Abstract
Objective
Increased vascular stiffness is central to the pathophysiology of aging, hypertension, diabetes and atherosclerosis. However, relatively few studies have examined vascular stiffness in both the thoracic and abdominal aorta with aging, despite major differences in anatomy, embryological origin and relation to aortic aneurysm.
Approach and Results
The two other unique features of this study were 1) to study young (9±1 years) and old (26±1 years) male monkeys, and 2) to study direct and continuous measurements of aortic pressure and thoracic and abdominal aortic diameters in conscious monkeys. As expected, aortic stiffness, β, was increased p<0.05, 2–3 fold, in old vs. young thoracic aorta, and augmented further with superimposition of acute hypertension with phenylephrine. Surprisingly, stiffness was not greater in old thoracic aorta than young abdominal aorta. These results can be explained in part by the collagen/elastin ratio, but more importantly, by disarray of collagen and elastin, which correlated best with vascular stiffness. However, vascular smooth muscle cell stiffness, was not different in thoracic vs. abdominal aorta in either young or old monkeys.
Conclusions
Thus, aortic stiffness increases with aging as expected, but the most severe increases in aortic stiffness observed in the abdominal aorta is novel, where values in young monkeys equaled, or even exceeded, values of thoracic aortic stiffness in old monkeys. These results can be explained by alterations in collagen/elastin ratio, but even more importantly by collagen and elastin disarray.
Keywords: hypertension, aortic stiffness, aging, collagen, elastin, non-human primates
INTRODUCTION
Increased vascular stiffness is not only an important mechanism for hypertension, but is also an important mechanism mediating some of the adverse outcomes in hypertension, aortic aneurysm, rupture and dissection1–4. However, increased aortic stiffness is most commonly associated with aging5–9. It is likely that all elderly individuals suffer from increased vascular stiffness to a more or less degree. Although aortic stiffness has been studied extensively in aging, and almost all studies agree that it is increased with aging, relatively few studies have studied vascular stiffness in both the thoracic and abdominal aorta, also examining the influences of aging. This comparison is important as there are major differences due to anatomy, wall thickness and even embryological origin, and clinical importance related to aneurysm development10–12. Furthermore, there is no consensus in the literature on the extent to which aging increases aortic stiffness in the thoracic vs. abdominal aorta. A major reason for this is that relatively few studies have been specifically designed to examine both thoracic and abdominal aortic stiffness in both young and old animals or clinical subjects.
There are also limitations in models and techniques used to assess the increase in vascular stiffness with aging. Whereas it is generally agreed that studies in human subjects are most relevant to understanding human pathophysiology, even these studies have limitations. First, most older human subjects have evidence of atherosclerosis on autopsy, which in itself is a major cause of increased aortic stiffness13, 14. Secondly, these studies utilize in vitro techniques from autopsied material or in vivo techniques based on echo or MRI, which allow only a snapshot in time for aortic stiffness measurement. These latter arguments also pertain to prior animal studies as well. In addition, for many experiments in animals, anesthesia is required for these measurements, which by itself, has a major impact on vascular stiffness.
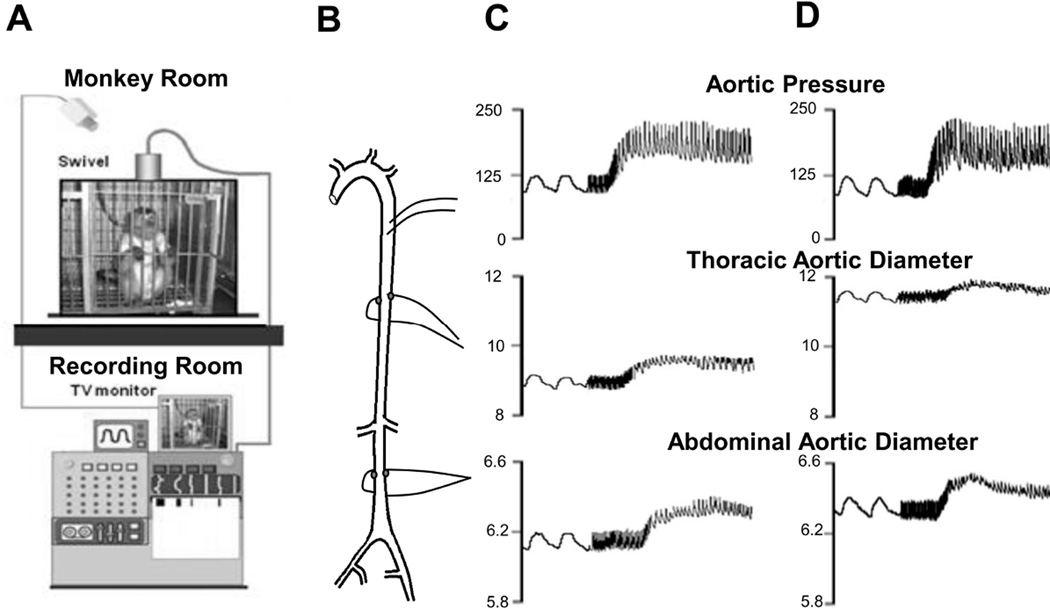
To obviate these problems, we developed techniques to measure vascular stiffness instantaneously and continuously in conscious animals without the complicating influences of recent surgery or anesthesia. To accomplish this, ultrasonic crystals were implanted on opposing surfaces of the thoracic and abdominal aorta and a pressure gauge was implanted in the aorta to provide beat by beat measurements of arterial pressure and diameter (Fig. 1), which are the critical variables required for assessing vascular stiffness, at baseline and in response to acute hypertension, induced by phenylephrine. An important feature of the current investigation was to measure both thoracic and abdominal aortic stiffness in the same animals, in order to address the lack of information and reconcile the controversy in the literature on differences in thoracic vs. abdominal aortic stiffness with aging. Furthermore, most prior studies in the literature have studied rodents, which is problematic for the study of aging, since rodents only live 2–3 years. Accordingly, we utilized non-human primates as the subjects in the current investigation, which not only are closer to humans phylogenetically, but also have lifespans of 25 to 40 years. An additional goal was to determine the mechanism of the increases, e.g., was it due to increased collagen or decreased elastin or to other factors. One important mechanism involves increases in stiffness of the vascular smooth muscle cells15, 16. This was accomplished by measuring the stiffness of isolated vascular smooth muscle cells with atomic force microscopy.
