Abstract
Background & Aims
Molecular events that lead to recurrence and/or metastasis after curative treatment of patients with colorectal cancers (CRCs) are poorly understood. Patients with stage II or III primary CRC with increased numbers of microsatellite alterations at selected tetra-nucleotide repeats (EMAST) and low levels of microsatellite instability (E/L) are more likely to have disease recurrence after treatment. Hypoxia and/or inflammation not only promote metastasis but also induce EMAST by causing deficiency of MSH3 in the cancer cell nucleus. We aimed to identify genetic alterations associated with metastasis of primary colorectal tumors to liver and to determine their effects on survival.
Methods
We obtained 4 sets of primary colorectal tumors and matched liver metastases from hospitals in Korea and Japan. Intragenic microsatellites with large repeats at 141 loci were examined for frame-shift mutations and/or loss of heterozygosity (LOH) as possible consequences of MSH3 deficiency. Highly altered loci were examined for association with E/L in liver metastases. We analyzed data from 156 of the patients with stage II or III primary colorectal tumors to determine outcomes and whether altered loci were associated with E/L.
Results
LOH at several loci at chromosome 9p24.2 (9p24.2-LOH) was associated with E/L in liver metastases (odds ratio, 10.5; 95% confidence interval [CI], 2.69–40.80; P=.0007). We found no significant difference in the frequency of E/L, 9p24.2-LOH, mutations in KRAS or BRAF, or the combination of E/L and 9p24.2-LOH between primary colorectal tumors and their matched metastases. Patients with stage II or III colorectal tumors with E/L and 9p24.2-LOH had increased survival following CRC recurrence (hazard ratio, 0.25; 95% CI, 0.12–0.50; P=.0001), compared to patients without with E/L and 9p24.2-LOH. E/L with 9p24.2-LOH appeared to be an independent prognostic factor for overall survival of patients with stage III CRC (hazard ratio, 0.06; 95% CI, 0.01–0.57; P=.01).
Conclusions
E/L with 9p24-LOH appears to be a biomarker for less aggressive metastasis from stage III primary colorectal tumors.
Keywords: microsatellite instability, colon cancer, progression, recurrence
Introduction
Recent advances in detection and treatment of colorectal cancer (CRC) have resulted in a significant increase in the overall survival (OS) rate and has reduced the rate of recurrence in CRC patients (1, 2, 3), especially strategies using flexible choices of general and/or target-specific chemotherapy plus surgery for treating metastasis from primary CRC. However, a substantial number of patients with Stage II and III CRC still experience disease recurrence with local or distant metastasis that eventually leads to death (3). This could be partially due to lack of a marker(s) that more accurately predicts patients’ outcomes than those presently used, which are based largely on lymph node metastasis. Although a quantitative assay based on the expressions of multiple molecular markers has been applied for the treatment decision of Stage II CRC (4), identification of genetic factors that may serve as a prognosticator and/or treatment target would be ideal to predict and/or control disease recurrence. Recent studies demonstrated that CRC is a heterogeneous disease that can be divided into several sub-groups with different prognoses according to their genetic and epigenetic abnormalities and gene expression patterns (5, 6, 7, 8, 9, 10, 11, 12). These observations suggest that there may be multiple pathways for recurrence and/or metastasis from primary CRC. Although the mutation status of KRAS/BRAF not only determines the outcome of Stage IV disease being treated by EGFR-specific therapy but also determines alternative choices for other target-specific therapy, there have not been general markers that distinguish patients with different prognoses following recurrence.
A defective mismatch repair (MMR) system leads to misrepair of slippage errors generated by DNA polymerase while copying microsatellite loci, resulting in microsatellite instability (MSI) (13). Tumor tissues derived in a setting of MMR deficiency exhibit a high level of MSI (MSI-H). When the NCI-endorsed microsatellite panels (consisting of 2 mononucleotide and 3 dinucleotide repeats) are utilized, the majority of CRCs show stable microsatellites (MSS), but some CRCs are found to have low levels of MSI (MSI-L) (14). MSI-L CRC does not occur because of defects in hMSH2 or hMLH1, and the molecular basis of MSI-L has been controversial (14, 15). In addition to MSI defined by the NCI markers, another type of mutation at microsatellite loci has been observed in human cancers including CRC (16). Among non-MSI-H CRC, some tumors show instability at loci with tetra-nucleotide repeats containing [AAAG] or [AGAT] but not at loci with mononucleotide repeats (17, 18, 19, 20). This type of microsatellite instability is called EMAST, for elevated microsatellite alterations at selected tetra-nucleotide repeats.
Recently, it has been demonstrated that EMAST and most likely MSI-L (abbreviated as E/L in this study) are a consequence of MSH3-deficiency and may be associated with the same pathological group of CRCs (17, 21, 22). About 50% of non-MSI-H primary CRCs exhibited EMAST (17, 18). Most but not all of MSI-L CRCs, and half of MSS CRCs defined by the standard NCI markers exhibit EMAST (17, 18). Loss of MSH3 in cultured human colon cancer cells results in frame shifts in the EMAST loci and those in the loci with di-nucleotide repeats at low frequency (17, 22, 23, unpublished data). Although an improved prognostic role of MSH3 loss in the setting of MLH1-deficiency in CRCs has been reported, it is not known whether loss of MSH3 has any impact on the prognosis of sporadic CRCs (24). However, a significant association between down-regulation of MSH3 expression and E/L has been demonstrated in sporadic CRC tissues (17). Furthermore, when a cohort of 167 primary CRCs was examined for MSI using standard NCI markers and EMAST markers, three groups of stage II and III CRCs were identified that differ according to the risk of recurrent distant metastasis (21). The highest risk group showed E/L and the lowest risk group exhibited MSI-H. An intermediate risk group did not show MSI at any of the NCI or EMAST markers. However, it remains to be determined how E/L is linked to recurrence and/or distant metastasis in CRC.
In this study, we aimed to identify genetic changes associated with recurrence and/or metastasis in patients with E/L CRCs. Our approach was based on the following assumptions: all the genetic and/or epigenetic changes necessary for recurrence and/or metastasis are recorded in metastasis tissues from primary CRC but not in primary CRC, and some of those changes could be the result of MSH3-deficiency and associated with E/L. Because recent studies demonstrated that MSH3-deficiency not only causes frame shift (FS) but also induces LOH due to impaired double-strand break repair (25), we selected a group of 141 gene loci with intragenic microsatellites by genome data mining, and screened them for FS mutations and LOH associated with E/L in liver metastasis from primary CRC. As a result, chromosome 9p24.2 LOH (9p24-LOH) was found to be associated with E/L in liver metastasis. The significance of E/L and 9p24-LOH for recurrence and metastasis in CRC was further examined.
