Abstract
Objective
Effective prevention and management strategies of intraplaque hemorrhage (IPH) remain elusive due to our limited knowledge regarding its etiology and contributing factors. This hypothesis-generating study aimed to investigate associations between cardiovascular risk factors and IPH for improved understanding of the pathogenesis of IPH.
Approach and Results
Asymptomatic subjects with 16–79% stenosis on ultrasound underwent carotid magnetic resonance imaging (MRI) using a large-coverage, three-dimensional MRI protocol. Individual plaques (maximum thickness>1.5 mm) in bilateral carotid arteries were identified and presence of IPH was determined. From 80 subjects, 176 de novo plaques were measured, of which 38 (21.6%) contained IPH. Blood pressure (BP), primarily low diastolic BP, was associated with IPH in multivariate analysis adjusted for age, sex, and plaque size (odds ratio [OR with 95% confidence interval] per 10 mmHg ↑: 0.51 [0.30–0.88]), which was little changed after adjusting for antihypertensive use and systemic atherosclerosis. Antiplatelet use was associated with IPH in age and sex-adjusted models (p=0.018), for which a trend remained after considering plaque size and past medical history (OR for aspirin alone vs. none: 3.1 [0.66–14.8]; OR for clopidogrel or dual therapy vs. none: 5.3 [0.80–35.0]; p=0.083).
Conclusions
Low diastolic BP was independently associated with IPH, which was unlikely due to treatment difference or BP changes from systemic atherosclerosis. Hemodynamic changes from lowering diastolic BP may be the pathophysiological link. Prospective serial studies are needed to assess whether BP and antiplatelet use are associated with the development of new or repeated IPH.
Keywords: atherosclerosis, intraplaque hemorrhage, magnetic resonance imaging, blood pressure
Introduction
Intraplaque hemorrhage (IPH) is a common finding in histological examination of advanced atherosclerotic plaques. It was largely neglected for decades, while research focus of vascular biology has been on lipid metabolism and plaque inflammation. However, the role of IPH in atherothrombosis is becoming increasingly recognized through mechanistic prospective studies that are recently made possible by in vivo imaging. It has been recognized that IPH promotes plaque progression,1–5 of which the pathways may involve iron-driven lipid oxidation,6 cholesterol deposition from erythrocyte membrane,7,8 blood-borne proteolytic activity,9,10 and more. In accord with such an effect, IPH was shown in various clinical settings to predict subsequent clinical outcomes.11,12
IPH-induced plaque progression may account for a substantial portion of the residual cardiovascular risk in the post-statin era, as indicated in prospective studies examining plaque progression patterns in contemporary cohorts.2,4 Effective prevention and management strategies targeting IPH are needed, which are currently lacking because of our limited knowledge regarding its pathogenesis and contributing factors. Both histopathology and imaging studies have observed associations between IPH and cardiovascular risk factors, of which most commonly noticed were with age, sex, and hypertension (Table I in the online-only Data Supplement).13–29 It remains unclear whether these observations have implications for the pathogenesis of IPH, as potential confounding factors may have played a role. Specifically, IPH is associated with larger plaques21,29 and more extensive systemic atherosclerosis,24,30 both of which have known relationships with traditional risk factors. Furthermore, prevalent medication use in subjects with atherosclerotic cardiovascular disease (ASCVD) may influence the relationships between IPH and modifiable risk factors.
This hypothesis-generating study aimed to investigate: 1) the associations of cardiovascular risk factors, particularly modifiable ones, with IPH; 2) whether the associations, with thorough inspection of potential confounding factors, may shed light on the pathogenesis of IPH.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
One subject withdrew from the study after the baseline MRI. Clinical characteristics of the remaining 80 subjects are shown in Table 1. This cohort consisted primarily of Caucasians (97.5%). The mean age was 70±8 years (range: 51–85). Fifty-three (66.2%) were males. Of the 160 carotid arteries, 15 (9.4%) were excluded due to either prior carotid endarterectomy or total occlusion while 7 (4.4%) had no plaque, 104 (65.0%) had one plaque, and 34 (21.3%) had ≥2 plaques (tandem lesions). A total of 176 plaques were measured.
Table 1.
Clinical characteristics of study population (n=80)
| Clinical variables | Mean ± standard deviation or n (%) | Range |
|---|---|---|
| Race* | ||
| Caucasian | 77 (97.5) | |
| Non-Caucasian | 2 (2.5) | |
| Sex | ||
| Male | 53 (66.2) | |
| Female | 27 (33.8) | |
| Age, years | 70 ± 8 | (51 – 85) |
| Body mass index – kg/m2 | 28 ± 6 | (18 – 58) |
| Smoker | 50 (62.5) | |
| Hypertension | 61 (76.2) | |
| Hyperlipidemia | 71 (88.8) | |
| Diabetes | 9 (11.2) | |
| Coronary artery disease | 22 (27.5) | |
| Peripheral artery disease | 18 (22.5) | |
| Antihypertensive use | 63 (78.8) | |
| Statin use | 65 (81.2) | |
| Antiplatelet use | 64 (80.0) | |
| Anticoagulant use | 1 (1.2) | |
One subject was excluded due to a missing value for race.
IPH was identified in 38 (21.6%) plaques, and was more likely found in males and subjects with lower diastolic blood pressure (BP) (Figure 1). Antiplatelet therapy was more potent on average in the IPH+ group (Table 2). Total cholesterol level tended to be lower in the IPH+ group, with borderline statistical significance. However, potential confounding factors were also different between the IPH+ and IPH− group. IPH+ plaques were significantly larger than IPH− plaques in terms of both wall thickness and plaque length. Furthermore, IPH was positively associated with markers of the extent of atherosclerosis, and medication use was numerically more prevalence in the IPH+ group.