Fig. 1.
(A) Chronically instrumented, conscious monkeys were connected to a tether, but otherwise unrestrained in their cage during recording. (B) The aorta was instrumented with descending thoracic aortic catheters for measurement of aortic pressure and ultrasonic dimension crystals on opposing surfaces of the thoracic and abdominal aorta for measurement of aortic diameters. Examples of responses to acute phenylephrine induced hypertension are shown in a young monkey (C) and in and old monkey (D). Stiffness reflected by reduced aortic diameter excursion was increased more in old monkeys with phenylephrine than in young monkeys.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
Hemodynamic Characteristics
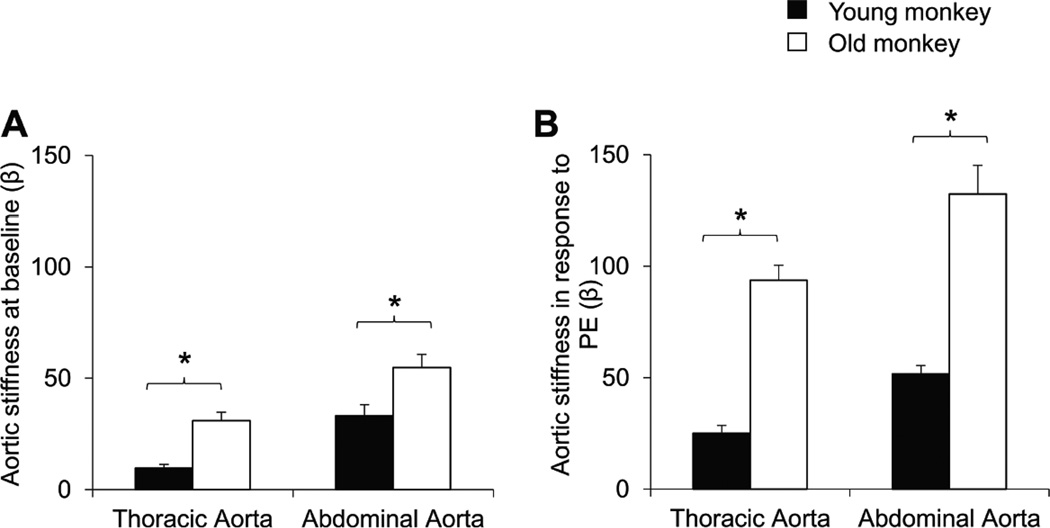
Although heart rate and mean arterial pressure were not different in old vs. young monkeys, the aortic pulse pressure was increased in the old monkeys (45±2.8mmHg), compared with young monkeys (38±2.3mmHg). In old monkeys, the aortic pulse diameters of both thoracic (0.19±0.02mm) and abdominal (0.06±0.01mm) aorta were markedly lower than those in the thoracic (0.47±0.12mm) and abdominal (0.09±0.01mm) aorta of young monkeys, suggesting both segments of the aorta were stiffer in aging monkeys (p<0.05). The calculated aortic stiffness index (β), based on aortic pressure and aortic diameter was increased in both the thoracic and abdominal aorta in old monkeys compared to young ones (p<0.05, Fig. 2A), it was also greater in the abdominal aorta compared with thoracic aorta in young as well as old monkeys (p<0.05, Fig. 2A). Interestingly, abdominal aortic stiffness in young monkeys (33±5.0) equaled that in thoracic aorta in old monkeys (31±3.8).
Fig. 2.
In chronically instrumented conscious monkeys, (A) baseline aortic stiffness was greater in old monkeys for both the thoracic and abdominal aorta, but interestingly abdominal aortic stiffness in young monkeys equaled thoracic aortic stiffness in old monkeys. (B) In response to phenylephrine (PE), aortic stiffness was dramatically increased in old monkeys compared to young monkeys. *p<0.05 by ANOVA.
Increased Aortic Stiffness with Acute Hypertension
Using phenylephrine to pharmacologically elevate aortic pressure, the continuous changes of mean arterial pressure and diameters and the corresponding aortic stiffness changes were monitored. Phenylephrine increased arterial pressure similarly in both young and old monkeys, but pulse diameters of both thoracic and abdominal aorta in old monkeys were significantly decreased more in old compared to those in young monkeys (Fig. 1). In addition, the reduction in pulse diameter of abdominal aorta of both young and old monkeys was more than in the thoracic aorta. The aortic stiffness, presented as stiffness index (β) increased more in response to phenylephrine in old monkeys as compared to young ones in both the abdominal and thoracic aorta (Fig. 2B).
