Abstract
There have been substantial advances in neonatal medical care over the past two decades that have resulted in the increased survival of very low birth weight infants, survival that in some centers extends to 22 weeks gestational age. Despite these advances, there continues to be significant morbidity associated with extreme preterm birth that includes both short-term and long-term pulmonary and neurologic consequences. No single therapy has proven to be effective in preventing or treating either developmental lung and brain injuries in preterm infants or the hypoxic-ischemic injury that can be inflicted on the full-term brain as a result of in utero or perinatal complications. Stem cell-based therapies are emerging as a potential paradigm-shifting approach for such complex diseases with multifactorial etiologies, but a great deal of work is still required to understand the role of stem/progenitor cells in normal development and in the repair of injured tissue. This review will summarize the biology of the various stem/progenitor cells, their effects on tissue repair in experimental models of lung and brain injury, the recent advances in our understanding of their mechanism of action, and the challenges that remain to be addressed before their eventual application to clinical care.
Introduction
Scientific, medical, and technological advances in the field of perinatal-neonatal medicine have resulted in increased survival rates for extremely low birth weight, near-term, and term infants treated in neonatal intensive care units. However, respiratory and neurologic impairments continue to constitute the major adverse outcomes of neonatal intensive care unit survivors, resulting in life-long morbidities that include bronchopulmonary dysplasia (BPD) and several forms of brain injury. Based on a recent study from the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network Centers reporting on the 20-year trend in survival and outcomes of preterm infants, there have been overall modest reductions in several morbidities; however, the rates of BPD have increased 1. Specifically, from 2009 to 2012, BPD rates increased for all gestational ages from 22 up to 27 weeks with an overall incidence rate of 45% in this age group. BPD affects at least 10,000 preterm infants in the United States each year.
The pathophysiologic features and underpinning of BPD have evolved over the last two decades such that the BPD of today is a result of ‘reprogramming’ of normal lung growth characterized by reduced numbers of alveoli and fewer blood vessels but with less prominent fibrosis and airway lesions than the “old” BPD originally described by Northway et al. 2. Nonetheless, BPD outcomes remain associated with significant long-term pulmonary morbidities, including airway hyperreactivity, abnormal pulmonary function test results, and, in some cases, emphysematous changes that persist into adulthood. Moreover, secondary pulmonary hypertension has been reported in moderate to severe cases of BPD and is associated with increased mortality 3, 4. All in all, BPD is not just a disease of the neonatal period or even of early childhood, but rather a condition that carries lifelong consequences including the development of chronic obstructive pulmonary disease of adulthood. Specific therapies of BPD are lacking, and this disease persists despite gentler ventilation strategies and improvements in neonatal intensive care. Further, newly adopted drugs or tested therapies such as inhaled nitric oxide, antioxidants, vitamin A, caffeine, and others have either failed or have minimal effect on BPD outcomes. Steroids can decrease BPD but are either linked to long-term adverse neurologic outcomes or potentially associated with increased death rate as reported recently by the Neonatal European Study of Inhaled Steroids Trial Group 5. Thus, the search for better treatment strategies to prevent and treat BPD continues.
Periventricular leukomalacia (PVL) is a consequence of the same perinatal insults of inflammation and oxidative damage on a developing brain that form the underpinnings of BPD. The new PVL, like the new BPD, is different, presenting with more diffuse damage in the central cerebral white matter with secondary decreases in cortical gray matter volume but without cystic changes more typical of the focal necrosis deep inside the periventricular white matter of the classic cystic PVL. In addition to the preterm brain, the full-term brain is susceptible to hypoxic-ischemic injury as a consequence of inadequate blood flow and oxygen delivery. Hypoxic-ischemic encephalopathy (HIE) occurs in 1-3 term births per 1000 6 and can result from acute blood loss secondary to placental abruption, fetal maternal hemorrhage, or prolapsed umbilical cord, among other insults. Although therapeutic hypothermia has become standard therapy for HIE, there are still a large number of infants who die or go on to develop severe neurologic impairments and cerebral palsy 7. We are in urgent need of additional new treatments for this devastating disease, as well as new strategies to treat PVL, BPD, and other related complications of preterm birth that adversely affect the long-term normal development of these children.
Evidence of Stem Cell Depletion in PVL and BPD
The preterm infant is exposed to several risk factors that result in tissue damage of multiple organs through common mechanisms that include inflammation, infection, and ischemia/reperfusion, all promoting the production of free oxygen radicals. In addition, the premature deprivation of protective and nutritive factors provided by the placenta and the maternal circulation play a key role in exacerbating the injury. The common pathway of such insults leads to altered programming of development that is characterized by arrest in normal organ growth with tissue simplification. Emerging evidence suggests that loss of endogenous stem/progenitor cells required for normal cell differentiation and tissue repair may underlie the pathobiology of such injury.
Stem cells are primitive cells that can undergo self-renewal and have the potential to differentiate into multiple cell types. They are critical for normal development and also for the maintenance of normal physiology by contributing to organ repair and regeneration throughout life. Depletion or dysfunction of endogenous stem cells underlies disease development and aging. The complex architecture of the lung is maintained by the presence of rare populations of multipotent endogenous stem cells that are regulated by specific environmental signals. Their specific identification, characterization, and role in normal development and repair are being actively investigated. Further, stem cells from the bone marrow, the peripheral blood, or other tissue sources are also thought to participate in organ repair by their recruitment to sites of injury. Experimental and clinical studies support the notion that loss of circulating and tissue progenitor cells could be a mechanism underlying the abnormal developmental growth pattern in diseases such as BPD. For example, some reports have linked BPD risk with decreased endothelial progenitors in the circulation as assessed through clonogenic analysis of late outgrowth endothelial colony-forming cells (ECFCs) 8, 9. In addition, lung resident mesenchymal stem cells (MSCs) isolated from the tracheal aspirates of preterm neonates display an altered, proinflammatory phenotype 10, 11 suggesting that both depletion and dysfunction of endogenous progenitor stem cells may predispose to the development of BPD.
As in the developing lung, the developing brain is composed of progenitor cells that are critical for normal growth and repair. Premyelinating oligodendrocytes (preoligodendrocytes) are progenitor cells that are present in great abundance in the preterm brain and progressively differentiate to mature oligodendrocytes between 28 to 40 weeks gestational age to form the myelin sheath. Preoligodendrocytes but not mature oligodendrocytes are highly vulnerable to inflammation and oxidative stress, and are preferentially lost in response to perinatal insults 12. Loss of preoligodendrocytes results in hypomyelination of the central cerebral white matter and associated complications of gray matter loss that form the hallmark of diffuse PVL.
Altogether, accumulating evidence suggests an important role for progenitor stem cells in both supporting and maintaining normal organ growth in development and disease. Endogenous progenitor cells have been isolated from several sources and are increasingly being tested in experimental models of disease and in the case of MSCs, in several human trials including BPD. This review will summarize current knowledge on the different types of progenitor stem cells with a particular focus on the biology of MSCs and their protective role in experimental models of brain and lung injury, as well as their clinical application to date. Further, we shall summarize emerging evidence on their mechanisms of action that includes the shedding of exosomes, extracellular vesicles that carry and transmit key mediators of the MSC biologic effect.
Mesenchymal Stem Cell Characterization
Mesenchymal stromal (stem) cells have been extensively studied in preclinical models of disease and in human transplantation studies. They have the ability to self-renew and their progeny has the ability to differentiate into a variety of cell lineages. They were originally described as a fibroblast-like population in bone marrow, and, as the field evolved, they were denoted as bone marrow mesenchymal stem cells or multipotent mesenchymal stromal cells. MSCs have subsequently been detected and isolated from a variety of tissues, including adipose tissue, umbilical cord blood, Wharton's jelly, placenta, and even adult lung tissue 13-17. Minimal criteria were published in 2006 by the Mesenchymal & Tissue Stem Cell Committee of the International Society for Cellular Therapy, mainly based on the presence or absence of cell surface markers, adherence to plastic, and differentiation potential 18. Progress in the field since then, and better understanding of the biology and functional attributes of MSCs, have generated a call for invigoration of the criteria and definition of MSCs in order to establish a more relevant framework for their study and their potential therapeutic uses 19.
MSCs of bone marrow origin were first proven to have efficacy in models of acute and fibrotic lung injury. Therapeutic properties were subsequently demonstrated in various disease models with MSCs isolated from other tissues, including the human adult lung and human embryonic stem cells (hESC) stimulated to differentiate into MSCs 20, 21. In general, MSCs are easy to isolate in large numbers from different sources. Adipose-derived MSCs, for instance, are available in large quantities from liposuction procedures, and are thus considered major candidates for future approaches to regenerative medicine 22. Wharton's jelly MSCs are easy to isolate from the umbilical cord, a tissue normally discarded at birth, and, importantly, have the potential to be used in an autologous fashion.
Unlike ES cells, MSCs can be easily expanded in vitro under standard culture conditions while maintaining their undifferentiated state and continuing to express MSC markers for several passages. In addition, they do not carry the teratogenic potential of ES cells after in vivo transplantation 23. MSCs are also thought to be immunoprivileged in that they evade clearance by the recipient immune system 24 due to low expression of the major histocompatibility complexes and to their ability to inhibit proliferation and function of immune cells, such as dendritic cells, NK cells, and T and B lymphocytes 25-27. MSCs can also be genetically-engineered without losing their stem cell properties and their differentiation potential.
