Abstract
Versican is an extracellular matrix (ECM) molecule that interacts with other ECM components to influence ECM organization, stability, composition, and cell behavior. Versican is known to increase in a number of cancers, but little is known about how versican influences the amount and organization of the ECM components in the tumor microenvironment. In the present study, we modulated versican expression using siRNAs in the human leiomyosarcoma (LMS) smooth muscle cell line SK-LMS-1, and observed the formation of elastin and elastic fibers in vitro and also in vivo in a nude mouse tumor model. Constitutive siRNA-directed knockdown of versican in LMS cells resulted in increased levels of elastin, as shown by immunohistochemical staining of the cells in vitro, and by mRNA and protein analyses. Moreover, versican siRNA LMS cells, when injected into nude mice, generated smaller tumors that had significantly greater immunohistochemical and histochemical staining for elastin when compared to control tumors. Additionally, microarray analyses were used to determine the influence of versican isoform modulation on gene expression profiles, and to identify genes that influence and relate to the process of elastogenesis. cDNA microarray analysis and TaqMan low density array validation identified previously unreported genes associated with downregulation of versican and increased elastogenesis. These results highlight an important role for the proteoglycan versican in regulating the expression and assembly of elastin and the phenotype of LMS cells.
Keywords: Extracellular matrix, versican, elastin, elastogenesis, proliferation, siRNA
Introduction
Versican is an extracellular matrix (ECM) proteoglycan found in the interstitium of most soft tissues. There are at least four naturally occurring versican isoforms that have been identified and characterized. These isoforms, designated V0, V1, V2, and V3, are generated by alternatively splicing the central α-glycosaminoglycan (α-GAG) and β-glycosaminoglycan (β-GAG) domains [1–3]. Important to the biology and function of versican are its chondroitin sulfate (CS) GAG side chains and the interaction of its protein core with other molecules [4, 5].
A number of studies have reported on an inverse relationship between elastin and versican expression [6–11]. Gene array studies of developing mice show coordinated expression patterns of elastin and its associated proteins, which underscore the critical spatial and temporal timing of protein expression required for the generation of elastic fibers [12]. In general, it has been observed that when versican expression and accumulation is high, tropoelastin expression and its polymerization is low [13]. For example, smooth muscle cells (SMCs) isolated from rat pups are typically elastogenic and do not express detectable versican in contrast to SMCs from adult rats, which typically produce little elastin and have measurable levels of versican [13–18]. Correspondingly, downregulation of versican in adult rat SMCs by the use of versican antisense mRNA results in increased deposition of elastin in vitro and in vivo [8]. Collectively, these studies provide support for the hypothesis that an inverse relationship exists between versican and elastin expression.
Numerous studies link versican to disease states (see recent reviews; [5, 19]). An inverse relationship between elastin and versican has been demonstrated in vascular and lung diseases. Versican is prominent in early and advanced stages of lesion formation in atherosclerotic plaques with loss or degradation of elastic lamellae [20]. Increased levels of versican are also found during restenosis, and in vascular grafts, and aneurysms [20, 21]. Furthermore, supravalvular aortic hypertrophic stenosis is observed in patients who have mutations and or deletions of the elastin gene [22]. In lung diseases such as lymphangioleiomyomatosis (LAM) and chronic obstructive pulmonary disease (COPD), increased elastin levels with decreased versican levels and vice versa have been observed [7, 23–25]. Interestingly, low levels of elastin expression are associated with high proliferation of skin fibroblasts in Costello syndrome [26] and this relationship could be reversed through modulation of versican isoform expression [6]. Furthermore, a relationship exists between elastin deposition and cell proliferation in that the elastin knockout mouse dies at birth due to excessive subendothelial proliferation of SMCs leading to obstruction and closure of the aorta [27]. Thus, there are significant data that suggest an important relationship between versican, elastin, and cell proliferation. However, the significance of this relationship has not been examined in cancer.
Versican is expressed in many types and grades of cancer [28–31] where it may influence tumor cell behavior. Leiomyosarcoma (LMS) cells, for example, produce a large amount of versican and, in previous experiments, we have shown that inhibiting versican synthesis in LMS cells inhibits both proliferation and tumor formation when these cells are injected into nude mice [32]. Correspondingly, we have found that the addition of exogenous versican restores the high proliferative rate of slow proliferating versican-depleted LMS cells while increasing migration and decreasing substrate adhesion [32].
Based on our observations of a relationship between versican and LMS cell behavior, we hypothesized that cell and tissue homeostatic control is exerted through a downregulation of versican and an upregulation of elastin. To further explore this relationship and determine if other ECM genes associated with elastogenesis were affected, we treated human LMS cells with siRNA directed at versican. Constitutive siRNA knockdown of versican in these cells resulted in increased expression of tropoelastin in vitro as assessed by immunohistochemistry (IHC) and Western blot analyses. Desmosine analysis of the deposited elastin confirmed a 70% increase over LMS controls. Microarray analysis identified 270 genes positively or negatively expressed in versican knockdown cells; a subset of which were selected for later validation by TaqMan low density microarray. Within the set of 96 genes analyzed by TaqMan low density array, tropoelastin was significantly upregulated as were elastin-associated genes that included fibulin-1, fibulin-5, and lysyl oxidase (LOX). The gene array and cell culture studies were further supported by in vivo studies, in which versican siRNA LMS tumor cells were injected into nude mice. In this system, the versican siRNA tumor cells deposited significantly more elastic fibers than did the control LMS cells, as shown by histochemical stain for elastic fibers and by IHC for tropoelastin. Collectively, the results in vitro and in vivo suggest an important role for versican in regulating elastogenesis in a tumor cell model.
Results
Reducing versican levels by siRNA effectively increases tropoelastin expression and its maturation into elastic fibers
In previous studies [32], LMS cells were treated with individual in vitro transcribed siRNAs specifically directed and spaced along the length of versican mRNA. Quantitative Real Time-Polymerase Chain Reaction (qRT-PCR) mRNA analyses revealed that the 5´-G1-directed siRNA was the most effective at inhibiting versican. Subsequently, the corresponding G1-directed siRNA cDNA was successfully subcloned into the pSilencer-3.1-H1neo plasmid expression vector with its neomycin selection cassette. The proper orientation and sequence of the insert was confirmed by nucleotide sequencing. Thus, when transfected into LMS cells, stable clones with constitutively reduced versican levels were successfully produced. These experiments used siRNA technology to knockdown versican expression (mRNA and protein) both transiently and constitutively for the purposes of modulating SMC phenotype and elastin synthesis.
To determine if reduced synthesis of the CS-rich versican variants by siRNA leads to enhanced tropoelastin synthesis, increased elastin deposition, and increased production of mature elastic fibers, we used the same RNA that was used to determine versican mRNA levels. We determined by qRT-PCR that tropoelastin mRNA levels were inversely related to the versican levels (Fig. 1). Media samples were taken to determine the protein levels of elastin by Western blot. We found that tropoelastin protein levels increased with increasing tropoelastin mRNA (Fig. 1C). To measure the levels of mature elastic fibers, the levels of desmosine cross-links were analyzed. The versican siRNA LMS cells produced approximately 63% more desmosine per mg protein in the hydrolysate compared to the LMS control cells (Fig. 1D). This indicated that the modulation of versican led not only to modulation of tropoelastin mRNA, but also to maturation of the tropoelastin into elastic fibers. Furthermore, IHC analysis of versican-modulated LMS cells demonstrated an enhanced tropoelastin signal along with a downregulated versican signal compared to unmodulated LMS controls (Fig. 2, A and B).
Figure 1. siRNA-mediated knockdown of versican leads to a corresponding increase in elastin expression, accumulation, and levels of mature elastin (desmosine).
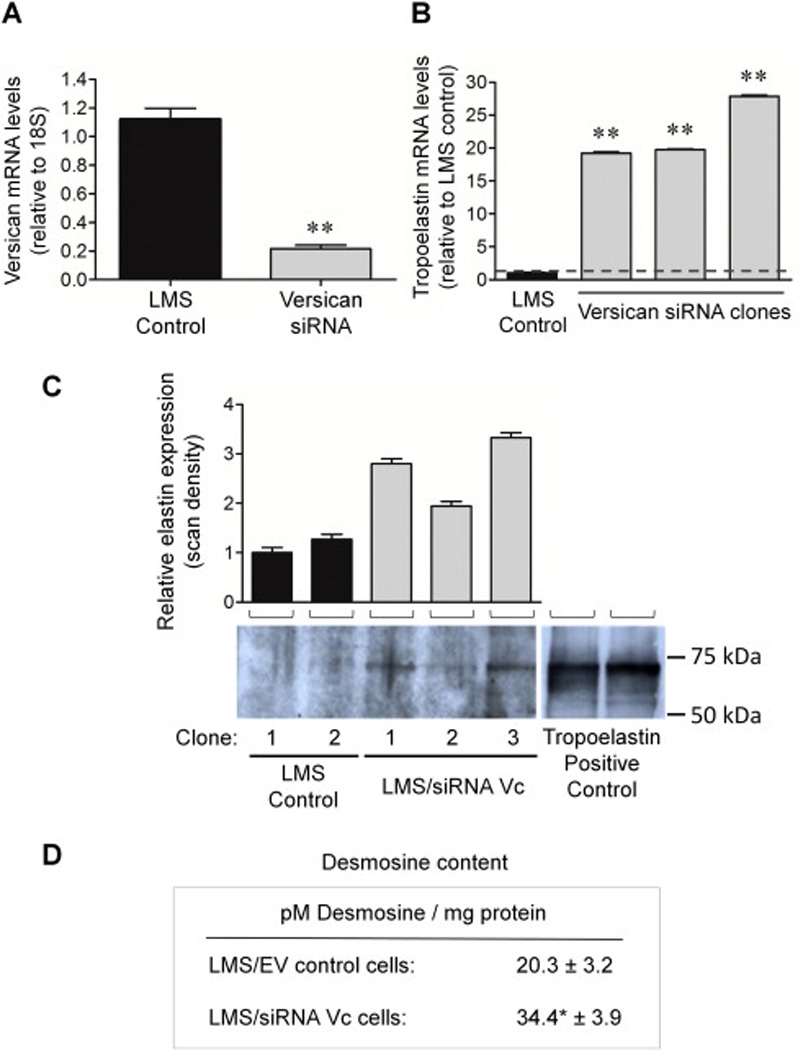
(A) qRT-PCR demonstrates on average a 79% knockdown of versican mRNA using versican-directed siRNA (n = 7; gray columns) compared to controls (n = 4; black columns). (B) A corresponding 12- to 29-fold increase in tropoelastin expression (gray columns) is observed in all the LMS clones constitutively expressing versican-directed siRNA compared to LMS/EV controls. The results of three clones are shown. ** p < 0.01. (C) Representative Western blot probed with anti-bovine tropoelastin detecting 70 kDa tropoelastin in lysates of empty vector clone and siRNA versican LMS cells in culture. All lanes were loaded with equal protein (20 µg). Lanes 1 and 2 show little or no elastin, while lanes 3, 4, and 5 show detectable elastin levels. Lanes 6 and 7 are positive controls of full-length tropoelastin which migrate at the same molecular weight as the cell culture sample extracts. Molecular weight range of ∼50 to 100 kDa is shown. Densitometry in upper panel represents the relative elastin expression ± SE; LMS control (black) vs. LMS/siRNA Vc cells (gray). The relative elastin expression level of each of the samples was made in comparison to the LMS/EV control in lane 1 (set to one). (D) Desmosine content was measured in LMS/EV control cells (n = 5) and compared to LMS/siRNA Vc cells (n = 5) and was approximately 70% greater in the versican siRNA cells vs. the control LMS cells. Mean ± SE; * p < 0.05.