Change in Extracellular Matrix
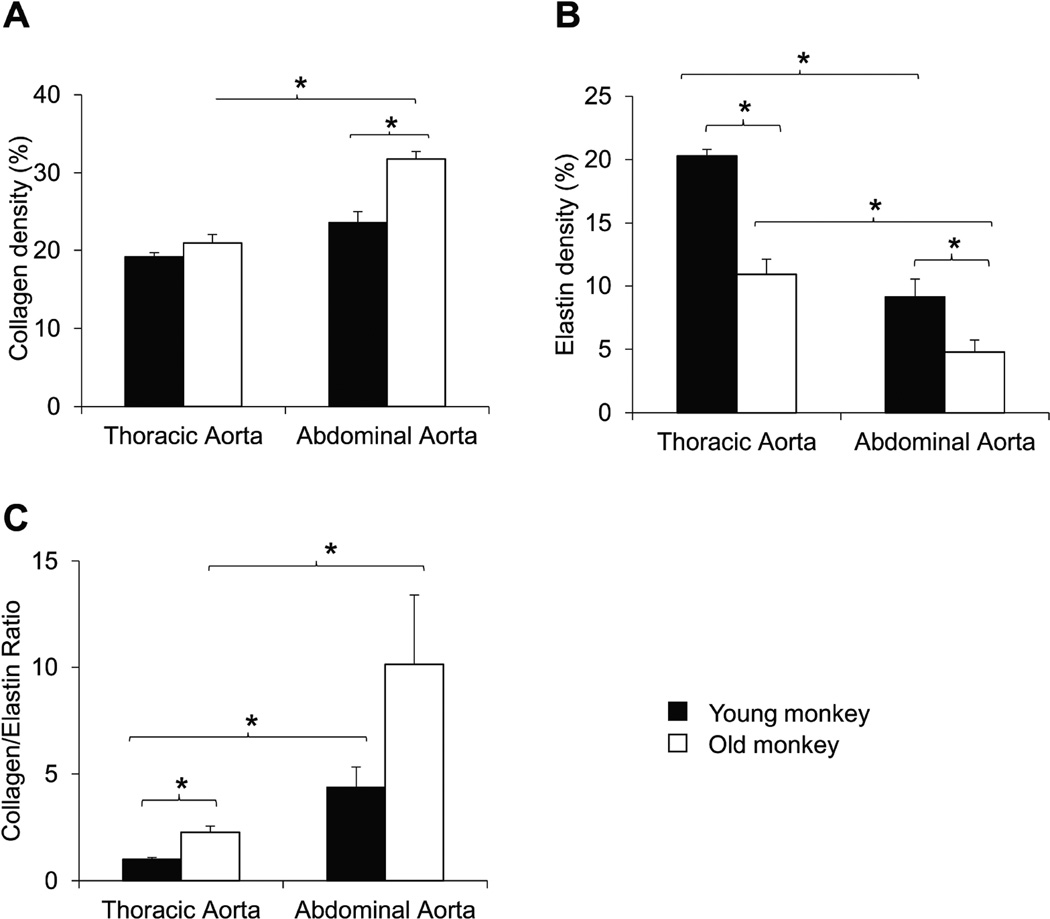
Aortic elastin and collagen were stained and compared. Collagen density was increased more in the abdominal aorta compared with thoracic aorta of old monkeys. Also, collagen density increased more in the abdominal aorta in old monkeys than abdominal aorta in young monkeys. These data indicate, that this is one mechanism mediating the greater stiffness in the abdominal aorta compared with the thoracic aorta in old monkeys and also, greater increases in stiffness in the abdominal aorta of old monkeys compared with abdominal aorta in young monkeys (Fig. 3A). The elastin density was decreased in both the abdominal and thoracic aorta in old monkeys, suggesting that this is an important mechanism underlying the increased stiffness in the abdominal, compared with the thoracic aorta (Fig. 3B). Furthermore, elastin density was lower in the abdominal vs. the thoracic aorta in both old and young monkeys, suggesting that this is an important mechanism underlying the increased stiffness in the aging abdominal, compared with the thoracic aorta (Fig. 3B).
Fig. 3.
(A) Using picrosirius red staining, collagen density was increased more in abdominal aorta of old monkeys, compared with abdominal aorta in young monkeys or thoracic aortic collagen in either old or young monkeys. (B) Using aldehyde fuchsin staining, elastin density was less in both abdominal aorta compared with the thoracic aorta in both old and young monkeys and decreases were greater in both abdominal and thoracic aorta in old monkeys. (C) Collagen to elastin ratio was increased with aging in both thoracic and abdominal aorta, but increases were greater in the abdominal aorta. *p<0.05 by ANOVA.
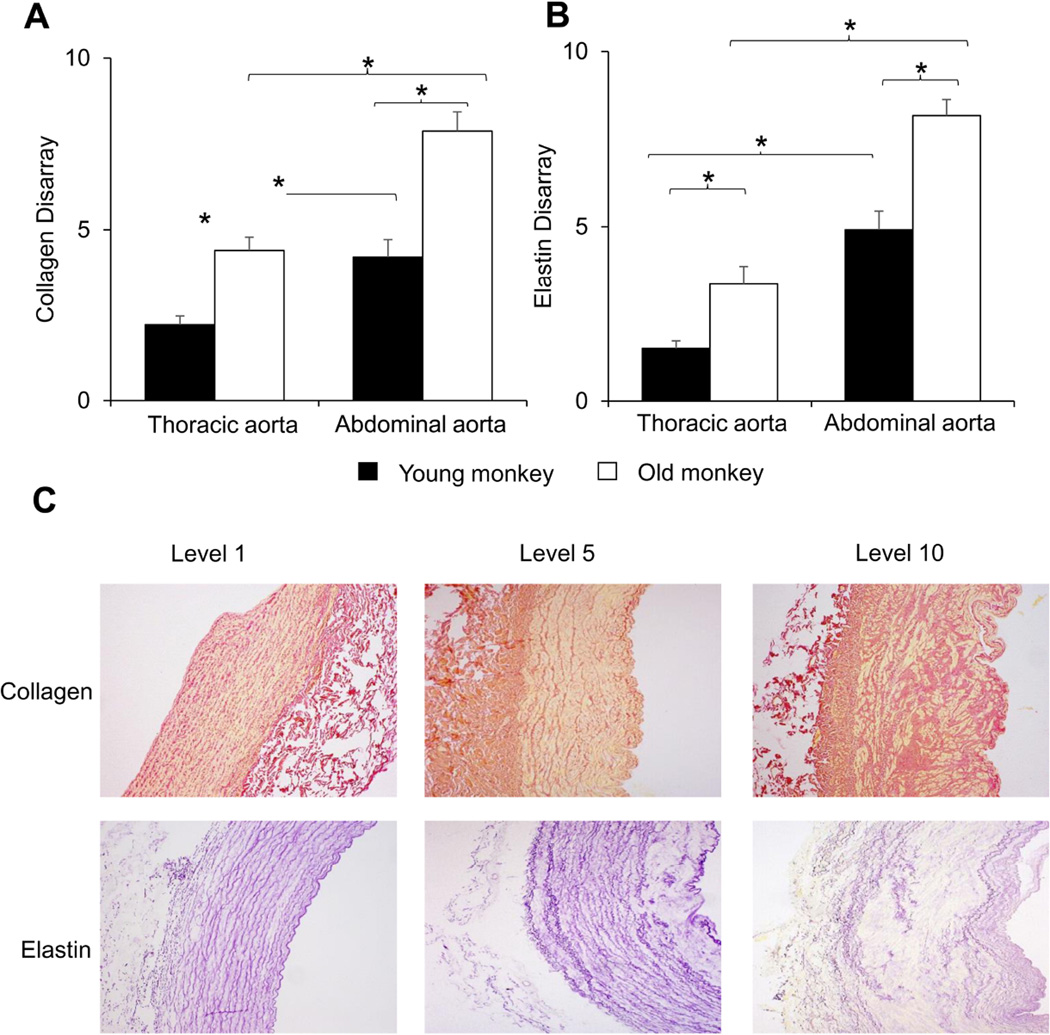
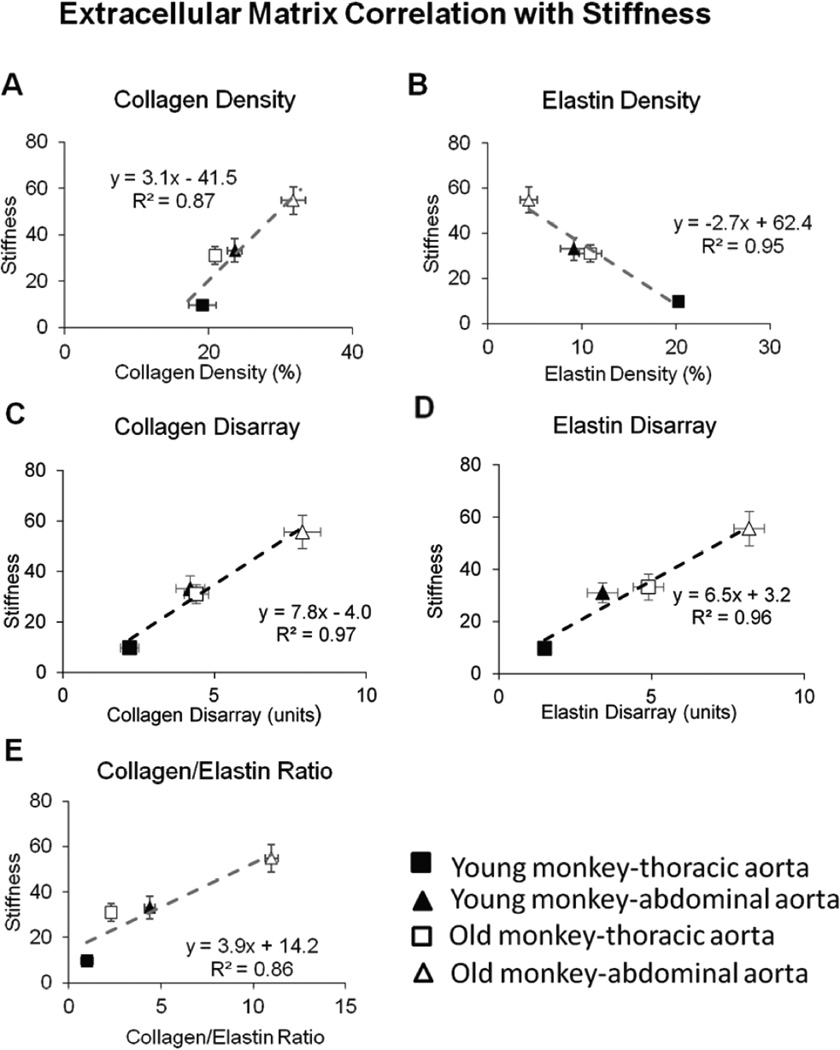