However, the role of MSCs in the field of regenerative medicine is not well defined and requires further study. Despite their ease of isolation and the existence of phenotypic markers for MSCs, no correlation between their phenotypic characteristics and immunomodulatory activity or lung regeneration capacity has been established. This is further complicated by the many sources of MSCs, the different culture techniques, as well as by species-specific properties. Although MSCs isolated from different sources express widely-accepted markers, adhere to plastic, and show baseline differentiation potential to osteoblast, chondroblast, and adipocyte lineage in vitro, there remains a large variation in differentiation capacity and gene transcription programs depending on the tissue source and species of these cells 28-30. Even if MSCs maintain their differentiation potential and differentiation markers after genetic manipulation or expansion in culture, these properties do not ensure immunomodulatory and regenerative capacity. Moreover, bone marrow-derived MSCs are the best characterized and have been shown to have cytoprotective effects; yet, they have also been reported to accumulate genetic mutations after extensive expansion 31-33. This genomic instability may account for the unusual in vitro behavior, with early cultures growing rapidly, but eventually giving rise to heterogeneous populations with variable properties that are often unable to reproduce the protective MSC effect.
Currently, our knowledge remains incomplete on the signals required to maintain the therapeutic/regenerative MSC phenotype upon expansion in vitro. In addition, because there is no one specific marker of MSC phenotype, we need greater insight into the cellular markers that define this phenotype, and the ISCT marker panel may not be sufficient in all cases. Further work is necessary to resolve the above ambiguities and develop rigorous standards to ensure reproducibility in preclinical studies. This hurdle is compounded by the fact that preclinical models of lung disease have diverse precipitating triggers, some more and some less relevant to human disease. Although lung inflammation is the most common denominator in these models, the choice of the particular model may be as important to a successful future translation to the clinic as the MSC phenotype used to treat the animals.
Mesenchymal Stem Cells in BPD—Preclinical Studies
Stem cell therapies have been extensively tested in preclinical models of BPD with encouraging results. Given the complex architecture of the lung, different cell therapy approaches have been implemented, including the use of 3D bioengineered and decellularized scaffolds populated with progenitor cells. To date, preclinical studies of BPD have focused on stem cells harvested from sources such as bone marrow, blood, umbilical cord, and adipose tissue and tested in the hyperoxia-induced neonatal murine and rat models, with results that show suppression of inflammation, improved survival, and marked attenuation in alveolar and lung vascular injury and the associated pulmonary hypertension 34-36. Administration of bone marrow MSCs on postnatal day 4 prevented the BPD changes in lung architecture and vascular remodeling evident at 14 days of hyperoxia in the control animals in both mouse 34 and rat models 36. Interestingly, the cell-free MSC-conditioned media (CM) afforded better protection than the cells in preserving the lung architecture and preventing alveolar loss in the Aslam et al., study 34. These experiments have been reproduced by several other groups using rodent bone marrow MSCs or MSCs derived from human cord blood and their cell-free CM 37-40. In this neonatal hyperoxia model, human umbilical cord blood (UCB)-derived MSCs delivered intratracheally (IT) were shown to confer significant protection only if they were administered in the early phase of inflammation, and, significantly, there were no synergies with combined early plus late MSC transplantation 41. Importantly, a single dose of bone marrow cell-free MSC-CM administered IV after 14 days of hyperoxia, when disease had already developed inhibited lung inflammation, reversed pulmonary hypertension and the pruning of the distal vascular tree, and markedly ameliorated lung fibrosis, collagen deposition, and alveolar destruction, although the lungs manifested some degree of residual emphysema compared with normoxic controls 42.
The active moieties that confer the therapeutic efficacy of MSCs remain elusive but likely include secreted proteins, nucleic acids, and membrane components, with, as discussed below, some potentially packaged in microvesicles released by MSCs. Use of the MSC secretome can represent an exciting and promising new approach to therapeutic interventions for certain lung diseases because it bypasses concerns associated with live cell treatments. Nevertheless, this is still a budding field; a recent paradigm that argues for the need to use live MSCs rather than merely their products demonstrates that mitochondrial transfer from MSCs to resident cells confers protection against lung injury 43. Beyond treating BPD pathology, administration of bone marrow-derived MSCs (BM-MSCs) or their secretome, has been shown to be effective in ameliorating injury and/or re-establishing homeostasis in several preclinical models of lung disease, including pulmonary hypertension, acute lung injury, pulmonary fibrosis, and asthma, among others 44-52.
Combined, the above studies support a protective role for bone marrow-derived MSCs as well as human cord blood MSCs or the MSC secretome in preventing or reversing injury in the neonatal rodent hyperoxic models of BPD. Long term follow-up of MSC-treated animals revealed a protective effect that is sustained at 100 days after a single dose of MSCs or their CM, both delivered IT, with cells having a better long lasting effect than the media 37. A six month follow-up evaluation after a single dose of IT MSCs or multiple intraperitoneal (IP) injections of MSC-CM for 17 days after hyperoxia in the neonatal rat resulted in improved exercise capacity and lung histology compared to untreated controls 40. The successful methods of delivery reported thus far include IV, IT, or IP, and all have shown therapeutic effects. The best route remains to be determined but one recent study suggested that IT administration of MSCs was more efficacious that IV using the neonatal rat hyperoxia model 53. In contrast, IV MSC injection halted lung deterioration whereas IT MSCs had no effect on lung histology or dysfunction in an adult mouse model of elastase-induced emphysema 54, pointing to potentially disease-specific and age-specific differential responses to the most optimal route for MSC delivery.
Other Stem Cell Therapies in Preclinical Models of BPD
The lung developmental reprogramming that underlies BPD is characterized by simplification of both the airway components as well as the vascular tree, with angiogenesis likely being the driver for both normal lung vascular and alveolar growth 55, 56. To specifically target the vascular aspects of lung growth, many investigators have examined vascular progenitor cells as potential treatment strategies for experimental BPD. In the past decade, endothelial progenitor cells (EPCs) have been investigated as biomarkers as well as cell therapy to promote angiogenesis and vascular repair in vivo. Asahara at al., revolutionized the field in 1997 with the identification of circulating EPCs in the peripheral blood that could differentiate into mature endothelial cells and be incorporated in the vessels of animal models of disease 57. Several subsequent studies showed the existence of more than one EPC phenotype, each with distinct growth and angiogenic characteristics. The isolated cells that form colonies within five days in culture (early out-growth EPCs) exhibit macrophage-like phagocytic activity, and promote angiogenesis but do not incorporate into blood vessels in vivo. The second population of putative EPCs isolated from peripheral blood is highly proliferative and generates colonies within 14 – 21 days (late outgrowth EPCs) 58, 59. It is these endothelial colony-forming cells (ECFCs) rather than the early out-growth, hematopoietic-like angiogenic progenitors that are able to incorporate in vivo and form chimeric vessels. Both early EPCs and ECFCs have been used as therapeutic agents in several preclinical models of systemic vascular disease as well as in models of BPD. Administration of bone marrow-derived angiogenic EPCs from healthy mice rescued the alveolar and vascular injury in hyperoxic mice 60. In a neonatal bleomycin model of lung injury, ECFCs and ECFC-CM prevented pulmonary hypertension but neither had an ameliorative effect on the architectural alveolar injury of this model 61. Intravenous delivery of human umbilical cord blood-derived ECFCs to immunocompromised mice prevented alveolar injury and pulmonary hypertension from hyperoxia exposure, both features of severe BPD 62. As was the case with MSCs, there was minimal engraftment of ECFCs in the recipient lungs and the reparative ECFC effect was recapitulated with the daily administration of cell-free media. The improvements in lung structure and function persisted for at least 10 months post-ECFC therapy in this model.
Cells isolated from the human amniotic epithelium (AECs) have also been explored in preclinical models of BPD. These cells are not well-characterized and likely constitute a mixed population with distinct regenerative and anti-inflammatory potential similar to MSCs. They have low immunogenic potential and are considered ‘stem-like’ as they are able to differentiate into mesodermal, ectodermal, and endodermal lineages in vitro 63. These cells were initially shown to have anti-fibrotic and anti-inflammatory effects in mice treated with bleomycin 64. Subsequently, using the fetal lamb model, intra-amniotic LPS-induced lung injury was not reduced with IV administration of AECs although there was significant reduction in inflammation 65. In a study using in utero ventilation of fetal sheep to induce BPD, IV plus IT administration of AECs successfully ameliorated lung structure with rare engraftment and transdifferentiation events pointing to paracrine mechanisms of protection 66.
For recent reviews on cell based therapies for the injured lung see 19, 67-72.
Lung Resident MSCs
Preclinical studies in lung injury models have predominantly investigated the effects of administration of exogenous stem cells derived from nonlung tissues. However, the lung harbors endogenous stem and progenitor cells which are rapidly mobilized upon cellular injury, proliferating and differentiating to repair and regenerate injured tissue. Among the epithelial progenitor cells that have been characterized in the mouse respiratory system are the basal cells of the proximal tracheobronchial region and submucosal glands, the secretory cells in the conducting airways as well as those of the bronchoalveolar duct junction. Such tissue resident progenitor cells are important regulators of pulmonary homeostasis and have crucial involvement in processes such as inflammation, angiogenesis, and fibrosis. Although the lung-resident MSCs represent a multipotent precursor population with reparative and regenerative functions, they may react in opposing ways to different environmental cues, and, under certain circumstances, actually participate in the pathology of disease (for recent reviews 70, 73).