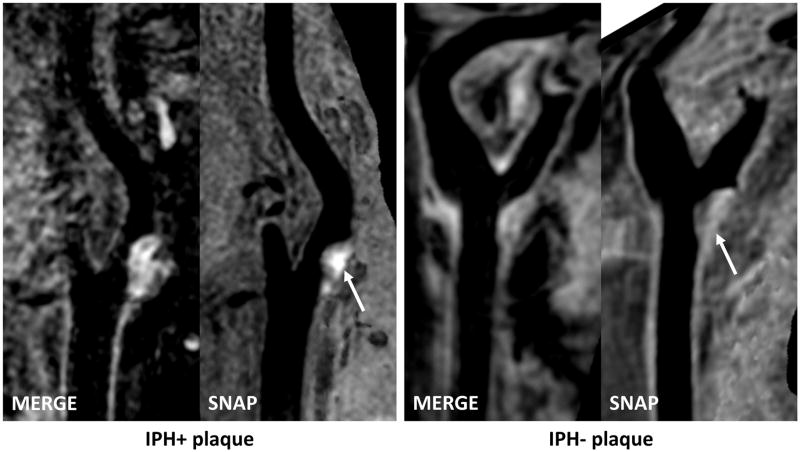
Figure 1. Plaques with and without IPH shown on isotropic three-dimensional MRI.
Left panel: Curved planar reformation (CPR) images show the left carotid artery of a 66-year old male (systolic blood pressure: 140 mmHg, diastolic blood pressure: 60 mmHg, antiplatelet use: dual therapy). Hyperintense signals on SNAP indicates IPH. Right panel: CPR images show right carotid artery of a 69-year old female (systolic blood pressure: 140 mmHg, diastolic blood pressure: 92.5 mmHg, antiplatelet use: aspirin alone).
Table 2.
Differences in potential risk factors between IPH+ and IPH− plaques
| IPH+ (n=38) | IPH− (n=138) | P value | |
|---|---|---|---|
| Potential risk factors
| |||
| Male sex | 33 (86.8) | 90 (65.2) | 0.008 |
| Age, years | 72 ± 8 | 71 ± 8 | 0.22 |
| Body mass index, kg/m2 | 27 ± 7 | 28 ± 6 | 0.64 |
| Smoker | 25 (65.8) | 87 (63.0) | 0.76 |
| Hypertension | 32 (84.2) | 101 (73.2) | 0.21 |
| Hyperlipidemia | 34 (89.5) | 121 (87.7) | 0.79 |
| Diabetes | 5 (13.2) | 12 (8.7) | 0.20 |
| Blood pressure* | |||
| Systolic blood pressure, mmHg | 141 ± 18 | 141 ± 18 | 0.88 |
| Diastolic blood pressure, mmHg | 75 ± 7 | 81 ± 10 | 0.001 |
| Pulse pressure, mmHg | 66 ± 16 | 61 ± 15 | 0.12 |
| Heart rate,* bpm | 63 ± 10 | 65 ± 12 | 0.16 |
| Total cholesterol, mg/dl | 146 ± 38 | 166 ± 37 | 0.048 |
| HDL cholesterol, mg/dl | 48 ± 12 | 50 ± 12 | 0.55 |
| eGFR, mL/min/1.73m2 | 79 ± 12 | 81 ± 11 | 0.61 |
| Antiplatelet use | 0.007† | ||
| None | 3 (7.9) | 31 (22.5) | |
| Aspirin alone | 28 (73.7) | 96 (69.6) | |
| Clopidogrel or dual therapy | 7 (18.4) | 11 (8.0) | |
|
| |||
| Potential confounding factors
| |||
| Plaque size | |||
| Plaque length, mm | 19.4 ± 8.5 | 11.1 ± 8.1 | <0.001 |
| Mean wall thickness, mm | 1.7 ± 0.0 | 1.4 ± 0.3 | 0.001 |
| Antihypertensive use | 0.15† | ||
| None | 4 (10.5) | 33 (23.9) | |
| Monotherapy | 11 (28.9) | 35 (25.3) | |
| Combination therapy | 23 (60.5) | 70 (50.7) | |
| Statin use | 34 (89.5) | 109 (79.0) | 0.22 |
| Systemic atherosclerosis | |||
| Clinically established ASCVD in other vascular beds | 24 (63.2) | 68 (49.3) | 0.048 |
| Ankle-brachial index<0.90 | 6 (15.8) | 14 (10.1) | 0.37 |
| Extent of atherosclerosis on MRI, % | 40.3 ± 14.7 | 33.6 ± 15.5 | 0.013 |
ASCVD indicates atherosclerotic cardiovascular disease; eGFR, estimated glomerular filtration rate.
Two plaques were excluded due to missing values for blood pressure and heart rate.
Trend test.
In age- and sex-adjusted models, male sex (odds ratio [OR with 95% confidence interval]: 3.5 [1.4–9.2]; p=0.010); diastolic BP (OR per 10 mmHg ↑: 0.49 [0.33–0.72]; p<0.001), and antiplatelet use (OR for aspirin alone vs. none: 2.4 [0.75–7.9]; OR for clopidogrel or dual therapy: 5.8 [1.3–24.6]; p=0.018) were significantly associated with IPH, while there was a trend for pulse pressure (OR per 10 mmHg ↑: 1.3 [0.97–1.8]; p=0.073) (Table 3). Adjusting for plaque morphology (mean wall thickness and plaque length) attenuated the associations with male sex and pulse pressure (Table 3). Both the strength and statistical significance of the association between IPH and diastolic BP remained after adjustments. A trend could still be seen for the association with antiplatelet use (p=0.07).