Materials and Methods
Tumor Samples
Four sets of tumor samples were used in this study (Fig. 1). Tumor Set #1 and #2: Collected from Department of Pathology at Chonnam National University (DPCNU, Kwanju, Korea) and from the Department of Pathology at Ohmori Hospital, Toho University (DPOHTU, Tokyo, Japan). Tumor Set #3: Collected from the DPCNU and from the Department of Surgery at Mie University (DSMU). Tumor Set #4: Collected from the DPCNU. All patients provided written informed consent, and the studies were approved by Baylor Research Institute Review Board (Approval number 003-180).
Fig.1. Tumor tissue sets used in this study and the purpose of their use.
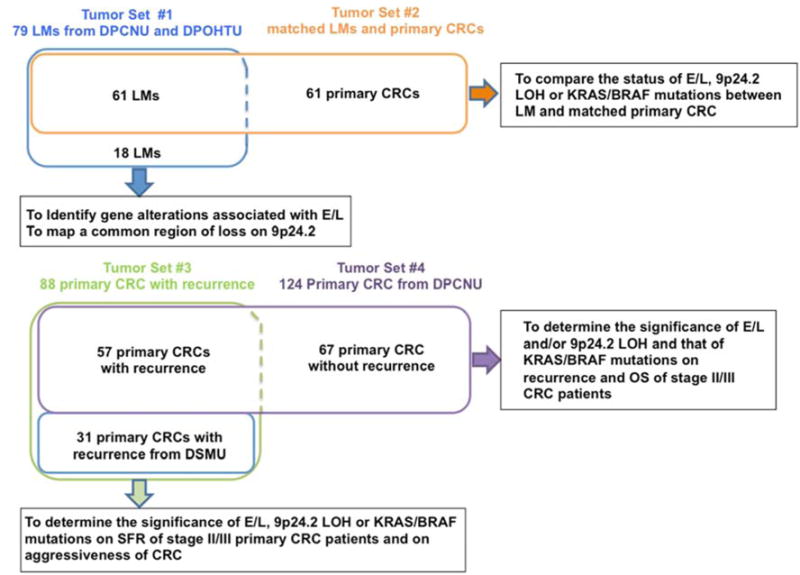
Tumor Set #1 (blue box) is made up of 79 liver metastasis tissues. This set was used for screening of 141 microsatellite genes to identify a locus whose FS and/or LOH is associated with E/L. The LOH at 9p24.2 (9p24.2-LOH) is found to be associated with E/L. Tumor Set #2 (orange box) was made up of 61 paired liver metastasis and matched primary CRC from which the liver metastases were derived. Status of E/L, 9p24.2-LOH and KRAS/BRAF mutations were compared between liver metastasis and matched primary CRC. Tumor Set #3 (green box) consisted of 88 stage II/III CRC that experienced recurrence or metastasis during a follow-up period of 5 years. Pathological significance of E/L and 9p24.2-LOH on recurrence and/or metastasis was examined. A sub-population of this set that developed liver metastatsis (50 cases) was also examined for a significance of E/L/9p24.2-LOH on receiving hepatectomy treatment and their aggressiveness. Tumor Set #4 (purple box) consisted of 124 stage II/III primary CRC. This set was used to determine whether E/L and/or 9p24.2-LOH could be determinants for DFS and/or OS for stage II/III primary CRC.
MSI/EMAST Assay and Categorization of CRCs
To determine the MSI status of primary CRC and liver metastasis tissues, PCR amplifications were performed from genomic DNA using fluorescently labeled 14 sets of primers (17). Tumors were categorized as follows: 1) Microsatellite instability-high (MSI-H): tumors exhibiting FS at three or more of the seven mono- (BAT25 and BAT26) or dinucleotide markers (D2S123, D5S346, D17S250, D18S64, and D18S69); 2) EMAST/MSI-L (E/L): non-MSI-H tumors exhibiting FS at any one or more than one of 14 markers. Thus, E/L includes EMAST tumors exhibiting FS at one or more locus in the seven EMAST markers (MYCL1, D20S82, D20S85, L17835, D8S321, D9S242 and D19S394) and/or MSI-L tumors exhibiting FS at one or two of the seven mono- or di-nucleotide markers; 3) Microsatellite stable (MS): tumors which did not exhibit FS at any of the 14 markers. In this study, we distinguish between a classical microsatellite stable CRC (MSS) defined by the reference NCI markers and MS defined by our categorization.
KRAS/BRAF Mutation Detection
KRAS (exons 2 and 3) and BRAF (V600E) mutations were analyzed by pyrosequencing as previously described (26). The list of primers used is found in Supplementary Materials and Methods. Reactions were run on a PyroMark Q96 ID system (Qiagen).
Statistical Analysis
The Chi-square test, Fisher’s exact test, McNemar’s test, and logistic regression analysis, were used to assess the association among variables. To estimate and compare the survival rate for a particular group of CRCs, the Kaplan-Meier method with the log rank test was used. Cox proportional hazards regression analysis was used to evaluate the significance of E/L/9p24− on survival following recurrence (SFR), OS and disease-free survival (DFS) of patients with stage II/III primary CRC. This factor was controlled for by patient’s age and sex, primary tumor’s stage (stage II vs III), location (proximal vs distal), grade (G1 vs G2/G3), cancer type (colon vs rectum), KRAS/BRAF mutation status and the presence or absence of adjuvant chemotherapy treatment. In tumor set #4 (Fig. 1), there is a sampling bias toward recurrence cases (57 cases) over non-recurrence cases (67 cases). We therefore calculated the inverse probability weight for each case according to the recurrence rate/year observed at DPCNU (Supplementary Materials and Methods). Then, the weighted data were subjected to Kaplan-Meier and Cox proportional hazard analyses. If a P value was less than 0.05, the difference was considered to be statistically significant. Statistical analyses were performed using R or JMP software.
Results
Identification of a microsatellite gene locus whose alteration is associated with EMAST/MSI-L (E/L) in resected liver metastasis from colorectal cancers
Figure 1 illustrates 4 sets of tumor samples and the purpose of their use.
To identify genetic changes associated with E/L and metastasis or recurrence, 79 resected liver metastases (Tumor Set #1, Fig. 1) were used. They were first analyzed for EMAST/MSI status and KRAS/BRAF mutations. As shown in Supplementary Table 1, 71% (56/79) and 29% (22/79) of them exhibited E/L and MS respectively. Only one case (1%) exhibited MSI-H. Thirty-three percent (26/77) of them exhibited KRAS (25 cases) and/or BRAF (2 cases) mutations. Neither E/L nor KRAS/BRAF mutation is associated with overall survival (OS) of the patients with these resected liver metastases (Supplementary Table 1). The 78 non-MSI-H liver metastases were used for further analysis.
We then searched human genome databases (Supplementary Materials and Methods) and selected a group of 141 genes containing di-, tri- or tetra-nucleotide repeats in intragenic sequences that would be the targets of MSH3-deficiency (Supplementary Table 2).