In a bleomycin-induced model of pulmonary fibrosis, the pathology was associated with the loss of endogenous lung-resident MSCs and transplantation of lung-resident MSCs, isolated as the “side population” from naïve animals, ameliorated disease, and restored effector T-cell responses 74. Conversely, using a mouse model of targeted depletion of extracellular superoxide dismutase, Chow et al. reported that the increased oxidative stress generated dysfunctionality in the resident lung MSCs, causing them to contribute to pulmonary microvascular remodeling 75. Hyperoxic injury increases the activity of the beta-catenin pathway in a mouse model of BPD, and this pathway activation was also reported in lung-resident MSCs isolated from tracheal aspirates of premature infants with respiratory distress. Because activation of the beta-catenin pathway leads to myofibroblastic differentiation, the authors advance the premise that the increased numbers of dysfunctional lung-resident MSCs present in BPD patients may contribute to the pathology of this disease 76. The proposition that exogenous, nonlung MSCs exert their therapeutic effect by facilitating the mobilization of the lung-resident progenitor cells, or by protecting them from injury, was investigated in the hyperoxia-induced murine model of BPD 77. The lungs of neonatal mice exposed to hyperoxia and treated with BM-MSCs or media conditioned by BM-MSCs had a significantly higher number of bronchoalveolar stem cells (BASCs), an adult lung stem cell population capable of self-renewal and differentiation in vitro, and shown to proliferate in response to bronchiolar and alveolar lung injury in vivo. This is an intriguing observation that needs to be further investigated and validated.
The recurrent theme in the above studies appears to be that the therapeutic action of exogenous MSC treatment on lung injury is predominantly an immunomodulatory paracrine function. The primary observation is the suppression of inflammatory responses and, in the models tested, media conditioned by MSCs appear to be as efficacious as or even more efficacious than cell transplantation. On the other hand, based on the current literature, the role of lung resident MSCs in repairing injured tissue or contributing to the injury itself remains unresolved.
MSC in Brain Injury—Pre clinical
Stem cell treatments for neonatal brain injury have used neural stem cells, embryonic stem cells, bone marrow-derived MSCs, umbilical cord blood (UCB) stem cells, and iPS cells. Such treatments have been effective for the most part in conferring significant neuroprotection, neurogeneration, and improvement of functional outcomes in animal models of neonatal hypoxia-ischemia, cerebral palsy, and stroke (for recent reviews see 71, 78-85). For the purposes of this review we will focus on the use of BM-MSCs and UCB-MSCs. The demonstration of efficacy of human UCB-MSCs in these models was an especially promising development, since hematologic malignancies and immunodeficiencies are routinely treated with UCB transplantation. This led to the initiation of a number of clinical trials to study the role of autologous or allogeneic UCB transplantation in traumatic brain injury in children (http://clinicaltrials.gov).
As in the case of the models of MSC treatment for lung diseases, a consensus has been formed that donor MSCs do not survive long-term in the recipient brain, and they do not engraft or differentiate to replace damaged tissue directly but rather home to the injured tissue and respond to signals of local injury by secreting trophic and immunomodulatory moieties. The paracrine action of MSCs facilitates the repair process made by the parenchymal cells of the recipient, and such processes may include blood vessel regeneration, replacement of damaged nerve cells, and improved survival of intrinsic neuronal cells. The repair process is facilitated by a significant dampening of inflammatory pathways. Indeed, on the basis of in vitro studies, it has been suggested that the therapeutic function of MSCs resides in their ability to reprogram brain microglia into a unique M2-like polarization state characterized by increased phagocytic activity and upregulated expression of anti-inflammatory mediators (Figure). It has been suggested that this phenotypic switch contributes to the resolution of inflammation and to tissue repair 86. Just as in the lung, there are also suggestions that the donor MSC secretome may actually activate the endogenous tissue-resident stem cells and enhance their function, although, as in the lung, more studies are necessary to firmly establish this crucial concept.
Figure. A schematic on the parallel actions of MSC treatment on injured lung or brain.
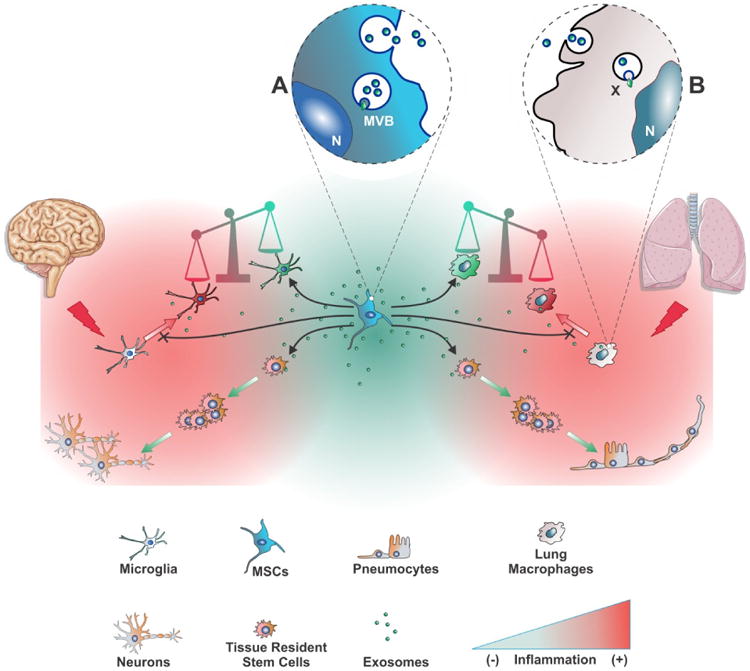
Tissue injury precipitates an inflammatory response by activating microglia and lung macrophages to a pro-inflammatory state (red icons). MSC transplantation restores homeostasis mainly though paracrine actions. Among such actions are the repression or reversal of the pro-inflammatory state, resulting in a shift in the balance towards an anti-inflammatory state (green icons). In the anti-inflammatory state, endogenous resident stem cells can repair tissue more efficiently. A possible parallel mechanism, as preliminary reports suggest, could be that MSC factors act directly on endogenous stem cells, mobilizing them to proliferate and differentiate. The main functional vector in the MSC secretome are extracellular vesicles, including exosomes, and the therapeutic effects of MSC treatment can be efficiently recapitulated by cell-free, exosomes-based treatment. (A): Biogenesis of exosomes in MSC multivesicular bodies (MVB) and release upon fusion of the MVB with the plasma membrane. (B): Uptake of MSC exosomes by a lung macrophage, and release of the exosomal cargo (X) into the recipient cell. N: nucleus.
Methods of delivery of donor MSCs to the injured brain include intraventricular injections, intravenous injections, and intranasal administration. The more complicated and direct intraventricular route is generally effective; for example, in a model of neonatal stroke with severe brain injury induced by middle cerebral artery occlusion, human UCB-MSCs transplanted into newborn rats six hours after the injury significantly improved the injury-associated abnormalities, including brain infarct volume, impaired functional tests, and histopathology (assessed up to 28 days post-injury) 87. Similarly, in the rat model of periventricular white matter injury induced by intracerebral injections of ibotenic acid at postnatal day five, transplantation of labeled neonatal rat MSCs resulted in significantly increased anti-myelin immunoreactivity in the corpus callosum, and improved reaching and retrieval skills 88. Intracardial injection has been used to deliver human BM-MSCs in a rat model of HIE with moderate efficacy, as indicated by significant improvement in lesion volume and moderate improvement in function 89. Studies in the same mouse model compared the IV route to the IP route in delivering MSCs to the brain, and, perhaps not surprisingly, found the former superior 90. The most efficient method of delivering MSCs to the injured brain appears to be the intranasal route. The long-term safety of intranasal administration of MSCs (0.5 million cells per animal), in the mouse model of HIE, was assessed by examining the recipient animals 14 months post-injection. Pathological analysis of 39 organs per animal revealed no significant increase in systemic pathological lesions or neoplasia in the nasal turbinates, brain, or other organs examined. The treated animals exhibited significant improvement of sensorimotor and cognitive functions compared with the group receiving the vehicle, and this improvement resulting from the MSC treatment was, in the authors' assessment, lifelong 91.
In the mouse model of neonatal HIE, mouse BM-MSC transplantation directly into the brain or through the intranasal route can improve functional outcomes, reduce lesion volume, increase differentiation of recently divided cells towards neurons and oligodendrocytes and decreased proliferating inflammatory cells, and can also significantly reduce the contralesional axonal remodeling induced by hypoxic-ischemic (HI) brain injury 92-94. A single dose was sufficient for a marked and long-lasting beneficial effect, and a minimum effective dosage of 0.5 million MSCs per animal was determined. The donor cells were observed to reach the lesion site 24 hours post-injection, and treatment at 10 days post HI was effective, but not at 17 days post-HI, indicating that an optimal therapeutic window exists. 95. Interestingly, the nature of protection depended on the time of delivery of MSCs to the injured brain. A single injection either at day three or day 10 post-insult increased neurogenesis, but in the case of two injections, one at day three and the second at day 10, the second MSC application did not increase neurogenesis but promoted corticospinal tract remodeling. This observation has led to the interesting suggestion that the function of MSCs is dictated by adaptive specific signals provided by the damaged and regenerating brain 96, 97.
