Abstract
Many cardiac electrophysiological abnormalities are accompanied by autonomic nervous system dysfunction. Here, we review mechanisms by which the cardiac nervous system controls normal and abnormal excitability and may contribute to atrial and ventricular tachyarrhythmias. Moreover, we explore the potential antiarrhythmic and/or arrhythmogenic effects of modulating the autonomic nervous system by several strategies, including ganglionated plexi ablation, vagal and spinal cord stimulations, and renal sympathetic denervation as therapies for atrial and ventricular arrhythmias.
1. Introduction
Autonomic regulation is essential for normal physiological functions and responses of the heart (i.e., inotropy, chronotropy, lusitropy, dromotropy). Importantly, autonomic dysfunction is increasingly recognized as playing a key role in adverse remodeling and malignant atrial and ventricular arrhythmias. A variety of pathological conditions may give rise to autonomic dysfunction, which may occur at various levels, including the intrinsic or extrinsic cardiac nervous systems or higher centers. Rather than focusing on pathology-dependent remodeling and dysfunction, we will describe more general mechanisms by which the intrinsic cardiac nervous system controls normal heart rate and may contribute to atrial and ventricular tachyarrhythmias. Specifically, we discuss the role of the intrinsic cardiac nervous system in normal and abnormal cardiac excitability and in contributing to atrial fibrillation (AF). We further detail two common forms of remodeling of the intrinsic ventricular sympathetic nerves (hyperinnervation and denervation) and discuss how this remodeling contributes to different forms of ventricular arrhythmia. We explore the potential antiarrhythmic and/or arrhythmogenic effects of modulating the autonomic nervous system by several strategies such as ganglionated plexi ablation, vagal nerve stimulation, and renal sympathetic denervation for the treatment of atrial and ventricular tachyarrhythmias.
2. Atrium
2.1 Neuroanatomy of the Atria
The cardiac nervous system can be divided into the intrinsic cardiac nervous system (ICNS) and extrinsic cardiac nervous system (ECNS). The ECNS axons mediate sympathetic connections between the myocardium and the cervical, stellate and thoracic ganglia, and parasympathetic connections between the atrial myocardium and the medulla oblongata. The ICNS forms a complex neural network composed of ganglionated plexi (GPs) and the interconnecting ganglia and axons (Armour et al., 1997; Singh et al., 1996). These ganglia are composed of diverse neuronal elements including cholinergic, adrenergic, afferent, and interconnecting neurons (Rysevaite et al., 2011b; Yuan et al., 1993). The ICNS is though to be a neuronal network that transduces local cardiac signals, as well as inputs from central neurons (Beaumont et al., 2013). Pauza et al. (2002a) delineated the neuroanatomy of the ICNS in the human adult and fetus, as well as mouse, rat, guinea pig, and dog. They concluded that the topographical organization of the ICNS is similar in these species (Pauza et al., 2002a; Pauza et al., 2002b; Rysevaite et al., 2011a; Vaitkevicius et al., 2009; Zarzoso et al., 2013). Armour et al. (1997) demonstrated that multiple large and small GPs are present in the atria. Hou et al. (2007a) used ablation and neural stimulation in anesthetized dogs and showed that pulmonary vein ganglia (PVG) serve as autonomic integration centers modulating cardiac excitability and AF inducibility. Moreover, Zarzoso et al. (2013) provided direct evidence for neuroanatomical connections between the PVG and the sinoatrial node (SAN) and observed similarities between the human and murine PVG neuroanatomy. Hence, intrinsic ganglia are abundant and more widely distributed than previously thought, and can play a role in normal and abnormal heart rhythm (Pauza et al., 2002a; Pauza et al., 2002b; Rysevaite et al., 2011a; Vaitkevicius et al., 2009; Zarzoso et al., 2013).
2.2 The Intrinsic Cardiac Nervous System in Sinus Rhythm
2.2.1 Basic Investigations
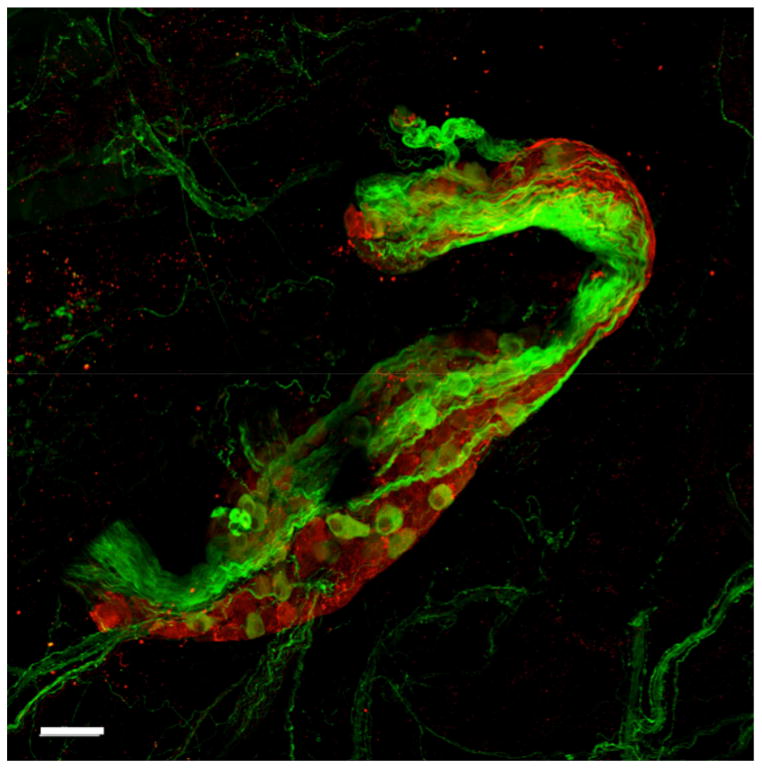
Zarzoso et al. (2013) studied the effect of GP stimulation on heart rate (HR) and SAN modulation. They demonstrated that GP influence on HR is dual, sympathetic and parasympathetic, in a time-dependent manner. Figure 1 is an immunofluorescence image of a mouse PVG stained for sympathetic (tyrosine hydroxylase- green) and parasympathetic (choline acetyltransferase- red) markers. The initial effect of PVG stimulation is predominantly parasympathetic, characterized by prolongation of the first three post-simulation P–P intervals. Additionally, it was demonstrated that PVG stimulation modulates the SAN activation pattern (Zarzoso et al., 2013). In whole-heart experiments, it was noted that PVG stimulation caused either inferior or superior displacement of the origin of SAN activation (Zarzoso et al., 2013). This is consistent with the effect of parasympathetic stimulation, which has been shown to produce either an inferior or superior shift in the origin of the pacemaker site (Glukhov et al., 2010; Mackaay et al., 1980; Shibata et al., 2001).
Figure 1.
Mouse pulmonary vein ganglion stained with tyrosine hydroxylase (green, sympathetic), and choline acetyltransferase (red, parasympathetic). The ganglion is composed of sympathetic and parasympathetic somas. Staining was done as described earlier (Zarzoso et al., 2013). Scale bar: 50 μm.
Sampaio et al., (2003) investigated HR and atrioventricular (AV) conduction control by two GP clusters in the rat. In an arterially perfused preparation, they electrically stimulated the epicardial sites that had a low threshold for causing changes in P–P and P–R intervals. The main stimulation sites correlated with the SAN ganglion located at the junction of the superior vena cava and right atrium, and the AV ganglion at the junction of the inferior PVs and left atrium. SAN ganglion stimulation consistently resulted in HR slowing without significant effect on conduction and AV ganglion stimulation resulted in conduction slowing without significant effect on HR. In the dog, high frequency stimulation of the GPs in epicardial fat pads induced AF and caused high grade AV block due to the strong parasympathetic effect on the AVN. This vagal response to stimulation was abolished by ablation of the GPs and thus it was concluded that cardiac GPs play an important role in AF initiation and maintenance but also in the regulation of AVN conduction (Scherlag et al., 2005). Hou et al. (2007b) investigated the effects of the interplay between the different GPs on sinus rate and AVN conduction. The study suggested that the GPs are interconnected and that they collectively modulate SAN and AVN response to ECNS stimuli and that these interconnections and their effect on SAN and AVN modulation should be kept in mind while identifying the targets for AF ablation (Hou et al., 2007b). Additionally, Randall et al. (2003) have demonstrated that multiple groups of neurons within the ICNS, at the right atrial ganglionated and posterior atrial ganglionated plexi, together modulated heart rate. The study found that while neurons of the right atrial ganglionated plexus directly lower heart rate, neurons in the posterior atrial ganglionated plexus are partly responsible for the interaction between sympathetic and parasympathetic control of chronotropy.
2.2.2 Clinical Investigations
In spite of clear data derived from animal research, suggesting a possible role for GP in SAN and AVN functions, extrapolation to the clinical setting is not straightforward. It is, for example, controversial whether radiofrequency delivery to the PVs during isolation procedures can stimulate PVGs and produce a decrease in ventricular HR, or whether a specific high-frequency stimulation protocol is necessary to accomplish this (Lemery et al., 2006; Pokushalov et al., 2009; Scherlag et al., 2005). The mechanism of this slowing is also controversial. It has been proposed that this phenomenon is caused by the activation of afferent fibers in the PVGs, thus evoking a vagal reflex response and resulting in the observed HR decrease (Haissaguerre et al., 1998; Lemery et al., 2006). On the other hand, Zarzoso et al. (2013) observed considerable and immediate HR slowing after radiofrequency delivery during PV isolation procedures in patients. They correlated their results with data from isolated mouse heart experiments that showed similar HR slowing upon PVG stimulation, suggesting that the local neuronal connections between PVGs and the SAN are sufficient to influence HR. Moreover, it has been demonstrated that PVG ablation may result in the degeneration of the ventricular innervation and may cause cardiac autonomic dysfunction that could last for up to a year and could predispose to ventricular arrhythmias (Bauer et al., 2006; Osman et al., 2010; Puodziukynas et al., 2012).
Bauer et al. (2006) studied the short- and long-term effects of segmental and circumferential PV ablation on the autonomic function of the heart. One hundred patients with AF were enrolled in this study, half of which received segmental ablation and the other half circumferential ablation. Cardiac acceleration and deceleration capacity significantly decreased immediately after ablation in the circumferential ablation group. This impairment lasted for one year after ablation. Acceleration and deceleration capacity also decreased immediately after ablation in the segmental ablation group. However, this function returned to baseline within one month. Consequently, it was apparent that PV ablation induces an immediate suppression of autonomic modulation of HR and this suppression seems to be more long term in circumferential PV ablation possibly because in circumferential ablation, more PVG are affected compared to segmental ablation.