Figure 2. Versican downregulation leads to elastin accumulation in vitro and in vivo.
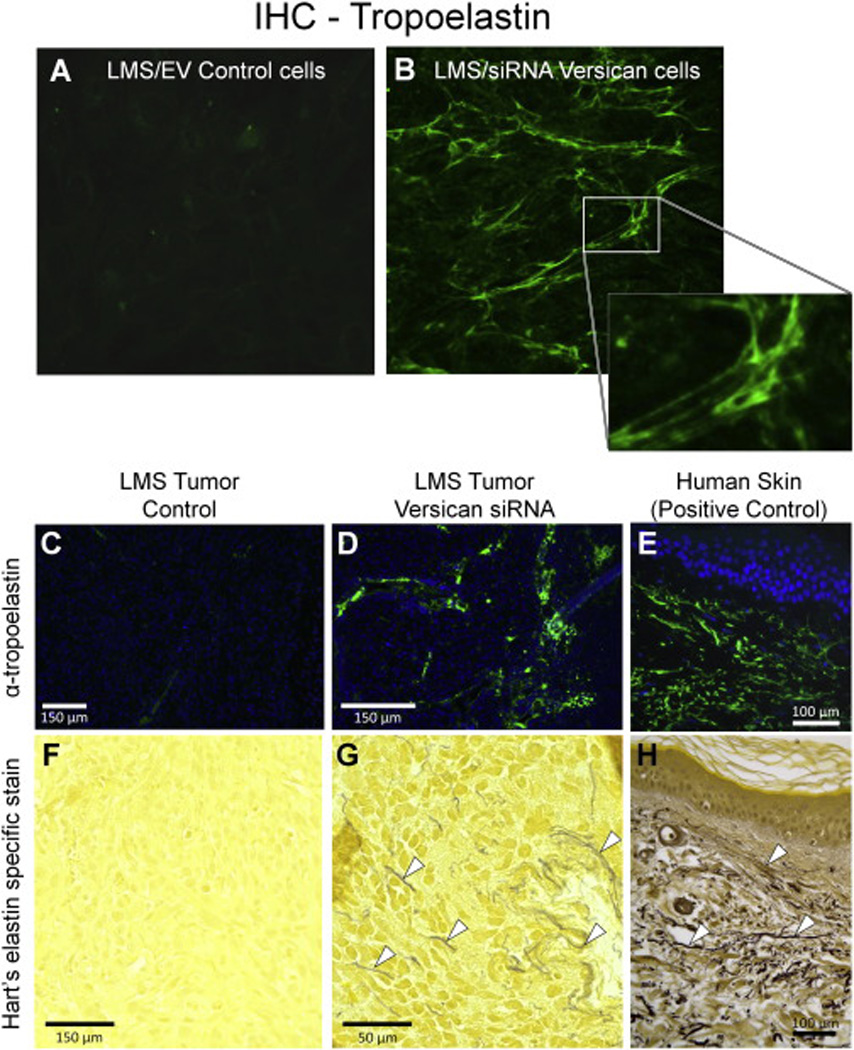
Representative images of (A) tropoelastin IHC analysis of LMS vs. (B) versican-downregulated LMS cells grown on glass coverslips after 7 days in culture (n = 8 in each experimental group). LMS/siRNA Vc cells display increased levels of tropoelastin signal (green) in fiber-like form (see expanded area insert) compared to LMS empty vector controls where no comparable fibers were observed. Images are of equal exposure. Elastin immunofluorescence (top panels; C, D, E) and elastin-specific Hart’s staining (bottom panels; F, G, H) of tumors formed from cells injected subcutaneously in BALB/c nude mice after 21 days. LMS/EV controls vs. LMS/siRNA Vc tumors stained for tropoelastin (green fluorescence) are of equal exposure. Normal human skin is shown at right as positive control. Bright yellow staining in the Hart’s stain panels is indicative of abundant ECM and cytoplasm. Arrowheads (⊲) indicate the presence of elastic fibers which appear black, dark brown or blue with the Hart’s stain. The positive control sections show how, in normal tissue, fine elastic fibers are present in high concentrations in the dermis and form an interconnecting network.
To test the impact of versican expression levels on tropoelastin synthesis and elastic fiber formation in vivo, either LMS/wild type (WT), LMS/empty vector (EV), LMS/siRNA scramble (Scr) controls, or LMS cells constitutively expressing versican siRNA (LMS/siRNA Vc) were injected into nude mice and examined over a three-week period. IHC staining of the LMS/WT, LMS/EV (shown), and LMS/siRNA Scr tumor control groups revealed no detectable tropoelastin staining nor did the elastin-specific Hart’s stain (Fig. 2, C and F). The Hart’s stain provided a good example of how some cancer cells produce an abundance of cytoplasm and ECM, characterized by bright yellow staining, and lack of elastic fiber staining (black). The LMS cells expressing limited versican levels did, however, form visible elastic fibers (as visualized by IHC and Hart’s specific elastin stain; Fig. 2, D and G) not characteristic of cancer cells. A normal (not diseased) human skin sample was provided as a positive control of elastin staining by IHC and Hart’s staining (Fig. 2, E and H). Our previous study showed that this treatment significantly reduced tumor volume over this three-week period in this animal model [32].
Gene array analysis identified genes differentially regulated by the reduction of versican expression
To evaluate the impact of versican downregulation on gene expression profiles, a microarray analysis was performed which identified 270 genes with ± 2-fold difference when versican was knocked down by siRNA in relation to control (Supplemental Table 1). Of interest were 129 cell surface receptor-linked signal transduction genes, 100 cell communication genes, 91 proliferation/cell cycle-related genes, 82 cytoskeletal genes, 57 ECM-related genes, and 20 protease/protease inhibitor genes (Supplemental Table 2). Additional genes differentially regulated by versican included 22 unidentified cDNAs including expressed sequence tags (ESTs) and cDNAs with unknown biological functions. Moreover, many of the genes differentially expressed had not previously been associated with changes in versican expression (Table 1).
Table 1.
Summary of top 15 up- and downregulated genes with the downregulation of versican expression.
| Gene | Differential Expression (vc siRNA/LMS) |
|---|---|
| Upregulated Genes | |
| Beta-actin | 7.75 |
| Calumenin | 6.41 |
| Adenylyl cyclase-associated protein | 5.15 |
| SWI/SNF (tumor suppressor) | 4.07 |
| Glyceraldehyde 3-phosphate dehydrogen ase | 3.90 |
| G protein α inhibiting activity polypeptide 2 | 3.71 |
| Calmodulin | 3.61 |
| ERF-1 | 3.41 |
| Nuclear ribonucleoprotein particle C protein | 3.29 |
| Gs alpha subunit | 3.28 |
| Cyclin dependent kinase 5, PSSALRE | 3.26 |
| Human transcriptional repressor, NAB1 | 3.10 |
| Parathymosin | 3.06 |
| Moesin | 2.97 |
| Metallothionein-Ie, MT1E | 2.93 |
| Downregulated Genes | |
| Aminoacyl-tRNA synthetase | 0.09 |
| EFS1, signal transduction modulating pro tein | 0.22 |
| Neuron specific X11 protein | 0.25 |
| Intercellular adhesion molecule 2 | 0.27 |
| CDC2-related protein kinase | 0.27 |
| Adipophilin | 0.28 |
| AF-6, RAS pathway activator | 0.29 |
| H2K binding factor 2 | 0.30 |
| RASAL2, RAS protein activator like 2 | 0.30 |
| Transcription factor 3 (E12/E47) | 0.30 |
| ZNF207, zinc finger transcription factor | 0.31 |
| DRES9, Phosphatidylinositol transfer protein | 0.31 |
| V-Raf-1, oncogene homologue 1 | 0.32 |
| Prostaglandin E receptor 2 (EP2) | 0.32 |
| SFFV, proviral integration oncogene | 0.33 |
Analysis by low density TaqMan array determined that 15 of the 21 genes significantly (± 2-fold change) up- or downregulated in the Research Genetics microarray were validated, i.e., 71% concurred in both the direction of change and relative magnitude. Furthermore, 6 of the 21 genes were elastin related (Supplemental Table 3). The analysis confirmed the upregulation of elastin with the downregulation of versican. A number of genes were very strongly upregulated (i.e., > 1000-fold) including hyaluronan and proteoglycan link protein-1 (HAPLN1), guanine nucleotide binding protein (G protein) alpha 12 (GNA12), N-deacetylase/N-sulfotransferase-3 (NDST3), and matrix metalloproteinase 12 (MMP12); while some were strongly downregulated, for example, hyaluronan synthase-3 (HAS3), biglycan (BGN), interleukin-1 (IL1), and matrix metalloproteinase-7 (MMP7) (Supplemental Table 3). Genes associated with the sulfation and biosynthesis of versican were also affected, for example, carbohydrate (chondroitin 4) sulfotransferase 11 (CHST11) message levels were decreased 20-fold and carbohydrate (chondroitin 6) sulfotransferase 3 (CHST3) decreased slightly; while chondroitin 6-sulfotransferase (CHST9) and carbohydrate (N-acetylglucosamine 6-O) sulfotransferase 7 (GST5) were elevated (39- and 4-fold, respectively). A number of matrix proteolytic genes were also affected, specifically, MMP12 (↑ 1205-fold); ADAMTS20 (↑423-fold); ADAMTS9 (↑7-fold); HYAL1 (↑3.6-fold); HYAL2 (↓2.3-fold); ADAMTS4 (↓2.5-fold); MMP7 (↓833-fold). Other gene products important in cell phenotype control were shown to be differentially expressed using TaqMan validation arrays. For example, the elastin cross-linking enzyme LOX was highly upregulated, as were the ECM proteoglycan decorin, and the elastin-associated proteins fibulin-1 and fibulin-5, while biglycan was significantly decreased with the reduction in versican (Fig. 3).