The changes in collagen and elastin resulted in a significantly increased ratio of collagen to elastin in aging monkeys, as well as in abdominal aorta, compared with thoracic aorta, in both age groups (Fig. 3C, p<0.05). Using a subjective grading system, the severity of disarray of elastic fibers was compared blindly between thoracic and abdominal aorta and between both young and old monkeys. Elastin showed more marked disarray in old monkeys than young monkeys for both the abdominal and thoracic aorta (Fig. 4A, p<0.05), and elastin disarray was even greater in the abdominal aorta of young monkeys than in the thoracic aorta of old monkeys (Fig. 4B, p<0.05). Examples of elastin and collagen disarray levels of 1, 5 and 10 are shown in Fig. 4C. Collagen disarray was also increased in old vs. young abdominal aorta and in old vs. young thoracic aorta. Regression analysis demonstrated that all parameters of the extracellular matrix correlated with the increased stiffness, but that the best correlations were for elastin density and elastin disarray (Fig. 5).
Fig. 4.
The disarray of elastin and collagen was subjectively graded in a blinded manner from level 1 as the best to level 10 as the most severe disarray, as shown in Table 1. (A) The elastin disarray was greater in abdominal aorta vs. thoracic aorta for both old and young monkeys and was greater in old monkeys vs. young monkeys for both the thoracic and abdominal aorta. (B) The collagen disarray was also greater in old vs. young monkeys. Examples of elastin and collagen disarray levels of 1, 5 and 10 are shown in (C). *p<0.05 by Mann-Whitney U-Test and Kruskal–Wallis test.
Fig. 5.
Correlation between stiffness and extracellular matrix was compared for (A) collagen density, (B) elastin density, (C) collagen disarray, (D) elastin disarray and (E) ratio of collagen to elastin density. All parameters showed a linear correlation between stiffness and the extracellular matrix. The best correlations were for elastin disarray and collagen disarray.