2.3 The Intrinsic Cardiac Nervous System in Atrial Fibrillation
It has been proposed that the autonomic nervous system plays an important role in the pathophysiology of AF (Coumel et al., 1978) since AF triggers were shown to originate from the densely innervated PV roots (Haissaguerre et al., 1998). On a more functional level, studies suggested that AF can be initiated by electrical stimulation of the GPs at the roots of the PVs and that ablation of these arrhythmogenic foci can abort AF (Lemola et al., 2008; Lin et al., 2008; Lin et al., 2007). Nevertheless, the exact role and mode of action of the GPs remains unclear. As mentioned above, while some investigators show that the GPs function as local centers of integration between the heart and the ECNS, others investigators showed that GPs can act independently of higher autonomic centers and can directly affect cardiac function such as SAN automaticity and myocardial contractility (Wickramasinghe and Patel, 2013). Choi et al. (2010) addressed this question by recording electrical activity from ECNS and ICNS in a dog model of AF. Although the majority of AF episodes were associated with bursts of activity in ECNS nerves, certain episodes of AF were associated with isolated activity of the GPs without concomitant activity in the ECNS, suggesting that ICNS can potentially trigger AF without input from higher autonomic centers.
Evidence supports the idea that both sympathetic and parasympathetic components play an important role in AF initiation and maintenance. The release of adrenergic neurotransmitters mobilizes excess Ca2+ intracellularly leading to early after depolarizations (EADs), which can trigger the ectopic firing of PV cells (see also Section 3.2). Additionally, acetylcholine (ACh) release by GP neurons projecting from PV roots to the atria can cause shortening of the atrial action potential duration (APD) contributing to AF stabilization (Male and Scherlag, 2014). A study by Patterson et al. (2005) addressed the single-cell electrophysiology behind these findings. They recorded intracellular action potentials from myocardial cells in an excised, superfused PV–atrial sleeve. PVG activation by low-level stimulation caused shortening of PV myocardial cell APD and refractory period, along with the initiation of rapid firing caused by EADs. Atrial APD shortening was prevented by atropine and EAD-induced rapid PV firing was suppressed by β1-blockade. This demonstrates the synergetic actions of the sympathetic and parasympathetic systems in the initiation and maintenance of AF.
2.4 Modulation of the autonomic nervous system to prevent AF
The demonstration by Haissaguerre et al. (1998) that focal electrical activity in the PVs played an important role in the initiation and maintenance of AF paved the way for interventional techniques targeting the PVs as a treatment for paroxysmal AF. Later on, strategies to modulate the autonomic nervous system were developed as stand-alone or adjunctive treatment approaches to prevent AF (Linz et al., 2014).
2.4.1 GP Ablation
Ablation of GPs to treat AF has been attempted, with highly variable success rates ranging from 25 to 78% AF freedom after ≥1 year of follow-up (Katritsis et al., 2008; Pokushalov et al., 2009; Scanavacca et al., 2006). Pappone et al. (2004) noted that the application of radiofrequency when inducing lesions to encircle the PVs, caused a vagal-like response characterized by the slowing of SAN firing rate and AVN conduction rate. This occurred in approximately one third of the patients studied and disappeared as radiofrequency ablation was continued. One year follow-up showed that the third of patients who manifested vagal response had a 99% freedom AF recurrence whereas the rest of the group had 85% freedom of recurrence, highlighting the importance of GP ablation to suppress AF. Katritsis et al. (2011) carried out a similar study in 3 randomly assigned groups of patients. The first group had PV isolation alone, the second had GP ablation alone, and the third had both PV isolation and GP ablation. The last group was shown to have a 20% greater success rate compared to the first two groups, thus confirming the importance of both the PVs and GPs in AF maintenance and initiation. The effect of GP ablation on ventricular arrhythmias is less clear and needs further investigation. In a dog model of acute myocardial ischemia, He et al. (2013) showed that GP ablation increased the risk of ventricular arrhythmias compared to control animals.
Verma et al. (2008) demonstrated the presence of complex fractionated atrial electrograms (CFAEs) in patients with AF. Ablation of the myocardial regions underlying the origins of these CFAEs terminated AF in 55% of the patients studied and the conjugate use of PV isolation and CFAE ablation further increased AF suppression rate. Although other studies further supported this ablation strategy (Arruda and Natale, 2008; Porter et al., 2008), the mechanisms underlying the origin and involvement of CFAEs in AF are not well understood. Furthermore, CFAEs can occur in the vicinity of the GPs, hence it is tempting to speculate that the hyperactivity of the plexi can participate in causing the formation of CFAEs (Katritsis et al., 2009; Lin et al., 2007). It was shown that CFAEs occur with the highest frequency at the sites where GPs cluster, and that patients who did not manifest CFAEs at anatomical GP sites were free of CFAEs anywhere else in the atria (Katritsis et al., 2009; Wickramasinghe and Patel, 2013). Additionally, a long-term follow up of patients following AF ablation reported AF freedom rates of 40, 37 and 29% after 1, 3 and 5 years following single catheter ablation. With a median of 2 repeat procedures per patient with AF recurrence, success rates increased to 87, 81 and 63%, over the same time frames (Weerasooriya et al., 2011).
2.4.2 Low-Level Vagal Nerve Stimulation
Vagal stimulation reduces heart rate and AV conduction and activates atrial ACh dependent potassium currents (IK,ACh), leading to a potent shortening of the effective refractory period, promoting atrial arrhythmias (Schotten et al., 2011). However, low-level vagal nerve stimulation (LL-VNS) not resulting in reduction of heart rate and AV conduction has been shown to result in antiarrhythmic effects. Rapid atrial pacing normally results in the shortening of the atrial effective refractory period. LL-VNS counters this effect by prolonging the atrial refractoriness, hence decreasing AF susceptibility (Lu et al., 2008; Sheng et al., 2011). Yu et al. (2013) performed low-level transcutaneous electrical stimulation of the auricular branch of vagus nerve at the targus of the ear in anaesthetized dogs and they were able to document decreased AF inducibility. Many mechanistic preclinical studies suggest the involvement of several integrative processes occurring at the intra- and extracardiac ganglion level. Left-sided LL-VNS suppressed stellate ganglion nerve activities and reduced the incidences of paroxysmal atrial tachyarrhythmias in ambulatory dogs. This was associated with a significant neural remodeling process of the left stellate ganglion (Pieroni et al., 2007). Li et al. (2009) and Yu et al. (2011) used atrial high frequency stimulation delivered during the refractory period of the atrium and PVs to induce local firing and AF. They demonstrated that bilateral LL-VNS could suppress this type of focal AF by inhibiting the neural activity of GPs within the ICNS. There are studies suggesting the involvement of local release of the neuromodulators vasostatin and its precursor chromogranin A during electrical stimulation to the autonomic nerves for the regulation of GPs (Pieroni et al., 2007). Injection of vasostatin into the major atrial GPs resulted in electrophysiological effects similar to those of LL-VNS (Stavrakis et al., 2012). Importantly, in the clinical setting, an electrode is placed around the right cervical vagus. Therefore, stimulation of the cervical vagosympathetic trunk contains postganglionic axons from at least the superior cervical ganglion activating both afferent and efferent axons. This may evoke complex responses in the lungs and the gastrointestinal systems, which could secondarily affect the heart. Anti-inflammatory effects, which may result indirectly from the stimulation of the gastrointestinal system or from a direct ANS-immune system connection are proposed to underlie some of the described beneficial effects of LL-VNS (Zhao et al., 2013). Even using low levels of stimulation intensity, many vagal axons with a low threshold for excitability will respond and may result in the above-mentioned effects. Another comparable strategy to modulate autonomic balance by sympathetic withdrawal and increased vagal activation is carotid baroreceptor stimulation. While strong carotid baroreceptor stimulation resulted in a pronounced increase in AF inducibility (Linz et al., 2013c), low-level carotid baroreceptor stimulation suppressed AF inducibility by inhibiting the activity of GPs (Liao et al., 2015).
2.4.3 Renal Denervation
Catheter-based renal denervation (RDN) involves section of the renal nerves running in the adventitia of the renal artery, while the neurons in the renal ganglia and much of the intra-renal innervation remain functional (Bohm et al., 2013). RDN is currently studied as a treatment option for drug-refractory hypertension (Bohm et al., 2013). Blood pressure reduction by RDN may reduce atrial structural arrhythmogenic remodeling processes thereby preventing the occurrence of atrial arrhythmias (Schotten et al. 2011). However, the results from the Symplicity-HTN3-trial did not meet the prespecified endpoint (Bhatt et al., 2014). Nevertheless, mechanistically, it has been observed that the procedure resulted in a 47% reduction of renal norepinephrine (NE) spillover measured with a radiochemical tracer methodology using 3H-norepinephrine (Krum et al., 2009) as well as in a 37% reduction in firing of single sympathetic vasoconstrictor fibers, measured by single muscle sympathetic nerve activity (Hering et al., 2013). These findings indicate a combined modulation of efferent and afferent signaling at the kidney by RDN. RDN results in a reduction in heart rate and AV-conduction velocity in pigs (Linz et al., 2013b). Accordingly, RDN reduces heart rate during AF in humans as well as in anesthetized pigs with AF induced by rapid atrial pacing (Linz et al., 2013b). Moreover, in goats RDN reduced cardiac sympathetic nerve sprouting, transcardiac NE concentrations and cardiac fibrosis formation, which was associated with a less complex fibrillatory conduction pattern during AF (Linz et al., 2015). In a pig model for sleep apnea, RDN attenuated the postapneic blood pressure rises as well as the renin angiotensin system activation, which may prevent the development of cardiac structural remodeling (Linz et al., 2013a). In a small study in humans, the atrial antiarrhythmic effects of circumferential PV isolation combined with RDN was investigated (Pokushalov et al., 2012). Patients who received both procedures showed significant reductions in average systolic and diastolic blood pressure, whereas those in the PV isolation-only group did not show any significant improvement in blood pressure (Pokushalov et al., 2012). At one-year follow-up, 69% of patients who received both procedures had no AF recurrences, compared to 29% of those in the PV isolation-only group (Pokushalov et al., 2012). In a case report, even RDN without PV isolation reduced blood pressure and attenuated paroxysmal AF episodes, which were symptomatic and drug-resistant before RDN (Vollmann et al., 2013).
2.4.4 Spinal Cord Stimulation
Stimulation of afferent spinal nerve fibers increases central vagal tone and decreases sympathetic tone via central reflex activation. Spinal cord stimulation (SCS) reduced peripheral sympathetic drive induced by right atrial pacing (Mannheimer et al., 1993) and suppressed activity generated by intrinsic afferent sensory cardiac neurons related to sympathetic excitation (Lopshire et al., 2009). SCS has been shown to suppress AF inducibility under certain conditions. In a canine model of AF induced by atrial tachypacing, SCS prolonged atrial effective refractory periods and reduced AF burden and inducibility (Bernstein et al., 2012). Rapid atrial pacing-induced AF was suppressed by SCS by inhibiting atrial autonomic remodeling in another canine model (Wang et al., 2016). There are data indicating that SCS may prevent episodic AF caused by rapid PV and non-PV firing through modulating GP activity (Yu et al., 2014). Interestingly, effectiveness of SCS in AF suppression increases with time. Chronic SCS therapy has been shown to modify intrinsic cardiac neuronal stochastic interconnectivity in AF suppression by altering synaptic function without directly targeting the transmembrane properties of individual intrinsic cardiac neuronal somata (Ardell et al., 2014). Although not directly related, it was shown in a dog model with rapid atrial pacing, that high thoracic epidural anesthesia inhibited atrial sympathetic nerve sprouting, which was associated with prevention of sustained AF (Yang et al., 2011).