Figure 3. Elastin and elastin-related genes are upregulated with the downregulation of versican.
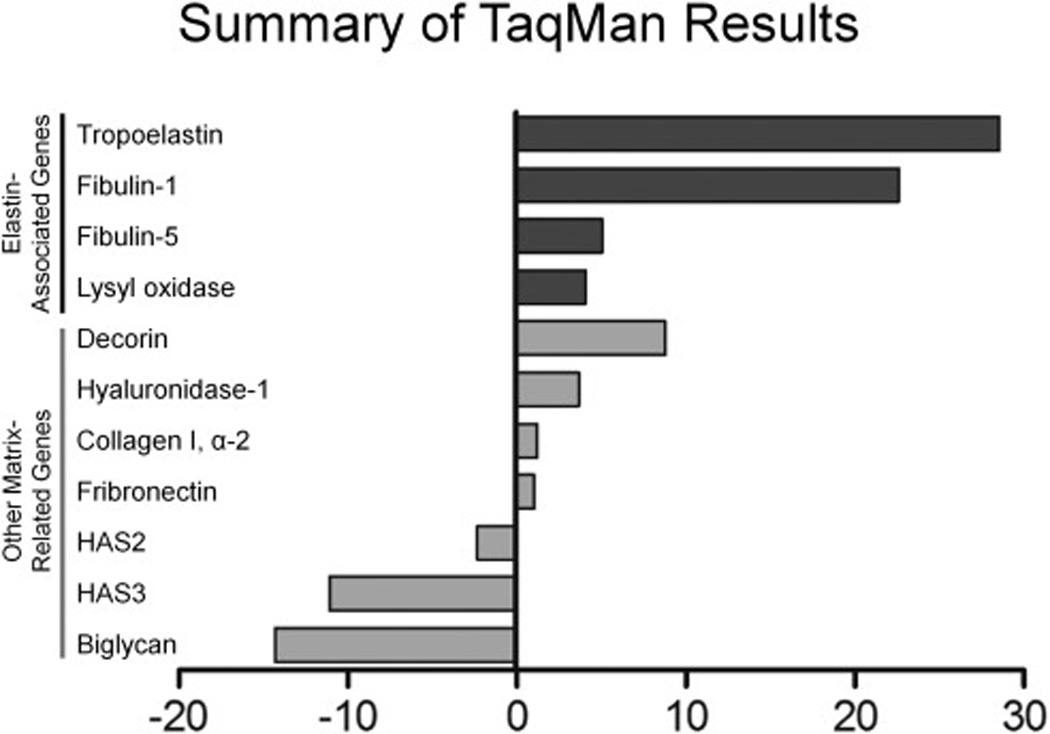
Fold differences relative to empty vector LMS controls are represented for each gene.
Using the Database for Annotation, Visualization and Integrated Discovery (DAVID) 6.7 (NIAID, National Institutes of Health) and the KEGG pathway map visualization tool, numerous pathway cluster changes were observed. Cancer (Supplement Fig. 1), actin cytoskeleton (Supplement Fig. 2), and focal adhesion (Supplement Fig. 3) pathways had a significant number of molecules up- or downregulated with the siRNA-specific knockdown of versican. The large number of molecules affected in these critical cell phenotype pathways provides evidence that versican is key to LMS phenotype, homeostasis, and ECM control of processes such as elastogenesis.
Discussion
This work builds on previous studies which demonstrated that a reduction of versican expression in SMCs led to an increase in elastin expression [7, 8] and dramatic changes in cell phenotype (morphology, migration, proliferation, adhesion, and inflammation) in blood vessels, skin, lung, and now in cancer [19, 32–35]. In the present study, we discovered that a specific knockdown of versican in LMS cancer cells with siRNA resulted in increases in elastin mRNA and protein, and in mRNAs for the elastin-associated proteins, fibulin, fibrillin, and LOX. Using microarray and TaqMan analyses, genes associated with the downregulation of versican were identified as significantly changed, including those for elastin, elastin-associated proteins, ECM, and ECM-modulating enzymes. The imbalance of ECM expression, stabilization and/or turnover has been demonstrated as a key mediating factor in the progression of diseases such as cancer and atherosclerosis [20, 36, 37]. To our knowledge, the downregulation of versican leading to an increase in elastogenesis accompanied by changes in cell phenotype in cancer has not been previously reported. The findings that cell proliferation is altered in systems exhibiting changes in elastic fiber deposition such as seen in Costello Syndrome [6, 26] and in the elastin knockout mouse [27] argue for a role of elastin in cell proliferation. Whether such a role exists in cancer development awaits further examination.
Elastic fibers are a critical component of a variety of tissues including blood vessels, heart valves, lungs, and skin. The principal component of elastic fibers, the protein elastin, is encoded by a single-copy gene and secreted as a soluble tropoelastin precursor (65 – 72 kDa) [38]. Once the tropoelastin transcript is synthesized, polymerization into a mature elastic fiber requires elastin-associated molecules, such as fibrillin and fibulin, and cross-linking catalyzed by enzymes such as LOX [13, 39]. There are numerous examples of mutations in elastin-associated molecules that lead to disruption in the organizational structure of elastic fibers which can result in a loss of tissue integrity and function [26, 40–49].
The downregulation of versican is accompanied by the loss of versican from the cell surface. By decreasing cell surface versican, cell surface elastin binding protein (EBP) increases [8]. Versican is thought to influence elastogenesis by sterically influencing the binding activity of EBP, thus preventing the polymerization of tropoelastin into elastic fibers [50]. Elastic fiber assembly involves the initial deposition of soluble, newly secreted tropoelastin monomers onto microfibrils, then the subsequent accretion of additional tropoelastin molecules that become covalently cross-linked by LOX, to form the insoluble elastin core [51]. This model of elastin synthesis is consistent with our results showing that when versican is reduced, elastin fibers are observed, along with increases in fibulin-1 and −5, LOX, and desmosine (a measure of the mature, cross-linked elastin fibers). Increasing amounts of negatively charged galactosugars, such as CS found on versican, cause what is postulated as premature release of the EBP component of the elastin receptor from the cell surface, thus disrupting the coordinate polymerization of tropoelastin monomer on the microfibrillar scaffold [50, 52, 53]. ECMs enriched in CS frequently exhibit delayed or defective elastic fiber assembly such as found in the subendothelial space of the ductus arteriosus during the closure of this vessel in fetal development [40, 41], in human vascular restenotic lesions [21], and in genetic diseases characterized by defective elastic fiber deposition [26, 42, 43]. One such genetic disease in which children are shown to have defective elastic fiber deposition is Costello syndrome [50]. When Costello syndrome patients’ cells are transduced with the versican V3 gene, the cell phenotype reverts to normal. In addition, results presented in the present study show that modulation of the CS chain-rich V0/V1 splice variants of versican changes the elastin content of the matrix. Such studies suggest that proteoglycans containing CS interfere with cell surface receptors critical for elastic fiber polymerization and assembly.
Our findings demonstrated that the gene expression of elastin-associated proteins fibulin-5, fibrillin-1, and LOX increases with decreasing versican (see Fig. 3). These elastin-associated proteins are critical components for the generation of elastic fibers in vivo [13, 39]. Knockout studies by Pereira et al. of fibrillin-1 closely reproduce the vascular aspects of Marfan syndrome [54]. In this model, the loss of fibrillin-1 (and the resulting discontiguous elastic fibers) is believed to be related to the disease process and the progression of ascending aortic aneurysms [49]. Fibulin-5 knockout mice produce normal levels of elastin, but the elastic fibers are aberrantly assembled and significantly less crosslinked [55]. LOX is known to be an important crosslinker of elastin and collagen fibers [56]. Electron microscopy of aortic walls of Lox−/−mouse fetuses showed highly fragmented elastic fibers and discontinuity in the SMC layers demonstrating the essential role of LOX in elastogenesis [57]. These findings, in combination with our findings that elastin and elastin-associated molecules increase with the downregulation of versican, underscore the importance of versican as a negative regulator of elastogenesis.
Versican and elastin are implicated as modulators of cancer progression and tumorigenesis. Numerous studies have shown that increased versican levels are correlated with elevated metastatic potential and poor disease prognosis [58–61]. In addition, we previously reported that in a nude mouse model, LMS tumor cells depleted in versican by siRNA resulted in lower mitotic indices and reduced tumor volumes compared to LMS/EV and Scr controls [32]. In the present study, we observed, using both IHC and histochemical methods, that in the smaller LMS/siRNA Vc tumors there was a greater amount of elastin staining (see Fig. 2). What is not entirely clear is whether the elastin or the versican levels are causing the change in tumor growth as there is some uncertainty in the literature of how elastin directly or indirectly influences the proliferation of tumor cells (mitogenic signaling). Early studies by Indik et al. (1990), demonstrated a chemotactic activity of tropoelastin monomer by fetal calf fibroblast cells [62]. Later, Timar et al. (1995) demonstrated that soluble elastin (75 kDa κ-elastin) inhibited in vitro proliferation of highly metastatic tumor cells at a low millimolar range [63]. On the other hand, it was observed that elastin degradation products [64] and soluble elastin [65] induced cell cycle entry and proliferation of human glioma cells. Given that the proliferative rate of cells grown on elastin is often low [66, 67], these results suggest that the expression of elastin and its form (polymerized or fragmented) may significantly influence the total mitogenic signaling the cell experiences, however, further study is needed.