In this model, it has been well established that the therapeutic action of the transplants is through secretion of trophic factors and stimulation of the endogenous repair processes 92, 93. Similarly, in the rat model of periventricular white matter injury, labeled donor rat BM-MSCs were observed to migrate from the injection site to the lesion area, but little evidence of differentiation of donor cells into neuronal phenotypes was found, indicating an indirect, probably paracrine mechanism for the MSC neuroprotective effect. 88. That this mechanism may involve enhancement of endogenous, tissue-resident stem cell function was suggested by in vitro studies where human BM-MSCs were shown to induce mouse neural stem cells to differentiate into neurons, leading the authors to suggest that this property represents a reflection of the neuroregenerative potential observed in vivo 98.
Genetically-modifying MSCs to overexpress protective molecules, such as trophic factors, can make them more efficacious, as shown by MSCs overexpressing and secreting brain derived neurotrophic factor (BDNF). Such modified cells were shown to regulate proliferation and differentiation of neural stem cells in vitro and in the mouse model of HIE, and to improve outcomes in vivo when compared with control (not BDNF-overexpressing) MSCs. This improvement was only partially or not at all recapitulated if other growth factors were overexpressed, instead of BDNF 99. Interestingly, in the rat model of neonatal stroke induced by transient middle cerebral artery occlusion, intranasal delivery of MSCs was efficacious in ameliorating white matter loss and improving function tests, but overexpression of BDNF by donor MSCs did not confer any additional long-lasting benefit 100, highlighting the restrictive range of the genetically-modified MSCs. A combination of hypothermia, the only clinically-available treatment currently for HIE, with human UBC-MSC transplantation was assessed and it was shown that the combination improves outcomes in the neonatal rat model of carotid artery ligation plus hypoxia. Although intraventricular MSC transplantation (0.1 million cells per animal) was more efficacious than hypothermia (target temperature at 32 degrees) at improving certain outcomes, a greater improvement in brain infarction volume, histopathology, cerebrospinal fluid cytokine levels, and function in behavioral tests was observed when the two therapies were combined 101.
Cell-Free Therapies--Exosomes
In the field of MSC research, once the consensus started to form that donor cells do not engraft long-term or differentiate in significant numbers in the injured recipient tissue, investigators, including our group, started to explore paracrine mechanisms to explain the robust cytoprotective effect of MSC treatment. It was soon demonstrated that administration of media conditioned by MSCs was as effective as the treatment with MSCs themselves in a number of animal models of disease 21, 34, 44, 102, 103, 104. Trophic factors and cytokines had been detected by proteomic analysis of media conditioned by MSCs, including VEGF, SDF-1, FGF, TGFβ, and IL-1ra, which appeared to be strong candidates for the promotion of angiogenesis, and cytoprotection against ischemic-hypoxic injury and tissue inflammation. None of these moieties, administered individually or in combination, was able to recapitulate the robust cytoprotective effect observed with MSCs or MSC-conditioned media treatment.45, 105-110. This is not surprising as the disease precipitated by the tissue injury is the result of dysfunction of complex multifactorial pathways. [Delivery of the active moieties of the MSC secretome to the right tissue compartment in a stable and bioavailable package is probably the key parameter of efficacy.]
Exosomes are a subset of the heterogeneous population of extracellular membrane-bound vesicles (EVs) produced by most, if not all, cell types, including MSCs, and they are also found in physiological fluids such as urine, plasma, cerebrospinal fluid, human milk, and exudates (reviewed in 111). They range in size from 30-150 nm in diameter, with a density of 1.13-1.19 g/mL as determined through ultracentrifugation on sucrose cushions or density gradients 112. Their biogenesis involves the endocytic pathway, where they are generated and stored within multivesicular bodies (MVBs) prior to their release into the environment through fusion of the MVB with the cell membrane 46, 113. In terms of their biogenesis and relatively uniform size, exosomes are quite distinct from other types of EVs, such as those shed directly from the plasma membrane by budding (often called ectosomes) and ranging in size from 50-1000 nm, and the much larger and heterogeneous population of apoptotic blebs (50-5000 nm) that are generated by disintegrating cells. As they are formed through the internal budding of the MVB membrane, exosomes can incorporate recycled cell membrane proteins, newly synthesized proteins directly from the Golgi and cytoplasmic moieties, functional mRNAs, miRNAs, and other types of nucleic acids.
Secreted exosomes can deliver their cargo to target cells, possibly guided by cell surface receptors such as tetraspanins, and modify the target's gene expression, signaling, and overall function. In the immune system, immature dendritic cells transfer MHC peptide molecules through exosomes to other dendritic cells to activate the immune response 114. Exosomes from a variety of sources have been reported to be engulfed by target cells, including macrophages, endothelial cells, or tumor cells 115-117, and the exosomal cargos have been postulated to represent a vector of genetic exchange and communication between cells 118.
Exosomes were originally described during reticulocyte maturation to mediate the selective externalization and removal of the transferrin receptor 119-121. Although the original and still major function of the endocytic pathway is a route to degrade in the lysosome, or to excrete and dispose of unwanted cellular components, it appears that, through evolution, components of the mechanism have assumed additional functions. Such functions may be deleterious, such as the hijacking of the system by retroviruses for their biogenesis 122-124, or beneficial, such as the development of a new level of intracellular signaling by the generation of the “signalosome,” an exosome harboring specific functional cargo that targets other cells (Figure).
Lung studies with exosomes
In proteomic analysis of the MSC secretome, our group identified many immunomodulators and matrix components, as well as proteins commonly associated with extracellular vesicles derived from different cell types. Many of these, including CD63, CD81, moesin, Alix, TSG101, and HSP70, are enriched in exosomes. The effect of exosomes in vitro has mostly been focused on their interaction with the immune system, including dendritic cell maturation, and Treg and B cell responses 111, 125, 126. Exosomes isolated from cardiac progenitor cells in culture protect from ischemia/reperfusion (I/R) injury when injected into the myocardium 103. Exosomes derived from dendritic cells modulate immune responses and inhibit rejection from heart transplantation 127. Lee et al. demonstrated that exosomes mediate the cytoprotective effect of bone marrow MSCs in hypoxia-induced pulmonary hypertension 128. In this model, administration of MSC-derived exosomes, identified through widely accepted exosomal markers and visualized by electron microscopy, protected against the elevation of right ventricular systolic pressure and the development of right ventricular hypertrophy after three weeks of hypoxic exposure, while microvesicle-depleted CM had no effect. Exosomal treatment was also able to abrogate early hypoxic macrophage influx and downregulate hypoxia-activated inflammatory pathways, thus mediating the anti-inflammatory properties of MSCs. Researchers have isolated and characterized exosomes from MSCs originating in almost all sources including from human embryonic stem cells, and the “MSC exosome” has been proposed as the alternative therapeutic vehicle for MSCs in many disease models 129-132. Such models of disease include a myocardial injury/reperfusion model in which MSC exosomes decreased infarct size and ameliorated reperfusion injury 133; a cisplatin-induced acute kidney injury model in the rat where adipose tissue MSC exosomes ameliorated oxidative stress and cell apoptosis, promoting cell proliferation in vivo and in vitro 46, 134; an acute kidney injury model where MSC exosomes activated a proliferative program in tubular cells 135, and an airway inflammation asthma model where MSC exosomes suppressed Th2/Th17-mediated allergic airway inflammation 52.
Exosomes released by MSCs may activate kinase pathways that are critical for ischemic preconditioning by increasing extracellular ATP levels and decreasing oxidative stress and inflammation 136-138. In a mouse model of hindlimb ischemia, microvesicles from endothelial progenitor cells improved neovascularization 139 by the transfer of miRNA or mRNA 140. The protection was lost with RNAse treatment or depletion of miR-126 and miR-296. The same mechanism is thought to protect the kidney from I/R through the miRNA-dependent reprogramming of renal resident cells imparted by the delivery of microvesicles released by EPCs or MSCs 141, 142. Mouse embryonic stem cell-derived exosomes were shown to enhance cardiac progenitor cell (CPC) function and to promote cardiac regeneration in a murine model of myocardial infarction 143. Enrichment of miR-294 in these exosomes was thought to be responsible for the enhanced CPC survival and proliferation.
Brain studies with exosomes
In the rat model of neonatal HIE, early reports suggested that CM of adipose-derived stem cells, delivered through the jugular vein, improved functional tests two months after the injury and significantly protected against hippocampal and cortical volume loss 104, although the exact therapeutic moiety was not identified. More recently, in adult animal models of brain injury, it is reported that the beneficial effects of MSC treatment resides with secreted exosomes/extracellular vesicles (EVs). The effect of treatment with MSC-derived EVs was assessed in a rat model of traumatic brain injury. The treatment involved a single administration through the tail vein of a preparation of EVs derived from rat MB-MSCs. The treated animals showed significant improvement in spatial learning and sensorimotor functional recovery assessed up to 34 days post-treatment. The authors observed a significant increase in the number of newly-generated endothelial cells in the lesion boundary zone and dentate gyrus, an increase in the number of newly-formed immature and mature neurons in the dentate gyrus, and a decrease in neuroinflammation, leading them to suggest that the protective effect of MSC exosomes was through immunomodulation and the promotion of endogenous angiogenesis and neurogenesis. 144. In another study, the effects of treatment by MSCs and MSC-derived EVs were systematically compared in a mouse stroke model of focal cerebral ischemia. MSCs were delivered to mice on day 1 post injury and EVs on days 1, 3, and 5 post-injury. Improvement in neurological impairment and long-term neuroprotection, associated with enhanced angioneurogenesis, was observed in animals receiving EVs, and the EV responses, which persisted throughout the observation period of 28 days, closely resembled responses to MSCs. Although the EVs did not affect cerebral immune cell infiltration, treatment resulted in attenuation in systemic immunosuppression at the earlier post-ischemia times 145. In summary, these and several other studies are emerging to support a key role for exosomes as messengers for intercellular signaling, with active investigation into their potential use as therapeutic agents.