3. Ventricle
Many cardiac diseases associated with ventricular arrhythmia are accompanied by disruption of the autonomic nervous system, which may involve increased central sympathetic drive and parasympathetic withdrawal along with changes to the density, distribution, excitability, and neurotransmitter content of the intrinsic efferent innervation of the ventricles. Although various forms of neuronal remodeling have been reported, we will focus on the mechanisms by which sympathetic hyperinnervation and sympathetic denervation contribute to ventricular arrhythmia. Interestingly, these seemingly opposite conditions share common mechanisms of arrhythmogenesis.
3.1 Ventricular Nerve Remodeling
Sympathetic hyperinnervation is one of the most well characterized forms of neural remodeling and has been clearly linked to ventricular arrhythmias in animal models and humans (Cao et al., 2000a; Cao et al., 2000b). Hyperinnervation occurs in a variety of pathological conditions including myocardial infarction (MI), heart failure, and hypercholesterolemia and is characterized by non-uniform increases in local nerve density compared to control tissue (Cao et al., 2000b; Li et al., 2004b; Liu et al., 2003). It is thought that increases in nerve growth factor (NGF) are primarily responsible for hyperinnervation (Cao et al., 2000a) and blocking NGF prevents nerve sprouting following MI (Hasan et al., 2006). Conversely, sympathetic denervation is also associated with ventricular arrhythmia (Boogers et al., 2010; Fallavollita et al., 2014) and occurs in MI, diabetic neuropathy, and heart failure (Gardner and Habecker, 2013; Gardner et al., 2015; Jacobson et al., 2010; Kimura et al., 2010). Interestingly, whereas NGF stimulates axon growth, its precursor, proNGF, triggers axon degeneration (Nykjaer et al., 2004) and may be involved in post-MI denervation (Siao et al., 2012). Recently, Gardner and Habecker (2013) also identified chondroitin sulfate proteoglycans, which are present in reperfused infarcts, as responsible for chronic denervation observed in reperfused post-MI hearts.
3.2 Cellular Responses to Sympathetic Stimulation
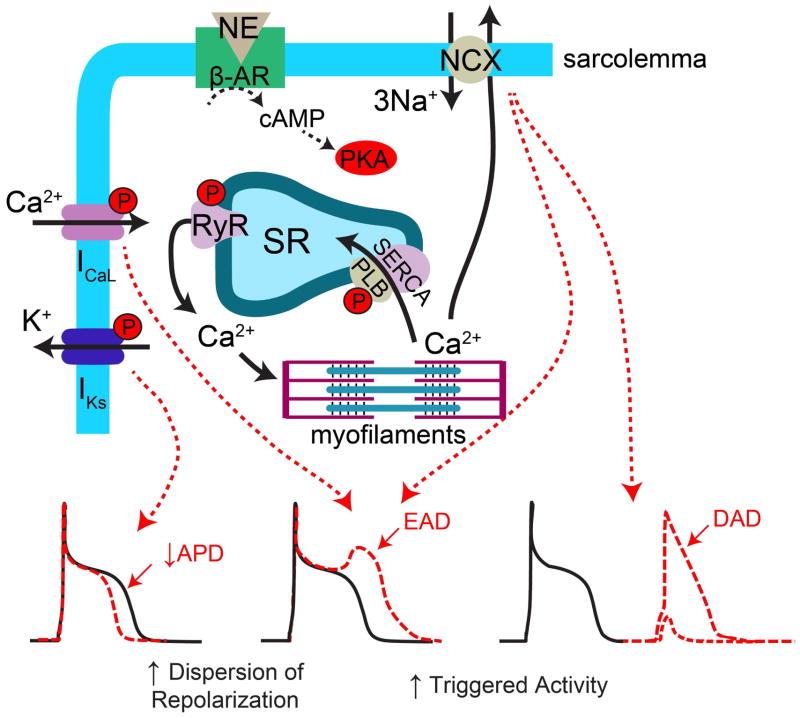
Release of NE from the nerve terminals (as well as epinephrine released from the adrenal medulla) during sympathetic activation leads to prototypical chronotropic and inotropic responses of the heart. Chronotropic responses are primarily mediated via increases in the rate of diastolic depolarization of pacemaker cells in the SAN (Bucchi et al., 2007; Vinogradova et al., 2006). Inotropic responses are mediated via alterations in myocyte Ca2+ handling (Figure 2). Briefly, NE (or Epi) binds to β-adrenergic receptors (AR) on cardiac myocytes and stimulates production of cAMP and consequent activation of protein kinase A (PKA). PKA phosphorylates several intracellular targets involved in excitation-contraction coupling, including phospholamban, L-type Ca2+ channels, and ryanodine receptors (RyR) (Bers, 2002). Phospholamban is an inhibitor of the sarcoplasmic reticulum (SR) Ca-ATPase (SERCA) pump and phosphorylation of phospholamban relieves this inhibition, leading to increased SERCA activity and a subsequent increase in the Ca2+ content of the SR. The amount of Ca2+ released from the SR during Ca2+-induced Ca2+ release (CICR) is highly dependent on SR Ca2+ load (Shannon et al., 2000); therefore, during sympathetic stimulation, more Ca2+ is released into the cytosol and is available to activate the myofilaments, increasing contractility. Furthermore, the β-AR mediated increase in L-type Ca2+ current also leads to enhanced triggering Ca2+ for CICR. PKA may also phosphorylate RyR, which can modulate the open probability of these channels (Marx et al., 2000; Valdivia et al., 1995), making Ca2+ release more likely for any given cytosolic and luminal Ca2+ concentration. However, the impact of PKA phosphorylation on RyR is currently unresolved, with mixed results in different experimental conditions (Song et al., 2001; Viatchenko-Karpinski and Györke, 2001).
Figure 2.
Myocyte electrophysiological responses to sympathetic stimulation. NE binding to β-ARs activates PKA, which phosphorylates several intracellular targets, including IKs, ICaL, RyR, and PLB. Phosphorylation of IKs leads to APD shortening that may increase the dispersion of repolarization due to non-uniform innervation and resulting spatially non-uniform β-AR activation. Phosphorylation of ICaL, RyR, and PLB lead to increased intracellular [Ca2+], increased SR Ca2+ leak, and increased SR Ca2+ load. Collectively, these conditions are favorable for EAD and DAD formation. EADs may contribute to increased dispersion of repolarization and both EADs and DADs contribute to ectopic activity and focal arrhythmia.
In addition to impacting intracellular Ca2+ handling, sympathetic stimulation also has effects on several ionic currents. NE binds to both α- and β- ARs and the effects of both of these signaling mechanisms on ion channels have been reviewed elsewhere (Gallego et al., 2014; Hartzell, 1988). One of the most well-known and well-studied features of β-AR stimulation is an increase in L-type Ca2+ current via phosphorylation of the channel Cav1.2 (Sperelakis, 1994). This effect, by itself, would be expected to increase the APD, but it is counterbalanced by the effect of β-AR on K+ currents, most notably an increase in IKs, but IKr may also be involved (Kagan et al., 2002; Marx et al., 2002). The net effect is typically a shortening of the APD, which is required to accommodate fast heart rates during sympathetic activity. Although the positive chronotropic and inotropic effects of sympathetic stimulation are necessary to meet increased cardiac demands during stress or exercise, sympathetic stimulation can also lead to pathological ventricular arrhythmias via focal (triggered) and/or reentrant mechanisms.
3.3 Focal Arrhythmia Mechanisms
At the cellular level, focal activity of ventricular myocytes during sympathetic activation is likely due to delayed afterdepolarizations (DADs) (Pogwizd and Bers, 2004), which are membrane depolarizations occurring during phase IV of the action potential. Increased cytosolic and SR Ca2+ levels can lead to SR Ca2+ overload, resulting in spontaneous opening of RyRs and Ca2+ release into the cytosol that is not in response to an action potential. This leads to Ca2+ extrusion from the cytosol via the Na+/Ca2+ exchanger (NCX) (Pogwizd et al., 2001). NCX is electrogenic, extruding one Ca2+ ion in exchange for 3 Na+ ions, which produces a net inward current. If the inward current is large enough, the cell membrane depolarizes and a triggered action potential occurs (Figure 2).
At the tissue level, however, several thousand cells must all experience DADs simultaneously in order to generate enough depolarizing current to produce a propagating action potential (called the ‘source-sink mismatch’) (Kumar et al., 1996; Xie et al., 2010). Localized application of β-AR agonists such as NE or isoproterenol can induce propagating premature ventricular complexes (PVCs) and sustained focal ventricular tachycardia (VT) in intact hearts (Doppalapudi et al., 2008; Myles et al., 2015; Nash et al., 2001), suggesting that hyperinnervation and localized release of NE can directly induce focal activity. Myles et al. (2012) further showed that this focal activity is due to synchronous SR Ca2+ overload and release and subsequent DADs among thousands of myocytes, providing a mechanistic link between the numerous experimental and clinical investigations that have found regional hyperinnervation and localized nerve sprouting accompanied by increased arrhythmia risk (Cao et al., 2000a; Cao et al., 2000b; Li et al., 2004b; Liu et al., 2003).
Recently, however, several clinical studies have revealed that the degree of sympathetic hypo-innervation or denervation (quantified with nuclear imaging) is a significant predictor of ventricular arrhythmia risk (Boogers et al., 2010), predicting cardiac arrest independent of infarct size and ejection fraction (Fallavollita et al., 2014). Chronic sympathetic denervation can lead to β-adrenergic super-sensitivity on myocytes, which may result from both an upregulation of β-ARs as well as increased responsiveness upon catecholamine binding (Vatner et al., 1985). The increased responsiveness may be attributable to a down-regulation of G-protein receptor kinase 2 (GRK2, also known as βARK1) that occurs in response to denervation (Yatani et al., 2006). GRK2 is normally activated by PKA during β-AR stimulation and acts to inhibit β-AR in a negative feedback loop. A loss of GRK2 may play an important role in denervation-induced β-AR supersensitivity, as GRK2 knockout mice also display β-AR supersensitivity (Raake et al., 2012).