Table 3.
Associations between risk factors and IPH after adjusting for age, sex, and plaque size (n=176)
| Risk factors | Model 1: adjusted for age and sex | Model 2: model 1 + mean wall thickness | Model 3: model 2 + plaque length | |||
|---|---|---|---|---|---|---|
|
| ||||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Male sex | 3.5 (1.4–9.2) | 0.010 | 2.3 (0.86–6.3) | 0.10 | 2.5 (0.84–7.4) | 0.10 |
| Age, per 5-year ↑ | 1.2 (0.93–1.4) | 0.19 | 1.1 (0.84–1.3) | 0.66 | 1.1 (0.82–1.4) | 0.69 |
| Body mass index, per 1-SD ↑ | 0.93 (0.65–1.3) | 0.69 | 0.95 (0.67–1.3) | 0.76 | 1.0 (0.71–1.5) | 0.89 |
| Smoker | 0.90 (0.40–2.0) | 0.79 | 0.67 (0.28–1.6) | 0.37 | 0.48 (0.18–1.3) | 0.15 |
| Hypertension | 1.8 (0.62–5.0) | 0.29 | 1.8 (0.60–5.4) | 0.30 | 1.7 (0.51–5.9) | 0.38 |
| Hyperlipidemia | 1.0 (0.27–3.8) | >0.99 | 0.68 (0.19–2.5) | 0.57 | 0.43 (0.12–1.5) | 0.19 |
| Diabetes | 1.8 (0.74–4.4) | 0.19 | 2.1 (0.74–6.2) | 0.16 | 2.0 (0.67–6.1) | 0.21 |
| Blood pressure,* per 10 mmHg ↑ | ||||||
| Systolic blood pressure | 0.95 (0.74–1.2) | 0.66 | 0.95 (0.74–1.2) | 0.68 | 0.93 (0.69–1.3) | 0.63 |
| Diastolic blood pressure | 0.49 (0.33–0.72) | <0.001 | 0.52 (0.33–0.81) | 0.004 | 0.51 (0.30–0.88) | 0.016 |
| Pulse pressure | 1.3 (0.97–1.8) | 0.073 | 1.3 (0.91–1.7) | 0.16 | 1.2 (0.8–1.7) | 0.30 |
| Heart rate,* per 1-SD ↑ | 0.82 (0.60–1.1) | 0.19 | 0.80 (0.58–1.1) | 0.19 | 0.91 (0.61–1.4) | 0.63 |
| Total cholesterol, per 1-SD ↑ | 0.65 (0.33–1.3) | 0.21 | 0.69 (0.34–1.4) | 0.30 | 0.68 (0.33–1.4) | 0.28 |
| HDL cholesterol, per 1-SD ↑ | 1.0 (0.68–1.5) | 0.93 | 0.99 (0.65–1.5) | 0.98 | 1.2 (0.73–1.9) | 0.50 |
| eGFR, per 1-SD ↑ | 0.95 (0.59–1.5) | 0.84 | 0.87 (0.53–1.4) | 0.60 | 0.97 (0.53–1.7) | 0.91 |
| Antiplatelet use | 0.018† | 0.025† | 0.070† | |||
| None | reference | reference | reference | |||
| Aspirin alone | 2.4 (0.75–7.9) | 2.6 (0.66–10.2) | 3.1 (0.72–13.2) | |||
| Clopidogrel or dual therapy | 5.8 (1.3–24.6) | 6.5 (1.3–33.2) | 5.2 (0.87–31.6) | |||
Associations between potential risk factors and IPH were progressively adjusted for age, sex, and plaque size. CI indicates confidence interval; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; OR, odds ratio; SD, standard deviation.
Two plaques were excluded due to missing values in blood pressure and heart rate.
Trend test.
The association between diastolic BP and IPH was little changed after further adjustment for antihypertensive use and available markers indicating the extent of atherosclerosis (Table 4). Subgroup analysis was performed and limited to only plaques in subjects treated with antihypertensive medications, which comprised the majority of study sample (n=137). IPH remained associated with lower diastolic BP (75.3 ± 7.6 mmHg vs 81.1 ± 10.7 mmHg in IPH+ and IPH− groups, respectively; adjusted OR per 10mmHg ↑: 0.43 [0.23–0.80]; p=0.008) whereas there was little difference in treatment intensity (monotherapy vs. combination therapy: 32.4 vs. 67.6% in IPH+ plaques, 32.0 vs. 68.0% in IPH− plaques; p=0.97). Systolic and diastolic BP are correlated and pulse pressure is simply their difference. Therefore, we built a model including both systolic and diastolic BP (adjusting for age, sex and plaque morphology) to account for their correlation. In this model, diastolic BP showed an even stronger association with IPH (OR per 10 mmHg ↑: 0.34 [0.16–0.73]; p=0.005) while systolic BP was still not significantly associated with IPH (OR per 10 mmHg ↑: 1.3 [0.92–1.9]; p=0.13).
Table 4.