First, FS and/or LOH at 141 loci were examined in 24 liver metastases exhibiting E/L (Supplementary Table 2). This analysis identified the loci with high frequencies of FS (5 loci) and/or those of LOH (11 loci) (Supplementary Table 3). Association of these alterations with E/L was further examined by analyzing the remaining 54 liver metastases consisting of 32 E/L and 22 MS (Supplementary Figs. 1A and 1B). As a result, FS at D21S11 on 21q21.1 (P=0.015) and ANKRD5 on 20p12.1 (P=0.015), and LOH at the SMARCA2 on 9p24.3 (P=0.006) were found to be associated with E/L by chi-square test, suggesting that these changes could be targets of E/L event (Fig. 2A). Because genome data from The Cancer Genome Atlas (TCGA) (http://www.cbioportal.org/public-portal/) showed a recurrent deletion at the 9p24.2 region in CRCs, we explored the possible association of LOH at the SMARCA2 or surrounding region with recurrence and/or metastasis from primary CRC in the subsequent experiments.
Fig. 2.
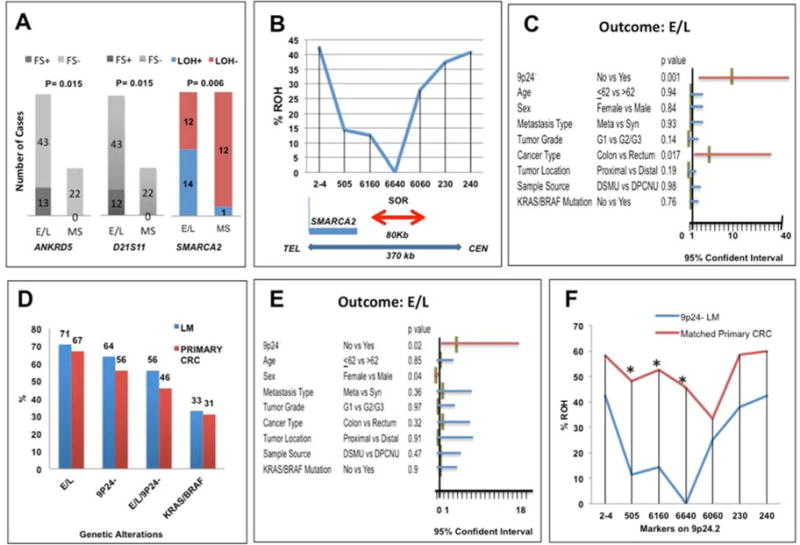
A: The loci associated with E/L in resected liver metastases. Frame shifts (FS) at ANKRD and D21S11, and LOH at SMARCA2 were associated with E/L.
B: Percentages of 9p24.2-LOH liver metastasis exhibiting ROH at each of 7 loci (2–4, 505, 6160, 6640, 6060 230 and 240) within 370kb (double-sided blue arrow) on 9p24.2 are shown. A thick red arrow indicates the smallest overlapping LOH region (SOR: ~80kb). The thin blue line indicates the 3′ terminus of the SMARCA2 gene. TEL: telomere, CEN: centromere.
C and E: Association between E/L and 9p24.2-LOH in resected liver metastases (P=0.007) (C) and in primary CRC that gave rise to liver metastasis (P=0.02) (E) was tested by logistic regression analysis. The association was adjusted by age, sex, metastasis type, primary tumor grades, cancer type, primary tumor sites, sample sources, and KRAS/BRAF mutations. The X-axis represents the range of the 95% Confidence Interval (CI). Each horizontal bar indicates the 95% CI range. A red or blue horizontal bar represents the significance or non-significance between each variable and E/L, respectively. The middle brown vertical bar represents a value for odd ratio. The P values were shown following each variable.
D: No difference in the frequency of E/L (P=0.68), 9p24.2-LOH (P=0.18), E/L/9p24.2-LOH (P=0.23) or KRAS/BRAF mutations (P=1) between paired liver metastasis and matched primary CRC was detected by McNemar’s test. Overall frequency of E/L, 9p24.2-LOH, E/L/9p24.2-LOH, or KRAS/BRAF mutations in liver metastasis (blue bars) and in matched primary CRC (red bars) is presented.
F: There were significant decreases in the frequency of ROH at 505, 6160 and 6640 loci in liver metastases compared to matching primary CRCs. P values were determined by McNemar’s (one-sided). Stars indicate a significant difference.
LOH at 9p24.2 in liver metastasis
To see whether SMARCA2 is the target of the observed LOH, we determined the smallest overlapping region of LOH (SOR) among the resected liver metastasis tissues. Seventy-four of the 78 liver metastases described above were analyzed for LOH. Detailed LOH data are presented in Supplementary Fig. 2A. Seven polymorphic microsatellite markers present within a 370kb region inside or near the SMARCA2 locus were used to determine SOR (Fig. 2B and Supplementary Fig. 2A). Sixty percent of them (44 of 74 cases) exhibited LOH in one or more of the 7 markers, and were defined as 9p24.2-LOH tumors (Supplementary Fig. 2A). The SOR was found in the ~80kb region bounded by markers 6160 and 6060 (Fig. 2B), eliminating SMARCA2 as the target of the LOH events. This conclusion was further supported by the following observations; 1) the expression levels of the SMARCA2 protein determined by immunohistochemical (IHC) staining (Supplementary Fig. 3A and 3B) or those of mRNA determined by qRT-PCR (Supplementary Fig. 3C) do not correlate with the presence or absence of 9p24.2 LOH in liver metastases; 2) deep targeted sequencing of the SMARCA2 locus did not detect any recurrent mutations in introns or exons of this gene in primary CRC tumors exhibiting 9p24.2 LOH (Supplementary Fig. 4).
No liver metastasis exhibiting 9p24.2-LOH retained heterozygosity at the 6640 locus (Fig. 2B), suggesting that one of the parental copies close to 6640 was lost in all of the liver metastases with 9p24.2-LOH. Strikingly, 84% of liver metastases with 9p24.2-LOH exhibited E/L (37/44, Supplementary Fig. 2). Thus, E/L and 9p24.2-LOH are associated in resected liver metastases in multivariate analyses (odds ratio: 10.5, 95% CI: 2.69–40.8, P=0.0007, Fig. 2C). E/L is also associated with liver metastases from rectum compared to liver metastases from colon (P=0.017).