Despite regional myocardial denervation, circulating plasma concentrations of NE and epinephrine are significantly higher in patients with heart disease due to increased cardiac and renal NE spillover and augmented adrenomedullary activity (Hasking et al., 1986). This increase in circulating catecholamines coupled with localized β-AR supersensitivity may be particularly arrhythmogenic. For example, Gardner et al. (2015) recently showed in a murine model that reperfused infarcts remain denervated and display β-AR supersensitivity, Ca2+ mishandling, and triggered arrhythmia in response to circulating β-AR agonists. Therefore, in much the same way that regional hyperinnervation and nerve sprouting lead to localized β-AR stimulation and triggered activity, denervation also leads to localized β-AR stimulation and triggered activity through localized supersensitivity.
3.4 Reentrant Arrhythmia Mechanisms
β-AR stimulation also has a significant impact on the ventricular APD and heterogeneous remodeling of the sympathetic nerves (either hyper- or denervation), may result in increased dispersion of repolarization (DOR), setting the stage for unidirectional conduction block, wavebreak, and reentrant arrhythmia. Indeed, even in the normal heart, the base-to-apex gradient of innervation may lead to an increase in DOR during sympathetic activity. For example, Mantravadi et al. (2007) showed an increase in DOR along with a complete reversal of the direction of repolarization during sympathetic nerve stimulation in the innervated Langendorff-perfused rabbit heart. These authors further observed that application of the β-AR agonist isoproterenol also increased the DOR, but the spatial distribution of APD shortening was completely different, suggesting that the dramatic changes in repolarization observed with sympathetic nerve stimulation are due to heterogeneous nerve distribution. Similar results have been obtained in the porcine ventricles in which dramatically different spatial patterns of activation-recovery intervals (ARIs, surrogate measure for APD) and repolarization were observed in response to sympathetic nerve stimulation compared to circulating NE (Ajijola et al., 2013a; Yagishita et al., 2015). Furthermore, these investigators also found that nerve stimulation, but not NE infusion, increased the T-peak to T-end interval, which is an independent marker of sudden cardiac death (Yagishita et al., 2015).
The differential electrophysiologic responses to physiological nerve stimulation versus circulating catecholamines are intriguing and highlight the shortcomings of using exogenously applied catecholamines to experimentally mimic nerve activity. A major contributor to these differences is likely the heterogeneous distribution of sympathetic fibers, which upon stimulation, results in heterogeneous β-AR activation compared to more uniform activation during catecholamine infusion. However, it is possible that there is also differential signaling between intra-junctional and extra-junctional cardiac β-ARs upon catecholamine binding, as well as the potential contribution of co-transmitter release during nerve stimulation that is not accounted for with catecholamine infusion. Interestingly, both ischemic (MI) and non-ischemic models of heart failure demonstrate transdifferentiation of sympathetic neurons, in which co-release of NE and ACh has been documented (Kanazawa et al., 2010; Olivas et al., 2016). The functional electrophysiological consequences of sympathetic transdifferentiation remain unknown and represent an important area for further study.
In addition to altered neurotransmitter release, maladaptive nerve remodeling may also include areas of hyperinnervation and/or denervation. Hence, in diseased hearts, physiological nerve activation may result in significantly greater heterogeneity of APD and DOR. Indeed, Ajijola and colleagues found that following MI, sympathetic stimulation not only caused increased DOR compared to control hearts, but that activation and propagation patterns were also significantly altered in MI hearts (Ajijola et al., 2013b). These results were confirmed in human patients with MI in which reflex sympathetic stimulation caused a 230% increase in ARI dispersion compared to patients with structurally normal hearts (Vaseghi et al., 2012).
In summary, both sympathetic hyper- and denervation of the ventricles can lead to heterogeneous β-AR activation, either through localized catecholamine release or localized β-AR supersensitivity, respectively. This non-uniform sympathetic activation increases the likelihood of focal arrhythmia triggers and creates gradients of repolarization, increasing the susceptibility to sustained reentrant arrhythmia (VT/VF). Together, these factors may lead to a ‘perfect storm’ of arrhythmia-provoking conditions during periods of high sympathetic drive. As discussed below, several clinical and pre-clinical studies are now demonstrating the powerful therapeutic effects of modulating autonomic activity to treat ventricular arrhythmias.
3.5 Modulation of the autonomic nervous system to prevent ventricular arrhythmia
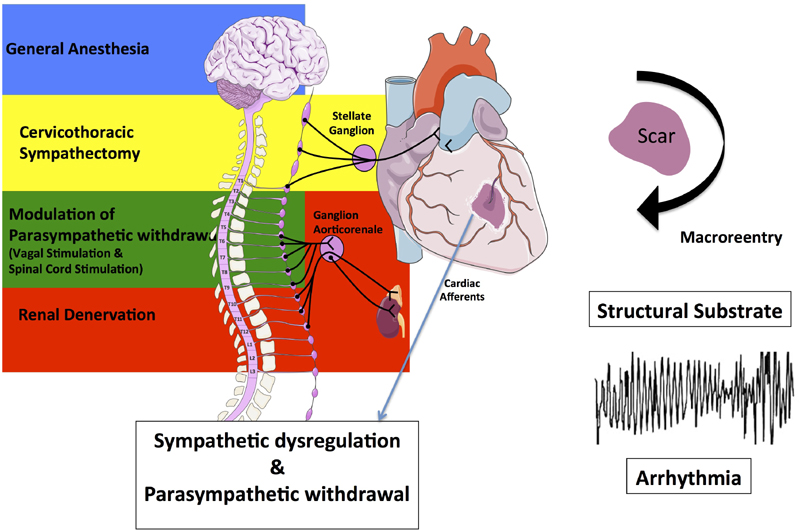
The effectiveness of implantable cardioverter-defibrillators (ICDs) for primary and secondary preventions of sudden cardiac death has resulted in an increasing number of patients presenting with recurrent, appropriate ICD shocks for VT (Moss et al., 1996). Because of the frequently insufficient success of pharmacologic therapy, catheter-based VT ablation, mainly targeting the structural arrhythmogenic substrate for VT, is commonly used in these patients but is associated with limited long-term efficacy and significant complications (Zipes et al., 2006). Despite structural substrates, which can be modified by VT-ablation (substrate modification), triggers and autonomic imbalance, characterized by increased sympathetic activation and parasympathetic withdrawal, may account for the timing of clinical presentation of the arrhythmia after ventricular injury (Figure 3). Therefore, modulation of the autonomic nervous system provides an adjunctive, in some cases even alternative treatment modality for VT. Attempts to therapeutically reduce sympathetic activation (e.g., by cardiac sympathetic denervation or renal denervation) or to increase the cardiac parasympathetic tone (e.g., by vagal stimulation and baroreceptor stimulation) may operate at any part of this integrative circuit.
Figure 3.
Impact of ventricular injury on scar generation and subsequent development of a structural substrate for macroreentries and ventricular arrhythmias as well as on cardiac afferents resulting in sympathetic dysregulation and parasympathetic withdrawal. Different regulatory centers of cardiac efferent sympathetic (brain, stellate ganglion, renal sympathetic nerves) and parasympathetic (vagus nerve, spinal cord) activation are shown on the left. Interventional strategies to reduce sympathetic activation (e.g. by cardiac sympathetic denervation or renal denervation) or to increase the cardiac parasympathetic tone (e.g. by vagal stimulation and baroreceptor stimulation) are provided.
3.5.1 Reduction of sympathetic activation
3.5.1.1 General anesthesia and thoracic epidural anesthesia
High thoracic epidural anesthesia and intubation with general anesthesia can reduce afferent and efferent sympathetic nerve impulse to the heart. Thoracic epidural anesthesia by intrathecal injection of clonidine was shown to reduce ischemia-induced ventricular arrhythmias in a canine model and in patients with electrical storm (Bourke et al., 2010).
3.5.1.2 Cardiac sympathetic denervation
The stellate ganglion (or cervicothoracic ganglion) is a sympathetic ganglion formed by the fusion of the inferior cervical ganglion and the first thoracic ganglion. It is located in front of the neck of the first rib and just below the subclavian artery. MI results in a persistant increase in synaptic density of bilateral stellate ganglia and is associated with increased stellate ganglionic nerve activity indicating remodeling of the extracardiac autonomic nerve activity and structure (Han et al., 2012). Left cardiac sympathetic denervation (surgical excision of stellate ganglion, together with second and third thoracic ganglia) reduces arrhythmia in high risk patients and canines following MI, in patients with long QT syndrome (Schwartz et al., 2004), catecholaminergic polymorphic VT (Wilde et al., 2008), and cardiomyopathy and refractory ventricular arrhythmias (Bourke et al., 2010). In patients with VT storm, particularly in those with structural heart disease, bilateral cardiac sympathetic denervation seems to be more beneficial than left cardiac sympathetic denervation alone as far as reduction in ICD shocks is concerned (Vaseghi et al., 2014).
3.5.1.3 Renal denervation (RDN)
In a pig model with acute MI, RDN has been shown to reduce ventricular ectopies and ventricular fibrillation, while arrhythmias and shortening of the monophasic APD during reperfusion were not affected by RDN (Linz et al., 2013d). Case series have provided evidence that in patients with dilated cardiomyopathy and electrical storm, RDN was able to reduce discharges from ICDs and ventricular ectopies (Remo et al., 2014; Ukena et al., 2012). In patients with ICDs and refractory ventricular arrhythmias, RDN was associated with reduced arrhythmic burden with no procedure-related complications (Armaganijan et al., 2015). Furthermore, by reducing circulating catecholamines, RDN has the potential to reduce the electrical heterogeneity between regions of scarred myocardium that demonstrate denervation supersensitivity to catecholamines and border zone regions with hyperinnervation due to nerve sprouting (Linz et al., 2014).
3.5.2 Modulation of parasympathetic withdrawal
3.5.2.1 Vagal Stimulation
Direct stimulation of cervical pre-ganglionic parasympathetic fibers activates overall cardiac vagal tone (Linz et al., 2014). Vagal stimulation, performed shortly after the onset of an acute ischemic episode in conscious animals with a healed MI can effectively prevent ventricular fibrillation independent from heart rate reduction (Vanoli et al., 1991). Additionally, vagal stimulation improved ventricular function (Li et al., 2004a), and most interestingly, has shown anti-inflammatory effects, which were associated with a reduction in ischemic injury during ischemia and reperfusion (Zhang et al., 2009) independent of heart rate changes.
3.5.2.2 Spinal Cord Stimulation
Increased central vagal tone and decreased sympathetic tone during thoracic SCS (Mannheimer et al., 1993) can protect against ischemic ventricular arrhythmias in a canine model of healed MI and heart failure (Issa et al., 2005). SCS had an antiarrhythmic effect on spontaneous non-sustained VT and sustained VT during ischemia-reperfusion in association with a reduction of repolarization alterations independent of any effect on infarct size (Odenstedt et al., 2011).