Vascular Smooth Muscle Cell Stiffness
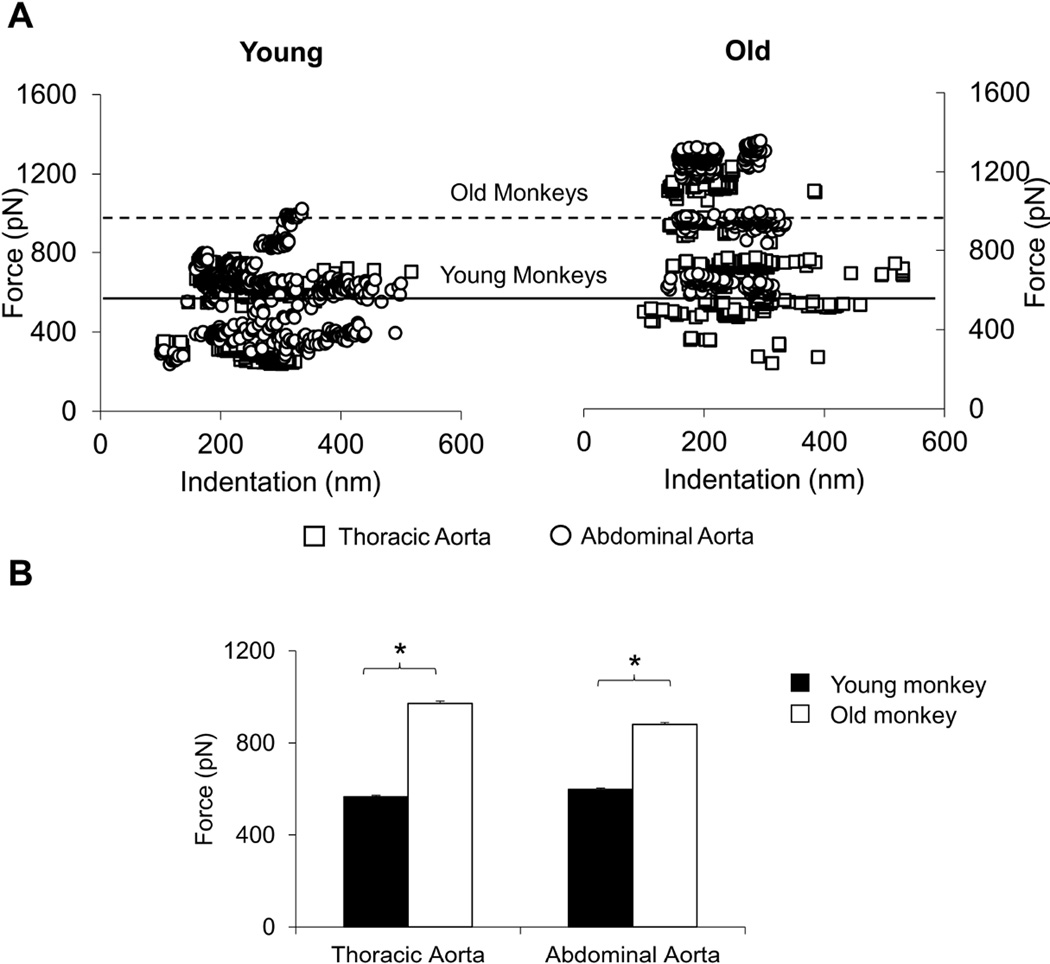
Vascular smooth muscle cells (VSMCs) were isolated from fresh thoracic and abdominal aorta, and using atomic force microscopy, the stiffness of single VSMCs was studied with force-indentation relationship by comparing required force for similar generated indentation. For both the thoracic aorta and abdominal aorta VSMC stiffness, directly relate to force-indentation, was increased in the old vs. the young monkeys, but there was no significant difference in abdominal vs. thoracic VSMC stiffness either in young or old monkeys (Fig. 6). It is suggesting the aging induced increases in aortic stiffness found in vivo was partially due to the increased intrinsic stiffness in VSMC, but that the regional differences in stiffness between the thoracic and abdominal aorta could not be explained by this mechanism.
Fig. 6.
Aortic vascular smooth muscle cells (VSMC) were isolated from young and old male monkeys. The AFM force-indentation measurements are directly related to VSMC stiffness. All the individual data are plotted in (A), with white squares representing thoracic aorta and white circles for abdominal aorta. The data for young monkeys are on the left and old monkeys on the right. The horizontal lines show the average data for old monkeys and young monkeys. VSMC stiffness was greater in old vs. young monkeys, but these differences were not greater in abdominal, compared with thoracic aorta, as reflected by the almost complete overlap of data, indicating that this mechanism was not responsible for the greater increases in abdominal aortic stiffness in old monkeys. The average data are shown in (B), *p<0.05 by ANOVA.
DISCUSSION
Increased vascular stiffness is a typical paradigm associated with aging9. Numerous studies on this topic have documented increased aortic stiffness with aging, but many of the studies did not examine the mechanism and there is controversy among those studies, which did examine mechanisms. There is also controversy about differences in thoracic vs. abdominal aortic stiffness with aging and the mechanisms involved6–8, 17–20, despite the clinical importance of this topic for renal perfusion or for abdominal aortic aneurysm, which exceeds thoracic in frequency21. There is even relatively little known about regional aortic stiffness and its mechanisms in the absence of aging17–19. The present investigation addressed these topics and the results were significant in two major areas; 1) the mechanisms of aortic stiffness that differ with aging, and 2) the differences in aortic stiffness and its mechanisms in the thoracic vs. abdominal aorta.
There are several features of this study that are novel and may be responsible for any differences from the literature. First of all measurements of aortic dimensions were recorded from implanted ultrasonic crystals on the thoracic and abdominal aorta to permit direct and continuous measurements of aortic diameters along with pressure for stiffness calculations. This is an important difference from the majority of prior work, where aortic stiffness was calculated from indirect measurements of pulse wave velocity or from in vitro specimens. Secondly, the current studies were conducted in non-human primates, a model phylogenetically closer to humans and quite different from the majority of prior studies conducted in rodents. Whereas, it would be best to study the effects of aging on human aortas, it is difficult to obtain measurements in older patients that are entirely free from atherosclerosis or some intimal changes, which can increase stiffness, whereas the aging monkey is free from these complicating effects.
We found, as expected, that aortic stiffness was increased in the old compared to the young monkey. We also found that abdominal aortic stiffness was greater than thoracic aortic stiffness, even in the young monkey. The magnitude of the difference was not expected, as reflected by the increased abdominal aortic stiffness in young monkeys matching, or exceeding, the levels of increased aortic stiffness observed in the thoracic aorta of old monkeys. Supporting these results the measurements of collagen and elastin density in the aorta were almost identical in the old thoracic and young abdominal aorta. These marked differences in thoracic vs. abdominal aortic stiffness with aging and even within the same age group help resolve the prior controversy in the literature on this topic.
Most studies attribute the increased aortic stiffness with aging to changes in the extracellular matrix, i.e., increases in collagen and/or decreases in elastin, which would both increase vascular stiffness22–24. Importantly, very few studies measured both aortic stiffness, in vivo, and extra cellular matrix changes, in vitro, as we did. Studies found that the mechanism for the increase in stiffness was an increase in collagen and decrease in elastin22, 25. We found no increase in collagen density in the thoracic aorta but an increase in collagen density in the abdominal aorta of old monkeys, but a clear decrease in elastin in both the old thoracic and abdominal aorta. Other studies measured collagen and elastin with aging, although they did not measure aortic stiffness, in vivo. However, even for those studies there is no consensus, with studies finding either an increase in collagen22, 25–28 or no change29 or a decrease in elastin22, 23, 30 or no change25. Interestingly some studies even showed an increase in elastin27, 31, which should act to decrease aortic stiffness. As noted above, fewer studies have examined both thoracic and abdominal aortic stiffness with aging, in vivo6–8, 17–20. Although isolated in vitro studies have found that abdominal stiffness is increased more with aging32, most prior studies, in vivo, found that increases in thoracic aortic stiffness with aging were greater than, or similar to that of abdominal aortic stiffness6–8, 17–20 which is the reverse of our data. Interestingly, hypertension without aging, an important disease associated with increased aortic stiffness, has been shown to exhibit greater abdominal than thoracic aortic stiffness33, 34.