Our data show that many of the molecules associated with disease pathologies, such as cancer, are reversed in their up- or downregulation when versican expression is suppressed. For example, G-protein alpha 12 (Gα12) is significantly overexpressed with the reduction of versican expression (Table 1 and Supplemental Table 3). Previously, we found that with the reduction of versican in LMS SMCs, cell shape, migration, and adhesiveness of these cancer cells changed [32]. The dramatic increase in the level of Gα12, which influences cell shape, motility, and adhesion, is consistent with these results. Also, we found that the synthesizing enzymes (HAS2 and HAS3) of hyaluronan, a molecule with which versican strongly interacts, are downregulated with the reduction of versican expression. Consistent with these observations, others have observed that the silencing of the HAS2 gene leads to a less aggressive phenotype of breast tumor cells [68]. Potentially related to the decrease in hyaluronan expression with decreasing versican was the dramatic increase in HAPLN1. The significance is not entirely clear, but HAPLN1 is associated with the condensation or contraction of the ECM [69] and may have a role in the complex process of elastogenesis. We also observed that another ECM proteoglycan, biglycan, was significantly decreased with the reduction in versican. This is consistent with previous studies which showed elevated levels of biglycan in highly proliferative prostate cancer cells [70]. Biglycan was also observed to progressively increase with severity of colorectal cancer and is being used as potential biomarker of disease progression [71]. In the present study, biglycan decreased by over 6-fold with the reduction of versican (Fig. 3 and Supplemental Table 3), indicating that a profound change in the ECM had occurred. Interestingly, when the GAG attachment sites of biglycan are mutated, elastogenesis is enhanced [72], indicating that GAG chains play a dynamic role in elastin synthesis and assembly.
Pathway analysis of the array data demonstrated that a significant number of components within the actin cytoskeleton, focal adhesion, and cancer pathways were impacted with the downregulation of versican, indicating that versican is a critical regulator of cellular phenotype by affecting cell-ECM interactions. Such changes in cell phenotype manifested at the cellular level concur with the observed changes in cell morphology (actin cytoskeleton), changes in cell-ECM signaling (focal adhesion), and cell proliferation, dedifferentiation, and metastasis (cancer phenotype) pathways. Rather than being highly proliferative, migratory, and non-adhesive, blocking versican expression transformed the LMS cells into cells that grew more slowly, were not migratory, were flattened in morphology, and expressed and accumulated elastin.
Gene products that were shown to be differentially expressed using the TaqMan validation array have also been shown to be important in cell phenotype control. For example, decorin increased with the downregulation of versican. Decorin, a component of connective tissue, binds to collagen and plays an important role in matrix assembly. Furthermore, decorin, like elastin, is capable of suppressing the growth of various tumor cell lines [73, 74]. Interestingly, with the reduction of versican expression, a number of sulfo-, acetylgalactosaminyl-, and xylotransferases associated with the biosynthesis of versican and other proteoglycans were elevated, while others were reduced. Not a great deal is known about the specific substrate kinetics and regulation of these enzymes, but the results suggest that the sulfotransferase CHST11 is especially affected by the reduction in versican. Notably, high CHST11 expression positively correlates with poor patient prognosis in astrocytomas [75] and ovarian cancer progression [76]. In contrast, the elevation of CHST7 and 9, chondroitin sulfate N-acetylgalactosaminyltransferase 1 (ChGn), and xylosyltransferase 1 and 2 (XYLT1 and 2) enzyme expression levels is potentially a compensatory mechanism by the cell to increase sulfation required for proper cell function.
The health or stability of ECM is also dependent on ECM turnover and its ability to remodel. With the reduction of versican, a number of ECM enzymes were also affected. MMP12, for example, was highly upregulated with the downregulation of versican. Significantly, MMP12 overexpression is associated with increased survival and decreased metastasis of colorectal cancers [77]. The major substrate for MMP12 is elastin, which is abundant in the lung and arterial wall [78]. The substantial increase in MMP expression may, therefore, be related to the increase in one of its primary substrates, elastin. In addition to elastolytic activity, MMP12 has been shown to be capable of degrading a broad spectrum of other ECM components, including type IV collagen, fibronectin, laminin, vitronectin, proteoglycans, CS, and myelin basic protein. Interestingly, we found that fibulin-5 expression was significantly upregulated with the reduction in versican. Fibulin-5 is known to inhibit hepatocellular carcinoma cell migration and invasion by downregulating MMP7 [79], which is an elastin digesting enzyme. Moreover, we found an over 800-fold decrease in MMP7 levels (Supplemental Table 3). This decrease in MMP7 may play a role in the elastic fiber accumulation we observed by limiting elastin degradation. We also observed elevated ADAMTS 9 and 20, and decreased ADAMTS 4 expression, suggesting that a more quiescent and stable matrix was being achieved. Loss of ADAMTS 9 and 20 enzymatic function is associated with renal and esophageal cancer development [80], while the overexpression of ADAMTS 4 and 5 is associated with glioblastomas. However, the relative importance for events mediated by ADAMTS and MMP enzymes in cell phenotype control in the context of versican modulation needs to be further clarified.
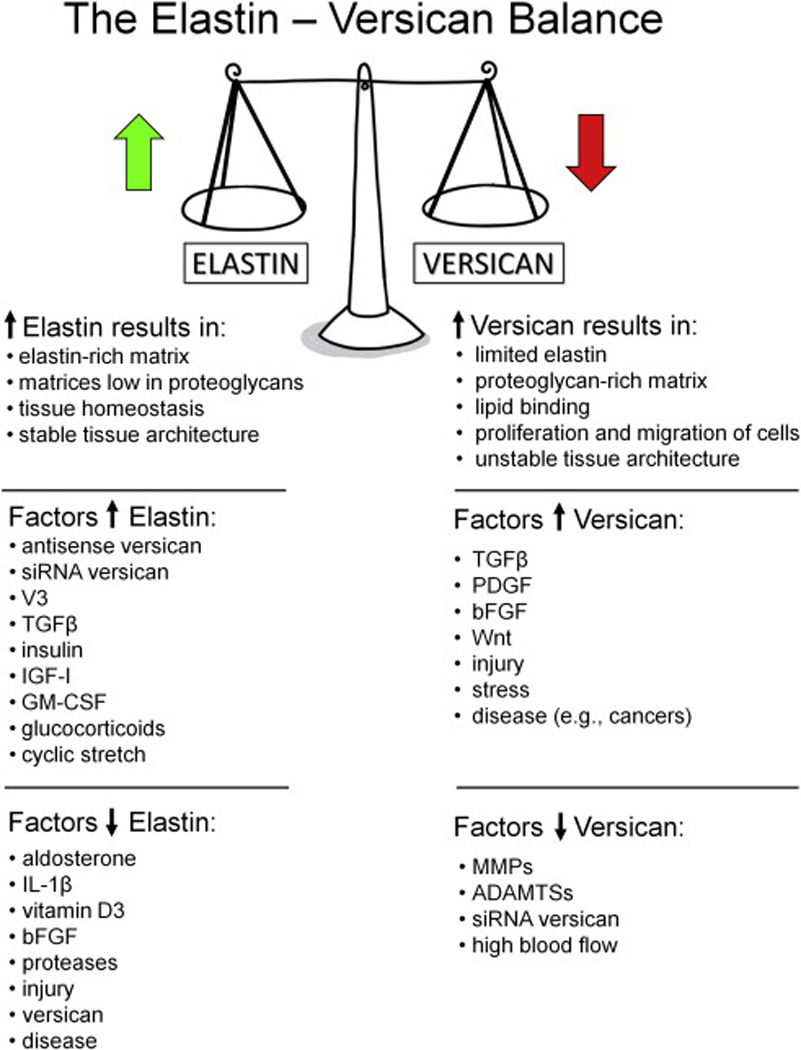
In conclusion, we demonstrated the existence of an inverse relationship between decreasing versican and increasing elastin expression (Fig. 4). With the increase in elastin levels, elastin-related proteins LOX, fibulin, and fibrillin also increased. Using microarray and TaqMan analyses, genes associated with the downregulation of versican were identified, including those for elastin and elastin-associated proteins. The significance of these findings is a better understanding of the relationships that exist between elastin and proteoglycans, such as versican, which are critical to the health and stability of the ECM. Future studies will further examine the influence of versican modulation on specific gene relationships.
Figure 4. Schematic diagram of the relationship between elastin and versican, summarizing how the elastin/versican balance influences cell phenotype and tissue stability.
Materials and Methods
Preparation of siRNA against versican
Previously, we determined that a siRNA targeted against the G1 domain of versican conferred the most effective knockdown of versican, and therefore, we developed stable clones which constitutively expressed versican-targeted shRNA to mediate a reduction in versican [32]. The versican G1 target sequence was subcloned into pSilencer-3.1-H1neo (Ambion) plasmid vector containing the H1 RNA pol III promoter and neomycin-selection cassette. Isolated cell clones of the human SK-LMS-1 (ATCC) cell line, which express an abundance of versican, were used [28, 32]. Individual LMS cell isolates were transfected with pSilencer-3.1-H1-versican-shRNA-neo plasmid for versican-directed siRNA production vs. empty vector or scramble sequence using FuGene 6 (Roche) and selected over 12–14 days using 600µg/mL neomycin (G418; Gibco).
qRT-PCR analysis
The LMS Vc siRNA-, Scr-, and EV-transfected cells were split and individual cell islands were selected and isolated, then grown and cultured separately. RNA from these stable cell lines was isolated as described [32], and qRT-PCR was performed using Assay-on-Demand (Applied Biosystems) primer/probe sets on the LMS/siRNA Vc vs. LMS/EV and LMS/siRNA Src control clones. Expression levels were all normalized to either β-actin or TATA-binding protein mRNA.