E/L and 9p24-LOH in liver metastases and in matched primary CRCs
To determine whether status of E/L, 9p24.2-LOH or KRAS/BRAF mutations differ between liver metastasis and primary CRC from which the liver metastases were derived, 61 of the matched liver metastasis and primary CRCs were analyzed (Tumor Set #2, Fig. 1 and Supplementary Fig. 2B). McNemar’s test showed that no significant difference in the frequency of E/L (P=0.68), 9p24.2-LOH (P=0.18), KRAS/BRAF mutations (P=1.00), or E/L/9p24.2-LOH (P=0.23) between paired liver metastases and their matched primary CRCs (Fig. 2D). Furthermore, 82% of primary CRCs with 9p24.2-LOH exhibited E/L (27/33, Supplementary Fig. 2). 9p24.2-LOH and E/L were associated with each other (odds ratio: 4.2, 95%CI: 1.3–13.9, P=0.02) in logistic regression analysis when adjusted by sample source, KRAS/BRAF mutation status, and stage (Fig. 2E). These results suggest that E/L and 9p24.2-LOH, and their associations are determined at the primary site. However, in contrast to the liver metastasis cases, no common LOH region was observed among primary CRCs with 9p24.2-LOH (Supplementary Fig. 2B). Additionally, when we compared the frequency of retention of heterozygosity (ROH) at each of 7 loci on 9p24.2 between matched primary CRC and liver metastasis cases in which the liver metastasis cases exhibited 9p24.2-LOH, a significant allelic loss was detected in liver metastases at loci 505 (P=0.0031), 6160 (P=0.002) and 6640 (P=0.0313) by McNemar’s test (Fig. 2F, one-sided P-values). These results suggest that the gene critical for metastasis may reside near the 505/6160/6640 region on 9p24.2.
The significance of E/L and 9p24.2-LOH on survival following recurrence (SFR) in stage II and III primary CRCs
To obtain further insight into the role of E/L and 9p24.2-LOH in recurrence and/or metastasis from primary CRC, we collected and analyzed another cohort consisting of 88 cases of originally staged II or III CRCs (Tumor Set #3, Fig 1) that ultimately gave rise to liver metastasis (50 cases), other distant metastasis (34 cases) and local recurrence (4 cases) for the incidence of E/L, 9p24.2-LOH, or KRAS/BRAF mutations, and examined their relationship to clinicopathological factors and patient outcomes (Table 1 and Supplementary Table 4).
Table 1.
Eighty-Eight Primary CRCs That Gave Rise to Recurrence During 5 year Follow-up Period
| Association withb | (OR, 95% CI) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variablesa | Number of Cases | E/L | 9p24− | E/L/9p24− | KRAS/BRAF | |
| Age | ≤62 vs >62 | 43 vs 45 | 0.54 | 0.67 | 0.61 | 0.77 |
| Sex | Female vs Male | 35 vs 53 | 0.82 | 0.7 | 0.88 | 0.34 |
| Stage | II vs III | 21 vs 67 | 0.57 | 0.71 | 0.4 | 0.54 |
| Tumor grade | G1 vs G2&G3 | 28 vs 60 | 0.58 | 0.76 | 0.95 | 0.03 (3.3, 1.1–9.8) |
| Tumor sites | Proximal vs Distal | 17 vs 71 | 0.81 | 0.053 | 0.26 | 0.49 |
| Cancer type | Colon vs Rectum | 40 vs 48 | 0.84 | 0.14 | 0.19 | 0.87 |
| Sites of metastasis | Non-liver vs Liver | 38 vs 50 | 0.24 | 0.02 (0.36, 0.15–0.86) | 0.001 (0.2, 0.08–0.52) | 0.98 |
| Sample source | Mie(DPMU) vs Korea (DPCNU) | 31 vs 57 | 0.64 | 0.2 | 0.33 | 0.8 |
| Adjuvant chemotherapy | Non-treated vs Treated | 13 vs 75 | 0.14 | 0.08 | 0.33 | 0.72 |
| MSI status | MS vs E/L | 34 vs 54 | N.A. | 0.046 (2.1, 1–6) | N.A. | 0.12 |
| 9p24.2 LOH status | 9p24r vs 9p24− | 45 vs 43 | 0.046 (2.1, 1–6) | N.A. | N.A. | 0.88 |
| E/L/9p24.2− | No vs Yes | 57 vs 31 | N.A. | N.A. | N.A. | 0.46 |
| KRAS/BRAF Mutation | Wild-type vs Mutated | 58 vs 30 | 0.12 | 0.88 | 0.46 | N.A. |
| DFS | ≤16 vs >16 | 60 vs 28 | 0.58 | 0.65 | 0.37 | 0.79 |
| SFR | ≤23 vs >23 | 53 vs 35 | 0.18 | 0.02 (2.9) | 0.003 (4.06) | 0.18 |
| OS | ≤39 vs >39 | 46 vs 42 | 0.23 | 0.09 | 0.01 (3.36) | 0.1 |
The average years of age in this cohort was 62, which was used as a cut-off criteria for dividing a chort into 2 groups according to the age.
The avarage months of DFS, SFR and OS was 16, 23 and 39 months respectively and was used as a cut-off criteria for dividing a cohort into 2 groups.
To determine the association between 2 variables, an univariate logistic regression analysis was used. Each number represents a P value.
When P <.05, the association is considered to be a significant (underlined bolded numbers). NA, Not applicable; OR, Odds Ratio.
As shown in Table 1, 54 of 88 cases (61%) and 43 of 88 cases (49%) exhibited E/L and 9p24-LOH respectively. Thirty of 88 cases (34%) had KRAS/BRAF mutations. 9p24-LOH is associated with non-liver-metastasis sites (P=0.02), E/L (p=0.046) and SFR (0.02) by univariate logistic regression analysis.
Because of the association between E/L and 9p24.2-LOH, this recurrence cohort was stratified by the E/L or MS group, and each group was analyzed for the association between 9p24.2-LOH and other variables including SFR, OS, and the sites of metastasis. As shown in Fig. 3A, patients with E/L showed improved SFR (P=0.005) and OS (P=0.006) compared to the patients with MS by Kaplan-Meier analysis. When E/L patients were grouped into the 9p24.2-LOH group (E/L/9p24.2-LOH) and the group with retention of 9p24 region (9p24r) (E/L/9p24r), the former showed improved SFR (P=0.0005) and OS (P=0.0005) compared to the latter (Fig. 3B). In contrast, there was no impact of 9p24.2 LOH status on SFR or OS of patients with MS (Fig. 3C). These results suggest that primary CRC with E/L/9p24.2-LOH has unique pathological characteristics compared to others. When SFR or OS was compared between E/L/9p24.2-LOH (31 cases) and the rest of CRC including E/L/9p24r (23 cases), MS/9p24.2-LOH (13 cases) and MS/9p24r CRC (21 cases), patients with E/L/9p24.2-LOH showed improved SFR (P<0.0001) and OS (P<0.0001) compared to the others (Fig. 3D). Furthermore, Cox proportional hazard analysis indicates that E/L/9p24.2-LOH may be an independent determinant for improved SFR (Fig. 3E, hazard ratio: 0.18, 95% CI: 0.08–0.4, P<0.0001) and OS (Fig. 3E, hazard ratio: 0.19, 95% CI: 0.09–0.42, P<0.0001) of stage II/III CRC patients that experienced recurrence. E/L/9p24.2-LOH is also associated with non-liver metastasis sites by univariate (P=0.001, Table 1) and multivariate logistic regression analysis (odds ratio: 0.06, 95%CI: 0.01–0.31, P=0.0007, Fig. 3E).