Impact of antihypertensive use and systemic atherosclerosis on the association between blood pressure and IPH (n=174*)
| Blood pressure parameters, per 10 mmHg ↑ | Model 4: model 3 + Antihypertensive use | Model 5: model 3 + Clinically established ASCVD in other vascular beds | Model 6: model 3 + ankle- brachial index<0.90 | Model 7: model 3 + extent of atherosclerosis on MRI | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Systolic blood pressure | 0.88 (0.65–1.2) | 0.41 | 0.93 (0.69–1.2) | 0.61 | 0.95 (0.70–1.3) | 0.74 | 0.97 (0.73–1.3) | 0.83 |
| Diastolic blood pressure | 0.47 (0.29–0.77) | 0.003 | 0.51 (0.29–0.88) | 0.017 | 0.52 (0.30–0.89) | 0.018 | 0.54 (0.32–0.90) | 0.018 |
| Pulse pressure | 1.2 (0.77–1.8) | 0.48 | 1.2 (0.86–1.7) | 0.26 | 1.3 (0.88–1.9) | 0.19 | 1.3 (0.91–1.8) | 0.15 |
Model 3 adjusted the association between blood pressure and IPH for age, sex, mean wall thickness and plaque length (See Table 3). The association between blood pressure and IPH was further adjusted for antihypertensive use (Model 4) and all available markers for the extent of atherosclerosis (Model 5–7). ASCVD indicates atherosclerotic cardiovascular disease; CI, confidence interval; OR, odds ratio; SD, standard deviation.
Two plaques were excluded due to missing blood pressure values.
For antiplatelet use, the question was whether the association was confounded by past medical history (i.e. clinical indications for antiplatelet agents). However, the trend remained after further adjusting for the presence of history of myocardial infarction, coronary artery bypass graft surgery, percutaneous coronary intervention, ischemic stroke, carotid endarterectomy, and peripheral artery disease (OR for aspirin alone vs. none: 3.1 [0.66–14.8]; OR for clopidogrel or dual therapy vs. none: 5.3 [0.80–35.0]; p=0.083).
Discussion
Several attempts have been made to explore associations between cardiovascular risk factors and IPH for various purposes. This study is one of the first to examine the associations specifically for understanding the pathogenesis of IPH. Accordingly, we sought to thoroughly inspect potential confounders that may influence the dependence and cause-effect direction of associations between cardiovascular risk factors and IPH in multivariate analysis. In an asymptomatic cohort with contemporary medical treatment, we noticed that IPH was more likely present in subjects with lower diastolic BP, of which the association remained statistically significant after several biologically motivated multivariate adjustments including antihypertensive use and markers for extent of systemic atherosclerosis. There was also an intriguing trend for the association between antiplatelet therapy and IPH, which was not explained by prior clinically established ASCVD. Collectively, our findings suggest a role of decreasing diastolic BP and neovessel rupture in the pathogenesis of IPH, which may extend our understanding of the complex relationship between BP and atherosclerosis.
As increasing evidence suggests that IPH is an undesirable pathologic event in the natural history of atherosclerosis,1–5,11,12 understanding the pathogenesis and contributing factors of IPH becomes of pivotal importance. Approaches to detect and measure IPH are limited. Due to the lack of established animal models of IPH, most studies have to be performed on human subjects, relying on either histopathological or MRI approaches. There are potential confounding factors in human studies which need to be considered to properly interpret associations between cardiovascular risk factors and IPH.
First, it is important to consider whether associations between risk factors and IPH are independent of plaque size. As a plaque feature that has been shown to promote plaque progression,1–3 IPH is expected to be seen more frequently in larger plaques. Most traditional risk factors have known associations with plaque burden. Indeed, the association of IPH with age was more often seen in studies that included a wider spectrum of plaque size, particularly if smaller plaques or normal arteries were included (Table I in the online-only Data Supplement). Our data, owing to the use of large-coverage 3D sequences, for the first time demonstrated that IPH is associated with longer and thicker plaques, highlighting the necessity of adjusting for plaque morphology in revealing pathophysiological relationships between cardiovascular risk factors and IPH. Second, the most suitable study populations for understanding associations between cardiovascular risk factors and IPH are those with distinct plaques on imaging, in which medication use is highly prevalent. Indications for certain medications can confound their associations with IPH, and associations between biomarkers and IPH need to be interpreted in the context of influential medications. For instance, both positive and negative associations between statin use and IPH have been observed in previous studies (Table I in the online-only Data Supplement). The former could be due to more clinical manifestations in the IPH+ group. Lastly, for BP in particular, it is necessary to examine whether the association with IPH is mediated by systemic atherosclerosis. Presence of IPH may indicate more extensive systemic atherosclerosis,20,24 to which our data have provided new supporting evidence. It is known that systemic atherosclerosis, particularly of central arteries, can cause increase in systolic BP and pulse pressure.31
To our knowledge, this is the first study to have thoroughly inspected all the above factors in multivariate analysis. The results support an independent association between diastolic BP and IPH. It is unlikely explained by systemic atherosclerosis, as adjusting for markers of the extent of atherosclerosis, including prior clinically established ASCVD in other vascular beds, low ankle-branchial index, and extent of atherosclerosis shown on large-coverage carotid MRI, had little effect on the observed association. Therefore, diastolic BP may be an unrecognized risk factor for IPH. The observation was made in a population of which the majority received antihypertensive treatment. Lower on-treatment diastolic BP but comparable treatment intensity between the IPH+ and IPH− group suggest two non-mutually exclusive possibilities: 1) pretreatment diastolic BP is lower in patients with IPH+ plaques; 2) patients whose diastolic BP has a larger decrease on antihypertensive therapy are more likely to develop IPH. The latter may be more likely in light of recent findings from the Rotterdam Study. In the Rotterdam Study, Selwaness et al26 also examined association between BP and IPH and noted that pulse pressure was the major component associated with IPH. Sample size difference between the two studies can explain the findings related to pulse pressure, but not those regarding diastolic BP. Differences in study population may have played a role. Although embedded in a population study, the Rotterdam study sample was enriched with plaques by applying a minimum wall thickness requirement (2 mm), and therefore was similar to the present study in most clinical characteristics. However, one notable difference is the lower rate of antihypertensive use in the Rotterdam sample (54% vs. 79%). No subgroup analysis in subjects with or without antihypertensive treatment was available in the study by Selwaness et al.26 Nonetheless, if patients whose diastolic BP has a larger decrease on antihypertensive treatment are more likely to develop IPH, increased antihypertensive use could contribute to a more distinct relationship. On the other hand, antihypertensives are expected to lower pulse pressure and may weaken the association of IPH with pulse pressure. Increase in systolic BP and decrease in diastolic BP can both lead to an increase in pulse pressure. When systolic and diastolic BP were both included in the multivariate model, it was seen that lower diastolic BP (at a fixed systolic BP) had a stronger association with IPH than higher systolic BP (at a fixed diastolic BP).