Tropoelastin mRNA, elastin protein, and mature elastic fibers in LMS/siRNA Vc cells
The levels of tropoelastin mRNA were determined by qRT-PCR using the same total mRNA that was used to determine versican message levels. Media samples were also taken in order to determine the protein levels of elastin by Western blot (see below). To measure the levels of mature elastic fibers, the levels of desmosine crosslinks were analyzed. This was performed by rigorous acid hydrolysis followed by radioimmunoassay analyses, as described [81]. Desmosine was expressed per mg protein in the hydrolysate. The protein content of tissue hydrolysates was determined from 2 µL by a ninhydrin-based method [82]. This indicated whether the modulation of versican led to not only the modulation of tropoelastin mRNA, but also the maturation of tropoelastin into elastic fibers.
Assessment of tropoelastin protein levels by Western blot
To further examine the inverse relationship between versican and elastin expression, LMS SMCs were transfected with empty 3.1neo (Ambion) (LMS/EV) or versican-specific siRNA (LMS/siRNA Vc) and were cultured to confluency in DMEM supplemented with 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin sulfate, 1 mM sodium pyruvate, and 1x non-essential amino acids (Invitrogen). Cells were plated in triplicate cultures and incubated for 10 days. At the end of the incubation period, the conditioned media was collected and the soluble proteins present in the intracellular compartments were extracted with 0.5 M acetic acid in the presence of proteinase inhibitors in the following final concentrations: 2 mM benzamidine, 2 mM E-amino-caproic acid, 2 mM PMSF, 1 mM EDTA, and 1 µg/ml aprotinin, as described [83]. Extraction was carried out for 2 h at 4 °C and the insoluble material was pelleted by centrifugation. The supernatant was dialyzed (3,500 Da cutoff membrane) at 4 °C against double deionized water containing proteinase inhibitors, then lyophilized. Concentrated preparations of the conditioned media and cell extracts from all analyzed cultures were analyzed for their protein content, and samples containing equal amounts of protein (20 µg/lane) were suspended in 4x NuPAGE LDS sample buffer (Invitrogen) with dithiothreitol, and electrophoresed on a 10% SDS-PAGE gel. The proteins were then transferred to a Protran BA83 nitrocellulose membrane (Schleicher & Schuell) for immunodetection using a Mini-Protean III transfer apparatus (BioRad Labs). After transfer, the membranes were blocked with 5% non-fat milk (Carnation) in tris-buffered saline (TBS) supplemented with 0.1% Tween 20 (T-TBS) for 1 h, then immunoblotted with rabbit anti-bovine tropoelastin antibody 1:1000. Membranes were washed with T-TBS followed by incubation with a 1:10,000 dilution of secondary goat anti-rabbit IgG conjugated alkaline phosphatase (Zymed Laboratories) in T-TBS. Membranes were thoroughly washed 3 × 5 min using T-TBS, followed by 2 × 5 min using ECL Assay buffer, then treated with Tropix® chemiluminescent reagents (Invitrogen) per manufacture’s specifications, and exposed to film (Kodak).
Analysis of elastin accumulation in LMS or LMS/siRNA Vc cells in a mouse tumor model
A well-established tumor cell growth model [84] was used combined with the clonal populations of the human tumor cell line, SK-LMS-1, described above. A total of 30 mice were treated. Twelve immunocompromised BALB/c nude mice (Charles River Laboratories) each received 2 × 106 LMS/siRNA Vc knockdown cells in one dorsal flank subcutaneously; 6 received 2 × 106 siRNA LMS/siRNA Scr control cells; 6 received 2 × 106 LMS/EV control cells; and 6 received 2 × 106 LMS wild type (LMS/WT) cells. The mice were observed daily after the initial injection, and tumor volumes and mitotic indicies were measured, as described [32]. At the end of the study, the animals were euthanized and the tumors were excised for further histological analyses to determine elastin accumulation levels. This study was approved by the Institutional Animal Care and Use Committee at the AAALAC-accredited Benaroya Research Institute.
Immunohistochemical and histochemical staining for elastin
50,000 empty vector control cells (LMS/EV) and 50,000 versican siRNA cells (LMS/siRNA Vc) were plated separately and cultured on 22 mm square glass coverslips and allowed to grow to confluence. Standard medium consisting of DMEM, 0.25 mM glucose (Gibco, Invitrogen) supplemented with 10% calf serum, 100 U/ml penicillin, 100 µg/ml streptomycin sulfate, 1 mM sodium pyruvate, and non-essential amino acids was used to maintain the cells while in culture. Seven-day confluent cultures were then fixed for 30 min in ice-cold 100% ethanol and immunostained with polyclonal bovine tropoelastin antibody 1:750 (generous gift from Dr. Robert Mecham, Washington University, St. Louis, MO).
For the IHC analysis of nude mouse tumors and human skin positive controls, the tropoelastin primary antibody (rabbit polyclonal anti-bovine elastin, 1:1000) was titered to provide optimum contrast and specificity without saturation. Biotinylated goat anti-rabbit antibody (Thermo Fisher, Catalog # B-2770) was used as the secondary. Controls for elastin immunostaining were performed in the presence of goat serum and absence of primary antibody to assure low background without false positive signal. All primary and secondary antibodies as well as binding molecules were diluted in JM buffer. JM buffer consisted of Modified Dulbecco’s PBS (Pierce #28374) in ddH20 with 0.01% Tween 20, 0.1% saponin (Fluka; Sigma), 1% normal goat serum, and 0.5% Tyramide Signal Amplification (TSA) block provided in the TSA Kit #22 (Alexa Fluor 488, Molecular Probes, Invitrogen) which was sterile filtered. DAPI (1 µg/ml) was added at the time of the secondary to visualize cell nuclei. The TSA Kit #22 was used per manufacturer’s directions to amplify the presence of epitope. Hart’s stain was used on paraffin embedded tissue sections by first deparaffinizing, then incubating at room temperature in rescorcin-fuchsin solution (Poly Scientific R&D) for 1 h, rinsing 5 min in tap water, then counterstaining with 2% tartrazine (acid yellow 23, CI 19140) in 0.5% acetic acid for 1 min, washing, dehydrating, clearing, and mounting.
cDNA microarray and multichannel TaqMan microfluidic analyses
mRNA was extracted from 2 LMS/EV controls and 3 LMS/siRNA Vc stables and analyzed for differential expression of genes by high density microarray. For the DNA microarray analyses, the mRNA was prepared by TRIzol extraction, followed by solubilization into aqueous phase using chloroform, precipitation of the RNA using isopropyl alcohol, washing with 80% ethanol, and reconstitution in RNAsecure. Any DNA contamination in the RNA extract was removed by treatment with DNAase enzyme. We performed microarray analysis to determine which gene expression profiles changed when comparing elastogenic (LMS/siRNA Vc) to non-elastogenic (LMS/EV) cells. The RNA was processed to labeled cDNA, hybridized to Research Genetics GF211 array platform, and run in quadruplicate. For hybridization to individual arrays, 1 µg of total RNA was used to synthesize 33P-dCTP-labeled first strand cDNA with Invitrogen Superscript II. Generally, each sample was labeled twice and hybridized to duplicate Research Genetics GF211 arrays. Each array was hybridized with 30–60 million cpm of probe for 18 h then washed extensively at 50 °C in 0.5X SSC + 0.1% SDS following the manufacturer’s protocol. Multiple exposures were collected on a Storm Phosphorimager (Molecular Dynamics). Images were imported using Research Genetics Pathways 3 software using all data point normalization, and processed as described below in “Data Normalization.” Filters from the same filter lot were used and reused, up to four times, with probe stripping (verified by image acquisition) between hybridizations. Multiple exposures were made of each hybridized filter.
Data normalization
There are many sources of systematic variation in cDNA microarray experiments and the term “normalization” refers to the process of removing such variation. We used a method we developed in which the median of log10-transformed expression values for each gene were calculated, giving a rough estimate of expression level for that gene [85]. Then, for each exposure of each filter, we computed a smooth “local” regression line between the log intensities for that exposure and the overall level estimated above, using the “loess” function (from the “modreg” library of R) with default parameters. These regression curves reflect broad trends in the data, but are insensitive to local deviations. The normalized log-level for each gene in the exposure is the value as predicted by the loess line from the measured value. Finally, normalized values for multiple exposures of a single hybridization on one filter are combined by taking the per gene medians of the multiple values computed above. Raw spot intensities varied substantially from exposure to exposure, even for genes showing no biological variability, principally due to varying exposure times. Variation was also introduced by labeling efficiency and multiple reuse of each filter. Nevertheless, scatter plots of one exposure against another generally show good consistency and a roughly linear correspondence. Pearson correlation between log-transformed pairs of exposures varied from 0.83 to 0.99 with the mean above 0.94.
Array statistical analysis
The normalized data were analyzed by t-test (Excel built-in function assuming unequal variances, i.e., heteroscedastic Welch test), SAM (http://www-stat.stanford.edu/~tibs/SAM/index.html), and software available at Genesifter.net (https://users.genesifter.net/users/). Values for differentially expressed genes were used to cluster all test groups using Genepilot software (http://www.genepilot.com) (TG Services). The functions of genes and the relationships between genes (clustering) that were statistically significant in their expression profiles were further evaluated using the Expression Analysis Systematic Explorer (EASE) software [86]. The experiments were run on five independent clones, each time with quadruplicate samples. Statistical significance between experimental and control samples was determined with a Student’s t-test (p < 0.05).
Pathway analysis
Functional annotation clustering and pathway analyses were performed using Database for Annotation, Visualization, and Integrated Discovery (DAVID) 6.7 (NIAID, National Institutes of Health, https://david.ncifcrf.gov). The results of the Research Genetics microarray for the genes differentially expressed (± 2-fold change; p < 0.05) were entered into the Kyoto Encylopedia of Genes and Genomes (KEGG; www.genome.jp/kegg/) database resource which provided significantly impacted, functional annotation clustering and pathway analyses.
