Abstract
Background
Lower integrity of cerebral gray matter is associated with higher gait variability. It is not known whether gray matter integrity is associated with higher lap time variation (LTV), a clinically accessible measure of gait variability, high levels of which have been associated with mortality. This study examines the cross-sectional association between gray matter mean diffusivity (MD) and LTV in community-dwelling older adults.
Methods
Study participants consisted of 449 high-functioning adults aged 50 and older (56.8% female) in the Baltimore Longitudinal Study of Aging, free of overt neurological disease. The magnitude of MD in the gray matter, a measure of impaired tissue integrity, was assessed by diffusion tensor imaging in 16 regions of interest (ROIs) involved with executive function, sensorimotor function, and memory. LTV was assessed as variability in lap time based on individual trajectories over ten 40-m laps. Age, sex, height, and weight were covariates. The model additionally adjusted for mean lap time and health conditions that may affect LTV.
Results
Higher levels of average MD across 16 ROIs were significantly associated with higher LTV after adjustment for covariates. Specifically, higher MD in the precuneus and the anterior and middle cingulate cortices was strongly associated with higher LTV, as compared to other ROIs. The association persisted after adjustment for mean lap time, hypertension, and diabetes.
Conclusions
Lower gray matter integrity in selected areas may underlie greater LTV in high-functioning community-dwelling older adults. Longitudinal studies are warranted to examine whether changes in gray matter integrity precede more variable gait.
Keywords: Gray matter integrity, diffusion tensor imaging, mean diffusivity, lap time variation, high-functioning older adults
1. Introduction
Age-related increases in gait variability are commonly seen in community-dwelling older adults even in the absence of overt neurological disease and mobility limitation (Callisaya et al., 2010). Gait variability escalates with advancing age and is an important marker of health deterioration associated with high risk of adverse physical and cognitive health outcomes, including falls (Hausdorff et al., 2001, Callisaya et al., 2011), mobility disability (Brach et al., 2007), and dementia (Verghese et al., 2007). Thus, gait variability may serve as an early marker of impending gait and cognitive abnormalities. It is increasingly recognized that gait is a complex motor task that requires executive function and attention, especially in a free-living environment. Thus, it is currently believed that gait variability is caused by neurological dysfunction (Yogev-Seligmann et al., 2008).
Research on the relationship between brain structure and gait variability is just beginning to emerge. The specific brain regions involved in gait variability have not been clearly identified. What brain morphological characteristics are associated with high gait variability remains unclear. Initial neuroimaging evidence suggests that the sensorimotor area, and the prefrontal, temporal, and parietal lobes are important (Rosano et al., 2007, Zimmerman et al., 2009, Shimada et al., 2013, Annweiler et al., 2014, Beauchet et al., 2014, Beauchet et al., 2015). However, prior studies have either focused on one specific region or relied on volumetric measures of brain structure, such as atrophy. Evidence is sparse on the relationship between structural integrity and gait variability. Conventional magnetic resonance imaging (MRI) is insensitive to age-related and pathologic changes at the microstructural level. Subtle alterations in structural integrity are detectable by diffusion tensor imaging (DTI). Indicators of microstructural integrity may be sufficiently sensitive to characterize early neurodegeneration in regions that appear normal on standard volumetric MR images (for review, see (Moseley, 2002, Chua et al., 2008). Mean diffusivity is a commonly used indicator of the degree of diffusion in gray matter due to the isotropic, non-directional structure of gray matter tissue. To date, only one study has examined the spatial distribution of gray matter integrity in relation to step length variability, uncovering strong associations in the hippocampus and the anterior cingulate cortex (Rosso et al., 2014).
While gait speed is routinely assessed in clinical and research settings, analyzing gait variability from one step to the next can be costly and time consuming as it requires complex motion systems. Prior studies have mainly focused on step-to-step gait variability in temporal (e.g. stride time) and spatial (e.g. step length) parameters. In contrast, lap time variation (LTV) is a simple, clinically accessible measure of variability in lap time that can be measured from repeated laps. It is easy to implement in large population-based studies and also can provide informative data on walking patterns shown to predict mortality (Vestergaard et al., 2009). The correlation between step-to-step variability and LTV is currently unknown.