Clinical Trials
For recent reviews on stem cell-based therapies in Clinical trials see 67, 146, 147
BPD Clinical Trials
Clinical trials that use MSCs for the treatment of a diverse number of diseases are increasing at a rapid pace (http://clinicaltrials.gov). The first clinical application of MSC therapy for human BPD was a single-center, phase I dose-escalation feasibility trial by Chang et al. 148. Nine preterm infants, born at 25.3 ± 0.9 weeks' gestational age assessed to be at highest risk of BPD, were treated with a single intratracheal dose of allogeneic, human UCB-MSCs at an average of 10.4 ± 2.6 postnatal days. The first three infants received 10 million cells/kg IT and since no immediate adverse events were noted, the second group of patients received a higher dose of 20 million cells/kg. The infants were monitored closely for signs of cardiorespiratory compromise around the time of MSC administration and followed for incidence and severity of BPD, markers of lung inflammation in tracheal aspirates, infections, and other multisystem complications. The treatments were well-tolerated without immediate side effects or dose-limiting toxicity, thus demonstrating both feasibility and short-term safety of allogeneic MSC administration in preterm neonates at the two tested doses. Because this is a phase I design, no conclusions can be drawn on the efficacy of MSC treatment in reducing the incidence and severity of BPD. There was reduction in inflammatory markers in the tracheal aspirates of treated infants, similar to the reported observations from several preclinical studies. These infants are currently being followed for long-term adverse outcomes and assessment of lung and neurologic health.
HIE Clinical Trial
No clinical trial has thus far been reported on the use of MSCs in neonatal brain injury. There is one report to date of autologous UCB transplantation tested in a phase I trial of 23 infants with HIE that met criteria and received concurrent therapeutic hypothermia 149. In this open-label study of noncryopreserved autologous volume- and red blood cell–reduced UCB cells, up to 4 doses of 10-50 million cells per dose were found to be both feasible and well-tolerated. Collection of UCB was successful even with small volumes of cord blood (range 3-178 mL) to achieve the goal dose for each infusion. No significant adverse reactions, cardiorespiratory compromise, or infections occurred as a result of the transfusions. Long-term outcomes are awaited from this trial and the anticipated phase II trial for both BPD and HIE.
Conclusions and Perspectives
It is clear that stem cell–based therapies represent the next breakthrough for diseases of the newborn where endogenous progenitor cells may be compromised from perinatal insults and thus contribute to the arrest in normal lung and neuronal growth underlying BPD, PVL, HIE, and other diseases of the developing neonate. MSCs are widely used in clinical trials on adults, and were approved in Canada for the treatment of graft-vs-host disease in children. Their isolation and production are relatively simple, and, unlike EPCs/ECFCs, their lack of immunogenicity makes MSCs an ideal stem cell candidate to be tested for the treatment of BPD and brain injury. However, unlike the adult population, additional precautions and long-term adverse consequences need to be considered for the immunocompromised, developing preterm infant. MSCs have the theoretical potential to enhance tumor growth or lead to malignant transformation, and this concern may be of particular significance in preterm infants due to their reduced immune surveillance. It is thus critical that all infants who receive MSCs or other stem cells, be monitored for several years for the theoretical risk of tumor formation. It is encouraging that a meta-analysis of MSC clinical trials from over 1000 patients showed no incidence of increased tumor risk up to 60 months post treatment 150. Administration of autologous stem cells obviates any concerns related to immunogenicity, however, stem cells derived from asphyxiated infants adds another level of concern regarding suboptimal efficacy. The better identification of cell populations in UCB that confer therapeutic effect, and the optimization of their isolation and delivery, in addition to determining the optimal dose response and number of doses to achieve efficacy without significant side effects, are unresolved issues. Further work is required to determine the appropriate time window for stem cell administration in order to maximize benefit while minimizing risk as well as to identify the best route and cell source or combination of stem cells to achieve maximal efficacy. Importantly, biomarkers of efficacy need to be developed and applied, including the use of imaging modalities and epigenetic and bioinformatic approaches to monitor therapeutic response and/or side effects.
Although each of these issues requires continued investigation, at the present time there is a pressing need to develop novel therapeutic modalities for these as yet incurable diseases that pose a huge burden to the patient, family, and society. Using the cancer field as a model, stem cell or cell-based transplantation should be widely investigated in well-designed, collaborative, multicenter clinical trials that have the power to demonstrate efficacy. In addition to MSCs or other stem cells, cell-free therapies obviating the theoretical tumorigenic potential of cell transplantation are on the horizon, including MSC-released extra-cellular vesicles/exosomes. As described above, these critical vectors of MSC action have the potential to deliver diverse signals, including proteins, lipids, and nucleic acid material such as mRNA or noncoding RNA, to the recipient cells and to alter signaling pathways to restore tissue function. Exosomes in peripheral blood likely reflect the state of the organ of exosomal origin and thus serve as biomarkers of disease, and as prognostic indicators of response to treatment or progression of disease. Exosomes from stem cells, such as MSCs, hold significant potential as therapeutic vehicles of epigenetic immunomodulatory signals to treat cardiovascular, pulmonary, renal, and neurologic injuries. The results from preclinical models are very promising, but a number of questions remain: is the production of therapeutic microvesicles a property of all MSCs, independent of their tissue of origin? This is important for future large-scale production and clinical translation, since adipose MSCs, umbilical cord blood, or Wharton's jelly MSCs can be obtained much easier than those from bone marrow. How do the MSC differentiation potential and passage in culture relate to microvesicle production? This reflects the hurdles associated with MSC characterization and phenotypic markers. Further critical issues will be the development of a ‘potency array’ and defining the effective therapeutic dose of MSC exosomes/microvesicles in the patient as well as the need for large-scale MSC cultures, which should be readily produced in bioreactors. In addition, we know very little about the microvesicle mechanism of action. What is the active cargo they contain and to what target cell in the injured lung or brain do they deliver it? Although the predominant effect appears to be immunomodulation, the pathways involved are not defined. Furthermore, the fact that a single dose of microvesicles has long lasting effects suggests epigenetic regulation of critical signaling pathways of immune function and organ homeostasis. Clearly, more work is required to better characterize the biology of MSC-released vesicles/exosomes, and the molecular and epigenetic mechanisms of their action on inflammatory pathways of injury and repair that underlie lung diseases such as BPD and the neurologic injuries affecting newborns such as PVL and HIE.
Acknowledgments
Supported in part by: the National Institute of Health Grants R01 HL055454 and R01 HL085446; and United Therapeutics Sponsored Research Grant
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
S. Alex Mitsialis, Email: Alex.mitsialis@childrens.harvard.edu.
Stella Kourembanas, Email: Stella.kourembanas@childrens.harvard.edu.