4. Conclusions and future perspectives
A complex interplay exists between the intrinsic and extrinsic cardiac nervous systems, and between their sympathetic and parasympathetic branches. These inputs are well documented to contribute to atrial and ventricular remodeling, creating an arrhythmogenic substrate. Despite such intricacies, we have highlighted key autonomic factors that play a role in normal sinus rhythm, and in atrial and ventricular tachyarrhythmias. Preclinical studies and first clinical observations suggest that modulation of the autonomic nervous system provides an adjunctive or maybe even alternative treatment modality for prevention of atrial and ventricular arrhythmias. Attempts to therapeutically modulate sympathetic and parasympathetic tone target the problem differently and consequently, it has become evident that the effect of autonomic nervous system modulation on cardiac arrhythmogenesis is not only highly complex, but also difficult to predict. Therefore, detailed studies from the molecular to the organismal levels, and from the bench to the bedside are becoming increasingly necessary to gain further insights into: 1) the roles of the extrinsic and intrinsic nervous systems in normal and abnormal cardiac electrophysiology; and 2) the interventional modulation of the autonomic nervous system for arrhythmia prevention.
Acknowledgments
CMR is supported in part by the US National Institutes of Health (R01 HL111600) and the American Heart Association (12SDG9010015). SFN is supported by the US National Institutes of Health (R00HL105574 and R01HL129136). DL is supported HOMFOR 2013/2014, the Else Kröner-Fresenius Foundation, and the Deutsche Gesellschaft für Kardiologie.
List of Abbreviations
- ACh
Acetylcholine
- AF
Atrial fibrillation
- APD
Action potential duration
- AR
Adrenergic receptor
- ARI
Activation-recovery interval
- AV
Atrioventricular
- AVN
Atrioventricular node
- CFAE
Complex fractionated atrial electrogram
- CICR
Ca2+-induced Ca2+ release
- DOR
Dispersion of repolarization
- DAD
Delayed afterdepolarization
- EAD
Early afterdepolarization
- ECNS
Extrinsic cardiac nervous system
- GP
Ganglionated plexi
- GRK
G-protein receptor kinase
- HR
Heart rate
- ICD
Implantable cardioverter-defibrillator
- ICNS
Intrinsic cardiac nervous system
- LL-VNS
Low-level vagal nerve stimulation
- MI
Myocardial infarction
- NCX
Na+/Ca2+ exchanger
- NE
Norepinephrine
- NGF
Nerve growth factor
- PKA
Protein kinase A
- PV
Pulmonary vein
- PVG
Pulmonary vein ganglia
- RDN
Renal denervation
- RyR
Ryanodine receptor
- SAN
Sinoatrial node
- SCS
Spinal cord stimulation
- SERCA
Sarcoplasmic reticulum Ca-ATPase
- SR
Sarcoplasmic reticulum
- VF
Ventricular fibrillation
- VT
Ventricular tachycardia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Crystal M. Ripplinger, Email: cripplinger@ucdavis.edu, .
Sami F. Noujaim, Email: snoujaim@health.usf.edu, .
Dominik Linz, Email: Dominik.Linz@uks.eu, .
References
- Ajijola OA, Vaseghi M, Zhou W, Yamakawa K, Benharash P, Hadaya J, Lux RL, Mahajan A, Shivkumar K. Functional differences between junctional and extrajunctional adrenergic receptor activation in mammalian ventricle. AJP: Heart and Circulatory Physiology. 2013a;304:H579–88. doi: 10.1152/ajpheart.00754.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajijola OA, Yagishita D, Patel KJ, Vaseghi M, Zhou W, Yamakawa K, So E, Lux RL, Mahajan A, Shivkumar K. Focal myocardial infarction induces global remodeling of cardiac sympathetic innervation: neural remodeling in a spatial context. AJP: Heart and Circulatory Physiology. 2013b;305:H1031–H1040. doi: 10.1152/ajpheart.00434.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardell JL, Cardinal R, Beaumont E, Vermeulen M, Smith FM, Andrew Armour J. Chronic spinal cord stimulation modifies intrinsic cardiac synaptic efficacy in the suppression of atrial fibrillation. Auton Neurosci. 2014;186:38–44. doi: 10.1016/j.autneu.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armaganijan LV, Staico R, Moreira DA, Lopes RD, Medeiros PT, Habib R, Melo Neto J, Katz M, Armaganijan D, Sousa AG, Mahfoud F, Abizaid A. 6-Month Outcomes in Patients With Implantable Cardioverter-Defibrillators Undergoing Renal Sympathetic Denervation for the Treatment of Refractory Ventricular Arrhythmias. JACC Cardiovasc Interv. 2015;8:984–90. doi: 10.1016/j.jcin.2015.03.012. [DOI] [PubMed] [Google Scholar]
- Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec. 1997;247:289–98. doi: 10.1002/(SICI)1097-0185(199702)247:2<289::AID-AR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Arruda M, Natale A. Ablation of permanent AF: adjunctive strategies to pulmonary veins isolation: targeting AF NEST in sinus rhythm and CFAE in AF. J Interv Card Electrophysiol. 2008;23:51–7. doi: 10.1007/s10840-008-9252-z. [DOI] [PubMed] [Google Scholar]
- Bauer A, Deisenhofer I, Schneider R, Zrenner B, Barthel P, Karch M, Wagenpfeil S, Schmitt C, Schmidt G. Effects of circumferential or segmental pulmonary vein ablation for paroxysmal atrial fibrillation on cardiac autonomic function. Heart Rhythm. 2006;3:1428–35. doi: 10.1016/j.hrthm.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Beaumont E, Salavatian S, Southerland EM, Vinet A, Jacquemet V, Armour JA, Ardell JL. Network interactions within the canine intrinsic cardiac nervous system: implications for reflex control of regional cardiac function. J Physiol. 2013;591:4515–33. doi: 10.1113/jphysiol.2013.259382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein SA, Wong B, Vasquez C, Rosenberg SP, Rooke R, Kuznekoff LM, Lader JM, Mahoney VM, Budylin T, Alvstrand M, Rakowski-Anderson T, Bharmi R, Shah R, Fowler S, Holmes D, Farazi TG, Chinitz LA, Morley GE. Spinal cord stimulation protects against atrial fibrillation induced by tachypacing. Heart Rhythm. 2012;9:1426–33 e3. doi: 10.1016/j.hrthm.2012.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL Investigators SH- A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- Bohm M, Linz D, Urban D, Mahfoud F, Ukena C. Renal sympathetic denervation: applications in hypertension and beyond. Nat Rev Cardiol. 2013;10:465–76. doi: 10.1038/nrcardio.2013.89. [DOI] [PubMed] [Google Scholar]
- Boogers MJ, Borleffs CJW, Henneman MM, van Bommel RJ, van Ramshorst J, Boersma E, Dibbets-Schneider P, Stokkel MP, van der Wall EE, Schalij MJ, Bax JJ. Cardiac sympathetic denervation assessed with 123-iodine metaiodobenzylguanidine imaging predicts ventricular arrhythmias in implantable cardioverter-defibrillator patients. Journal of the American College of Cardiology. 2010;55:2769–2777. doi: 10.1016/j.jacc.2009.12.066. [DOI] [PubMed] [Google Scholar]
- Bourke T, Vaseghi M, Michowitz Y, Sankhla V, Shah M, Swapna N, Boyle NG, Mahajan A, Narasimhan C, Lokhandwala Y, Shivkumar K. Neuraxial modulation for refractory ventricular arrhythmias: value of thoracic epidural anesthesia and surgical left cardiac sympathetic denervation. Circulation. 2010;121:2255–62. doi: 10.1161/CIRCULATIONAHA.109.929703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucchi A, Baruscotti M, Robinson RB, DiFrancesco D. Modulation of rate by autonomic agonists in SAN cells involves changes in diastolic depolarization and the pacemaker current. Journal of Molecular and Cellular Cardiology. 2007;43:39–48. doi: 10.1016/j.yjmcc.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Cao JM, Chen LS, KenKnight BH, Ohara T, Lee MH, Tsai J, Lai WW, Karagueuzian HS, Wolf PL, Fishbein MC, Chen PS. Nerve sprouting and sudden cardiac death. Circulation Research. 2000a;86:816–821. doi: 10.1161/01.res.86.7.816. [DOI] [PubMed] [Google Scholar]
- Cao JM, Fishbein MC, Han JB, Lai WW, Lai AC, Wu TJ, Czer L, Wolf PL, Denton TA, Shintaku IP, Chen PS, Chen LS. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation. 2000b;101:1960–1969. doi: 10.1161/01.cir.101.16.1960. [DOI] [PubMed] [Google Scholar]
- Choi EK, Shen MJ, Han S, Kim D, Hwang S, Sayfo S, Piccirillo G, Frick K, Fishbein MC, Hwang C, Lin SF, Chen PS. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation. 2010;121:2615–23. doi: 10.1161/CIRCULATIONAHA.109.919829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coumel P, Attuel P, Lavallee J, Flammang D, Leclercq JF, Slama R. The atrial arrhythmia syndrome of vagal origin. Arch Mal Coeur Vaiss. 1978;71:645–56. [PubMed] [Google Scholar]
- Doppalapudi H, Jin Q, Dosdall DJ, Qin H, Walcott GP, Killingsworth CR, Smith WM, Ideker RE, Huang J. Intracoronary infusion of catecholamines causes focal arrhythmias in pigs. Journal of cardiovascular electrophysiology. 2008;19:963–970. doi: 10.1111/j.1540-8167.2008.01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallavollita JA, Heavey BM, Luisi AJ, Michalek SM, Baldwa S, Mashtare TL, Hutson AD, Dekemp RA, Haka MS, Sajjad M, Cimato TR, Curtis AB, Cain ME, Canty JM. Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. Journal of the American College of Cardiology. 2014;63:141–149. doi: 10.1016/j.jacc.2013.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego M, Alday A, Alonso H, Casis O. Adrenergic regulation of cardiac ionic channels: role of membrane microdomains in the regulation of kv4 channels. Biochimica et biophysica acta. 2014;1838:692–699. doi: 10.1016/j.bbamem.2013.06.025. [DOI] [PubMed] [Google Scholar]