This study aimed to quantify the relationship between gray matter structural integrity, assessed by mean diffusivity (MD) on DTI MR, and LTV in community-dwelling older adults. We hypothesized that lower gray matter integrity would be associated with higher LTV. This study also explored the spatial distribution of gray matter structural integrity in relation to LTV. Because high gait variability is linked to poor sensorimotor and executive function (Rosano et al., 2008), we hypothesized that the association with LTV would be localized to regions important for sensorimotor and executive function.
2. Methods
2.1 Study population
This study used data from the ongoing Baltimore Longitudinal Study of Aging (BLSA) (Shock et al., 1984). Four hundred and seventy participants aged 50 and older underwent a brain MRI with DTI on a 3 Tesla scanner at the National Institute on Aging between 2009 and 2013. These participants were free of cognitive impairment, dementia, and Parkinson’s disease, and they did not have a history of clinical stroke. Diagnoses of dementia and Alzheimer’s disease were determined by the Diagnostic and Statistical Manual (DSM)-III-R and the National Institute of Neurological and Communication Disorders—Alzheimer’s Disease and Related Disorders Association criteria (McKhann et al., 1984), respectively. Among the 470 participants with a brain MRI, 449 men and women were healthy enough to complete the Long Distance Corridor Walk (LDCW). The study protocol was approved by the institutional review board of record at the time of data collection. All participants provided written informed consent.
2.2 Imaging data acquisition
Imaging data were acquired on 3 Tesla Philips Achieva scanner at the National Institute on Aging Clinical Research Unit in Baltimore, Maryland. Imaging evaluations for each participant included a T1-weighted magnetization-prepared rapid gradient-recalled echo (MPRAGE) scan, and two DTI scans.
The MPRAGE protocol was as follows: number of slices=170, voxel size=1mm×1mm×1.2mm, reconstruction matrix=256×256, flip angle=8 degrees and TR/TE=6.5ms/3.1ms.
The DTI protocol included two acquisitions of the following: number of gradients=32, max b-factor=700 s/mm2, TR/TE=7454ms/75ms, number of slices=70, voxel size=0.81mm×0.81mm×2.2mm, reconstruction matrix=320×320, acquisition matrix=116×115, field of view=260mm×260mm, flip angle=90 degrees. Each DTI acquisition included two b0 images, which were averaged in k-space. The two separate DTI acquisitions with NSA=1 were obtained and then combined offline (as explained in Image processing below) for an effective NSA=2 to improve signal-to-noise ratio.
2.3 Image processing
DTI processing follows standard practice for tensor fitting and quality assessment and is explained in detail in earlier publications (Lauzon et al., 2013). Briefly, the individual diffusion weighted volumes were affine co-registered to a minimally weighted (b0) target to compensate for eddy current effects and physiological motion. The gradient tables were corrected for the identified rotational component using finite strain (Alexander et al., 2001). To combine the two DTI sessions with different and unknown intensity normalization constants, each diffusion-weighted image was normalized by its own reference image prior to tensor fitting.
To segment gray matter regions, we used multi-atlas registration with 35 manually labeled atlases from NeuroMorphometrics with the BrainCOLOR protocol (Klein et al., 2010). The labels of regions of interest (ROIs) obtained from the T1 image for each visit were affine registered to the diffusion image and used to extract region-specific average MD measures. Quality control (QC) was performed to remove scans with either excessive motion or images that had globally high diffusion measure bias by reviewing the distributions of QC summary statistics generated by our pipeline (Lauzon et al., 2013).
The MD in each individual region was computed as a weighted average of left and right hemispheres using regional volumes. 16 ROIs were selected a priori based on their known associations with mobility. These ROIs were grouped into three domains based on their predominant roles in behavioral functions, consisting of sensorimotor (precentral and postcentral gryus, supplementary motor cortex, putamen, caudate, thalamus proper) and executive function (middle frontal gyrus, superior parietal lobe) and memory (hippocampus, parahippocampus, entorhinal cortex, amygdala) (Seidler et al., 2010). In addition, two regions were considered multimodal based on their roles in all three functions noted above (precuneus, posterior cingulate cortex). Another two regions were considered to be integrating areas (anterior and middle cingulate cortices). The anterior cingulate cortex was involved with memory and executive function and the middle cingulate cortex was involved with sensorimotor and executive function. The average MD across 16 ROIs selected in this study was computed as a summary score.