References
- 1.Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314:1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967;276:357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 3.Khemani E, McElhinney DB, Rhein L, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: Clinical features and outcomes in the surfactant era. Pediatrics. 2007;120:1260–1269. doi: 10.1542/peds.2007-0971. [DOI] [PubMed] [Google Scholar]
- 4.del Cerro MJ, Sabate Rotes A, Carton A, et al. Pulmonary hypertension in bronchopulmonary dysplasia: Clinical findings, cardiovascular anomalies and outcomes. Pediatr Pulmonol. 2014;49:49–59. doi: 10.1002/ppul.22797. [DOI] [PubMed] [Google Scholar]
- 5.Bassler D, Plavka R, Shinwell ES, et al. Early inhaled budesonide for the prevention of bronchopulmonary dysplasia. N Engl J Med. 2015;373:1497–1506. doi: 10.1056/NEJMoa1501917. [DOI] [PubMed] [Google Scholar]
- 6.Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86:329–338. doi: 10.1016/j.earlhumdev.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 8.Baker CD, Balasubramaniam V, Mourani PM, et al. Cord blood angiogenic progenitor cells are decreased in bronchopulmonary dysplasia. Eur Respir J. 2012;40:1516–1522. doi: 10.1183/09031936.00017312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borghesi A, Massa M, Campanelli R, et al. Circulating endothelial progenitor cells in preterm infants with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2009;180:540–546. doi: 10.1164/rccm.200812-1949OC. [DOI] [PubMed] [Google Scholar]
- 10.Popova AP, Bozyk PD, Bentley JK, et al. Isolation of tracheal aspirate mesenchymal stromal cells predicts bronchopulmonary dysplasia. Pediatrics. 2010;126:e1127–1133. doi: 10.1542/peds.2009-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bozyk PD, Popova AP, Bentley JK, et al. Mesenchymal stromal cells from neonatal tracheal aspirates demonstrate a pattern of lung-specific gene expression. Stem Cells Dev. 2011;20:1995–2007. doi: 10.1089/scd.2010.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93:F153–161. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romanov YA, Darevskaya AN, Merzlikina NV, Buravkova LB. Mesenchymal stem cells from human bone marrow and adipose tissue: Isolation, characterization, and differentiation potentialities. Bull Exp Biol Med. 2005;140:138–143. doi: 10.1007/s10517-005-0430-z. [DOI] [PubMed] [Google Scholar]
- 14.Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: Candidate msc-like cells from umbilical cord. Stem Cells. 2003;21:105–110. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- 15.Secco M, Zucconi E, Vieira NM, et al. Multipotent stem cells from umbilical cord: Cord is richer than blood! Stem Cells. 2008;26:146–150. doi: 10.1634/stemcells.2007-0381. [DOI] [PubMed] [Google Scholar]
- 16.Musina RA, Bekchanova ES, Sukhikh GT. Comparison of mesenchymal stem cells obtained from different human tissues. Bull Exp Biol Med. 2005;139:504–509. doi: 10.1007/s10517-005-0331-1. [DOI] [PubMed] [Google Scholar]
- 17.Peng L, Jia Z, Yin X, et al. Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev. 2008;17:761–773. doi: 10.1089/scd.2007.0217. [DOI] [PubMed] [Google Scholar]
- 18.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 19.Weiss DJ, Chambers D, Giangreco A, et al. An official american thoracic society workshop report: Stem cells and cell therapies in lung biology and diseases. Ann Am Thorac Soc. 2015;12:S79–97. doi: 10.1513/AnnalsATS.201502-086ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarvinen L, Badri L, Wettlaufer S, et al. Lung resident mesenchymal stem cells isolated from human lung allografts inhibit t cell proliferation via a soluble mediator. J Immunol. 2008;181:4389–4396. doi: 10.4049/jimmunol.181.6.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Koppen A, Joles JA, van Balkom BW, et al. Human embryonic mesenchymal stem cell-derived conditioned medium rescues kidney function in rats with established chronic kidney disease. PLoS One. 2012;7:e38746. doi: 10.1371/journal.pone.0038746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreml S, Babilas P, Fruth S, et al. Harvesting human adipose tissue-derived adult stem cells: Resection versus liposuction. Cytotherapy. 2009;11:947–957. doi: 10.3109/14653240903204322. [DOI] [PubMed] [Google Scholar]
- 23.de Sa Silva F, Almeida PN, Rettore JV, et al. Toward personalized cell therapies by using stem cells: Seven relevant topics for safety and success in stem cell therapy. J Biomed Biotechnol. 2012;2012:758102. doi: 10.1155/2012/758102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Blanc K, Ringden O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:321–334. doi: 10.1016/j.bbmt.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce t-cell unresponsiveness. Blood. 2005;105:2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 26.Duffy MM, Ritter T, Ceredig R, Griffin MD. Mesenchymal stem cell effects on t-cell effector pathways. Stem Cell Res Ther. 2011;2:34. doi: 10.1186/scrt75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: Evidence that activated nk cells are capable of killing mscs, whereas mscs can inhibit il-2-induced nk-cell proliferation. Blood. 2006;107:1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 28.Jansen BJ, Gilissen C, Roelofs H, et al. Functional differences between mesenchymal stem cell populations are reflected by their transcriptome. Stem Cells Dev. 2010;19:481–490. doi: 10.1089/scd.2009.0288. [DOI] [PubMed] [Google Scholar]
- 29.Kuang PP, Lucey E, Rishikof DC, Humphries DE, Bronsnick D, Goldstein RH. Engraftment of neonatal lung fibroblasts into the normal and elastase-injured lung. Am J Respir Cell Mol Biol. 2005;33:371–377. doi: 10.1165/rcmb.2004-0319OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai MS, Hwang SM, Chen KD, et al. Functional network analysis of the transcriptomes of mesenchymal stem cells derived from amniotic fluid, amniotic membrane, cord blood, and bone marrow. Stem Cells. 2007;25:2511–2523. doi: 10.1634/stemcells.2007-0023. [DOI] [PubMed] [Google Scholar]
- 31.Binato R, de Souza Fernandez T, Lazzarotto-Silva C, et al. Stability of human mesenchymal stem cells during in vitro culture: Considerations for cell therapy. Cell Prolif. 2013;46:10–22. doi: 10.1111/cpr.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foudah D, Redaelli S, Donzelli E, et al. Monitoring the genomic stability of in vitro cultured rat bone-marrow-derived mesenchymal stem cells. Chromosome Res. 2009;17:1025–1039. doi: 10.1007/s10577-009-9090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou YF, Bosch-Marce M, Okuyama H, et al. Spontaneous transformation of cultured mouse bone marrow-derived stromal cells. Cancer Res. 2006;66:10849–10854. doi: 10.1158/0008-5472.CAN-06-2146. [DOI] [PubMed] [Google Scholar]
- 34.Aslam M, Baveja R, Liang OD, et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009;180:1122–1130. doi: 10.1164/rccm.200902-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang YS, Choi SJ, Sung DK, et al. Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells dose-dependently attenuates hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2011;20:1843–1854. doi: 10.3727/096368911X565038. [DOI] [PubMed] [Google Scholar]
- 36.van Haaften T, Byrne R, Bonnet S, et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med. 2009;180:1131–1142. doi: 10.1164/rccm.200902-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sutsko RP, Young KC, Ribeiro A, et al. Long-term reparative effects of mesenchymal stem cell therapy following neonatal hyperoxia-induced lung injury. Pediatr Res. 2013;73:46–53. doi: 10.1038/pr.2012.152. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Wang H, Shi Y, et al. Role of bone marrow-derived mesenchymal stem cells in the prevention of hyperoxia-induced lung injury in newborn mice. Cell Biol Int. 2012;36:589–594. doi: 10.1042/CBI20110447. [DOI] [PubMed] [Google Scholar]
- 39.Chang YS, Oh W, Choi SJ, et al. Human umbilical cord blood-derived mesenchymal stem cells attenuate hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2009;18:869–886. doi: 10.3727/096368909X471189. [DOI] [PubMed] [Google Scholar]
- 40.Pierro M, Ionescu L, Montemurro T, et al. Short-term, long-term and paracrine effect of human umbilical cord-derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax. 2013;68:475–484. doi: 10.1136/thoraxjnl-2012-202323. [DOI] [PubMed] [Google Scholar]
- 41.Chang YS, Choi SJ, Ahn SY, et al. Timing of umbilical cord blood derived mesenchymal stem cells transplantation determines therapeutic efficacy in the neonatal hyperoxic lung injury. PLoS One. 2013;8:e52419. doi: 10.1371/journal.pone.0052419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansmann G, Fernandez-Gonzalez A, Aslam M, et al. Mesenchymal stem cell-mediated reversal of bronchopulmonary dysplasia and associated pulmonary hypertension. Pulm Circ. 2012;2:170–181. doi: 10.4103/2045-8932.97603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Timmers L, Lim SK, Arslan F, et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007;1:129–137. doi: 10.1016/j.scr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Kinnaird T, Stabile E, Burnett MS, Epstein SE. Bone-marrow-derived cells for enhancing collateral development: Mechanisms, animal data, and initial clinical experiences. Circ Res. 2004;95:354–363. doi: 10.1161/01.RES.0000137878.26174.66. [DOI] [PubMed] [Google Scholar]
- 46.Dorronsoro A, Robbins PD. Regenerating the injured kidney with human umbilical cord mesenchymal stem cell-derived exosomes. Stem Cell Res Ther. 2013;4:39. doi: 10.1186/scrt187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tetta C, Bruno S, Fonsato V, Deregibus MC, Camussi G. The role of microvesicles in tissue repair. Organogenesis. 2011;7:105–115. doi: 10.4161/org.7.2.15782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A. 2004;101:13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pant S, Hilton H, Burczynski ME. The multifaceted exosome: Biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol. 2012;83:1484–1494. doi: 10.1016/j.bcp.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rana S, Zoller M. Exosome target cell selection and the importance of exosomal tetraspanins: A hypothesis. Biochem Soc Trans. 2011;39:559–562. doi: 10.1042/BST0390559. [DOI] [PubMed] [Google Scholar]