Our finding is in line with the notion that neovessel rupture is the primary source for IPH.32,33 Physiologically, hydrostatic pressure in capillaries such as vasa vasorum is controlled by arterioles according to local oxygen tension and therefore minimally influenced by systemic BP fluctuation.34 With an acute decrease in BP in the lumen, intraplaque hydrostatic pressure is suddenly decreased, resulting in increased pressure gradient across capillary walls of vasa vasorum. Before a new steady state is established gradually under the new BP level, the increased hydrostatic pressure gradient across capillary walls of vasa vasorum is expected to compromise microvascular integrity and increase the risk for IPH. Diastolic BP should be more relevant than systolic BP, as the highest hydrostatic pressure gradient is reached during diastole and the perfusion of vasa vasorum also mainly occur during diastole.35 The magnitude of acute decrease in diastolic BP is expected to be more critical than the absolute level it reaches. Some arterioles during neoangiogenesis may have weaker muscular wall (thus limited capability to control blood flow), in which high pulse pressure may exert some similar effect to cause IPH.
High BP, particularly systolic BP, is doubtlessly one important risk factor for ischemic stroke, which can be attributed to its role in the initiation and progression of atherosclerosis. However, the interplay between BP and atherosclerosis may be complex and vary at different stages of the disease process. In the early stage of atherosclerosis, the relationship appears to be monotonic and higher systolic BP promotes the progression of atherosclerosis.36,37 In the later stage of atherosclerosis when some distinct plaques have been well-vascularized by the proliferative vasa vasorum, an acute decrease in diastolic BP may adversely affect prognosis by causing IPH. Our findings highlight the need to understand the pathophysiology in managing cardiovascular risk factors, as the effects of risk factors may be multifold and what is seen in the general population may not be applicable to specific patient populations. Notably, when systolic and diastolic BP were both included into the model, higher systolic BP tended to increase the risk for IPH rather than being protective (OR per 10 mmHg ↑: 1.3 [0.92–1.9]; p=0.13) at the same level of diastolic BP. The detrimental effect of increasing systolic BP may still be there, but not as strong as decreasing diastolic BP.
Diabetes is associated with broad microvascular and macrovascular complications. Its exact effect on vasa vasorum and how such effect is related to atherosclerotic plaque progression remain to be studied. In an autopsy study on aortic atherosclerosis, Purushothaman et al38 found that plaques in diabetic patients had a higher density of neovessels and also more severe IPH. In our study, the multivariate adjusted association between diabetes and IPH showed a consistent signal but was not statistically significant (OR: 2.0 [0.67, 6.1]; p=0.21). Among all traditional risk factors, diabetes had the lowest prevalence in this study population, which could have led to wide confidence intervals and limited our ability to study the potential effect of diabetes. Moreover, diabetes duration is unknown in previous studies as well as in this one. From increased angiogenesis to IPH, it may require a longer period of exposure compared to the more straightforward relationship between BP and IPH. However, it is notable that a spectrum of structural changes, both promoting and damaging microvascular integrity, have been observed in diabetes in previous studies on diabetic retinopathy, such as basement membrane thickening, pericyte demise, and capillary occlusion.39 Whether similar changes occur in vasa vasorum and what the aggregate effect is on IPH remain to be examined.
The association between antiplatelet use and IPH showed an intriguing trend, which was not confounded by prior clinical conditions that could lead to antiplatelet use. Four other studies have reported an association of IPH with antiplatelet or anticoagulant use (Table I in the online-only Data Supplement), in two of which the data were adjusted for age and sex. Derksen et al14 found that coumarin derivative use was associated with IPH in carotid endarterectomy specimens (OR: 1.99 [1.05, 3.74]; p=0.03). Note that 93% of the subjects were on antiplatelet therapy as the study population were surgery patients. Liem et al28 recently reported in recently symptomatic patients that prior antiplatelet use was associated with IPH on carotid MRI (OR: 2.71 [1.12, 6.61]; p=0.03). Our finding, by examining the potency of antiplatelet therapy and adjusting for plaque size and clinical conditions in asymptomatic subjects, extends previous observations and reveals unrecognized potential risk of antiplatelet agents. Individualized anti-clotting therapy may be needed as risks may outweigh benefits in patients with IPH+ or well-vascularized plaques.