2.4 Mobility measures
LTV was obtained from the LDCW. As administered in the BLSA, the LDCW consists of a 2.5-minute walk done at a normal pace followed immediately by a 400-meter walk done as quickly as possible. The course is 20 meters long in an uncarpeted corridor marked by orange traffic cones at either end. Participants are instructed to walk to the far cone and back for ten 40-meter laps (Simonsick et al., 2001). Participants receive encouragement and feedback on laps remaining after completion of each lap.
In order to control for the effect of fatigue-related slowing on variability, a detrended SD of lap time was computed as a more accurate measure of variability than the SD of mean lap time alone (Simonsick et al., 2014, Tian et al., 2015). For each participant, mean lap time (in seconds) was computed as the arithmetic mean of the time to complete each of the ten 40-meter laps. LTV was measured as standard deviation of the residuals of lap time over ten laps. First, the individual trajectory was computed from a participant-specific regression of lap time on lap number using linear random-effects models with random intercepts and slopes. Then, the residual was computed as the difference between the lap time from each lap and the predicted lap time based on participant-specific regression of lap time on lap number. Finally, the SD of the residuals from ten laps was obtained and used in the analysis.
2.5 Other measures of interest
Demographic information was collected at the time of brain imaging and the 400-meter walk, including age, sex, race, years of education, height, and weight. Health-related conditions considered as potential confounders included smoking status, heart attack, hypertension, and diabetes. Disease status was obtained by self-reported diagnoses during medical interviews.
Rapid gait speed was measured at a fast pace over 6 meters in an uncarpeted corridor. Participants were asked to walk at their fast pace. Time to complete the 6 meter course was measured. Two trials were completed and the mean of two trials was used for analysis in meters/second.
2.6 Statistical analysis
Bivariate associations of sample characteristics and MD with LTV were examined using Pearson correlation coefficients or independent t-tests as appropriate. To examine whether MD in selected ROIs was associated with LTV independent of covariates, regression analysis was performed relating average MD across selected ROIs to LTV with adjustment for initial entry of age, sex, height, and weight. A p<0.05 indicated the average MD in ROIs was significantly related to LTV.
Cross-sectional associations between MD in each ROI and LTV were analyzed using multiple linear regression models. Models included MD in each ROI as the independent variable and LTV as the dependent variable, adjusted for age, sex, height, and weight. LTV was log transformed due to its skewed distribution. Since this was an exploratory analysis, we report raw p-values and define the statistical significance as p<0.05. However, to facilitate multiple comparisons adjustment for the 16 ROIs, we also report the Bonferroni cut-off of 0.05/16 = 0.0031.
To examine the strength of the relationships, models were additionally adjusted for mean lap time as well as health conditions that were related to LTV. Sensitivity analyses were performed by excluding 6 participants who used a walking aid during the 400-meter walk.
As a sensitivity analysis, we performed additional voxel-based morphometry (VBM) analyses (Ashburner and Friston, 2000) to check the pattern of the associations examined by the current ROI approach. All VBM analyses were conducted using Statistical Parametric Mapping 8 (Penny et al., 2011) and VBM toolbox (Draganski et al., 2006). In the preprocessing, the MD image from each participant was co-registered to three-dimensional T1-weighted image. Then, the T1-weighted image was normalized to the standard space using the “New Segment” toolbox (Weiskopf et al., 2011) and the normalization parameter was applied to co-registered MD image. Lastly, the normalized MD maps were smoothed using 8-mm isotropic Gaussian Kernels. In statistical analyses, we investigated the correlation between MD and lap time variation using the general linear model with adjustment for all covariates (age, sex, height, weight, mean lap time, hypertension, and diabetes) Significance was reported as family discovery rate (FDR)-corrected p<0.05 with a cluster size greater than 60 voxels.
3 Results
Table 1 describes sample characteristics, MD in each ROI, and mobility measures as well as their bivariate associations with LTV. Older age and shorter height were associated with higher LTV. Caucasians had higher LTV than non-white as did persons with hypertension. Those with diabetes had marginally significantly higher LTV than those without, but neither smoking status nor past heart attack were associated with LTV. In bivariate analysis, MD in selected ROIs was positively associated with LTV. Longer mean lap time and slower rapid gait speed were associated with higher LTV.
Table 1.