These conclusions, quite different from ours, might be explained by baseline differences. If baseline abdominal aortic stiffness is greater than thoracic in young subjects, then the change in old compared to the young expressed as a change from baseline would necessarily be less for the abdominal aorta, due to the difference in the initial baseline values (neglecting that absolute values are greater in the aging abdominal aortas). For example, with our data, the % increase in abdominal aortic stiffness with aging in the abdominal aorta (71%) was less than in the thoracic aorta (204%), even though the absolute values for stiffness were greater in the aging abdominal aorta (55±5.9), compared with absolute values for stiffness in the thoracic aorta (31±3.8). However, almost none had looked at extracellular matrix mechanisms at the same time comparing regional aortic stiffness measurements within the same age group.
As noted above, we found significantly greater increases in abdominal compared to thoracic aortic stiffness, both with aging and even in young animals. In contrast to the thoracic aorta, collagen rose by 34% in the abdominal aorta with aging, and was greater than that observed in the thoracic aorta. Elastin was decreased in the abdominal compared to the thoracic aorta in young animals, but was decreased to even lower levels with aging. Consistent with our finding that abdominal aortic stiffness in the young monkeys equaled that of thoracic stiffness in old monkeys, the abdominal aortic collagen and elastin levels in young monkeys equaled those values observed in the thoracic aorta for old monkeys, emphasizing the importance for studying regional aortic stiffness changes with aging.
Simply, basal levels of collagen and elastin were not the only mechanism explaining why stiffness was greater in the abdominal aorta both in young and old monkeys. We also observed marked disarray of both collagen and elastin with aging and more in abdominal vs. thoracic aorta. In fact, the marked disarray of elastin and collagen in the young abdominal aorta is likely responsible for the unexpected increased aortic stiffness observed even in the absence of aging. The elastin and collagen disarray correlated better with stiffness than did elastin and collagen content. It is surprising that these marked architectural disarray changes we observed in the aging aorta with increased stiffness have not been noted extensively in the past, even though isolated observations have previously found disarray in aortas related to aneurysm35–37, hypertension38 and aging39.
Since increased aortic stiffness, even in the absence of aging, is almost uniformly observed in hypertension, we also utilized a hypertensive challenge with phenylephrine to examine how increasing arterial pressure affected thoracic and abdominal aortic stiffness with aging. The increases in stiffness with hypertension were significantly greater in the old vs. the young monkey in both the abdominal and thoracic aorta, despite equivalent increases in aortic pressure. These findings help explain why combined age and hypertension affects aortic stiffness more severely than in young animals40.
One limitation to the regional measurements of aortic stiffness is that the chronically instrumented monkeys only had an implanted thoracic aortic catheter to measure pressure, but diameters were measured in both the thoracic and abdominal aorta. Accordingly, in terminal experiments after sedation we also measured regional thoracic and abdominal aortic pressures using a Millar micromanometer so that thoracic and abdominal stiffness could be calculated using the regional measurement of aortic pressure. The data from these experiments plotted against those when only thoracic aortic pressure was measured in the conscious animals showed a highly linear relationship with an r2 of 0.97 (Supplemental Fig. I).
There are other mechanisms mediating increased vascular stiffness with aging, e.g., at the level of the endothelium or VSMC15, 40. We have previously shown, using atomic force microscopy to measure isolated VSMC elasticity, that this is an important mechanism mediating the increased aortic stiffness in aging and hypertension15, 40. Although we cannot conclude the extent to which this mechanism was important, since we did not perform any experiments eliminating VSMC stiffness and showing less total aortic stiffness, there are several lines of reasoning that support a role for the VSMC stiffness mechanism. First, it is logical that if one component of the aortic wall becomes stiffer, e.g., VSMCs, it likely contributes to the aggregate increased aortic stiffness, which results from several mechanisms. Secondly, our prior studies showed that VSMC stiffness is an important mechanism mediating the increased aortic stiffness in aging and hypertension15, 40. We also used these techniques in the current investigation and confirmed the increases in VSMC stiffness with aging in the thoracic aorta and abdominal aorta. However, there was no significant difference in thoracic vs. abdominal aortic VSMC stiffness, indicating that this mechanism could not explain the increased stiffness in the abdominal aorta and that the extracellular matrix mechanisms were more important. It is also likely that the mechanism of increased VSMC stiffness contributed to the augmented stiffness observed with phenylephrine in the present investigation, as we previously demonstrated that angiotensin increased VSMC stiffness in VSMCs from both SHR and WKY control rats16.
Supplementary Material
SIGNIFICANCE.
-
*
Demonstration that increases in abdominal aortic stiffness are more important than those in the thoracic aorta with aging and that abdominal aortic stiffness in the young animals was equal to or greater than that of thoracic aorta in aging animals.
-
*
Demonstration that the mechanism of the differences with aging and the differences in the thoracic and abdominal aorta were due to changes in collagen and elastin density and more novel, in collagen and elastin disarray.
-
*
Novelty also due to use of the non-human primate model, which is more relevant to human aging than rodent studies and the combined study of direct and continuous measurement of aortic diameters in both the thoracic and abdominal aorta along with aortic pressure in vivo followed by in vitro studies of isolated vascular smooth muscle stiffness and collagen and elastin histology.
-
*
The results of this study are directly relevant not only to the pathophysiology of aging, but also other diseases involving vascular stiffness, in particular hypertension, diabetes and atherosclerosis.
-
*
The results are also relevant to the understanding of aortic aneurysm.
Acknowledgments
We thank Dr. Yimin Tian and Ms. Grace Lee for their work on the histology studies.