Fig. 3.
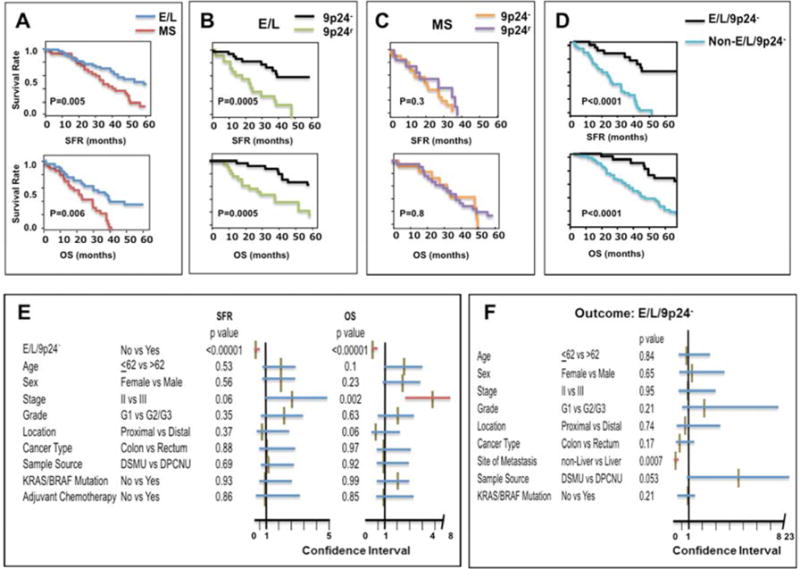
A–D: Kaplan-Meier curves for SFR (upper) and for OS (bottom) of patients with recurrent CRCs. SFR or OS was compared between patients with primary CRC exhibiting E/L (dark blue line, N=54) and MS (red line, N=34) (A), between E/L/9p24.2-LOH (black line, N=31) and E/L/9p24r (green line, N=23) (B), between MS/9p24.2-LOH (orange line, N=13) and MS/9p24r (purple line, N=21) (C) and between E/L/9p24.2-LOH (black line, N=31) and non-E/L/9p24.2-LOH (light blue line, N=57) (D). The X-axis represents SFR or OS in months. The Y-axis represents survival rates. P values were determined by log-rank test.
E: Cox proportional hazard test for SFR (left) and OS (right) of 88 patients with recurrent CRCs. The effect of E/L/9p24.2-LOH on SFR or OS was adjusted by the 9 variables indicated. The X-axis represents a range of the 95% Confidence Interval (CI). Each horizontal red (significant) or blue (non-significant) bar indicates the 95% CI range for a variable. The middle brown vertical bar represents a value for hazard risk. The P values were shown following to each variable.
F: Logistic regression test for association between E/L/9p24.2-LOH exhibited by primary CRC and each of 9 variables in 88 cases with recurrence. The X-axis represents a range of the 95% Confidence Interval (CI). Each horizontal bar indicates the 95% CI for a variable. A red or blue bar represents the significance or non-significance between each variable and E/L. respectively. The middle brown vertical bar represents a value for odd ratio. The P values were shown following to each variable.
Taken together, these results indicate that Stage II/III primary CRC exhibiting E/L is a heterogeneous population. E/L CRC with 9p24.2-LOH exhibits improved SFR and OS and has a tendency to metastasize to a variety of sites including liver (10 cases), lung (10 cases) and other tissues (11 cases) (Supplementary Table 4). On the other hand, E/L CRC with 9p24r exhibits negative SFR and OS, and more often metastasizes to the liver (19/24 cases) (Supplementary Table 4). The above results also suggest that combinations of the presence and/or absence of E/L and of 9p24.2-LOH are potential markers for prognosis of Stage II/III primary CRC following recurrence. When we analyzed Stage II (21 cases) and Stage III (67 cases) separately by Kaplan-Meier with log-rank test, a significant association was found between E/L/9p24.2-LOH and SFR (P<0.0001) or E/L/9p24.2-LOH and OS (P<0.0001) in Stage III cases but not in Stage II cases (SFR: P=0.13, OS: P=0.66) (Supplementary Fig. 5). The results suggest that E/L/9p24.2-LOH could be a marker for improved SFR or OS of recurrent Stage III. However, a number of Stage II cases with E/L/9p24.2-LOH and non- E/L/9p24.2-LOH in Stage II are too small (N=9 vs N=12) to draw any decisive conclusions about stage II cases. A further study is needed to confirm these observations using a larger cohort.
Enrichment of E/L/9p24.2-LOH in resected liver metastasis
In tumor set #3, 50 cases experienced liver metastasis (Supplementary Tables 5). Twenty percent (10 of 50) of such primary CRCs exhibited E/L/9p24.2-LOH; in tumor set #1, 59.4% of resected metachronous liver metastasis exhibited E/L/9p24.2-LOH (22/37, Supplementary Fig. 2A), suggesting that there is an enrichment of tumors exhibiting E/L/9p24.2-LOH in our liver metastasis cohort (Fisher’s exact test, P<0.0001, Fig. 4A). Considering that the liver metastases used in this study were selected for and treated by hepatectomy, our liver metastasis cohort must be biased as a cohort that exhibited less aggressive behavior amenable to hepatectomy compared to cohorts that could not be treated by hepatectomy (27). Interestingly, thirteen of the 50 liver metastases derived from primary CRC in Tumor Set #3 were treated with hepatectomy, and 61.5% of these primary CRCs (8/13) exhibited E/L/9p24.2-LOH, whereas only 5.4% (2/37) of primary CRCs that gave rise to liver metastasis not treated with hepatectomy exhibited E/L/9p24.2-LOH (P=0.0001, Fig. 4B). Thus, E/L/9p24.2-LOH primary CRC gave rise to the liver metastasis which are likely subjected to hepatectomy (Supplementary Table 4), supporting the idea that primary CRCs exhibiting E/L/9p24.2-LOH may give rise to less aggressive liver metastasis suitable for resection. In fact, E/L/9p24.2-LOH but not hepatectomy per se is an independent determinant for improved SFR (hazard ratio: 0.13, 95%CI: 0.03–0.91, P=0.04) and OS (hazard ratio: 0.12, 95%CI: 0.02–0.59, P=0.01) of 50 patients who developed recurrent liver metastases (Fig. 4C). Furthermore, primary CRC exhibiting E/L/9p24.2-LOH frequently recurs as liver metastases with a lack of simultaneous extra-hepatic metastasis and/or a small amount of metastasis within a liver (<3) (defied as less aggressiveness in this study), which is one of the major criteria for choosing hepatectomy for liver metastasis treatment (27). Less aggressiveness of recurrent liver metastasis is associated with E/L/9p24.2-LOH (odds ratio: 0.15, 95%CI: 0.03–0.8, P=0.03) but not with KRAS/BRAF mutations (odds ratio: 1.3, 95%CI: 0.4–4.6, P=0.67) exhibited by primary CRC from which these liver metastases were derived (Fig. 4D).