Sample characteristics and mobility measures and their associations with lap time variation (n=449)
| Mean ± SD or n (%) | Range | Correlations with lap time variation, r (p) or MD±SE (p) | |
|---|---|---|---|
| Demographics | |||
| Age, yrs | 70.8±9.7 | 50.2–94.9 | 0.342 (<0.001) |
| Female | 256 (56.8) | - | 0.024±0.038 (0.531) |
| White | 284 (63.3) | - | 0.086±0.039 (0.029) |
| Education, yrs | 17.0±2.7 | 8–32 | 0.011 (0.809) |
| Height, cm | 167.4±9.3 | 141.6–192.4 | −0.176 (<0.001) |
| Weight, kg | 76.3±15.1 | 43.1–138.6 | 0.028 (0.548) |
| Health conditions | |||
| Former or current smokers | 167 (37.2) | - | 0.020±0.039 (0.614) |
| Heart attack | 13 (2.9) | - | −0.104±0.067 (0.360) |
| Hypertension | 221 (49.2) | - | 0.085±0.038 (0.026) |
| Diabetes | 85 (18.9) | - | 0.114±0.060 (0.060) |
| Mean diffusivity in ROIs^, unitless | |||
| Precentral gyrus | 1.107±0.117 | 0.872–1.831 | 0.159 (0.001) |
| Postcentral gyrus | 1.143±0.119 | 0.907–2.116 | 0.192 (<0.001) |
| Supplementary motor cortex | 1.041±0.110 | 0.812–1.918 | 0.217 (<0.001) |
| Precuneus | 1.146±0.126 | 0.865–1.858 | 0.335 (<0.001) |
| Putamen | 0.811±0.108 | 0.603–1.968 | 0.253 (<0.001) |
| Caudate | 0.857±0.126 | 0.653–2.176 | 0.232 (<0.001) |
| Thalamus Proper | 0.992±0.096 | 0.809–2.093 | 0.287 (<0.001) |
| Middle frontal gyrus | 1.125±0.090 | 0.916–1.415 | 0.204 (<0.001) |
| Superior parietal lobe | 1.085±0.116 | 0.801–2.146 | 0.231 (<0.001) |
| Anterior cingulate gyrus | 1.224±0.140 | 0.415–1.828 | 0.315 (<0.001) |
| Middle cingulate gyrus | 1.183±0.120 | 0.924–1.855 | 0.291 (<0.001) |
| Posterior cingulate gyrus | 1.236±0.133 | 0.925–1.710 | 0.333 (<0.001) |
| Hippocampus | 1.320±0.170 | 0.970–2.365 | 0.289 (<0.001) |
| Parahippocampal gyrus | 1.353±0.164 | 1.020–2.511 | 0.297 (<0.001) |
| Entorhinal area | 1.388±0.287 | 0.903–2.844 | 0.178 (<0.001) |
| Amygdala | 1.001±0.154 | 0.764–2.463 | 0.189 (<0.001) |
| Mobility measures | |||
| Lap time variation, sec | 0.81±0.40 | 0.20–3.46 | - |
| Use of walking aid | 6 (1.3) | - | - |
| Mean lap time, sec | 27.5±6.4 | 15.7–72.8 | 0.621 (<0.001) |
| Rapid gait speed, m/sec | 3.7±0.9 | 1.9–8.7 | 0.383 (<0.001) |
Note:
multiplied by 1,000.
With the initial entry of age, sex, height, and weight in the model, there was a significant model change after adding average MD across selected ROIs (ΔR=0.016, p=0.004). Average MD across selected ROIs was associated with LTV after adjustment for covariates in the model (β=0.080, 95%CI: (0.025, 0.135), p=0.004).
Among 16 ROIs, higher MD in the precuneus, the cingulate cortex, the hippocampus, and the parahippocampal gyrus was associated with greater LTV after adjustment for age, sex, height, and weight (Table 2, Model 1). The association of MD in the precuneus, and the anterior and middle cingulate cortices persisted after further adjustment for mean lap time, hypertension, and diabetes (Table 1, Model 2; Figure 1). The association of MD in the posterior cingulate cortex, the hippocampus, and the parahippocampal gyrus attenuated after further adjustment (Table 2, Model 3).
Table 2.