FUNDING RESOURCES
Study was supported by National Institute of Health grants 5R01HL102472, 6T32HL069752, 5R01HL119464, 3P01HL069020, 6T32HL069752, 6R01HL093481, 5R01HL106511, 1R01HL124282, R01HL130848
ABBREVIATIONS
- VSMCs
Vascular smooth muscle cells
- AFM
Atomic force microscopy
- PE
Phenylephrine
- MRI
Magnetic Resonance Imaging
Footnotes
DISCLOSURES: None
REFERENCES
- 1.Pfeffer MA, Pfeffer JM, Frohlich ED. Pumping ability of the hypertrophying left ventricle of the spontaneously hypertensive rat. Circulation research. 1976;38:423–429. doi: 10.1161/01.res.38.5.423. [DOI] [PubMed] [Google Scholar]
- 2.McNulty M, Mahmud A, Feely J. Advanced glycation end-products and arterial stiffness in hypertension. American journal of hypertension. 2007;20:242–247. doi: 10.1016/j.amjhyper.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Williams IM, Hughes OD, Townsend E, Winter RK, Lewis MH. Prevalence of abdominal aortic aneurysm in a hypertensive population. Annals of the Royal College of Surgeons of England. 1996;78:501–504. [PMC free article] [PubMed] [Google Scholar]
- 4.Isselbacher EM. Epidemiology of thoracic aortic aneurysms, aortic dissection, intramural hematoma, and penetrating atherosclerotic ulcers. Aortic Dissection and Related Syndromes Developments in Cardiovascular Medicine. 2007;260:3–15. [Google Scholar]
- 5.Lee HY, Oh BH. Aging and arterial stiffness. Circulation journal : official journal of the Japanese Circulation Society. 2010;74:2257–2262. doi: 10.1253/circj.cj-10-0910. [DOI] [PubMed] [Google Scholar]
- 6.Rogers WJ, Hu YL, Coast D, Vido DA, Kramer CM, Pyeritz RE, Reichek N. Age-associated changes in regional aortic pulse wave velocity. Journal of the American College of Cardiology. 2001;38:1123–1129. doi: 10.1016/s0735-1097(01)01504-2. [DOI] [PubMed] [Google Scholar]
- 7.Taviani V, Hickson SS, Hardy CJ, McEniery CM, Patterson AJ, Gillard JH, Wilkinson IB, Graves MJ. Age-related changes of regional pulse wave velocity in the descending aorta using fourier velocity encoded m-mode. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2011;65:261–268. doi: 10.1002/mrm.22590. [DOI] [PubMed] [Google Scholar]
- 8.Westenberg JJ, Scholte AJ, Vaskova Z, van der Geest RJ, Groenink M, Labadie G, van den Boogaard PJ, Radonic T, Hilhorst-Hofstee Y, Mulder BJ, Kroft LJ, Reiber JH, de Roos A. Age-related and regional changes of aortic stiffness in the marfan syndrome: Assessment with velocity-encoded mri. Journal of magnetic resonance imaging : JMRI. 2011;34:526–531. doi: 10.1002/jmri.22646. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: The framingham heart study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 10.Erbel R, Eggebrecht H. Aortic dimensions and the risk of dissection. Heart. 2006;92:137–142. doi: 10.1136/hrt.2004.055111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolinsky H, Glagov S. Comparison of abdominal and thoracic aortic medial structure in mammals. Deviation of man from the usual pattern. Circulation research. 1969;25:677–686. doi: 10.1161/01.res.25.6.677. [DOI] [PubMed] [Google Scholar]
- 12.Kau T, Sinzig M, Gasser J, Lesnik G, Rabitsch E, Celedin S, Eicher W, Illiasch H, Hausegger KA. Aortic development and anomalies. Seminars in interventional radiology. 2007;24:141–152. doi: 10.1055/s-2007-980040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, Hoeks AP, van der Kuip DA, Hofman A, Witteman JC. Association between arterial stiffness and atherosclerosis: The rotterdam study. Stroke; a journal of cerebral circulation. 2001;32:454–460. doi: 10.1161/01.str.32.2.454. [DOI] [PubMed] [Google Scholar]
- 14.Wykretowicz A, Gerstenberger P, Guzik P, Milewska A, Krauze T, Adamska K, Rutkowska A, Wysocki H. Arterial stiffness in relation to subclinical atherosclerosis. European journal of clinical investigation. 2009;39:11–16. doi: 10.1111/j.1365-2362.2008.02057.x. [DOI] [PubMed] [Google Scholar]
- 15.Qiu H, Zhu Y, Sun Z, Trzeciakowski JP, Gansner M, Depre C, Resuello RR, Natividad FF, Hunter WC, Genin GM, Elson EL, Vatner DE, Meininger GA, Vatner SF. Short communication: Vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circulation research. 2010;107:615–619. doi: 10.1161/CIRCRESAHA.110.221846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sehgel NL, Zhu Y, Sun Z, Trzeciakowski JP, Hong Z, Hunter WC, Vatner DE, Meininger GA, Vatner SF. Increased vascular smooth muscle cell stiffness: A novel mechanism for aortic stiffness in hypertension. American journal of physiology. Heart and circulatory physiology. 2013;305:H1281–H1287. doi: 10.1152/ajpheart.00232.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devos DG, Rietzschel E, Heyse C, Vandemaele P, Van Bortel L, Babin D, Segers P, Westenberg JM, Achten R. Mr pulse wave velocity increases with age faster in the thoracic aorta than in the abdominal aorta. Journal of magnetic resonance imaging : JMRI. 2015;41:765–772. doi: 10.1002/jmri.24592. [DOI] [PubMed] [Google Scholar]
- 18.Nelson AJ, Worthley SG, Cameron JD, Willoughby SR, Piantadosi C, Carbone A, Dundon BK, Leung MC, Hope SA, Meredith IT, Worthley MI. Cardiovascular magnetic resonance-derived aortic distensibility: Validation and observed regional differences in the elderly. Journal of hypertension. 2009;27:535–542. doi: 10.1097/hjh.0b013e32831e4599. [DOI] [PubMed] [Google Scholar]
- 19.Hickson SS, Butlin M, Graves M, Taviani V, Avolio AP, McEniery CM, Wilkinson IB. The relationship of age with regional aortic stiffness and diameter. JACC. Cardiovascular imaging. 2010;3:1247–1255. doi: 10.1016/j.jcmg.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Farrar DJ, Bond MG, Sawyer JK, Green HD. Pulse wave velocity and morphological changes associated with early atherosclerosis progression in the aortas of cynomolgus monkeys. Cardiovascular research. 1984;18:107–118. doi: 10.1093/cvr/18.2.107. [DOI] [PubMed] [Google Scholar]