- 51.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of sirna to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 52.Cruz FF, Borg ZD, Goodwin M, et al. Systemic administration of human bone marrow-derived mesenchymal stromal cell extracellular vesicles ameliorates aspergillus hyphal extract-induced allergic airway inflammation in immunocompetent mice. Stem Cells Transl Med. 2015;4:1302–1316. doi: 10.5966/sctm.2014-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sung DK, Chang YS, Ahn SY, et al. Optimal route for human umbilical cord blood-derived mesenchymal stem cell transplantation to protect against neonatal hyperoxic lung injury: Gene expression profiles and histopathology. PLoS One. 2015;10:e0135574. doi: 10.1371/journal.pone.0135574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tibboel J, Keijzer R, Reiss I, de Jongste JC, Post M. Intravenous and intratracheal mesenchymal stromal cell injection in a mouse model of pulmonary emphysema. COPD. 2014;11:310–318. doi: 10.3109/15412555.2013.854322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, flt-1, and tie-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;164:1971–1980. doi: 10.1164/ajrccm.164.10.2101140. [DOI] [PubMed] [Google Scholar]
- 56.Thebaud B, Ladha F, Michelakis ED, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: Evidence that angiogenesis participates in alveolarization. Circulation. 2005;112:2477–2486. doi: 10.1161/CIRCULATIONAHA.105.541524. [DOI] [PubMed] [Google Scholar]
- 57.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 58.Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 59.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balasubramaniam V, Ryan SL, Seedorf GJ, et al. Bone marrow-derived angiogenic cells restore lung alveolar and vascular structure after neonatal hyperoxia in infant mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L315–323. doi: 10.1152/ajplung.00089.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baker CD, Seedorf GJ, Wisniewski BL, et al. Endothelial colony-forming cell conditioned media promote angiogenesis in vitro and prevent pulmonary hypertension in experimental bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2013;305:L73–81. doi: 10.1152/ajplung.00400.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alphonse RS, Vadivel A, Fung M, et al. Existence, functional impairment, and lung repair potential of endothelial colony-forming cells in oxygen-induced arrested alveolar growth. Circulation. 2014;129:2144–2157. doi: 10.1161/CIRCULATIONAHA.114.009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, Manuelpillai U. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod. 2007;77:577–588. doi: 10.1095/biolreprod.106.055244. [DOI] [PubMed] [Google Scholar]
- 64.Moodley Y, Ilancheran S, Samuel C, et al. Human amnion epithelial cell transplantation abrogates lung fibrosis and augments repair. Am J Respir Crit Care Med. 2010;182:643–651. doi: 10.1164/rccm.201001-0014OC. [DOI] [PubMed] [Google Scholar]
- 65.Vosdoganes P, Hodges RJ, Lim R, et al. Human amnion epithelial cells as a treatment for inflammation-induced fetal lung injury in sheep. Am J Obstet Gynecol. 2011;205:156 e126–133. doi: 10.1016/j.ajog.2011.03.054. [DOI] [PubMed] [Google Scholar]
- 66.Hodges RJ, Jenkin G, Hooper SB, et al. Human amnion epithelial cells reduce ventilation-induced preterm lung injury in fetal sheep. Am J Obstet Gynecol. 2012;206:448 e448–415. doi: 10.1016/j.ajog.2012.02.038. [DOI] [PubMed] [Google Scholar]
- 67.Pawelec K, Gladysz D, Demkow U, Boruczkowski D. Stem cell experiments moves into clinic: New hope for children with bronchopulmonary dysplasia. Adv Exp Med Biol. 2015;839:47–53. doi: 10.1007/5584_2014_27. [DOI] [PubMed] [Google Scholar]
- 68.Sdrimas K, Kourembanas S. Msc microvesicles for the treatment of lung disease: A new paradigm for cell-free therapy. Antioxid Redox Signal. 2014;21:1905–1915. doi: 10.1089/ars.2013.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weiss DJ. Stem cells, cell therapies, and bioengineering in lung biology and diseases. Comprehensive review of the recent literature 2010-2012. Ann Am Thorac Soc. 2013;10:S45–97. doi: 10.1513/AnnalsATS.201304-090AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wansleeben C, Barkauskas CE, Rock JR, Hogan BL. Stem cells of the adult lung: Their development and role in homeostasis, regeneration, and disease. Wiley Interdiscip Rev Dev Biol. 2013;2:131–148. doi: 10.1002/wdev.58. [DOI] [PubMed] [Google Scholar]
- 71.Borghesi A, Cova C, Gazzolo D, Stronati M. Stem cell therapy for neonatal diseases associated with preterm birth. J Clin Neonatol. 2013;2:1–7. doi: 10.4103/2249-4847.109230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fung ME, Thebaud B. Stem cell-based therapy for neonatal lung disease: It is in the juice. Pediatr Res. 2014;75:2–7. doi: 10.1038/pr.2013.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Foronjy RF, Majka SM. The potential for resident lung mesenchymal stem cells to promote functional tissue regeneration: Understanding microenvironmental cues. Cells. 2012;1:874. doi: 10.3390/cells1040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jun D, Garat C, West J, et al. The pathology of bleomycin-induced fibrosis is associated with loss of resident lung mesenchymal stem cells that regulate effector t-cell proliferation. Stem Cells. 2011;29:725–735. doi: 10.1002/stem.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chow K, Fessel JP, Kaoriihida S, et al. Dysfunctional resident lung mesenchymal stem cells contribute to pulmonary microvascular remodeling. Pulm Circ. 2013;3:31–49. doi: 10.4103/2045-8932.109912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Popova AP, Bentley JK, Anyanwu AC, et al. Glycogen synthase kinase-3beta/beta-catenin signaling regulates neonatal lung mesenchymal stromal cell myofibroblastic differentiation. Am J Physiol Lung Cell Mol Physiol. 2012;303:L439–448. doi: 10.1152/ajplung.00408.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tropea KA, Leder E, Aslam M, et al. Bronchioalveolar stem cells increase after mesenchymal stromal cell treatment in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2012;302:L829–837. doi: 10.1152/ajplung.00347.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Titomanlio L, Kavelaars A, Dalous J, et al. Stem cell therapy for neonatal brain injury: Perspectives and challenges. Ann Neurol. 2011;70:698–712. doi: 10.1002/ana.22518. [DOI] [PubMed] [Google Scholar]
- 79.Verina T, Fatemi A, Johnston MV, Comi AM. Pluripotent possibilities: Human umbilical cord blood cell treatment after neonatal brain injury. Pediatr Neurol. 2013;48:346–354. doi: 10.1016/j.pediatrneurol.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 80.Chicha L, Smith T, Guzman R. Stem cells for brain repair in neonatal hypoxia-ischemia. Childs Nerv Syst. 2014;30:37–46. doi: 10.1007/s00381-013-2304-4. [DOI] [PubMed] [Google Scholar]
- 81.Carroll J. Human cord blood for the hypoxic-ischemic neonate. Pediatr Res. 2012;71:459–463. doi: 10.1038/pr.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gonzales-Portillo GS, Reyes S, Aguirre D, Pabon MM, Borlongan CV. Stem cell therapy for neonatal hypoxic-ischemic encephalopathy. Front Neurol. 2014;5:147. doi: 10.3389/fneur.2014.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fleiss B, Guillot PV, Titomanlio L, Baud O, Hagberg H, Gressens P. Stem cell therapy for neonatal brain injury. Clin Perinatol. 2014;41:133–148. doi: 10.1016/j.clp.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 84.Bennet L, Tan S, Van den Heuij L, et al. Cell therapy for neonatal hypoxia-ischemia and cerebral palsy. Ann Neurol. 2012;71:589–600. doi: 10.1002/ana.22670. [DOI] [PubMed] [Google Scholar]
- 85.van Velthoven CT, Kavelaars A, Heijnen CJ. Mesenchymal stem cells as a treatment for neonatal ischemic brain damage. Pediatr Res. 2012;71:474–481. doi: 10.1038/pr.2011.64. [DOI] [PubMed] [Google Scholar]
- 86.Hegyi B, Kornyei Z, Ferenczi S, et al. Regulation of mouse microglia activation and effector functions by bone marrow-derived mesenchymal stem cells. Stem Cells Dev. 2014;23:2600–2612. doi: 10.1089/scd.2014.0088. [DOI] [PubMed] [Google Scholar]
- 87.Kim ES, Ahn SY, Im GH, et al. Human umbilical cord blood-derived mesenchymal stem cell transplantation attenuates severe brain injury by permanent middle cerebral artery occlusion in newborn rats. Pediatr Res. 2012;72:277–284. doi: 10.1038/pr.2012.71. [DOI] [PubMed] [Google Scholar]
- 88.Chen A, Siow B, Blamire AM, Lako M, Clowry GJ. Transplantation of magnetically labeled mesenchymal stem cells in a model of perinatal brain injury. Stem Cell Res. 2010;5:255–266. doi: 10.1016/j.scr.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 89.Lee JA, Kim BI, Jo CH, et al. Mesenchymal stem-cell transplantation for hypoxic-ischemic brain injury in neonatal rat model. Pediatr Res. 2010;67:42–46. doi: 10.1203/PDR.0b013e3181bf594b. [DOI] [PubMed] [Google Scholar]
- 90.Ohshima M, Taguchi A, Tsuda H, et al. Intraperitoneal and intravenous deliveries are not comparable in terms of drug efficacy and cell distribution in neonatal mice with hypoxia-ischemia. Brain Dev. 2015;37:376–386. doi: 10.1016/j.braindev.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 91.Donega V, Nijboer CH, van Velthoven CT, et al. Assessment of long-term safety and efficacy of intranasal mesenchymal stem cell treatment for neonatal brain injury in the mouse. Pediatr Res. 2015;78:520–526. doi: 10.1038/pr.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Mesenchymal stem cell treatment after neonatal hypoxic-ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav Immun. 2010;24:387–393. doi: 10.1016/j.bbi.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 93.van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Nasal administration of stem cells: A promising novel route to treat neonatal ischemic brain damage. Pediatr Res. 2010;68:419–422. doi: 10.1203/PDR.0b013e3181f1c289. [DOI] [PubMed] [Google Scholar]
- 94.van Velthoven CT, van de Looij Y, Kavelaars A, et al. Mesenchymal stem cells restore cortical rewiring after neonatal ischemia in mice. Ann Neurol. 2012;71:785–796. doi: 10.1002/ana.23543. [DOI] [PubMed] [Google Scholar]