Limitations
To date, all studies examining the associations between risk factors and IPH, including the present study, are cross-sectional. These findings are intriguing and may have implications for BP control and antiplatelet therapy. However, prospective serial studies are needed to determine whether these factors are associated with the development of new or repeated IPH. Carotid MRI has the capability to characterize change in IPH and is thus compatible with prospective studies. Another limitation is the relatively small sample size. Although the statistical signal is strong, particularly regarding the relationship between BP and IPH, our findings should be considered hypothesis-generating and need to be tested in larger cohorts. A larger study would also be helpful in determining whether pulse pressure increase resulting from an increase in systolic BP poses additional risk for IPH at a given level of diastolic BP. Furthermore, various markers for the extent of atherosclerosis that were available were used to test if the observed low diastolic BP and high pulse pressure in IPH+ plaques were simply the result of more extensive systemic atherosclerosis. Although our data rejected this hypothesis, future studies should consider assessment of more direct measures of aortic atherosclerosis, such as plaque burden or pulse wave velocity. Lastly, as our data have pointed to plaque neovessels as the source of IPH, future mechanistic studies may incorporate techniques to directly image the extent of vasa vasorum and plaque neovasculature.
Conclusions
In a study population with high prevalence of antihypertensive use, IPH was found to be independently associated with low diastolic BP that was unlikely due to treatment difference or systemic atherosclerosis. BP appears to be a major modifiable risk factor implicated in the pathogenesis of IPH. If the time sequence of this relationship is confirmed in prospective studies, BP control may need to target for a more precise and stable level of diastolic BP to prevent new or repeated IPH.
Supplementary Material
Significance.
Atherosclerotic plaque progression is the major underlying cause for cardiovascular disease. Intraplaque hemorrhage is a common finding in human plaques and has been shown to accelerate plaque progression. Effective prevention and management strategies targeting intraplaque hemorrhage requires understanding of its pathogenesis. In a cohort in which 78.8% were on antihypertensive therapy, we found an independent pathophysiological relationship between lower diastolic blood pressure and intraplaque hemorrhage. There was also a trend indicating antiplatelet therapy increases the risk for intraplaque hemorrhage. Putting the findings into the perspective of hemodynamics, an acute decrease in diastolic blood pressure increases hydrostatic pressure gradient across capillary walls of plaque neovessels, which may compromise microvascular integrity and cause intraplaque hemorrhage. The new hypothesis established in this study, which highlights an unrecognized pathophysiological mechanism and has broad implications for vascular medicine, awaits to be tested in serial studies.
Acknowledgments
Sources of Funding: NIH R01 HL103609.
Abbreviations
- ASCVD
atherosclerotic cardiovascular disease
- BP
blood pressure
- IPH
intraplaque hemorrhage
- OR
odds ratio
Footnotes
Disclosures: DSH: research grants from Philips Healthcare and GE Healthcare. NB: research grant from Philips Healthcare. TH: research grants from Philips Healthcare. CY: research grants and advisory board of Philips Healthcare. All other authors have no conflict of interest to disclose.
References
- 1.Takaya N, Yuan C, Chu BC, Saam T, Polissar NL, Jarvik GP, Isaac C, McDonough J, Natiello C, Small R, Ferguson MS, Hatsukami TS. Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: a high-resolution magnetic resonance imaging study. Circulation. 2005;111:2768–2775. doi: 10.1161/CIRCULATIONAHA.104.504167. [DOI] [PubMed] [Google Scholar]
- 2.Underhill HR, Yuan C, Yarnykh VL, Chu B, Oikawa M, Polissar NL, Schwartz SM, Jarvik GP, Hatsukami TS. Arterial remodeling in the subclinical carotid artery disease. J Am Coll Cardiol Img. 2009;2:1381–1389. doi: 10.1016/j.jcmg.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun J, Underhill HR, Hippe DS, Xue Y, Yuan C, Hatsukami TS. Sustained acceleration in carotid atherosclerotic plaque progression with intraplaque hemorrhage: a long-term time course study. J Am Coll Cardiol Img. 2012;5:798–804. doi: 10.1016/j.jcmg.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun J, Balu N, Hippe DS, Xue Y, Dong L, Zhao X, Li F, Xu D, Hatsukami TS, Yuan C. Subclinical carotid atherosclerosis: short-term natural history of lipid-rich necrotic core--a multicenter study with MR imaging. Radiology. 2013;268:61–68. doi: 10.1148/radiol.13121702. [DOI] [PubMed] [Google Scholar]