TaqMan low density array analysis
Total RNA was isolated from frozen tissues using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. RNA was treated with DNAase and purified by organic/aqueous phase separation [87]. RNA was eluted in 30 µl of RNAlater (Ambion) and quantified by UV-spectroscopy. Samples were stored at −80 °C. cDNA synthesis was conducted using the High Capacity cDNA Archive Kit (Applied Biosystems) according to the manufacturer’s instructions. These cDNA samples were then used for TaqMan low density array analyses.
The Gene Expression Micro Fluidic Card (Applied Biosystems) contains eight sample-loading ports each connected by a microchannel to 48 miniature reaction chambers for a total of 384 wells per card. Primers and probes were chosen online from Applied Biosystems Assays-on-Demand™ Gene Expression Products. Each well of the card contains specific, user-defined primers and probes, capable of detecting a single gene. The primers and probes were factory loaded and dried down in the micro fluidic card.
In this study, the TaqMan low density array card was configured into four identical 96-gene sets (four replicates per assay). The genes were chosen based on the previously performed Research Genetics differential display DNA microarray and included 74 matrix-specific genes. Each set of 96 genes also contained one housekeeping gene, GAPDH, a mandatory control designed into each card by the manufacturer. First, 100 µl of each cDNA sample was added to an equal volume of TaqMan Universal PCR Master Mix (2X; Applied Biosystems) and mixed by inversion. After brief centrifugation, the mixture was transferred into each of the eight loading ports of the micro fluidic card. Each of the 74 matrix-specific genes and 21 genes from cDNA microarray differential display were run in quadruplicate on each card. Five cards in total were run; 2 LMS/EV control clones and 3 LMS/siRNA Vc clones. The cards were placed in Sorvall/Heraeus Custom Buckets (Applied Biosystems) and centrifuged in a Sorvall Legend™ centrifuge (Kendro Scientific) for 1 min at 12,000 rpm (306 × g) to distribute the samples from the loading port into each well. Following centrifugation, the cards were sealed with a TaqMan low density array sealer (Applied Biosystems) to prevent cross-contamination. Each card was placed in the micro fluidic card sample block of an ABI Prism® 7900HT sequence detection system (Applied Biosystems), and the PCR amplifications were run. Thermal cycling conditions were as follows: 2 min at 50 °C to activate uracil-DNA glycosylase (UNG), 10 min at 94.5 °C (activation), 40 cycles of denaturation at 97 °C for 30 s, and annealing and extension at 59.7 °C for 1 min. Each sample was measured three times.
The gene expression values were calculated by the comparative threshold cycle (Ct) method [88], which uses the formula 2κ−ΔΔCt to calculate the expression of target genes normalized to a calibrator. The Ct indicates the cycle number by which the amount of amplified target reaches a fixed threshold. The Ct data for all genes and GAPDH in each sample were used to create ΔCt values [ΔCt = Ct (target gene) – Ct (GAPDH)]. Thereafter, ΔΔCt values were calculated by subtracting the calibrator from the ΔCt value of each target. The relative quantity (RQ) values were calculated with the equation: RQ = 2− ΔΔCt. For calculating the RQ of matrix-specific genes in the versican knockdown vs. the control, the mean ΔCt values of LMS/EV control cDNAs were designated as calibrators. All gene expression values were assigned a relative value of 1.00 in the control samples, which determined comparative gene expression such that ΔΔCt = ΔCt (LMS/Vc siRNA) - ΔCt (LMS/EV) [89]. qRT-PCR data were quantified using the SDS 2.2 software package (Applied Biosystems).
Statistical analyses
A one-sample, two-tailed t-test was applied to compare the average expression level of 95 genes in LMS/siRNA Vc cells to those in the normalized LMS/EV controls (which was assigned an expression level of 1.00 for each of the genes examined). The expression differences among different versican siRNA clones were evaluated using an independent-samples t-test.
All assays were performed in triplicate (except the TaqMan analysis which was performed in quadruplicate on one plate) and repeated at least twice to confirm the initial results. Data were statistically analyzed using unpaired t-tests (Microsoft Excel; Redmond, WA). The results were considered statistically significant when the p value was < 0.05 (*p < 0.05, **p < 0.01, ***p < 0.001). All data are shown as the mean value ± standard deviation of the mean (SDM).
Supplementary Material
Highlights.
Downregulation of versican in leiomyosarcoma cells leads to elastic fiber formation.
Microarray analyses identified an increase in ECM- and elastin-related genes.
Combining cDNA and TaqMan low density arrays was useful for discovery and validation.
Results highlight a role for versican in regulating elastin fiber assembly in cancer.
Acknowledgments
We thank Drs. Aleksander Hinek, Michael Kinsella, Lena Kjellen, and Robert Vernon for helpful discussions; Dr. John Miller for technical assistance; and Dr. Virginia M. Green for careful editing and preparation of the manuscript.
Financial Support: This work was supported by National Institutes of Health grants HL098067 (to T.N.W.) and HL72262 (to E.M.), and by an American Heart Association Pre-Doctoral Fellowship 0310062Z (to P.A.K.).
Abbreviations used
- ECM
extracellular matrix
- α-GAG
α-glycosaminoglycan
- β-GAG
β-glycosaminoglycan
- CS
chondroitin sulfate
- SMCs
smooth muscle cells
- LAM
lymphangioleiomyomatosis
- COPD
chronic obstructive pulmonary disease
- LMS
leiomyosarcoma
- IHC
immunohistochemistry
- LOX
lysyl oxidase
- qRT-PCR
quantitative Real Time-Polymerase Chain Reaction
- WT, LMS/wild type
non-transfected wild type leiomyosarcoma cells
- EV, LMS/empty vector
leiomyosarcoma cells transfected with empty vector plasmid
- Scr, LMS/siRNA scramble
leiomyosarcoma cells transfected with siRNA scrambled expression sequence without any known genetically transcribed target
- LMS/siRNA Vc
leiomyosarcoma cells transfected with short hairpin sequence specifically targeting versican
- HAPLN1
hyaluronan and proteoglycan link protein-1
- GNA12 (Gα12)
guanine nucleotide binding protein (G-protein) alpha 12
- NDST3
N-deacetylase/N-sulfotransferase-3
- MMP12
matrix metalloproteinase 12
- HAS3
hyaluronan synthase-3
- BGN
biglycan
- IL1
interleukin-1
- MMP7
matrix metalloproteinase-7
- CHST11
carbohydrate (chondroitin 4) sulfotransferase 11
- CHST3
carbohydrate (chondroitin 6) sulfotransferase 3
- CHST9
carbohydrate (N-acetylgalactosamine 4-0) sulfotransferase 9
- ChGn
chondroitin sulfate N-acetylgalactosaminyltransferase 1
- GST5(CHST7, C6ST-2)
carbohydrate (N-acetylglucosamine 6-O) sulfotransferase
- EBP
elastin binding protein
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- RQ
relative quantity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ito K, Shinomura T, Zako M, Ujita M, Kimata K. Multiple forms of mouse PG-M, a large chondroitin sulfate proteoglycan generated by alternative splicing. J Biol Chem. 1995;270:958–965. doi: 10.1074/jbc.270.2.958. [DOI] [PubMed] [Google Scholar]
- 2.Zimmermann D, Versican . In: Proteoglycans: Structure, Biology and Molecular Interactions. Iozzo R, editor. New York: Marcel Dekker, Inc; 2000. pp. 327–341. [Google Scholar]
- 3.Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol. 2002;14:617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- 4.Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15:483–494. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- 5.Wight TN, Kinsella MG, Evanko SP, Potter-Perigo S, Merrilees MJ. Versican and the regulation of cell phenotype in disease. Biochim Biophys Acta. 2014;1840:2441–2451. doi: 10.1016/j.bbagen.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinek A, Braun KR, Liu K, Wang Y, Wight TN. Retrovirally mediated overexpression of versican v3 reverses impaired elastogenesis and heightened proliferation exhibited by fibroblasts from Costello syndrome and Hurler disease patients. Am J Pathol. 2004;164:119–131. doi: 10.1016/S0002-9440(10)63103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merrilees M, Ching P, Beaumont B, Hinek A, Wight T, Black P. Changes in elastin, elastin binding protein and versican in alveoli in chronic obstructive pulmonary disease. Respir Res. 2008;18:41–50. doi: 10.1186/1465-9921-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang R, Merrilees MJ, Braun K, Beaumont B, Lemire J, Clowes AW, et al. Inhibition of versican synthesis by antisense alters smooth muscle cell phenotype and induces elastic fiber formation in vitro and in neointima after vessel injury. Circ Res. 2006;98:370–377. doi: 10.1161/01.RES.0000202051.28319.c8. [DOI] [PubMed] [Google Scholar]
- 9.Merrilees MJ, Lemire JM, Fischer JW, Kinsella MG, Braun KR, Clowes AW, et al. Retrovirally mediated overexpression of versican v3 by arterial smooth muscle cells induces tropoelastin synthesis and elastic fiber formation in vitro and in neointima after vascular injury. Circ Res. 2002;90:481–487. doi: 10.1161/hh0402.105791. [DOI] [PubMed] [Google Scholar]