- 95.Donega V, van Velthoven CT, Nijboer CH, et al. Intranasal mesenchymal stem cell treatment for neonatal brain damage: Long-term cognitive and sensorimotor improvement. PLoS One. 2013;8:e51253. doi: 10.1371/journal.pone.0051253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Repeated mesenchymal stem cell treatment after neonatal hypoxia-ischemia has distinct effects on formation and maturation of new neurons and oligodendrocytes leading to restoration of damage, corticospinal motor tract activity, and sensorimotor function. J Neurosci. 2010;30:9603–9611. doi: 10.1523/JNEUROSCI.1835-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Mesenchymal stem cell transplantation changes the gene expression profile of the neonatal ischemic brain. Brain Behav Immun. 2011;25:1342–1348. doi: 10.1016/j.bbi.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 98.Donega V, Nijboer CH, Braccioli L, et al. Intranasal administration of human msc for ischemic brain injury in the mouse: In vitro and in vivo neuroregenerative functions. PLoS One. 2014;9:e112339. doi: 10.1371/journal.pone.0112339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van Velthoven CT, Braccioli L, Willemen HL, Kavelaars A, Heijnen CJ. Therapeutic potential of genetically modified mesenchymal stem cells after neonatal hypoxicischemic brain damage. Mol Ther. 2014;22:645–654. doi: 10.1038/mt.2013.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van Velthoven CT, Sheldon RA, Kavelaars A, et al. Mesenchymal stem cell transplantation attenuates brain injury after neonatal stroke. Stroke. 2013;44:1426–1432. doi: 10.1161/STROKEAHA.111.000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Park WS, Sung SI, Ahn SY, et al. Hypothermia augments neuroprotective activity of mesenchymal stem cells for neonatal hypoxic-ischemic encephalopathy. PLoS One. 2015;10:e0120893. doi: 10.1371/journal.pone.0120893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abman SH, Matthay MA. Mesenchymal stem cells for the prevention of bronchopulmonary dysplasia: Delivering the secretome. Am J Respir Crit Care Med. 2009;180:1039–1041. doi: 10.1164/rccm.200909-1330ED. [DOI] [PubMed] [Google Scholar]
- 103.Chen L, Wang Y, Pan Y, et al. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun. 2013;431:566–571. doi: 10.1016/j.bbrc.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wei X, Du Z, Zhao L, et al. Ifats collection: The conditioned media of adipose stromal cells protect against hypoxia-ischemia-induced brain damage in neonatal rats. Stem Cells. 2009;27:478–488. doi: 10.1634/stemcells.2008-0333. [DOI] [PubMed] [Google Scholar]
- 105.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 106.Nakagami H, Maeda K, Morishita R, et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25:2542–2547. doi: 10.1161/01.ATV.0000190701.92007.6d. [DOI] [PubMed] [Google Scholar]
- 107.Van Overstraeten-Schlogel N, Beguin Y, Gothot A. Role of stromal-derived factor-1 in the hematopoietic-supporting activity of human mesenchymal stem cells. Eur J Haematol. 2006;76:488–493. doi: 10.1111/j.1600-0609.2006.00633.x. [DOI] [PubMed] [Google Scholar]
- 108.Ortiz LA, Dutreil M, Fattman C, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 2007;104:11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kupcova Skalnikova H. Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie. 2013;95:2196–2211. doi: 10.1016/j.biochi.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 110.Lavoie JR, Rosu-Myles M. Uncovering the secretes of mesenchymal stem cells. Biochimie. 2013;95:2212–2221. doi: 10.1016/j.biochi.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 111.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 112.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3 doi: 10.1002/0471143030.cb0322s30. Unit 3 22. [DOI] [PubMed] [Google Scholar]
- 113.Thery C, Zitvogel L, Amigorena S. Exosomes: Composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 114.Segura E, Amigorena S, Thery C. Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood Cells Mol Dis. 2005;35:89–93. doi: 10.1016/j.bcmd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 115.Barres C, Blanc L, Bette-Bobillo P, et al. Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood. 2010;115:696–705. doi: 10.1182/blood-2009-07-231449. [DOI] [PubMed] [Google Scholar]
- 116.Tian T, Wang Y, Wang H, Zhu Z, Xiao Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J Cell Biochem. 2010;111:488–496. doi: 10.1002/jcb.22733. [DOI] [PubMed] [Google Scholar]
- 117.Skog J, Wurdinger T, van Rijn S, et al. Glioblastoma microvesicles transport rna and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mrnas and micrornas is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 119.Trams EG, Lauter CJ, Salem N, Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645:63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 120.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell. 1983;33:967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 121.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- 122.Gould SJ, Booth AM, Hildreth JE. The trojan exosome hypothesis. Proc Natl Acad Sci U S A. 2003;100:10592–10597. doi: 10.1073/pnas.1831413100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kramer B, Pelchen-Matthews A, Deneka M, Garcia E, Piguet V, Marsh M. Hiv interaction with endosomes in macrophages and dendritic cells. Blood Cells Mol Dis. 2005;35:136–142. doi: 10.1016/j.bcmd.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 124.Wurdinger T, Gatson NN, Balaj L, Kaur B, Breakefield XO, Pegtel DM. Extracellular vesicles and their convergence with viral pathways. Adv Virol. 2012;2012:767694. doi: 10.1155/2012/767694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kitazawa Y, Li XK, Xie L, Zhu P, Kimura H, Takahara S. Bone marrow-derived conventional, but not cloned, mesenchymal stem cells suppress lymphocyte proliferation and prevent graft-versus-host disease in rats. Cell Transplant. 2012;21:581–590. doi: 10.3727/096368911X605510. [DOI] [PubMed] [Google Scholar]
- 126.Budoni M, Fierabracci A, Luciano R, Petrini S, Di Ciommo V, Muraca M. The immunosuppressive effect of mesenchymal stromal cells on b lymphocytes is mediated by membrane vesicles. Cell Transplant. 2013;22:369–379. doi: 10.3727/096368911X582769. [DOI] [PubMed] [Google Scholar]
- 127.Peche H, Heslan M, Usal C, Amigorena S, Cuturi MC. Presentation of donor major histocompatibility complex antigens by bone marrow dendritic cell-derived exosomes modulates allograft rejection. Transplantation. 2003;76:1503–1510. doi: 10.1097/01.TP.0000092494.75313.38. [DOI] [PubMed] [Google Scholar]
- 128.Lee C, Mitsialis SA, Aslam M, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601–2611. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Parekkadan B, van Poll D, Suganuma K, et al. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2:e941. doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012;3:359. doi: 10.3389/fphys.2012.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yeo RW, Lai RC, Zhang B, et al. Mesenchymal stem cell: An efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65:336–341. doi: 10.1016/j.addr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 133.Lai RC, Arslan F, Lee MM, et al. Exosome secreted by msc reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 134.Zhou Y, Xu H, Xu W, et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013;4:34. doi: 10.1186/scrt194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bruno S, Grange C, Deregibus MC, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu GS, Thornton J, Van Winkle DM, Stanley AW, Olsson RA, Downey JM. Protection against infarction afforded by preconditioning is mediated by a1 adenosine receptors in rabbit heart. Circulation. 1991;84:350–356. doi: 10.1161/01.cir.84.1.350. [DOI] [PubMed] [Google Scholar]
- 137.Kitakaze M, Hori M, Takashima S, Sato H, Inoue M, Kamada T. Ischemic preconditioning increases adenosine release and 5′-nucleotidase activity during myocardial ischemia and reperfusion in dogs. Implications for myocardial salvage. Circulation. 1993;87:208–215. doi: 10.1161/01.cir.87.1.208. [DOI] [PubMed] [Google Scholar]
- 138.Arslan F, Lai RC, Smeets MB, et al. Mesenchymal stem cell-derived exosomes increase atp levels, decrease oxidative stress and activate pi3k/akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 139.Ranghino A, Cantaluppi V, Grange C, et al. Endothelial progenitor cell-derived microvesicles improve neovascularization in a murine model of hindlimb ischemia. Int J Immunopathol Pharmacol. 2012;25:75–85. doi: 10.1177/039463201202500110. [DOI] [PubMed] [Google Scholar]
- 140.Deregibus MC, Cantaluppi V, Calogero R, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mrna. Blood. 2007;110:2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 141.Gatti S, Bruno S, Deregibus MC, et al. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant. 2011;26:1474–1483. doi: 10.1093/ndt/gfr015. [DOI] [PubMed] [Google Scholar]
- 142.Cantaluppi V, Gatti S, Medica D, et al. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microrna-dependent reprogramming of resident renal cells. Kidney Int. 2012;82:412–427. doi: 10.1038/ki.2012.105. [DOI] [PubMed] [Google Scholar]
- 143.Khan M, Nickoloff E, Abramova T, et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res. 2015;117:52–64. doi: 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang Y, Chopp M, Meng Y, et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122:856–867. doi: 10.3171/2014.11.JNS14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Doeppner TR, Herz J, Gorgens A, et al. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med. 2015;4:1131–1143. doi: 10.5966/sctm.2015-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Trounson A, McDonald C. Stem cell therapies in clinical trials: Progress and challenges. Cell Stem Cell. 2015;17:11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 147.Ahn SY, Chang YS, Park WS. Stem cell therapy for bronchopulmonary dysplasia: Bench to bedside translation. J Korean Med Sci. 2015;30:509–513. doi: 10.3346/jkms.2015.30.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chang YS, Ahn SY, Yoo HS, et al. Mesenchymal stem cells for bronchopulmonary dysplasia: Phase 1 dose-escalation clinical trial. J Pediatr. 2014;164:966–972 e966. doi: 10.1016/j.jpeds.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 149.Cotten CM, Murtha AP, Goldberg RN, et al. Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. J Pediatr. 2014;164:973–979 e971. doi: 10.1016/j.jpeds.2013.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lalu MM, McIntyre L, Pugliese C, et al. Safety of cell therapy with mesenchymal stromal cells (safecell): A systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]