- 5.Simpson RJ, Akwei S, Hosseini AA, MacSweeney ST, Auer DP, Altaf N. MR Imaging-Detected Carotid Plaque Hemorrhage Is Stable for 2 Years and a Marker for Stenosis Progression. AJNR Am J Neuroradiol. 2015;36:1171–1175. doi: 10.3174/ajnr.A4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagy E, Eaton JW, Jeney V, Soares MP, Varga Z, Galajda Z, Szentmiklosi J, Mehes G, Csonka T, Smith A, Vercellotti GM, Balla G, Balla J. Red cells, hemoglobin, heme, iron, and atherogenesis. Arterioscler Thromb Vasc Biol. 2010;30:1347–1353. doi: 10.1161/ATVBAHA.110.206433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, Narula J, Finn AV, Virmani R. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349:2316–2325. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- 8.Giannoglou GD, Koskinas KC, Tziakas DN, Ziakas AG, Antoniadis AP, Tentes IK, Parcharidis GE. Total cholesterol content of erythrocyte membranes and coronary atherosclerosis: an intravascular ultrasound pilot study. Angiology. 2009;60:676–682. doi: 10.1177/0003319709337307. [DOI] [PubMed] [Google Scholar]
- 9.Michel JB, Virmani R, Arbustini E, Pasterkamp G. Intraplaque haemorrhages as the trigger of plaque vulnerability. Eur Heart J. 2011;32:1977–1985. doi: 10.1093/eurheartj/ehr054. 1985a 1985b 1985c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leclercq A, Houard X, Philippe M, Ollivier V, Sebbag U, Meilhac O, Michel JB. Involvement of intraplaque hemorrhage in atherothrombosis evolution via neutrophil protease enrichment. J Leukoc Biol. 2007;82:1420–1429. doi: 10.1189/jlb.1106671. [DOI] [PubMed] [Google Scholar]
- 11.Saam T, Hetterich H, Hoffmann V, Yuan C, Dichgans M, Poppert H, Koeppel T, Hoffmann U, Reiser MF, Bamberg F. Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. J Am Coll Cardiol. 2013;62:1081–1091. doi: 10.1016/j.jacc.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Noguchi T, Kawasaki T, Tanaka A, Yasuda S, Goto Y, Ishihara M, Nishimura K, Miyamoto Y, Node K, Koga N. High-Intensity Signals in Coronary Plaques on Noncontrast T1-Weighted Magnetic Resonance Imaging as a Novel Determinant of Coronary Events. J Am Coll Cardiol. 2014;63:989–999. doi: 10.1016/j.jacc.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 13.Hellings WE, Peeters W, Moll FL, Piers SR, van Setten J, Van der Spek PJ, de Vries JP, Seldenrijk KA, De Bruin PC, Vink A, Velema E, de Kleijn DP, Pasterkamp G. Composition of carotid atherosclerotic plaque is associated with cardiovascular outcome: a prognostic study. Circulation. 2010;121:1941–1950. doi: 10.1161/CIRCULATIONAHA.109.887497. [DOI] [PubMed] [Google Scholar]
- 14.Derksen WJ, Peeters W, Tersteeg C, de Vries JP, de Kleijn DP, Moll FL, van der Wal AC, Pasterkamp G, Vink A. Age and coumarin-type anticoagulation are associated with the occurrence of intraplaque hemorrhage, while statins are associated less with intraplaque hemorrhage: a large histopathological study in carotid and femoral plaques. Atherosclerosis. 2011;214:139–143. doi: 10.1016/j.atherosclerosis.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 15.Altaf N, Daniels L, Morgan PS, Auer D, MacSweeney ST, Moody AR, Gladman JR. Detection of intraplaque hemorrhage by magnetic resonance imaging in symptomatic patients with mild to moderate carotid stenosis predicts recurrent neurological events. J Vasc Surg. 2008;47:337–342. doi: 10.1016/j.jvs.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 16.Singh N, Moody AR, Gladstone DJ, Leung G, Ravikumar R, Zhan J, Maggisano R. Moderate carotid artery stenosis: MR imaging-depicted intraplaque hemorrhage predicts risk of cerebrovascular ischemic events in asymptomatic men. Radiology. 2009;252:502–508. doi: 10.1148/radiol.2522080792. [DOI] [PubMed] [Google Scholar]
- 17.Kwee RM, van Oostenbrugge RJ, Prins MH, Ter Berg JW, Franke CL, Korten AG, Meems BJ, van Engelshoven JM, Wildberger JE, Mess WH, Kooi ME. Symptomatic patients with mild and moderate carotid stenosis: plaque features at MRI and association with cardiovascular risk factors and statin use. Stroke. 2010;41:1389–1393. doi: 10.1161/STROKEAHA.109.575670. [DOI] [PubMed] [Google Scholar]
- 18.Altaf N, Goode SD, Beech A, Gladman JR, Morgan PS, MacSweeney ST, Auer DP. Plaque hemorrhage is a marker of thromboembolic activity in patients with symptomatic carotid disease. Radiology. 2011;258:538–545. doi: 10.1148/radiol.10100198. [DOI] [PubMed] [Google Scholar]
- 19.Cheung HM, Moody AR, Singh N, Bitar R, Zhan J, Leung G. Late stage complicated atheroma in low-grade stenotic carotid disease: MR imaging depiction--prevalence and risk factors. Radiology. 2011;260:841–847. doi: 10.1148/radiol.11101652. [DOI] [PubMed] [Google Scholar]
- 20.Noguchi T, Yamada N, Higashi M, Goto Y, Naito H. High-Intensity Signals in Carotid Plaques on T1-Weighted Magnetic Resonance Imaging Predict Coronary Events in Patients With Coronary Artery Disease. J Am Coll Cardiol. 2011;58:416–422. doi: 10.1016/j.jacc.2011.01.056. [DOI] [PubMed] [Google Scholar]
- 21.Turc G, Oppenheim C, Naggara O, Eker OF, Calvet D, Lacour JC, Crozier S, Guegan-Massardier E, Henon H, Neau JP, Toussaint JF, Mas JL, Meder JF, Touze E. Relationships between recent intraplaque hemorrhage and stroke risk factors in patients with carotid stenosis: the HIRISC study. Arterioscler Thromb Vasc Biol. 2012;32:492–499. doi: 10.1161/ATVBAHA.111.239335. [DOI] [PubMed] [Google Scholar]