- 21.Multicentre aneurysm screening study (mass) Cost effectiveness analysis of screening for abdominal aortic aneurysms based on four year results from randomised controlled trial. BMJ. 2002;325:1135. doi: 10.1136/bmj.325.7373.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nosaka T, Tanaka H, Watanabe I, Sato M, Matsuda M. Influence of regular exercise on age-related changes in arterial elasticity: Mechanistic insights from wall compositions in rat aorta. Canadian journal of applied physiology = Revue canadienne de physiologie appliquee. 2003;28:204–212. doi: 10.1139/h03-016. [DOI] [PubMed] [Google Scholar]
- 23.Qiu H, Depre C, Ghosh K, Resuello RG, Natividad FF, Rossi F, Peppas A, Shen YT, Vatner DE, Vatner SF. Mechanism of gender-specific differences in aortic stiffness with aging in nonhuman primates. Circulation. 2007;116:669–676. doi: 10.1161/CIRCULATIONAHA.107.689208. [DOI] [PubMed] [Google Scholar]
- 24.Avolio A, Jones D, Tafazzoli-Shadpour M. Quantification of alterations in structure and function of elastin in the arterial media. Hypertension. 1998;32:170–175. doi: 10.1161/01.hyp.32.1.170. [DOI] [PubMed] [Google Scholar]
- 25.Wheeler JB, Mukherjee R, Stroud RE, Jones JA, Ikonomidis JS. Relation of murine thoracic aortic structural and cellular changes with aging to passive and active mechanical properties. Journal of the American Heart Association. 2015;4:e001744. doi: 10.1161/JAHA.114.001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. Journal of hypertension. 2003;21:3–12. doi: 10.1097/00004872-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Chamiot-Clerc P, Renaud JF, Safar ME. Pulse pressure, aortic reactivity, and endothelium dysfunction in old hypertensive rats. Hypertension. 2001;37:313–321. doi: 10.1161/01.hyp.37.2.313. [DOI] [PubMed] [Google Scholar]
- 28.Faber M, Oller-Hou G. The human aorta. V. Collagen and elastin in the normal and hypertensive aorta. Acta pathologica et microbiologica Scandinavica. 1952;31:377–382. [PubMed] [Google Scholar]
- 29.Hosoda Y, Kawano K, Yamasawa F, Ishii T, Shibata T, Inayama S. Age-dependent changes of collagen and elastin content in human aorta and pulmonary artery. Angiology. 1984;35:615–621. doi: 10.1177/000331978403501001. [DOI] [PubMed] [Google Scholar]
- 30.Atanasova M, Dimitrova A, Ruseva B, Stoyanova A, Georgieva M, Konova E. Quantification of elastin, collagen and advanced glycation end products as functions of age and hypertension. 2012;Chapter 21 [Google Scholar]
- 31.Sauvage M, Hinglais N, Mandet C, Badier C, Deslandes F, Michel JB, Jacob MP. Localization of elastin mrna and tgf-beta1 in rat aorta and caudal artery as a function of age. Cell and tissue research. 1998;291:305–314. doi: 10.1007/s004410051000. [DOI] [PubMed] [Google Scholar]
- 32.Haskett D, Johnson G, Zhou A, Utzinger U, Vande Geest J. Microstructural and biomechanical alterations of the human aorta as a function of age and location. Biomechanics and modeling in mechanobiology. 2010;9:725–736. doi: 10.1007/s10237-010-0209-7. [DOI] [PubMed] [Google Scholar]
- 33.Ameer OZ, Salman IM, Avolio AP, Phillips JK, Butlin M. Opposing changes in thoracic and abdominal aortic biomechanical properties in rodent models of vascular calcification and hypertension. American journal of physiology. Heart and circulatory physiology. 2014;307:H143–H151. doi: 10.1152/ajpheart.00139.2014. [DOI] [PubMed] [Google Scholar]
- 34.Ng K, Butlin M, Avolio AP. Persistent effect of early, brief angiotensin-converting enzyme inhibition on segmental pressure dependency of aortic stiffness in spontaneously hypertensive rats. Journal of hypertension. 2012;30:1782–1790. doi: 10.1097/HJH.0b013e3283562e35. [DOI] [PubMed] [Google Scholar]
- 35.Lee J, Shen M, Parajuli N, Oudit GY, McMurtry MS, Kassiri Z. Gender-dependent aortic remodelling in patients with bicuspid aortic valve-associated thoracic aortic aneurysm. Journal of molecular medicine. 2014;92:939–949. doi: 10.1007/s00109-014-1178-6. [DOI] [PubMed] [Google Scholar]
- 36.Hofmann Bowman M, Wilk J, Heydemann A, Kim G, Rehman J, Lodato JA, Raman J, McNally EM. S100a12 mediates aortic wall remodeling and aortic aneurysm. Circulation research. 2010;106:145–154. doi: 10.1161/CIRCRESAHA.109.209486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pezzini A, Del Zotto E, Giossi A, Volonghi I, Costa P, Padovani A. Transforming growth factor beta signaling perturbation in the loeys-dietz syndrome. Current medicinal chemistry. 2012;19:454–460. doi: 10.2174/092986712803414286. [DOI] [PubMed] [Google Scholar]
- 38.Sans M, Moragas A. Mathematical morphologic analysis of the aortic medial structure. Biomechanical implications. Analytical and quantitative cytology and histology / the International Academy of Cytology [and] American Society of Cytology. 1993;15:93–100. [PubMed] [Google Scholar]
- 39.Fornieri C, Quaglino D, Jr, Mori G. Role of the extracellular matrix in age-related modifications of the rat aorta. Ultrastructural, morphometric, and enzymatic evaluations. Arteriosclerosis and thrombosis : a journal of vascular biology / American Heart Association. 1992;12:1008–1016. doi: 10.1161/01.atv.12.9.1008. [DOI] [PubMed] [Google Scholar]
- 40.Sehgel NL, Sun Z, Hong Z, Hunter WC, Hill MA, Vatner DE, Vatner SF, Meininger GA. Augmented vascular smooth muscle cell stiffness and adhesion when hypertension is superimposed on aging. Hypertension. 2015;65:370–377. doi: 10.1161/HYPERTENSIONAHA.114.04456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.