(7):M393–M399. doi: 10.1093/gerona/55.7.m393. [DOI] [PubMed] [Google Scholar]
- 26.Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, et al. Studying sex and gender differences in pain and analgesia: A consensus report. PAIN. 2007;132(Suppl 1):S26–S45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riley JL, III, Gilbert GH. Orofacial pain symptoms: An interaction between age and sex. PAIN. 2001;90(3):245–256. doi: 10.1016/S0304-3959(00)00408-5. [DOI] [PubMed] [Google Scholar]
- 28.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis: Classification of osteoarthritis of the knee. Arthritis & Rheumatism. 1986;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 29.Bellamy N. Pain assessment in osteoarthritis: Experience with the WOMAC osteoarthritis index. Seminars in Arthritis and Rheumatism. 1989;18(4, Supplement 2):14–17. doi: 10.1016/0049-0172(89)90010-3. [DOI] [PubMed] [Google Scholar]
- 30.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of the WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. Journal of Rheumatology. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 31.Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50(2):133–149. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 32.Smith BH, Penny KI, Purves AM, Munro C, Wilson B, Grimshaw J, et al. The Chronic Pain Grade questionnaire: validation and reliability in postal research. PAIN. 1997;71(2):141–147. doi: 10.1016/s0304-3959(97)03347-2. [DOI] [PubMed] [Google Scholar]
- 33.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. Journal of Gerontology. 1994;49(2):M85–M89. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 34.Riley JL, III, Cruz-Almeida Y, Glover TL, King CD, Goodin BR, Sibille KT, et al. Age and race effects on pain sensitivity and modulation among middle-aged and older adults. The Journal of Pain. 2014;15(3):272–282. doi: 10.1016/j.jpain.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larivière M, Goffaux P, Marchand S, Julien N. Changes in pain perception and descending inhibitory controls start at middle age in healthy adults. The Clinical Journal of Pain. 2007;23(6):506–510. doi: 10.1097/AJP.0b013e31806a23e8. [DOI] [PubMed] [Google Scholar]
- 36.Lautenbacher S, Kunz M, Strate P, Nielsen J, Arendt-Nielsen L. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. PAIN. 2005;115(3):410–418. doi: 10.1016/j.pain.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 37.Edwards RR, Fillingim RB. Age-associated differences in responses to noxious stimuli. Journals of Gerontology Series A. 2001;56(3):M180–M185. doi: 10.1093/gerona/56.3.m180. [DOI] [PubMed] [Google Scholar]
- 38.Lautenbacher S. Experimental approaches in the study of pain in the elderly. Pain Medicine. 2012;13:S44–S50. doi: 10.1111/j.1526-4637.2012.01326.x. [DOI] [PubMed] [Google Scholar]
- 39.Sun F, Norman I, While A. Physical activity in older people: A systematic review. BMC Public Health. 2013;13(1):449. doi: 10.1186/1471-2458-13-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naugle KM, Riley JL. Self-reported physical activity predicts pain inhibitory and facilitatory function. Medicine and science in sports and exercise. 2014;46(3):622–629. doi: 10.1249/MSS.0b013e3182a69cf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pickering G, Jourdan D, Eschalier A, Dubray C. Impact of age, gender and cognitive functioning on pain perception. Gerontology. 2002;48(2):112–118. doi: 10.1159/000048937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.