Fig. 4.
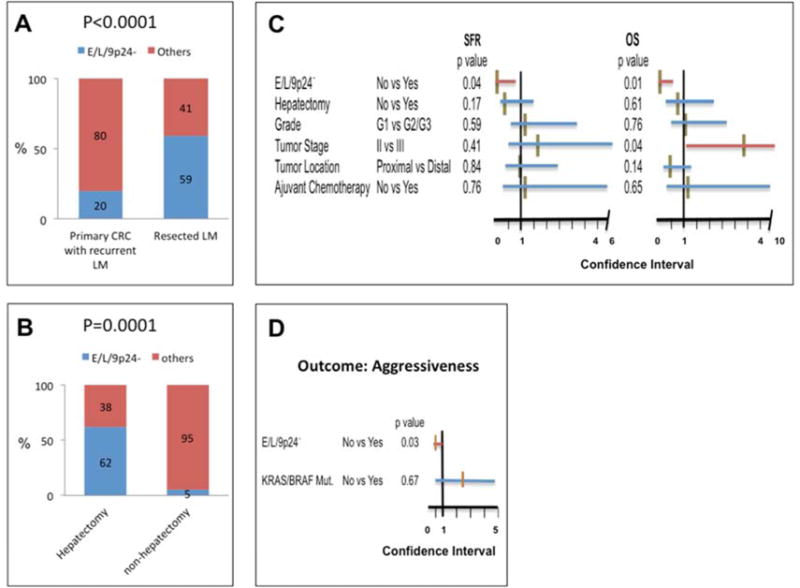
A: Significant enrichment of E/L/9p24.2-LOH tumors in resected metachronous liver metastases (22 of 37 cases) compared to primary CRC that gave rise to metachronous liver metastases (10 of 50 cases). P value was calculated by the Fisher’s exact test.
B: Significant association between E/L/9p24.2-LOH in stage II/III primary CRC that gave rise to liver metastasis and the resulting liver metastasis’ incidence of being treated by hepatectomy. P value was calculated by the Fisher’s exact test.
C: Cox proportional hazard test for SFR (left) and OS (right) of 50 patients with recurrent liver metastases. The effect of E/L/9p24.2-LOH on SFR or OS was adjusted by 5 variables including incidence of being treated by hepatectomy, primary tumor grade, stage and location and incidence of being treated by adjuvant chemotherapy. The X-axis represents the range of the 95% Confidence Interval (CI). Each horizontal red (significant) or blue (non-significant) bar indicates 95% CI. The middle brown vertical bar represents the value for the hazard risk. The P values were shown following to each variable.
D: Association between E/L/9p24.2-LOH or between KRAS/BRAF mutations in 50 stage II/III primary CRCs that gave rise to liver metastasis and aggressiveness of the resulting liver metastases was tested by logistic regression analysis. A positive for aggressiveness of liver metastasis was defined as when liver metastasis showed the presence of simultaneous extra-hepatic metastasis and/or more than 3 metastases within the liver (N=27). Liver metastasis showing a lack of simultaneous extra-hepatic metastasis and/or having fewer than 3 metastases within the liver (≤3) was considered negative for aggressiveness (N=23). The X-axis represents the range of the 95% confidence Interval (CI). The red (significant) or blue (non-significant) bar indicates range of 95% CI. The middle brown vertical bar represents a value for odd ratio. No: no mutation for both KRAS and BRAF. The P values were shown following each variable.
Significance of E/L and/or 9p24.2-LOH on recurrence-free and overall survival in stage II/III primary CRC
We next determined whether E/L, 9p24.2-LOH, E/L/9p24.2-LOH or KRAS/BRAF mutations has an impact on DFS and/or OS of the patients with stage II and/or III primary CRC. We used Tumor Set #4, consisting of 50 Stage II and 74 Stage III primary CRC – with (57 cases) and without (67 cases) – recurrence (Fig.1 and Table 2).
Table 2.
Characteristics of 124 Primary CRCs That Were Followed up to 5 Year After Diagnosis
| Association withb | (OR, 95% CI) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variablesa | Number of Cases | E/Lc | 9p24−d | E/L/9p24−e | KRAS/BRAF | |
| Age | ≤62 vs >62 | 57 vs 67 | 0.69 | 0.1 | 0.18 | 0.5 |
| Sex | Female vs Male | 49 vs 75 | 0.18 | 0.81 | 0.88 | 0.02 (0.41, 0.2–0.9) |
| Stage | II vs III | 50 vs 74 | 0.42 | 0.78 | 0.97 | 0.37 |
| Tumor grade | G1 vs G2&G3 | 53 vs 71 | 0.94 | 0.98 | 0.89 | 0.81 |
| Tumor sites | Proximal vs Distal | 18 vs 106 | 0.43 | 0.14 | 0.18 | 0.69 |
| Cancer type | Colon vs Rectum | 52 vs 72 | 0.93 | 0.59 | 0.28 | 0.13 |
| Adjuvant chemotherapy | Non-treated vs Treated | 19 vs 105 | 0.6 | 0.013 (6.89, 1.5–31.3) | 0.12 | 0.76 |
| MSI status | MS vs E/L | 59 vs 65 | N.A. | 0.02 (2.4, 1.1–5.0) | N.A. | 0.038 (0.45, 0.2–0.95) |
| 9p24.2 LOH status | 9p24r vs 9p24− | 75 vs 49 | 0.02 (2.4, 1.1–5.0) | N.A. | N.A. | 0.25 |
| E/L/9p24− | No vs Yes | 92 vs 32 | N.A. | N.A. | N.A. | 0.64 |
| KRAS/BRAF Mutations | Wild-type vs Mutated | 81 vs 43 | 0.038 (0.45, 0.2–0.95) | 0.25 | 0.64 | N.A. |
| Recurrence | No vs Yes | 67 vs 57 | 0.028 (2.25, 1.1–4.6) | 0.21 | 0.18 | 0.93 |
| Surval | Alive vs Dead | 86 vs 38 | 0.98 | 0.42 | 0.04 (0.33, 0.1–0.9) | 0.74 |
The average years of age in this cohort was 62, which was used as a cut-off criteria for dividing a chort into 2 groups according to the age.
To determine the association between 2 variables, an univariate logistic regression analysis was used. The each number represents a p value.
When p value is less than 0.05, the association is considered to be a significant (underlined bolted numbers). NA, Not applicable; OR, Odds Ratio; 95% CI, 95% Confidence Interval.