- 22.McNally JS, Kim S, Yoon H, Findeiss LK, Roberts JA, Nightingale DR, Narra KK, Parker DL, Treiman GS. Carotid Magnetization-Prepared Rapid Acquisition With Gradient-Echo Signal Is Associated With Acute Territorial Cerebral Ischemic Events Detected by Diffusion-Weighted MRI. Circ Cardiovasc Imaging. 2012;5:376–382. doi: 10.1161/CIRCIMAGING.111.967398. [DOI] [PubMed] [Google Scholar]
- 23.van den Bouwhuijsen QJ, Vernooij MW, Hofman A, Krestin GP, van der Lugt A, Witteman JC. Determinants of magnetic resonance imaging detected carotid plaque components: the Rotterdam Study. Eur Heart J. 2012;33:221–229. doi: 10.1093/eurheartj/ehr227. [DOI] [PubMed] [Google Scholar]
- 24.Singh N, Moody AR, Rochon-Terry G, Kiss A, Zavodni A. Identifying a high risk cardiovascular phenotype by carotid MRI-depicted intraplaque hemorrhage. Int J Cardiovasc Imaging. 2013;29:1477–1483. doi: 10.1007/s10554-013-0229-3. [DOI] [PubMed] [Google Scholar]
- 25.Hosseini AA, Kandiyil N, Macsweeney ST, Altaf N, Auer DP. Carotid plaque hemorrhage on magnetic resonance imaging strongly predicts recurrent ischemia and stroke. Ann Neurol. 2013;73:774–784. doi: 10.1002/ana.23876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selwaness M, van den Bouwhuijsen QJ, Verwoert GC, Dehghan A, Mattace-Raso FU, Vernooij M, Franco OH, Hofman A, van der Lugt A, Wentzel JJ, Witteman JC. Blood pressure parameters and carotid intraplaque hemorrhage as measured by magnetic resonance imaging: The Rotterdam Study. Hypertension. 2013;61:76–81. doi: 10.1161/HYPERTENSIONAHA.112.198267. [DOI] [PubMed] [Google Scholar]
- 27.Gupta A, Baradaran H, Kamel H, Mangla A, Pandya A, Fodera V, Dunning A, Sanelli PC. Intraplaque High-Intensity Signal on 3D Time-of-Flight MR Angiography Is Strongly Associated with Symptomatic Carotid Artery Stenosis. AJNR Am J Neuroradiol. 2014;35:557–561. doi: 10.3174/ajnr.A3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liem MI, Schreuder FHBM, van Dijk AC, de Rotte AAJ, Truijman MTB, Daemen MJAP, van der Steen AFW, Hendrikse J, Nederveen AJ, van der Lugt A, Kooi ME, Nederkoorn PJ. Use of Antiplatelet Agents Is Associated With Intraplaque Hemorrhage on Carotid Magnetic Resonance Imaging: The Plaque at Risk Study. Stroke. 2015;46:3411–3415. doi: 10.1161/STROKEAHA.115.008906. [DOI] [PubMed] [Google Scholar]
- 29.McLaughlin A, Hinckley PJ, Treiman SM, Kim SE, Stoddard GJ, Parker DL, Treiman GS, McNally JS. Optimal Prediction of Carotid Intraplaque Hemorrhage Using Clinical and Lumen Imaging Markers. AJNR Am J Neuroradiol. 2015 doi: 10.3174/ajnr.A4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun J, Hippe D, Underhill H, Chu B, Saam T, Takaya N, Cai J, Oikawa-Wakayama M, Yu W, Dong L, Yuan C, Hatsukami T. Incremental value of carotid intraplaque hemorrhage for discriminating prior coronary events. Neurovascular Imaging. 2015;1:1–7. [Google Scholar]
- 31.Safar ME, Blacher J, Mourad JJ, London GM. Stiffness of carotid artery wall material and blood pressure in humans: application to antihypertensive therapy and stroke prevention. Stroke. 2000;31:782–790. doi: 10.1161/01.str.31.3.782. [DOI] [PubMed] [Google Scholar]
- 32.Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–2061. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- 33.Sun J, Song Y, Chen H, Kerwin WS, Hippe DS, Dong L, Chen M, Zhou C, Hatsukami TS, Yuan C. Adventitial perfusion and intraplaque hemorrhage: a dynamic contrast-enhanced MRI study in the carotid artery. Stroke. 2013;44:1031–1036. doi: 10.1161/STROKEAHA.111.000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall JE, Guyton AC. Guyton and Hall textbook of medical physiology. 12. Philadelphia: Saunders/Elsevier; 2011. [Google Scholar]
- 35.Scotland RS, Vallance PJ, Ahluwalia A. Endogenous factors involved in regulation of tone of arterial vasa vasorum: implications for conduit vessel physiology. Cardiovasc Res. 2000;46:403–411. doi: 10.1016/s0008-6363(00)00023-7. [DOI] [PubMed] [Google Scholar]
- 36.Salonen R, Salonen JT. Carotid atherosclerosis in relation to systolic and diastolic blood pressure: Kuopio Ischaemic Heart Disease Risk Factor Study. Ann Med. 1991;23:23–27. doi: 10.3109/07853899109147926. [DOI] [PubMed] [Google Scholar]
- 37.Lakka TA, Salonen R, Kaplan GA, Salonen JT. Blood pressure and the progression of carotid atherosclerosis in middle-aged men. Hypertension. 1999;34:51–56. doi: 10.1161/01.hyp.34.1.51. [DOI] [PubMed] [Google Scholar]
- 38.Purushothaman KR, Purushothaman M, Muntner P, Lento PA, O’Connor WN, Sharma SK, Fuster V, Moreno PR. Inflammation, neovascularization and intra-plaque hemorrhage are associated with increased reparative collagen content: implication for plaque progression in diabetic atherosclerosis. Vasc Med. 2011;16:103–108. doi: 10.1177/1358863X11402249. [DOI] [PubMed] [Google Scholar]
- 39.Curtis TM, Gardiner TA, Stitt AW. Microvascular lesions of diabetic retinopathy: clues towards understanding pathogenesis? Eye (Lond) 2009;23:1496–1508. doi: 10.1038/eye.2009.108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.