The association between mean diffusivity and lap time variation (n=449)
| Functional domain | Regions of interest | Model 1: adjusted for age, sex, height, and weight | Model 2: model 1+ mean lap time, hypertension, and diabetes |
|---|---|---|---|
|
| |||
| β (95% CI), p-value | |||
|
|
|||
| Multi-modal areas | Precuneus | 0.088** (0.035, 0.140) 0.001 |
0.053* (0.003, 0.103) 0.038 |
| Posterior cingulate gyrus | 0.069* (0.012, 0.125) 0.017 |
0.037 (−0.017, 0.091) 0.175 |
|
| Sensori-motor function | Precentral gyrus | 0.022 (−0.021, 0.064) 0.322 |
0.011 (−0.029, 0.052) 0.577 |
| Postcentral gyrus | 0.033 (−0.011, 0.076) 0.144 |
0.016 (−0.026, 0.058) 0.447 |
|
| Supplementary motor cortex | 0.040 (−0.004, 0.084) 0.072 |
0.029 (−0.012, 0.070) 0.169 |
|
| Putamen | 0.025 (−0.020, 0.071) 0.274 |
0.007 (−0.036, 0.051) 0.739 |
|
| Caudate | 0.038 (−0.006, 0.082) 0.091 |
0.022 (−0.019, 0.063) 0.298 |
|
| Thalamus Proper | 0.046 (−0.001, 0.092) 0.053 |
0.014 (−0.030, 0.058) 0.525 |
|
| Middle cingulate gyrus | 0.077** (0.029, 0.126) 0.002 |
0.055* (0.009, 0.101) 0.019 |
|
| Executive function | Middle frontal gyrus | 0.014 (−0.033, 0.062) 0.553 |
0.008 (−0.037, 0.053) 0.726 |
| Superior parietal lobe | 0.042 (−0.001, 0.085) 0.053 |
0.026 (−0.015, 0.067) 0.216 |
|
| Anterior cingulate gyrus | 0.075** (0.027, 0.123) 0.002 |
0.059* (0.014, 0.104) 0.011 |
|
| Memory | Hippocampus | 0.062* (0.003, 0.121) 0.038 |
0.043 (−0.012, 0.099) 0.128 |
| Parahippocampal gyrus | 0.057* (0.007, 0.106) 0.026 |
0.031 (−0.016, 0.078) 0.198 |
|
| Entorhinal area | 0.028 (−0.017, 0.073) 0.230 |
0.021 (−0.021, 0.064) 0.317 |
|
| Amygdala | 0.043 (−0.001, 0.087) 0.058 |
0.027 (−0.014, 0.069) 0.198 |
|
Note:
p<0.05;
p≤0.0031 (Bonferroni adjusted). Lap time variation was log transformed due to skewed distribution. Mean diffusivity in bilateral region were weighted average of bilateral gray matter volume and computed in standardized units.
Figure 1.
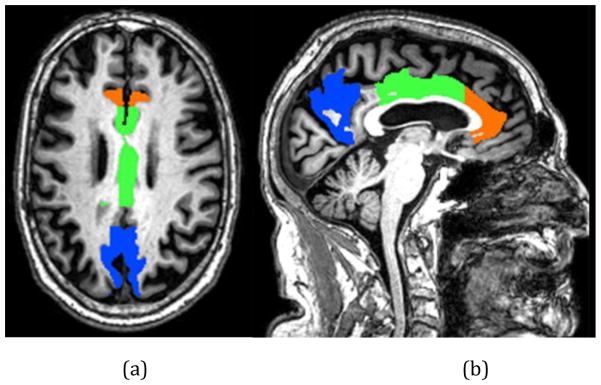
Lower mean diffusivity was associated with higher lap time variation, independent of demographics, mean lap time, and health conditions, in the anterior cingulate cortex (p=0.011; orange), the middle cingulate cortex (p=0.019; green), and the precuneus (p=0.038; blue) in the axial (a) and sagittal view (b).
Results remained largely unchanged when analyses were limited to those who did not use the walking aid during the 400-meter walk (data not shown).
In the sensitivity analyses using VBM analyses, consistent with the ROI approach, there were significant associations between LTV and higher MD in regions of precuneus, anterior cingulate cortex, and middle cingulate cortex (Supplementary Figure 1). There were also additional significant associations in regions of the frontal lobe (bilateral superior orbital gyrus, right middle orbital gyrus, left superior medial frontal gyrus, bilateral rectal gyrus), parietal lobe (right angular gyrus, bilateral precuneus, temporal lobe (left inferior temporal gyrus, left middle temporal gyrus, left fusiform gyrus, right olfactory cortex), occipital lobe (bilateral cuneus, left middle occipital gyrus, left inferior occipital gyrus, left superior occipital gyrus, left calcarine gyrus), and limbic lobe (bilateral anterior cingulate cortex, and right middle cingulate cortex). All were significant after adjustment for all covariates (all FDR-corrected p<0.05; Supplementary Table 1; Supplementary Figure 2).