E/L is associated with 9p24−, Wild-type KRAS/BRAF, and recurrence.
9p24− is associated with E/L, and receiving adjuvant chemotherapy.
E/L/9p24− is associated with improved survival.
MutatedKRAS/BRAF is associated with female patients and MS.
Following sample weighting (see Materials and Methods), we first compared E/L and MS patients for OS by Kaplan-Meier analysis. There is no difference in OS between E/L and MS in Stage II/III, Stage III or Stage II CRCs (Fig. 5A). However, when the E/L were divided into 9p24.2-LOH and 9p24r groups, E/L/9p24.2-LOH is significantly associated with improved OS compared to E/L/9p24r in stage II/III (P=0.03) and stage III (P=0.01) but not in stage II (P=0.83) primary CRC (Fig. 5B). On the other hand, there is no significant difference in OS between MS/9p24.2-LOH and MS/9p24r in stage II and/or stage III CRCs (Fig. 5C). Thus, 9p24.2-LOH has a significant impact on OS of stage III E/L compared to stage II E/L and MS CRCs. To determine whether E/L/9p24.2-LOH is associated with improved OS in stage II/III primary CRC, OS of E/L/9p24.2-LOH and that of non-E/L/9p24.2-LOH patients was compared. As shown in Fig. 5D, E/L/9p24.2-LOH showed a significant improvement in OS compared to non-E/L/9p24.2-LOH in stage III primary CRC (P=0.03) but not in stage II plus III (P=0.1) or stage II CRC (P=0.64). We then determined the effect of E/L/9p24.2-LOH on OS of stage III CRCs by Cox proportional hazard analysis controlled by 7 other covariates. The results showed that E/L/9p24.2-LOH might be an independent determinant for improved OS of patients with stage III CRC (hazard ratio: 0.1, 95%CI: 0.01–0.64, P=0.01) (Fig. 5E).
Fig. 5.
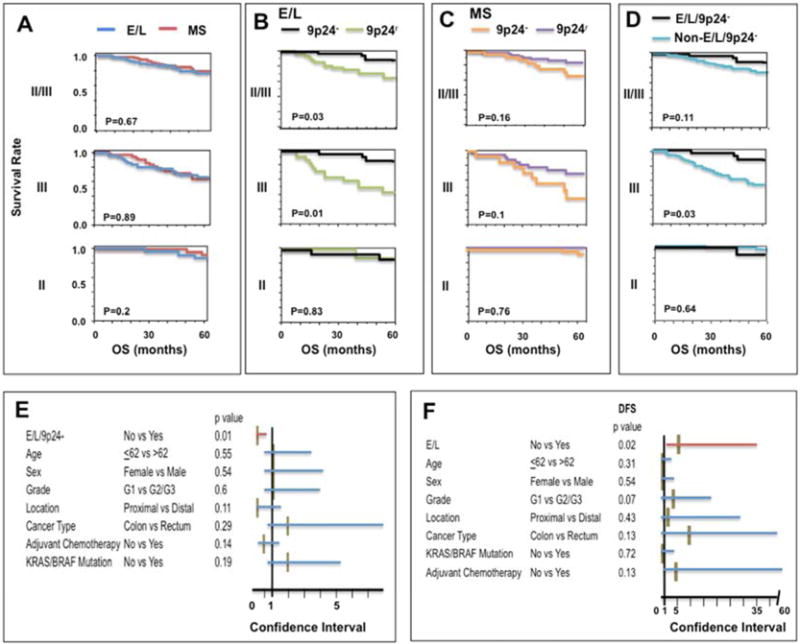
A–D: Kaplan-Meier curves for OS of patients with stage II plus III CRCs (II/III, upper panel, n=124), those of patients with stage III alone (III, middle panel, n=74) or patients with stage II alone (II, bottom panel, n=50). OS was compared between patients with E/L (dark blue line) and MS (red line) in stage II/III (E/L: N=65 vs MS: N=59), stage III (E/L: N=41 vs MS: N=33) and stage II (E/L: N=24 vs MS: N=26). (A), OS was compared between patients with E/L/9p24.2-LOH (black line) and E/L/9p24r (green line) in stage II/III (E/L/9p24.2-LOH: N=32 vs E/L/9p24r N=33) in stage III (E/L/9p24.2-LOH: N=19 vs E/L/9p24r N=22) and stage II (E/L/9p24.2-LOH: N=13 vs E/L/9p24r N=11) (B), OS was compared between MS/9p24.2-LOH (orange line) and MS/9p24r (purple line) in stage II/III (MS/9p24.2-LOH N=17 vs MS/9p24r: N=42), in stage III (MS/9p24.2-LOH N=11 vs MS/9p24r: N=22) and in stage II (MS/9p24.2-LOH N=6 vs MS/9p24r: N=20) (C), OS was compared between E/L/9p24.2-LOH (black line) and non-E/L/9p24.2-LOH (light blue line) in stage II/III (E/L/9p24.2-LOH: N=32 vs non-E/L/9p24.2-LOH: N=92) in stage III (E/L/9p24.2-LOH: N=19 vs non-E/L/9p24.2-LOH: N=55) and in stage II (E/L/9p24.2-LOH: N=13 vs non-E/L/9p24.2-LOH: N=37) (D). The X-axis represents OS in months. The Y-axis represents survival rates. P values were determined by log-rank test.
E: Cox proportional hazard test for OS of 74 patients with stage III primary CRCs. The effect of E/L/9p24.2-LOH on OS was adjusted by the 7 variables indicated. The X-axis represents the range of the 95% Confidence Interval (CI). Each horizontal red (significant) or blue (non-significant) bar indicates 95% CI. The middle brown vertical bar represents the value for hazard risk. The P values were shown following to each variable.
F: Cox proportional hazard test for DFS of 50 patients with stage II primary CRC, the effect of E/L on DFS was adjusted by the 7 variables indicated. The X-axis represents the range of the 95% Confidence Interval (CI). Each horizontal red (significant) or blue (non-significant) bar indicates 95% CI. The middle brown vertical bar represents the value for hazard risk. The P values were shown following to each variable.
As previously described (21), E/L is associated with reduced DFS compared to MS in stage II/III CRC by Kaplan-Meier analysis (P=0.03, Supplementary Fig. 6). Further analysis showed that E/L in Stage II (P=0.02) but not stage III (P=0.3) is associated with reduced DFS (Supplementary Fig. 6). Cox proportional hazard analysis showed that E/L might be an independent determinant for reduced DFS in stage II CRC (hazard ratio: 7.01, 95%CI: 1.3–37, P=0.02, Fig. 5F). Neither KRAS/BRAF mutations nor 9p24.2-LOH has significant impact on OS or DFS of patients with stage II/III primary CRC (Table 2).