- Gardner RT, Habecker BA. Infarct-derived chondroitin sulfate proteoglycans prevent sympathetic reinnervation after cardiac ischemia-reperfusion injury. Journal of Neuroscience. 2013;33:7175–7183. doi: 10.1523/JNEUROSCI.5866-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RT, Wang L, Lang BT, Cregg JM, Dunbar CL, Woodward WR, Silver J, Ripplinger CM, Habecker BA. Targeting protein tyrosine phosphatase σ after myocardial infarction restores cardiac sympathetic innervation and prevents arrhythmias. Nature communications. 2015;6:6235. doi: 10.1038/ncomms7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glukhov AV, Fedorov VV, Anderson ME, Mohler PJ, Efimov IR. Functional anatomy of the murine sinus node: high-resolution optical mapping of ankyrin-B heterozygous mice. Am J Physiol Heart Circ Physiol. 2010;299:H482–91. doi: 10.1152/ajpheart.00756.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- Han S, Kobayashi K, Joung B, Piccirillo G, Maruyama M, Vinters HV, March K, Lin SF, Shen C, Fishbein MC, Chen PS, Chen LS. Electroanatomic remodeling of the left stellate ganglion after myocardial infarction. J Am Coll Cardiol. 2012;59:954–61. doi: 10.1016/j.jacc.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell HC. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52:165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Hasan W, Jama A, Donohue T, Wernli G, Onyszchuk G, Al-Hafez B, Bilgen M, Smith PG. Sympathetic hyperinnervation and inflammatory cell NGF synthesis following myocardial infarction in rats. Brain research. 2006;1124:142–154. doi: 10.1016/j.brainres.2006.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasking GJ, Esler MD, Jennings GL, Burton D, Johns JA, Korner PI. Norepinephrine spillover to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation. 1986;73:615–21. doi: 10.1161/01.cir.73.4.615. [DOI] [PubMed] [Google Scholar]
- He B, Lu Z, He W, Wu L, Cui B, Hu X, Yu L, Huang C, Jiang H. Effects of ganglionated plexi ablation on ventricular electrophysiological properties in normal hearts and after acute myocardial ischemia. Int J Cardiol. 2013;168:86–93. doi: 10.1016/j.ijcard.2012.09.067. [DOI] [PubMed] [Google Scholar]
- Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, Esler MD, Schlaich MP. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension. 2013;61:457–64. doi: 10.1161/HYPERTENSIONAHA.111.00194. [DOI] [PubMed] [Google Scholar]
- Hou Y, Scherlag BJ, Lin J, Zhang Y, Lu Z, Truong K, Patterson E, Lazzara R, Jackman WM, Po SS. Ganglionated plexi modulate extrinsic cardiac autonomic nerve input: effects on sinus rate, atrioventricular conduction, refractoriness, and inducibility of atrial fibrillation. J Am Coll Cardiol. 2007a;50:61–8. doi: 10.1016/j.jacc.2007.02.066. [DOI] [PubMed] [Google Scholar]
- Hou Y, Scherlag BJ, Lin J, Zhou J, Song J, Zhang Y, Patterson E, Lazzara R, Jackman WM, Po SS. Interactive atrial neural network: Determining the connections between ganglionated plexi. Heart Rhythm. 2007b;4:56–63. doi: 10.1016/j.hrthm.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Issa ZF, Zhou X, Ujhelyi MR, Rosenberger J, Bhakta D, Groh WJ, Miller JM, Zipes DP. Thoracic spinal cord stimulation reduces the risk of ischemic ventricular arrhythmias in a postinfarction heart failure canine model. Circulation. 2005;111:3217–20. doi: 10.1161/CIRCULATIONAHA.104.507897. [DOI] [PubMed] [Google Scholar]
- Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, Agostini D, Weiland F, Chandna H, Narula J Investigators A-H. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. Journal of the American College of Cardiology. 2010;55:2212–2221. doi: 10.1016/j.jacc.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Kagan A, Melman YF, Krumerman A, McDonald TV. 14-3-3 amplifies and prolongs adrenergic stimulation of HERG K+ channel activity. The EMBO journal. 2002;21:1889–1898. doi: 10.1093/emboj/21.8.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa H, Ieda M, Kimura K, Arai T, Kawaguchi-Manabe H, Matsuhashi T, Endo J, Sano M, Kawakami T, Kimura T, Monkawa T, Hayashi M, Iwanami A, Okano H, Okada Y, Ishibashi-Ueda H, Ogawa S, Fukuda K. Heart failure causes cholinergic transdifferentiation of cardiac sympathetic nerves via gp130-signaling cytokines in rodents. J Clin Invest. 2010;120:408–21. doi: 10.1172/JCI39778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katritsis D, Giazitzoglou E, Sougiannis D, Goumas N, Paxinos G, Camm AJ. Anatomic approach for ganglionic plexi ablation in patients with paroxysmal atrial fibrillation. Am J Cardiol. 2008;102:330–4. doi: 10.1016/j.amjcard.2008.03.062. [DOI] [PubMed] [Google Scholar]
- Katritsis D, Giazitzoglou E, Sougiannis D, Voridis E, Po SS. Complex fractionated atrial electrograms at anatomic sites of ganglionated plexi in atrial fibrillation. Europace. 2009;11:308–15. doi: 10.1093/europace/eup036. [DOI] [PubMed] [Google Scholar]
- Katritsis DG, Giazitzoglou E, Zografos T, Pokushalov E, Po SS, Camm AJ. Rapid pulmonary vein isolation combined with autonomic ganglia modification: a randomized study. Heart Rhythm. 2011;8:672–8. doi: 10.1016/j.hrthm.2010.12.047. [DOI] [PubMed] [Google Scholar]
- Kimura K, Kanazawa H, Ieda M, Kawaguchi-Manabe H, Miyake Y, Yagi T, Arai T, Sano M, Fukuda K. Norepinephrine-induced nerve growth factor depletion causes cardiac sympathetic denervation in severe heart failure. Autonomic neuroscience: basic & clinical. 2010;156:27–35. doi: 10.1016/j.autneu.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–81. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- Kumar R, Wilders R, Joyner RW, Jongsma HJ, Verheijck EE, Golod DA, van Ginneken AC, Goolsby WN. Experimental model for an ectopic focus coupled to ventricular cells. Circulation. 1996;94:833–841. doi: 10.1161/01.cir.94.4.833. [DOI] [PubMed] [Google Scholar]
- Lemery R, Birnie D, Tang AS, Green M, Gollob M. Feasibility study of endocardial mapping of ganglionated plexuses during catheter ablation of atrial fibrillation. Heart Rhythm. 2006;3:387–96. doi: 10.1016/j.hrthm.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Lemola K, Chartier D, Yeh YH, Dubuc M, Cartier R, Armour A, Ting M, Sakabe M, Shiroshita-Takeshita A, Comtois P, Nattel S. Pulmonary vein region ablation in experimental vagal atrial fibrillation: role of pulmonary veins versus autonomic ganglia. Circulation. 2008;117:470–7. doi: 10.1161/CIRCULATIONAHA.107.737023. [DOI] [PubMed] [Google Scholar]
- Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004a;109:120–4. doi: 10.1161/01.CIR.0000105721.71640.DA. [DOI] [PubMed] [Google Scholar]
- Li S, Scherlag BJ, Yu L, Sheng X, Zhang Y, Ali R, Dong Y, Ghias M, Po SS. Low-level vagosympathetic stimulation: a paradox and potential new modality for the treatment of focal atrial fibrillation. Circ Arrhythm Electrophysiol. 2009;2:645–51. doi: 10.1161/CIRCEP.109.868331. [DOI] [PubMed] [Google Scholar]
- Li W, Knowlton D, Van Winkle DM, Habecker BA. Infarction alters both the distribution and noradrenergic properties of cardiac sympathetic neurons. American journal of physiology Heart and circulatory physiology. 2004b;286:H2229–36. doi: 10.1152/ajpheart.00768.2003. [DOI] [PubMed] [Google Scholar]
- Liao K, Yu L, Zhou X, Saren G, Wang S, Wang Z, Huang B, Yang K, Jiang H. Low-level baroreceptor stimulation suppresses atrial fibrillation by inhibiting ganglionated plexus activity. Can J Cardiol. 2015;31:767–74. doi: 10.1016/j.cjca.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Lin J, Scherlag BJ, Lu Z, Zhang Y, Liu S, Patterson E, Jackman WM, Lazzara R, Po SS. Inducibility of atrial and ventricular arrhythmias along the ligament of marshall: role of autonomic factors. J Cardiovasc Electrophysiol. 2008;19:955–62. doi: 10.1111/j.1540-8167.2008.01159.x. [DOI] [PubMed] [Google Scholar]
- Lin J, Scherlag BJ, Zhou J, Lu Z, Patterson E, Jackman WM, Lazzara R, Po SS. Autonomic mechanism to explain complex fractionated atrial electrograms (CFAE) J Cardiovasc Electrophysiol. 2007;18:1197–205. doi: 10.1111/j.1540-8167.2007.00976.x. [DOI] [PubMed] [Google Scholar]
- Linz D, Hohl M, Nickel A, Mahfoud F, Wagner M, Ewen S, Schotten U, Maack C, Wirth K, Bohm M. Effect of renal denervation on neurohumoral activation triggering atrial fibrillation in obstructive sleep apnea. Hypertension. 2013a;62:767–74. doi: 10.1161/HYPERTENSIONAHA.113.01728. [DOI] [PubMed] [Google Scholar]
- Linz D, Mahfoud F, Schotten U, Ukena C, Hohl M, Neuberger HR, Wirth K, Bohm M. Renal sympathetic denervation provides ventricular rate control but does not prevent atrial electrical remodeling during atrial fibrillation. Hypertension. 2013b;61:225–31. doi: 10.1161/HYPERTENSIONAHA.111.00182. [DOI] [PubMed] [Google Scholar]
- Linz D, Mahfoud F, Schotten U, Ukena C, Neuberger HR, Wirth K, Bohm M. Effects of electrical stimulation of carotid baroreflex and renal denervation on atrial electrophysiology. J Cardiovasc Electrophysiol. 2013c;24:1028–33. doi: 10.1111/jce.12171. [DOI] [PubMed] [Google Scholar]
- Linz D, Ukena C, Mahfoud F, Neuberger HR, Bohm M. Atrial autonomic innervation: a target for interventional antiarrhythmic therapy? J Am Coll Cardiol. 2014;63:215–24. doi: 10.1016/j.jacc.2013.09.020. [DOI] [PubMed] [Google Scholar]
- Linz D, van Hunnik A, Hohl M, Mahfoud F, Wolf M, Neuberger HR, Casadei B, Reilly SN, Verheule S, Bohm M, Schotten U. Catheter-based renal denervation reduces atrial nerve sprouting and complexity of atrial fibrillation in goats. Circ Arrhythm Electrophysiol. 2015;8:466–74. doi: 10.1161/CIRCEP.114.002453. [DOI] [PubMed] [Google Scholar]
- Linz D, Wirth K, Ukena C, Mahfoud F, Poss J, Linz B, Bohm M, Neuberger HR. Renal denervation suppresses ventricular arrhythmias during acute ventricular ischemia in pigs. Heart Rhythm. 2013d;10:1525–30. doi: 10.1016/j.hrthm.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Liu Y-B, Wu C-C, Lu L-S, Su M-J, Lin C-W, Lin S-F, Chen LS, Fishbein MC, Chen P-S, Lee Y-T. Sympathetic nerve sprouting, electrical remodeling, and increased vulnerability to ventricular fibrillation in hypercholesterolemic rabbits. Circulation Research. 2003;92:1145–1152. doi: 10.1161/01.RES.0000072999.51484.92. [DOI] [PubMed] [Google Scholar]
- Lopshire JC, Zhou X, Dusa C, Ueyama T, Rosenberger J, Courtney N, Ujhelyi M, Mullen T, Das M, Zipes DP. Spinal cord stimulation improves ventricular function and reduces ventricular arrhythmias in a canine postinfarction heart failure model. Circulation. 2009;120:286–94. doi: 10.1161/CIRCULATIONAHA.108.812412. [DOI] [PubMed] [Google Scholar]