4 Discussion
In a sample of high-functioning community-dwelling older adults free of overt neurological disease, higher magnitude of MD in the overall gray matter was associated with greater LTV, after controlling for age, sex, height, and weight. The association was particularly evident for the precuneus and the anterior and middle cingulate cortices, and was independent of health conditions that were related to LTV. These associations persisted after adjustment for mean lap time.
The neural correlates of gait variability have been previously observed in healthy elderly (Annweiler et al. 2014; Beauchet et al. 2014 and 2015; Rosso et al. 2014). In line with prior findings, we found a consistent pattern linking gray matter integrity to LTV over and beyond covariates. It is worth pointing out that our study sample consisted of high-functioning older adults as they were able to independently complete the 400-meter walk. In contrast, Annweiler et al’s study excluded only those unable to walk independently (Annweiler et al. 2014), and Rosso et al.’s study excluded only those unable to walk 20 meters and was performed in a much older population (Rosso et al. 2014).
Quantifying gray matter microstructural integrity using MD provides new insights in identifying early markers of neurodegenerative processes, especially in healthy elderly without overt neurological disease. MD in gray matter describes the magnitude of diffusivity and can be quantified in specific regions. Although the underlying mechanisms of subtle alterations in MD are not fully understood, elevated MD suggests loss of neurons and dendrites. Our finding of an overall association between gray matter diffusivity and LTV is consistent with a prior report (Rosso et al. 2014), and further supports the hypothesis in a sample of high-functioning older adults. It is worth noting that MD in each region was computed as the weighted average by volumes in two hemispheres. It accounts for the potential effect of brain atrophy on estimates of gray matter microstructure.
One novel aspect of this study is the examination of LTV by using a simple, clinically accessible approach based on the 400-meter walk. LTV captures a more global measure of variability in walking pace as compared to step-to-step gait variability. It is easy to assess in large epidemiologic studies and provides informative characteristics of gait variability. While LTV was used in one prior study in a format of coefficient of variance, that measure did not account for possible fatigability during repeated laps of the 400-meter walk (Vestergaard et al., 2009). In the present study, LTV was computed based on individual trajectories. This measure ruled out a possible increasing trend in lap time across laps.
We identified that the microstructural integrity in three regions, the precuneus, and the anterior and middle cingulate cortices, was strongly associated with LTV. The importance of the anterior cingulate cortex in gait variability has been previously reported (Rosso et al. 2014), while the precuneus and the middle cingulate cortex have not been previously reported. Prior studies examined the spatial distribution of gray matter volume and MD in relation to gait variability, but did not find an association in the precuneus and the middle cingulate cortex (Beauchet et al., 2014, Rosso et al., 2014). The fact that the previous study did not find an association with the precuneus may stem from the use of different measures of gait variability and different neuroimaging markers in different samples. Interestingly, the precuneus and the anterior and middle cingulate cortices were all thought to be integrating areas, involved with multimodal functions. The precuneus is considered a “hot spot” or cortical hub, interconnecting a variety of cortical and subcortical regions (Cavanna and Trimble, 2006). It is known to be involved in visuospatial information processing with the interconnection to the caudal parietal operculum, the inferior and superior parietal lobe, as well as the intraparietal sulcus. It is also involved with executive and sensorimotor function because of its strong projections to the prefrontal cortex, the dorsal premotor area, and the supplementary motor area. The precuneus thus may subserve multiple behavioral functions. The anterior cingulate cortex plays a key role in several behavioral functions, such as attention, performance monitoring, decision making and learning (Shenhav et al., 2013). Different parts of the anterior cingulate cortex project to widespread brain regions. The dorsal anterior cingulate cortex is interconnected to the prefrontal cortex, parietal lobe, and the motor area, while the ventral anterior cingulate cortex projects to the amygdala, hypothalamus, and anterior insula. The middle cingulate cortex is connected to sensorimotor areas, including premotor cortex, putamen, caudate, and is also interconnected to inferior parietal lobe, as revealed by functional MRI (Hoffstaedter et al., 2012). Besides the strong associations in the precuneus and the anterior and middle cingulate cortices, there was also a trend toward associations with LTV in other brain regions, including the posterior cingulate cortex, the hippocampus, and the parahippocampal gyrus. Interestingly, we did not observe a strong association between MD and LTV in regions that play a predominant role in one particular functional domain, either sensorimotor, executive function, or memory. Instead, we found the integrating regions, projecting to multiple brain regions and responsible for a diversity of behavior functions, to be strongly associated with LTV. The precuneus and the anterior and middle cingulate cortices also fall into default mode network. The default mode network is characterized as a group of brain areas that are active when the brain is awake and at rest as compared with active engagement in task-related activity. It is associated with activation in brain areas important for attention, executive function, as well as memory (Greicius et al. 2009). Future functional connectivity studies may be necessary to further identify the role of the precuneus, the anterior and middle cingulate cortices in gait variability.