- 10.Kang I, Yoon DW, Braun KR, Wight TN. Expression of versican V3 by arterial smooth muscle cells alters TGFβ-, EGF-, and NFκB-dependent signaling pathways, creating a microenvironment that resists monocyte adhesion. J Biol Chem. 2014;289:15393–15404. doi: 10.1074/jbc.M113.544338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merrilees M, Kang I, Hinek A, Wight T. Regulating elastogenesis using proteoglycans. In: Ramamurthi A, Koth C, editors. Elastic Fiber Matrices: Biomemetic Approaches to Regeneration and Repair. Boca Raton, FL: CRC Press/Taylor & Francis; 2015. In press. [Google Scholar]
- 12.Wagenseil JE, Mecham RP. New insights into elastic fiber assembly. Birth Defects Res C Embryo Today. 2007;81:229–240. doi: 10.1002/bdrc.20111. [DOI] [PubMed] [Google Scholar]
- 13.Kelleher C, McLean S, Mecham R. Current Topics in Developmental Biology. New York: Elsevier Inc; 2004. Vascular extracellular matrix and aortic development; pp. 153–188. [DOI] [PubMed] [Google Scholar]
- 14.Lemire JM, Potter-Perigo S, Hall KL, Wight TN, Schwartz SM. Distinct rat aortic smooth muscle cells differ in versican/PG-M expression. Arterioscler Thromb Vasc Biol. 1996;16:821–829. doi: 10.1161/01.atv.16.6.821. [DOI] [PubMed] [Google Scholar]
- 15.Faris B, Tan OT, Toselli P, Franzblau C. Long-term neonatal rat aortic smooth muscle cell cultures: a model for the tunica media of a blood vessel. Matrix. 1992;12:185–188. doi: 10.1016/s0934-8832(11)80060-0. [DOI] [PubMed] [Google Scholar]
- 16.Toselli P, Faris B, Sassoon D, Jackson BA, Franzblau C. In-situ hybridization of tropoelastin mRNA during the development of the multilayered neonatal rat aortic smooth muscle cell culture. Matrix. 1992;12:321–332. doi: 10.1016/s0934-8832(11)80084-3. [DOI] [PubMed] [Google Scholar]
- 17.Ross JJ, Tranquillo RT. ECM gene expression correlates with in vitro tissue growth and development in fibrin gel remodeled by neonatal smooth muscle cells. Matrix Biol. 2003;22:477–490. doi: 10.1016/s0945-053x(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 18.Long JL, Tranquillo RT. Elastic fiber production in cardiovascular tissue-equivalents. Matrix Biol. 2003;22:339–350. doi: 10.1016/s0945-053x(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 19.Wight TN, Kang I, Merrilees MJ. Versican and the control of inflammation. Matrix Biol. 2014;35:152–161. doi: 10.1016/j.matbio.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wight TN, Merrilees MJ. Proteoglycans in atherosclerosis and restenosis: key roles for versican. Circ Res. 2004;94:1158–1167. doi: 10.1161/01.RES.0000126921.29919.51. [DOI] [PubMed] [Google Scholar]
- 21.Wight TN, Lara S, Reissen R, LeBaron R, Isner J. Selective deposits of versican in the extracellular matrix of restenotic lesions from human peripheral arteries. Am J Pathol. 1997;151:963–973. [PMC free article] [PubMed] [Google Scholar]
- 22.Morris CA. Genetic aspects of supravalvular aortic stenosis. Curr Opin Cardiol. 1998;13:214–249. [PubMed] [Google Scholar]
- 23.Merrilees MJ, Hankin EJ, Black JL, Beaumont B. Matrix proteoglycans and remodelling of interstitial lung tissue in lymphangioleiomyomatosis. J Pathol. 2004;203:653–660. doi: 10.1002/path.1577. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Wu L, Qu JM, Bai CX, Merrilees MJ, Black PN. Pro-inflammatory phenotype of COPD fibroblasts not compatible with repair in COPD lung. J Cell Mol Med. 2012;16:1522–1532. doi: 10.1111/j.1582-4934.2011.01492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersson-Sjoland A, Hallgren O, Rolandsson S, Weitoft M, Tykesson E, Larsson-Callerfelt AK, et al. Versican in inflammation and tissue remodeling: the impact on lung disorders. Glycobiology. 2015;25:243–251. doi: 10.1093/glycob/cwu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinek A, Smith AC, Cutiongco EM, Callahan JW, Gripp KW, Weksberg R. Decreased elastin deposition and high proliferation of fibroblasts from Costello syndrome are related to functional deficiency in the 67-kD elastin-binding protein. Am J Hum Genet. 2000;66:859–872. doi: 10.1086/302829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, et al. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276–280. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 28.Cattaruzza S, Schiappacassi M, Kimata K, Colombatti A, Perris R. The globular domains of PG-M/versican modulate the proliferation-apoptosis equilibrium and invasive capabilities of tumor cells. FASEB J. 2004;18:779–781. doi: 10.1096/fj.03-0660fje. [DOI] [PubMed] [Google Scholar]
- 29.Theocharis AD, Tsolakis I, Tzanakakis GN, Karamanos NK. Chondroitin sulfate as a key molecule in the development of atherosclerosis and cancer progression. Adv Pharmacol. 2006;53:281–295. doi: 10.1016/S1054-3589(05)53013-8. [DOI] [PubMed] [Google Scholar]
- 30.Wegrowski Y, Maquart FX. Chondroitin sulfate proteoglycans in tumor progression. Adv Pharmacol. 2006;53:297–321. doi: 10.1016/S1054-3589(05)53014-X. [DOI] [PubMed] [Google Scholar]
- 31.Ricciardelli C, Sakko AJ, Ween MP, Russell DL, Horsfall DJ. The biological role and regulation of versican levels in cancer. Cancer Metastasis Rev. 2009;28:233–245. doi: 10.1007/s10555-009-9182-y. [DOI] [PubMed] [Google Scholar]
- 32.Keire PA, Bressler SL, Lemire JM, Edris B, Rubin BP, Rahmani M, et al. A role for versican in the development of leiomyosarcoma. J Biol Chem. 2014;289:34089–34103. doi: 10.1074/jbc.M114.607168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li F, Li S, Cheng T. TGF-beta1 promotes osteosarcoma cell migration and invasion through the miR-143-versican pathway. Cell Physiol Biochem. 2014;34:2169–2179. doi: 10.1159/000369660. [DOI] [PubMed] [Google Scholar]
- 34.Desjardins M, Xie J, Gurler H, Muralidhar GG, Sacks JD, Burdette JE, et al. Versican regulates metastasis of epithelial ovarian carcinoma cells and spheroids. J Ovarian Res. 2014;7:70. doi: 10.1186/1757-2215-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bu P, Yang P. MicroRNA-203 inhibits malignant melanoma cell migration by targeting versican. Exp Ther Med. 2014;8:309–315. doi: 10.3892/etm.2014.1708. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Bergamaschi A, Tagliabue E, Sorlie T, Naume B, Triulzi T, Orlandi R, et al. Extracellular matrix signature identifies breast cancer subgroups with different clinical outcome. J Pathol. 2008;214:357–367. doi: 10.1002/path.2278. [DOI] [PubMed] [Google Scholar]
- 37.Wagsater D, Bjork H, Zhu C, Bjorkegren J, Valen G, Hamsten A, et al. ADAMTS-4 and −8 are inflammatory regulated enzymes expressed in macrophage-rich areas of human atherosclerotic plaques. Atherosclerosis. 2008;196:514–522. doi: 10.1016/j.atherosclerosis.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 38.Fazio MJ, Mattei MG, Passage E, Chu ML, Black D, Solomon E, et al. Human elastin gene: new evidence for localization to the long arm of chromosome 7. Am J Hum Genet. 1991;48:696–703. [PMC free article] [PubMed] [Google Scholar]
- 39.Kielty CM. Elastic fibres in health and disease. Expert Rev Mol Med. 2006;8:1–23. doi: 10.1017/S146239940600007X. [DOI] [PubMed] [Google Scholar]
- 40.Hinek A, Mecham RP, Keeley F, Rabinovitch M. Impaired elastin fiber assembly related to reduced 67-kD elastin-binding protein in fetal lamb ductus arteriosus and in cultured aortic smooth muscle cells treated with chondroitin sulfate. J Clin Invest. 1991;88:2083–2094. doi: 10.1172/JCI115538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hinek A, Boyle J, Rabinovitch M. Vascular smooth muscle cell detachment from elastin and migration through elastic laminae is promoted by chondroitin sulfate-induced “shedding” of the 67-kDa cell surface elastin binding protein. Exp Cell Res. 1992;203:344–353. doi: 10.1016/0014-4827(92)90008-v. [DOI] [PubMed] [Google Scholar]
- 42.Hinek A, Wilson SE. Impaired elastogenesis in Hurler disease: dermatan sulfate accumulation linked to deficiency in elastin-binding protein and elastic fiber assembly. Am J Pathol. 2000;156:925–938. doi: 10.1016/S0002-9440(10)64961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hinek A, Zhang S, Smith AC, Callahan JW. Impaired elastic-fiber assembly by fibroblasts from patients with either Morquio B disease or infantile GM1-gangliosidosis is linked to deficiency in the 67-kD spliced variant of beta-galactosidase. Am J Hum Genet. 2000;67:23–36. doi: 10.1086/302968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tassabehji M, Metcalfe K, Hurst J, Ashcroft GS, Kielty C, Wilmot C, et al. An elastin gene mutation producing abnormal tropoelastin and abnormal elastic fibres in a patient with autosomal dominant cutis laxa. Hum Mol Genet. 1998;7:1021–1028. doi: 10.1093/hmg/7.6.1021. [DOI] [PubMed] [Google Scholar]
- 45.Tassabehji M, Urban Z. Congenital heart disease: Molecular diagnostics of supravalvular aortic stenosis. Methods Mol Med. 2006;126:129–156. doi: 10.1385/1-59745-088-X:129. [DOI] [PubMed] [Google Scholar]
- 46.Gray V, Karmiloff-Smith A, Funnell E, Tassabehji M. In-depth analysis of spatial cognition in Williams syndrome: A critical assessment of the role of the LIMK1 gene. Neuropsychologia. 2006;44:679–685. doi: 10.1016/j.neuropsychologia.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 47.Bergen AA, Plomp AS, Schuurman EJ, Terry S, Breuning M, Dauwerse H, et al. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat Genet. 2000;25:228–231. doi: 10.1038/76109. [DOI] [PubMed] [Google Scholar]
- 48.Milewicz DM, Urban Z, Boyd C. Genetic disorders of the elastic fiber system. Matrix Biol. 2000;19:471–480. doi: 10.1016/s0945-053x(00)00099-8. [DOI] [PubMed] [Google Scholar]