- Lu Z, Scherlag BJ, Lin J, Niu G, Fung KM, Zhao L, Ghias M, Jackman WM, Lazzara R, Jiang H, Po SS. Atrial fibrillation begets atrial fibrillation: autonomic mechanism for atrial electrical remodeling induced by short-term rapid atrial pacing. Circ Arrhythm Electrophysiol. 2008;1:184–92. doi: 10.1161/CIRCEP.108.784272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaay AJ, Op’t Hof T, Bleeker WK, Jongsma HJ, Bouman LN. Interaction of adrenaline and acetylcholine on cardiac pacemaker function. Functional inhomogeneity of the rabbit sinus node. J Pharmacol Exp Ther. 1980;214:417–22. [PubMed] [Google Scholar]
- Male S, Scherlag BJ. Role of neural modulation in the pathophysiology of atrial fibrillation. Indian J Med Res. 2014;139:512–22. [PMC free article] [PubMed] [Google Scholar]
- Mannheimer C, Eliasson T, Andersson B, Bergh CH, Augustinsson LE, Emanuelsson H, Waagstein F. Effects of spinal cord stimulation in angina pectoris induced by pacing and possible mechanisms of action. BMJ. 1993;307:477–80. doi: 10.1136/bmj.307.6902.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantravadi R, Gabris B, Liu T, Choi B-R, de Groat WC, Ng GA, Salama G. Autonomic nerve stimulation reverses ventricular repolarization sequence in rabbit hearts. Circulation Research. 2007;100:e72–80. doi: 10.1161/01.RES.0000264101.06417.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx SO, Kurokawa J, Reiken S, Motoike H, D'Armiento J, Marks AR, Kass RS. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science (New York, NY) 2002;295:496–499. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–40. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- Myles RC, Wang L, Bers DM, Ripplinger CM. Decreased inward rectifying K+ current and increased ryanodine receptor sensitivity synergistically contribute to sustained focal arrhythmia in the intact rabbit heart. J Physiol (Lond) 2015;593(6):1479–1493. doi: 10.1113/jphysiol.2014.279638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles RC, Wang L, Kang C, Bers DM, Ripplinger CM. Local β-adrenergic stimulation overcomes source-sink mismatch to generate focal arrhythmia. Circulation Research. 2012;110:1454–1464. doi: 10.1161/CIRCRESAHA.111.262345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash MP, Thornton JM, Sears CE, Varghese A, O'Neill M, Paterson DJ. Ventricular activation during sympathetic imbalance and its computational reconstruction. Journal of applied physiology (Bethesda, Md: 1985) 2001;90:287–298. doi: 10.1152/jappl.2001.90.1.287. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, Hempstead BL, Petersen CM. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- Odenstedt J, Linderoth B, Bergfeldt L, Ekre O, Grip L, Mannheimer C, Andrell P. Spinal cord stimulation effects on myocardial ischemia, infarct size, ventricular arrhythmia, and noninvasive electrophysiology in a porcine ischemia-reperfusion model. Heart Rhythm. 2011;8:892–8. doi: 10.1016/j.hrthm.2011.01.029. [DOI] [PubMed] [Google Scholar]
- Olivas A, Gardner RT, Wang L, Ripplinger CM, Woodward WR, Habecker BA. Myocardial Infarction Causes Transient Cholinergic Transdifferentiation of Cardiac Sympathetic Nerves via gp130. Journal of Neuroscience. 2016;36(2):479–488. doi: 10.1523/JNEUROSCI.3556-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman F, Kundu S, Tuan J, Jeilan M, Stafford PJ, Ng GA. Ganglionic plexus ablation during pulmonary vein isolation--predisposing to ventricular arrhythmias? Indian Pacing Electrophysiol J. 2010;10:104–7. [PMC free article] [PubMed] [Google Scholar]
- Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, Mazzone P, Tortoriello V, Landoni G, Zangrillo A, Lang C, Tomita T, Mesas C, Mastella E, Alfieri O. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004;109:327–34. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624–31. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Pauza DH, Pauziene N, Pakeltyte G, Stropus R. Comparative quantitative study of the intrinsic cardiac ganglia and neurons in the rat, guinea pig, dog and human as revealed by histochemical staining for acetylcholinesterase. Ann Anat. 2002a;184:125–36. doi: 10.1016/S0940-9602(02)80005-X. [DOI] [PubMed] [Google Scholar]
- Pauza DH, Skripka V, Pauziene N. Morphology of the intrinsic cardiac nervous system in the dog: a whole-mount study employing histochemical staining with acetylcholinesterase. Cells Tissues Organs. 2002b;172:297–320. doi: 10.1159/000067198. [DOI] [PubMed] [Google Scholar]
- Pieroni M, Corti A, Tota B, Curnis F, Angelone T, Colombo B, Cerra MC, Bellocci F, Crea F, Maseri A. Myocardial production of chromogranin A in human heart: a new regulatory peptide of cardiac function. Eur Heart J. 2007;28:1117–27. doi: 10.1093/eurheartj/ehm022. [DOI] [PubMed] [Google Scholar]
- Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends in cardiovascular medicine. 2004;14:61–66. doi: 10.1016/j.tcm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circulation Research. 2001;88:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Baranova V, Turov A, Shirokova N, Karaskov A, Mittal S, Steinberg JS. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol. 2012;60:1163–70. doi: 10.1016/j.jacc.2012.05.036. [DOI] [PubMed] [Google Scholar]
- Pokushalov E, Romanov A, Shugayev P, Artyomenko S, Shirokova N, Turov A, Katritsis DG. Selective ganglionated plexi ablation for paroxysmal atrial fibrillation. Heart Rhythm. 2009;6:1257–64. doi: 10.1016/j.hrthm.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Porter M, Spear W, Akar JG, Helms R, Brysiewicz N, Santucci P, Wilber DJ. Prospective study of atrial fibrillation termination during ablation guided by automated detection of fractionated electrograms. J Cardiovasc Electrophysiol. 2008;19:613–20. doi: 10.1111/j.1540-8167.2008.01189.x. [DOI] [PubMed] [Google Scholar]
- Puodziukynas A, Kazakevicius T, Vaitkevicius R, Rysevaite K, Jokubauskas M, Saburkina I, Sladkeviciute-Dirzinauskiene V, Dirzinauskas E, Zabiela V, Sileikis V, Plisiene J, Pauziene N, Zaliunas R, Jalife J, Pauza DH. Radiofrequency catheter ablation of pulmonary vein roots results in axonal degeneration of distal epicardial nerves. Auton Neurosci. 2012;167:61–5. doi: 10.1016/j.autneu.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Raake PW, Zhang X, Vinge LE, Brinks H, Gao E, Jaleel N, Li Y, Tang M, Most P, Dorn GW, Houser SR, Katus HA, Chen X, Koch WJ. Cardiac G-protein-coupled receptor kinase 2 ablation induces a novel Ca2+ handling phenotype resistant to adverse alterations and remodeling after myocardial infarction. Circulation. 2012;125:2108–2118. doi: 10.1161/CIRCULATIONAHA.111.044255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall DC, Brown DR, McGuirt AS, Thompson GW, Armour JA, Ardell JL. Interactions within the intrinsic cardiac nervous system contribute to chronotropic regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1066–75. doi: 10.1152/ajpregu.00167.2003. [DOI] [PubMed] [Google Scholar]
- Remo BF, Preminger M, Bradfield J, Mittal S, Boyle N, Gupta A, Shivkumar K, Steinberg JS, Dickfeld T. Safety and efficacy of renal denervation as a novel treatment of ventricular tachycardia storm in patients with cardiomyopathy. Heart Rhythm. 2014;11:541–6. doi: 10.1016/j.hrthm.2013.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rysevaite K, Saburkina I, Pauziene N, Noujaim SF, Jalife J, Pauza DH. Morphologic pattern of the intrinsic ganglionated nerve plexus in mouse heart. Heart Rhythm. 2011a;8:448–54. doi: 10.1016/j.hrthm.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rysevaite K, Saburkina I, Pauziene N, Vaitkevicius R, Noujaim SF, Jalife J, Pauza DH. Immunohistochemical characterization of the intrinsic cardiac neural plexus in whole-mount mouse heart preparations. Heart Rhythm. 2011b;8:731–8. doi: 10.1016/j.hrthm.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio KN, Mauad H, Spyer KM, Ford TW. Differential chronotropic and dromotropic responses to focal stimulation of cardiac vagal ganglia in the rat. Exp Physiol. 2003;88:315–27. doi: 10.1113/eph8802525. [DOI] [PubMed] [Google Scholar]
- Scanavacca M, Pisani CF, Hachul D, Lara S, Hardy C, Darrieux F, Trombetta I, Negrao CE, Sosa E. Selective atrial vagal denervation guided by evoked vagal reflex to treat patients with paroxysmal atrial fibrillation. Circulation. 2006;114:876–85. doi: 10.1161/CIRCULATIONAHA.106.633560. [DOI] [PubMed] [Google Scholar]
- Scherlag BJ, Nakagawa H, Jackman WM, Yamanashi WS, Patterson E, Po S, Lazzara R. Electrical stimulation to identify neural elements on the heart: their role in atrial fibrillation. J Interv Card Electrophysiol. 2005;13(Suppl 1):37–42. doi: 10.1007/s10840-005-2492-2. [DOI] [PubMed] [Google Scholar]
- Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev. 2011;91:265–325. doi: 10.1152/physrev.00031.2009. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ, Priori SG, Cerrone M, Spazzolini C, Odero A, Napolitano C, Bloise R, De Ferrari GM, Klersy C, Moss AJ, Zareba W, Robinson JL, Hall WJ, Brink PA, Toivonen L, Epstein AE, Li C, Hu D. Left cardiac sympathetic denervation in the management of high-risk patients affected by the long-QT syndrome. Circulation. 2004;109:1826–33. doi: 10.1161/01.CIR.0000125523.14403.1E. [DOI] [PubMed] [Google Scholar]
- Shannon TR, Ginsburg KS, Bers DM. Potentiation of fractional sarcoplasmic reticulum calcium release by total and free intra-sarcoplasmic reticulum calcium concentration. Biophysical journal. 2000;78:334–343. doi: 10.1016/S0006-3495(00)76596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng X, Scherlag BJ, Yu L, Li S, Ali R, Zhang Y, Fu G, Nakagawa H, Jackman WM, Lazzara R, Po SS. Prevention and reversal of atrial fibrillation inducibility and autonomic remodeling by low-level vagosympathetic nerve stimulation. J Am Coll Cardiol. 2011;57:563–71. doi: 10.1016/j.jacc.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Shibata N, Inada S, Mitsui K, Honjo H, Yamamoto M, Niwa R, Boyett MR, Kodama I. Pacemaker shift in the rabbit sinoatrial node in response to vagal nerve stimulation. Exp Physiol. 2001;86:177–84. doi: 10.1113/eph8602100. [DOI] [PubMed] [Google Scholar]