The observed associations were robust, even after adjustment for hypertension and diabetes. Most prior studies examining brain structural characteristics and gait variability did not account for health-related conditions. We also adjusted for mean lap time because it was highly correlated with LTV. The associations in the precuneus, and the anterior and middle cingulate cortices persisted in fully adjusted models, although they did not survive the Bonferroni-corrected p-value. This attenuation is possibly due to a collinearity between MD and mean lap time.
This study has limitations. Due to the cross-sectional study design, it is uncertain whether subtle disruption in gray matter microstructure precedes altered gait variability. Longitudinal studies may provide insight for understanding the temporal sequence. Participants in this study are volunteers and may be heathier than the general population.
In conclusion, gray matter diffusivity averaged over 16 ROIs and specifically in the precuneus and in the anterior and middle cingulate cortices, was positively associated with LTV in high-functioning older adults free of overt neurological disease. It suggests that subtle variations in regional gray matter structural integrity may underlie the observed age-related increases in gait variability. Attention to gait variability may thus serve as an early marker of declining gray matter integrity.
Supplementary Material
Highlights.
Lower gray matter integrity was associated with greater lap time variation.
The relationship was found in high-functioning and cognitively intact older adults.
They were localized in the precuneus, the anterior and middle cingulate cortices.
The associations were independent of walking speed and medical conditions.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Aging. The authors would like to acknowledge the editorial assistance from the National Institutes of Health Fellows Editorial Board.
Footnotes
Conflict of interest
The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander DC, Pierpaoli C, Basser PJ, Gee JC. Spatial transformations of diffusion tensor magnetic resonance images. IEEE transactions on medical imaging. 2001;20:1131–1139. doi: 10.1109/42.963816. [DOI] [PubMed] [Google Scholar]
- Annweiler C, Montero-Odasso M, Bartha R, Drozd J, Hachinski V, Beauchet O. Association between gait variability and brain ventricle attributes: a brain mapping study. Experimental gerontology. 2014;57:256–263. doi: 10.1016/j.exger.2014.06.015. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Beauchet O, Annweiler C, Celle S, Bartha R, Barthelemy JC, Roche F. Higher gait variability is associated with decreased parietal gray matter volume among healthy older adults. Brain topography. 2014;27:293–295. doi: 10.1007/s10548-013-0293-y. [DOI] [PubMed] [Google Scholar]
- Beauchet O, Launay CP, Annweiler C, Allali G. Hippocampal volume, early cognitive decline and gait variability: which association? Experimental gerontology. 2015;61:98–104. doi: 10.1016/j.exger.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Brach JS, Studenski SA, Perera S, VanSwearingen JM, Newman AB. Gait variability and the risk of incident mobility disability in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2007;62:983–988. doi: 10.1093/gerona/62.9.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callisaya ML, Blizzard L, Schmidt MD, Martin KL, McGinley JL, Sanders LM, Srikanth VK. Gait, gait variability and the risk of multiple incident falls in older people: a population-based study. Age and ageing. 2011;40:481–487. doi: 10.1093/ageing/afr055. [DOI] [PubMed] [Google Scholar]
- Callisaya ML, Blizzard L, Schmidt MD, McGinley JL, Srikanth VK. Ageing and gait variability--a population-based study of older people. Age and ageing. 2010;39:191–197. doi: 10.1093/ageing/afp250. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain: a journal of neurology. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chua TC, Wen W, Slavin MJ, Sachdev PS. Diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease: a review. Current opinion in neurology. 2008;21:83–92. doi: 10.1097/WCO.0b013e3282f4594b. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Buchel C, May A. Temporal and spatial dynamics of brain structure changes during extensive learning. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82:1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- Hoffstaedter F, Grefkes C, Caspers S, Roski C, Fox PT, Zilles K, Eickhoff SB. Functional connectivity of the mid-cingulate cortex. Klinische Neurophysiologie. 2012;43:128. [Google Scholar]