- 49.Robinson PN, Godfrey M. The molecular genetics of Marfan syndrome and related microfibrillopathies. J Med Genet. 2000;37:9–25. doi: 10.1136/jmg.37.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hinek A, Pshezhetsky AV, von Itzstein M, Starcher B. Lysosomal sialidase (neuraminidase-1) is targeted to the cell surface in a multiprotein complex that facilitates elastic fiber assembly. J Biol Chem. 2006;281:3698–3710. doi: 10.1074/jbc.M508736200. [DOI] [PubMed] [Google Scholar]
- 51.Starcher B, d’Azzo A, Keller PW, Rao GK, Nadarajah D, Hinek A. Neuraminidase-1 is required for the normal assembly of elastic fibers. Am J Physiol Lung Cell Mol Physiol. 2008;295:L637–L647. doi: 10.1152/ajplung.90346.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hinek A, Wrenn DS, Mecham RP, Barondes SH. The elastin receptor: a galactoside-binding protein. Science. 1988;239:1539–1541. doi: 10.1126/science.2832941. [DOI] [PubMed] [Google Scholar]
- 53.Hinek A. Biological roles of the non-integrin elastin/laminin receptor. Biol Chem. 1996;377:471–480. [PubMed] [Google Scholar]
- 54.Pereira L, Andrikopoulos K, Tian J, Lee SY, Keene DR, Ono R, et al. Targetting of the gene encoding fibrillin-1 recapitulates the vascular aspect of Marfan syndrome. Nat Genet. 1997;17:218–222. doi: 10.1038/ng1097-218. [DOI] [PubMed] [Google Scholar]
- 55.Choi J, Bergdahl A, Zheng Q, Starcher B, Yanagisawa H, Davis EC. Analysis of dermal elastic fibers in the absence of fibulin-5 reveals potential roles for fibulin-5 in elastic fiber assembly. Matrix Biol. 2009;28:211–220. doi: 10.1016/j.matbio.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siegel RC, Pinnell SR, Martin GR. Cross-linking of collagen and elastin. Properties of lysyl oxidase. Biochemistry. 1970;9:4486–4492. doi: 10.1021/bi00825a004. [DOI] [PubMed] [Google Scholar]
- 57.Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, et al. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106:2503–2509. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- 58.Labropoulou VT, Theocharis AD, Ravazoula P, Perimenis P, Hjerpe A, Karamanos NK, et al. Versican but not decorin accumulation is related to metastatic potential and neovascularization in testicular germ cell tumours. Histopathology. 2006;49:582–593. doi: 10.1111/j.1365-2559.2006.02558.x. [DOI] [PubMed] [Google Scholar]
- 59.Nikitovic D, Zafiropoulos A, Katonis P, Tsatsakis A, Theocharis AD, Karamanos NK, et al. Transforming growth factor-β as a key molecule triggering the expression of versican isoforms v0 and v1, hyaluronan synthase-2 and synthesis of hyaluronan in malignant osteosarcoma cells. IUBMB Life. 2006;58:47–53. doi: 10.1080/15216540500531713. [DOI] [PubMed] [Google Scholar]
- 60.Sakko AJ, Ricciardelli C, Mayne K, Suwiwat S, LeBaron RG, Marshall VR, et al. Modulation of prostate cancer cell attachment to matrix by versican. Cancer Res. 2003;63:4786–4791. [PubMed] [Google Scholar]
- 61.Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Indik Z, Abrams WR, Kucich U, Gibson CW, Mecham RP, Rosenbloom J. Production of recombinant human tropoelastin: characterization and demonstration of immunologic and chemotactic activity. Arch Biochem Biophys. 1990;280:80–86. doi: 10.1016/0003-9861(90)90521-y. [DOI] [PubMed] [Google Scholar]
- 63.Timar J, Diczhazi C, Ladanyi A, Raso E, Hornebeck W, Robert L, et al. Interaction of tumour cells with elastin and the metastatic phenotype. Ciba Found Symp. 1995;192:321–335. discussion 35–7. [PubMed] [Google Scholar]
- 64.Jung S, Rutka JT, Hinek A. Tropoelastin and elastin degradation products promote proliferation of human astrocytoma cell lines. J Neuropathol Exp Neurol. 1998;57:439–448. doi: 10.1097/00005072-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 65.Hinek A, Jung S, Rutka JT. Cell surface aggregation of elastin receptor molecules caused by suramin amplified signals leading to proliferation of human glioma cells. Acta Neuropathol. 1999;97:399–407. doi: 10.1007/s004010051004. [DOI] [PubMed] [Google Scholar]
- 66.Sosa-Melgarejo JA, Berry CL. Vascular smooth muscle cells cultured on elastin membranes. Arch Med Res. 1996;27:77–82. [PubMed] [Google Scholar]
- 67.Wilson E, Sudhir K, Ives HE. Mechanical strain of rat vascular smooth muscle cells is sensed by specific extracellular matrix/integrin interactions. J Clin Invest. 1995;96:2364–2372. doi: 10.1172/JCI118293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y, Li L, Brown TJ, Heldin P. Silencing of hyaluronan synthase 2 suppresses the malignant phenotype of invasive breast cancer cells. Int J Cancer. 2007;120:2557–2567. doi: 10.1002/ijc.22550. [DOI] [PubMed] [Google Scholar]
- 69.Morgelin M, Paulsson M, Heinegard D, Aebi U, Engel J. Evidence of a defined spatial arrangement of hyaluronate in the central filament of cartilage proteoglycan aggregates. Biochem J. 1995;307(Pt 2):595–601. doi: 10.1042/bj3070595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang J, Levenson AS, Satcher RL., Jr Identification of a unique set of genes altered during cell-cell contact in an in vitro model of prostate cancer bone metastasis. Int J Mol Med. 2006;17:849–856. [PubMed] [Google Scholar]
- 71.Galamb O, Sipos F, Spisak S, Galamb B, Krenacs T, Valcz G, et al. Potential biomarkers of colorectal adenoma-dysplasia-carcinoma progression: mRNA expression profiling and in situ protein detection on TMAs reveal 15 sequentially upregulated and 2 downregulated genes. Cell Oncol. 2009;31:19–29. doi: 10.3233/CLO-2009-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hwang JY, Johnson PY, Braun KR, Hinek A, Fischer JW, O’Brien KD, et al. Retrovirally mediated overexpression of glycosaminoglycan-deficient biglycan in arterial smooth muscle cells induces tropoelastin synthesis and elastic fiber formation in vitro and in neointimae after vascular injury. Am J Pathol. 2008;173:1919–1928. doi: 10.2353/ajpath.2008.070875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seidler DG, Goldoni S, Agnew C, Cardi C, Thakur ML, Owens RT, et al. Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase-3 activation. J Biol Chem. 2006;281:26408–26418. doi: 10.1074/jbc.M602853200. [DOI] [PubMed] [Google Scholar]
- 74.Neill T, Schaefer L, Iozzo RV. Decorin as a multivalent therapeutic agent against cancer. Adv Drug Deliv Rev. 2015 doi: 10.1016/j.addr.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kobayashi T, Yan H, Kurahashi Y, Ito Y, Maeda H, Tada T, et al. Role of GalNAc4S-6ST in astrocytic tumor progression. PLoS ONE. 2013;8:e54278. doi: 10.1371/journal.pone.0054278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oliveira-Ferrer L, Heβling A, Trillsch F, Mahner S, Milde-Langosch K. Prognostic impact of chondroitin-4-sulfotransferase CHST11 in ovarian cancer. Tumour Biol. 2015 doi: 10.1007/s13277-015-3652-3. In press. [DOI] [PubMed] [Google Scholar]
- 77.Zucker S, Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 2004;23:101–117. doi: 10.1023/a:1025867130437. [DOI] [PubMed] [Google Scholar]
- 78.Chen YE. MMP-12, an old enzyme plays a new role in the pathogenesis of rheumatoid arthritis? Am J Pathol. 2004;165:1069–1070. doi: 10.1016/S0002-9440(10)63368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tu K, Dou C, Zheng X, Li C, Yang W, Yao Y, et al. Fibulin-5 inhibits hepatocellular carcinoma cell migration and invasion by down-regulating matrix metalloproteinase-7 expression. BMC Cancer. 2014;14:938. doi: 10.1186/1471-2407-14-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lo PH, Leung AC, Kwok CY, Cheung WS, Ko JM, Yang LC, et al. Identification of a tumor suppressive critical region mapping to 3p14.2 in esophageal squamous cell carcinoma and studies of a candidate tumor suppressor gene, ADAMTS9. Oncogene. 2007;26:148–157. doi: 10.1038/sj.onc.1209767. [DOI] [PubMed] [Google Scholar]
- 81.Starcher B, Conrad M. A role for neutrophil elastase in solar elastosis. Ciba Found Symp. 1995;192:338–346. [PubMed] [Google Scholar]
- 82.Starcher B. A ninhydrin-based assay to quantitate the total protein content of tissue samples. Anal Biochem. 2001;292:125–129. doi: 10.1006/abio.2001.5050. [DOI] [PubMed] [Google Scholar]
- 83.Hinek A, Rabinovitch M. The ductus arteriosus migratory smooth muscle cell phenotype processes tropoelastin to a 52-kDa product associated with impaired assembly of elastic laminae. J Biol Chem. 1993;268:1405–1413. [PubMed] [Google Scholar]
- 84.Horiuchi A, Nikaido T, Mitsushita J, Toki T, Konishi I, Fujii S. Enhancement of antitumor effect of bleomycin by low-voltage in vivo electroporation: a study of human uterine leiomyosarcomas in nude mice. Int J Cancer. 2000;88:640–644. doi: 10.1002/1097-0215(20001115)88:4<640::aid-ijc19>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 85.Mulvihill ER, Jaeger J, Sengupta R, Ruzzo WL, Reimer C, Lukito S, et al. Atherosclerotic plaque smooth muscle cells have a distinct phenotype. Arterioscler Thromb Vasc Biol. 2004;24:1283–1289. doi: 10.1161/01.ATV.0000132401.12275.0c. [DOI] [PubMed] [Google Scholar]
- 86.Hosack DA, Dennis G, Sherman BT, Lane HC, Lempicki RA., Jr Identifying biological themes within lists of genes with EASE. Genome Biol. 2003;4:R70. doi: 10.1186/gb-2003-4-10-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993;15:532–534. 6–7. [PubMed] [Google Scholar]
- 88.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 89.Steg A, Wang W, Blanquicett C, Grunda JM, Eltoum IA, Wang K, et al. Multiple gene expression analyses in paraffin-embedded tissues by TaqMan low-density array: Application to hedgehog and Wnt pathway analysis in ovarian endometrioid adenocarcinoma. J Mol Diagn. 2006;8:76–83. doi: 10.2353/jmoldx.2006.040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