- Siao C-J, Lorentz CU, Kermani P, Marinic T, Carter J, McGrath K, Padow VA, Mark W, Falcone DJ, Cohen-Gould L, Parrish DC, Habecker BA, Nykjaer A, Ellenson LH, Tessarollo L, Hempstead BL. ProNGF, a cytokine induced after myocardial infarction in humans, targets pericytes to promote microvascular damage and activation. The Journal of experimental medicine. 2012;209:2291–2305. doi: 10.1084/jem.20111749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Johnson PI, Lee RE, Orfei E, Lonchyna VA, Sullivan HJ, Montoya A, Tran H, Wehrmacher WH, Wurster RD. Topography of cardiac ganglia in the adult human heart. J Thorac Cardiovasc Surg. 1996;112:943–53. doi: 10.1016/S0022-5223(96)70094-6. [DOI] [PubMed] [Google Scholar]
- Song LS, Wang SQ, Xiao RP, Spurgeon H, Lakatta EG, Cheng H. beta-Adrenergic stimulation synchronizes intracellular Ca(2+) release during excitation-contraction coupling in cardiac myocytes. Circulation Research. 2001;88:794–801. doi: 10.1161/hh0801.090461. [DOI] [PubMed] [Google Scholar]
- Sperelakis N. Regulation of calcium slow channels of heart by cyclic nucleotides and effects of ischemia. Advances in pharmacology (San Diego, Calif) 1994;31:1–24. doi: 10.1016/s1054-3589(08)60605-5. [DOI] [PubMed] [Google Scholar]
- Stavrakis S, Scherlag BJ, Fan Y, Liu Y, Liu Q, Mao J, Cai H, Lazzara R, Po SS. Antiarrhythmic effects of vasostatin-1 in a canine model of atrial fibrillation. J Cardiovasc Electrophysiol. 2012;23:771–7. doi: 10.1111/j.1540-8167.2012.02317.x. [DOI] [PubMed] [Google Scholar]
- Ukena C, Bauer A, Mahfoud F, Schreieck J, Neuberger HR, Eick C, Sobotka PA, Gawaz M, Bohm M. Renal sympathetic denervation for treatment of electrical storm: first-in-man experience. Clin Res Cardiol. 2012;101:63–7. doi: 10.1007/s00392-011-0365-5. [DOI] [PubMed] [Google Scholar]
- Vaitkevicius R, Saburkina I, Rysevaite K, Vaitkeviciene I, Pauziene N, Zaliunas R, Schauerte P, Jalife J, Pauza DH. Nerve supply of the human pulmonary veins: an anatomical study. Heart Rhythm. 2009;6:221–8. doi: 10.1016/j.hrthm.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Valdivia HH, Kaplan JH, Ellis-Davies GC, Lederer WJ. Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation. Science (New York, NY) 1995;267:1997–2000. doi: 10.1126/science.7701323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS, Jr, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res. 1991;68:1471–81. doi: 10.1161/01.res.68.5.1471. [DOI] [PubMed] [Google Scholar]
- Vaseghi M, Gima J, Kanaan C, Ajijola OA, Marmureanu A, Mahajan A, Shivkumar K. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and long-term follow-up. Heart Rhythm. 2014;11:360–6. doi: 10.1016/j.hrthm.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaseghi M, Lux RL, Mahajan A, Shivkumar K. Sympathetic stimulation increases dispersion of repolarization in humans with myocardial infarction. AJP: Heart and Circulatory Physiology. 2012;302:H1838–46. doi: 10.1152/ajpheart.01106.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatner DE, Lavallee M, Amano J, Finizola A, Homcy CJ, Vatner SF. Mechanisms of supersensitivity to sympathomimetic amines in the chronically denervated heart of the conscious dog. Circulation Research. 1985;57:55–64. doi: 10.1161/01.res.57.1.55. [DOI] [PubMed] [Google Scholar]
- Verma A, Novak P, Macle L, Whaley B, Beardsall M, Wulffhart Z, Khaykin Y. A prospective, multicenter evaluation of ablating complex fractionated electrograms (CFEs) during atrial fibrillation (AF) identified by an automated mapping algorithm: acute effects on AF and efficacy as an adjuvant strategy. Heart Rhythm. 2008;5:198–205. doi: 10.1016/j.hrthm.2007.09.027. [DOI] [PubMed] [Google Scholar]
- Viatchenko-Karpinski S, Györke S. Modulation of the Ca(2+)-induced Ca(2+) release cascade by beta-adrenergic stimulation in rat ventricular myocytes. The Journal of Physiology. 2001;533:837–848. doi: 10.1111/j.1469-7793.2001.t01-1-00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, Deo S, Barlow M, Johnson S, Caffrey JL, Zhou Y-Y, Xiao R-P, Cheng H, Stern MD, Maltsev VA, Lakatta EG. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circulation Research. 2006;98:505–514. doi: 10.1161/01.RES.0000204575.94040.d1. [DOI] [PubMed] [Google Scholar]
- Vollmann D, Sossalla S, Schroeter MR, Zabel M. Renal artery ablation instead of pulmonary vein ablation in a hypertensive patient with symptomatic, drug-resistant, persistent atrial fibrillation. Clin Res Cardiol. 2013;102:315–8. doi: 10.1007/s00392-012-0529-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhou X, Huang B, Wang Z, Zhou L, Chen M, Yu L, Jiang H. Spinal cord stimulation suppresses atrial fibrillation by inhibiting autonomic remodeling. Heart Rhythm. 2016;13(1):274–281. doi: 10.1016/j.hrthm.2015.08.018. [DOI] [PubMed] [Google Scholar]
- Weerasooriya R, Khairy P, Litalien J, Macle L, Hocini M, Sacher F, Lellouche N, Knecht S, Wright M, Nault I, Miyazaki S, Scavee C, Clementy J, Haissaguerre M, Jais P. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57:160–6. doi: 10.1016/j.jacc.2010.05.061. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe SR, Patel VV. Local innervation and atrial fibrillation. Circulation. 2013;128:1566–75. doi: 10.1161/CIRCULATIONAHA.113.001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde AA, Bhuiyan ZA, Crotti L, Facchini M, De Ferrari GM, Paul T, Ferrandi C, Koolbergen DR, Odero A, Schwartz PJ. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N Engl J Med. 2008;358:2024–9. doi: 10.1056/NEJMoa0708006. [DOI] [PubMed] [Google Scholar]
- Xie Y, Sato D, Garfinkel A, Qu Z, Weiss JN. So little source, so much sink: requirements for afterdepolarizations to propagate in tissue. Biophysical journal. 2010;99:1408–1415. doi: 10.1016/j.bpj.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagishita D, Chui RW, Yamakawa K, Rajendran PS, Ajijola OA, Nakamura K, So EL, Mahajan A, Shivkumar K, Vaseghi M. Sympathetic nerve stimulation, not circulating norepinephrine, modulates T-peak to T-end interval by increasing global dispersion of repolarization. Circulation: Arrhythmia and Electrophysiology. 2015;8:174–185. doi: 10.1161/CIRCEP.114.002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SS, Han W, Cao Y, Dong G, Zhou G, Li WM, Gan RT, Chang HY, Wang Z. Effects of high thoracic epidural anesthesia on atrial electrophysiological characteristics and sympathetic nerve sprouting in a canine model of atrial fibrillation. Basic Res Cardiol. 2011;106:495–506. doi: 10.1007/s00395-011-0154-3. [DOI] [PubMed] [Google Scholar]
- Yatani A, Shen Y-T, Yan L, Chen W, Kim S-J, Sano K, Irie K, Vatner SF, Vatner DE. Down regulation of the L-type Ca2+ channel, GRK2, and phosphorylated phospholamban: protective mechanisms for the denervated failing heart. Journal of Molecular and Cellular Cardiology. 2006;40:619–628. doi: 10.1016/j.yjmcc.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Yu L, Huang B, He W, Wang S, Liao K, Zhou X, He B, Lu Z, Jiang H. Spinal cord stimulation suppresses focal rapid firing-induced atrial fibrillation by inhibiting atrial ganglionated plexus activity. J Cardiovasc Pharmacol. 2014;64:554–9. doi: 10.1097/FJC.0000000000000154. [DOI] [PubMed] [Google Scholar]
- Yu L, Scherlag BJ, Li S, Fan Y, Dyer J, Male S, Varma V, Sha Y, Stavrakis S, Po SS. Low-level transcutaneous electrical stimulation of the auricular branch of the vagus nerve: a noninvasive approach to treat the initial phase of atrial fibrillation. Heart Rhythm. 2013;10:428–35. doi: 10.1016/j.hrthm.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Yu L, Scherlag BJ, Li S, Sheng X, Lu Z, Nakagawa H, Zhang Y, Jackman WM, Lazzara R, Jiang H, Po SS. Low-level vagosympathetic nerve stimulation inhibits atrial fibrillation inducibility: direct evidence by neural recordings from intrinsic cardiac ganglia. J Cardiovasc Electrophysiol. 2011;22:455–63. doi: 10.1111/j.1540-8167.2010.01908.x. [DOI] [PubMed] [Google Scholar]
- Yuan BX, Ardell JL, Hopkins DA, Armour JA. Differential cardiac responses induced by nicotine sensitive canine atrial and ventricular neurones. Cardiovasc Res. 1993;27:760–9. doi: 10.1093/cvr/27.5.760. [DOI] [PubMed] [Google Scholar]
- Zarzoso M, Rysevaite K, Milstein ML, Calvo CJ, Kean AC, Atienza F, Pauza DH, Jalife J, Noujaim SF. Nerves projecting from the intrinsic cardiac ganglia of the pulmonary veins modulate sinoatrial node pacemaker function. Cardiovasc Res. 2013;99(3):566–575. doi: 10.1093/cvr/cvt081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Popovic ZB, Bibevski S, Fakhry I, Sica DA, Van Wagoner DR, Mazgalev TN. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail. 2009;2:692–9. doi: 10.1161/CIRCHEARTFAILURE.109.873968. [DOI] [PubMed] [Google Scholar]
- Zhao M, He X, Bi XY, Yu XJ, Gil Wier W, Zang WJ. Vagal stimulation triggers peripheral vascular protection through the cholinergic anti-inflammatory pathway in a rat model of myocardial ischemia/reperfusion. Basic Res Cardiol. 2013;108:345. doi: 10.1007/s00395-013-0345-1. [DOI] [PubMed] [Google Scholar]
- Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL, Smith SC, Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B American College of Cardiology/American Heart Association Task F, European Society of Cardiology Committee for Practice G, European Heart Rhythm A, the Heart Rhythm S. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death--executive summary: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Eur Heart J. 2006;27:2099–140. doi: 10.1093/eurheartj/ehl199. [DOI] [PubMed] [Google Scholar]