- Lauzon CB, Asman AJ, Esparza ML, Burns SS, Fan Q, Gao Y, Anderson AW, Davis N, Cutting LE, Landman BA. Simultaneous analysis and quality assurance for diffusion tensor imaging. PloS one. 2013;8:e61737. doi: 10.1371/journal.pone.0061737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley M. Diffusion tensor imaging and aging - a review. NMR in biomedicine. 2002;15:553–560. doi: 10.1002/nbm.785. [DOI] [PubMed] [Google Scholar]
- Penny WD, Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE. Statistical parametric mapping: the analysis of functional brain images: the analysis of functional brain images. Academic press; 2011. [Google Scholar]
- Rosano C, Aizenstein H, Brach J, Longenberger A, Studenski S, Newman AB. Special article: gait measures indicate underlying focal gray matter atrophy in the brain of older adults. The journals of gerontology Series A, Biological sciences and medical sciences. 2008;63:1380–1388. doi: 10.1093/gerona/63.12.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C, Brach J, Studenski S, Longstreth WT, Jr, Newman AB. Gait variability is associated with subclinical brain vascular abnormalities in high-functioning older adults. Neuroepidemiology. 2007;29:193–200. doi: 10.1159/000111582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso AL, Olson Hunt MJ, Yang M, Brach JS, Harris TB, Newman AB, Satterfield S, Studenski SA, Yaffe K, Aizenstein HJ, Rosano C Health ABCs. Higher step length variability indicates lower gray matter integrity of selected regions in older adults. Gait & posture. 2014;40:225–230. doi: 10.1016/j.gaitpost.2014.03.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neuroscience and biobehavioral reviews. 2010;34:721–733. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79:217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada H, Ishii K, Ishiwata K, Oda K, Suzukawa M, Makizako H, Doi T, Suzuki T. Gait adaptability and brain activity during unaccustomed treadmill walking in healthy elderly females. Gait & posture. 2013;38:203–208. doi: 10.1016/j.gaitpost.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Shock N, Greulich R, Andres R, Arenberg D, Costa P, Lakatta E, Tobin J. Normal human aging: The Baltimore longitudinal study of aging. 1984. [Google Scholar]
- Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: the Health ABC Long Distance Corridor Walk. Journal of the American Geriatrics Society. 2001;49:1544–1548. doi: 10.1046/j.1532-5415.2001.4911247.x. [DOI] [PubMed] [Google Scholar]
- Simonsick EM, Schrack JA, Glynn NW, Ferrucci L. Assessing fatigability in mobility-intact older adults. Journal of the American Geriatrics Society. 2014;62:347–351. doi: 10.1111/jgs.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Simonsick EM, Resnick SM, Shardell MD, Ferrucci L, Studenski SA. Lap time variation and executive function in older adults: the Baltimore Longitudinal Study of Aging. Age and ageing. 2015;44:796–800. doi: 10.1093/ageing/afv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. Journal of neurology, neurosurgery, and psychiatry. 2007;78:929–935. doi: 10.1136/jnnp.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard S, Patel KV, Bandinelli S, Ferrucci L, Guralnik JM. Characteristics of 400-meter walk test performance and subsequent mortality in older adults. Rejuvenation research. 2009;12:177–184. doi: 10.1089/rej.2009.0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf N, Lutti A, Helms G, Novak M, Ashburner J, Hutton C. Unified segmentation based correction of R1 brain maps for RF transmit field inhomogeneities (UNICORT) NeuroImage. 2011;54:2116–2124. doi: 10.1016/j.neuroimage.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23:329–342. doi: 10.1002/mds.21720. quiz 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman ME, Lipton RB, Pan JW, Hetherington HP, Verghese J. MRI- and MRS-derived hippocampal correlates of quantitative locomotor function in older adults. Brain research. 2009;1291:73–81. doi: 10.1016/j.brainres.2009.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.