Discussion
Recent studies suggest that E/L in non-MSI-H CRC may be caused by loss of MSH3 in a cancer cell due to the effects of tumor micro-environmental factors, such as hypoxia and/or inflammation, rather than genetic causes (17, 21, 28, 29, 30). Previously, we demonstrated that E/L in Stage II/III primary CRC is associated with a high risk for recurrent distant metastasis (21). To further understand the significance of E/L for recurrence and/or metastasis at a molecular level in CRC, here we sought loci containing microsatellite repeats exhibiting FS mutations and/or allelic loss associated with E/L. We assumed that some of the microsatellite loci might be mutated through hypoxia- or inflammation-induced MSH3-deficiencies and play a role in recurrence and/or metastasis. We therefore selected and screened 141 microsatellite loci for FS or LOH that are associated with E/L in liver metastases.
Among the loci screened, LOH at SMARCA2 on 9p24 was significantly associated with E/L. However, our data for the SOR, IHC and deep sequencing of the SMARCA2 suggested that a novel locus near SMARCA2 is likely a target of the LOH observed on 9p24.2 and that allelic loss of this putative locus might contribute to metastasis from primary CRC. An intense investigation is underway to identify candidate transcribed gene(s) involved in 9p24.2-LOH events and in metastasis from primary CRC.
One of the major findings of this study is that E/L/9p24.2-LOH identified in the resected liver metastases from primary CRC is also detected in matched primary CRC (Fig. 2D) and in both recurrent and non-recurrent stage II/III primary CRCs (Tables 1 and 2). Furthermore, E/L/9p24.2-LOH is associated with an improved OS of the patients with stage III but not stage II CRCs (Figs. 5C and 5E). A significant association of E/L/9p24.2-LOH with improved SFR and OS was also detected in stage II plus III and stage III CRC that later gave rise to distant metastasis (Fig. 4E and Supplementary Fig. 5). These results strongly support the idea that E/L/9p24.2-LOH is a specific marker for less aggressive type of stage III CRC. The results also suggest that E/L/9p24.2-LOH may be involved in the metastatic process after dissemination from the primary sites.
What could be the mechanism by which patients with E/L/9p24.2-LOH stage III primary CRC have improved OS? One possibility is that the spread of E/L/9p24.2-LOH tumors is limited by immunological and/or inflammatory factors. In fact, EMAST-positive CRC has been associated with infiltration by CD8+ T cells (19, 31). Other studies have reported the presence of a subgroup of non-MSI-H CRC cases with infiltrating T cells expressing CD8+, CD45R01 or FOXP3, which show a better prognosis (32, 33, 34). Another possibility is that E/L/9p24.2-LOH stage III primary CRC may be moderately sensitive to 5-FU based chemotherapy compared to non- E/L/9p24.2-LOH stage III primary CRC. We are currently exploring these possibilities.
In contrast to E/L/9p24.2-LOH primary CRC, E/L without 9p24.2-LOH (termed E/L/9p24r in this study) in stage III CRC exhibits more aggressive behavior compared to the E/L/9p24.2-LOH counterparts both in the recurrent cohort (Tumor Set# 3, hazard ratio: 0.14, 95%CI: 0.04–0.4, P=0.0008) and in the mixed cohort (Tumor Set# 4, hazard ratio: 0.1, 95%CI: 0.01–0.5, P=0.006). When E/L/9p24.2-LOH or E/L/9p24r CRC metastasizes, the former tends to spreads not only to the liver but also to other sites (Fig. 3) whereas the latter tends to spread to the liver (P=0.02, Fisher’s exact test). These results suggest that there may be a genetic or epigenetic change other than 9p24.2 LOH that is associated with E/L and may promote metastasis to the liver. A recent study by Enquist et al suggested that metastasis to the liver from primary CRC is mediated through hematogenous but not lymphangiogenous spread (35). Therefore, it could be that the presence or absence of 9p24.2 LOH in the E/L background in stage III CRC may also determines the route of dissemination.
In this study, we also found that E/L is an independent determinant for reduced DFS in stage II but not in stage III CRC (Supplementary Fig. 6 and Fig. 5F). These results suggest that the unknown event (not 9p24.2-LOH) associated with E/L in stage II primary CRC may facilitate an invasion of CRC cells from the primary sites to the nearby lymph nodes.
From the clinical point of view, our findings raise the following questions. First, although our previous study showed that there is no difference in benefit of the 5-FU-based adjuvant chemotherapy between the patients with E/L and MS in stage II/III primary CRC (36), it is important to determine whether E/L/9p24.2-LOH or E/L/9p24r in stage III CRCs responds differently to this treatment. E/L alone in stage II CRCs may be associated with invasion from primary sites to lymph node, and these E/L-positive patients should be considered for adjuvant chemotherapy. Second, although prognostic value of KRAS/BRAF mutations in CRC is controversial (11, 37, 38), we did not find any significant prognostic effects of KRAS/BRAF mutations on patients’ outcomes in this study. However, a fraction of E/L/9p24.2-LOH-positive CRCs had KRAS/BRAF mutations (Supplementary Table 7). Considering that KRAS/BRAF mutations are resistance markers for anti-EGFR treatment, it is undetermined whether other target-specific therapies such as anti-VEGF affect the prognosis of E/L/9p24.2-LOH CRCs with KRAS/BRAF mutations. Third, the mean age of our CRC cohorts from Korea and Japan is 62 years old, younger than that of the typical American CRC population (~68 years old). The reason for this difference is not clear. CRC cohorts from some Asian countries tend to be younger than American cohorts, and in our non-population based cohort, the number of MSI-H cancer patients is very low, removing a demographic that is typically older for sporadic CRC. A recent population-based cohort of CRCs demonstrated that MSI-H patients were 2–3 years older in mean age over MS patients (39). Thus, a study using a different race as well as a larger cohort and/or randomized population is necessary to evaluate the predictive and/or prognostic values of E/L in stage II and E/L/9p24.2-LOH in stage III CRCs.
In conclusion, our results indicate that stage II primary CRCs exhibiting elevated microsatellite alterations such as EMAST/MSI-L are prone to recur but aggressiveness of the EMAST/MSI-L-positive Stage III CRC depends upon the presence or absence of 9p24.2 LOH, suggesting that EMAST/MSI-L and 9p24.2 LOH could be useful as a potential prognostic markers for stage II and/or III CRC.
Supplementary Material
Acknowledgments
The bio-specimens and their associated data used for this study were provided by the Biobank of Chonnam National University Hwasun Hospital, a member of the Korea Biobank Network.
Grant Support: This work was financed by a pilot grant from Sammons Cancer Center, Baylor University Medical Center (M.K.), NIH grants R01 CA72851 (C.R.B.), Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (24591985) (H.H), and RO1 DK067287, UO1 CA162147 and A. Alfred Taubman Institute of the University of Michigan (J.M.C.).
Abbreviations
- CRC
colorectal cancer
- DFS
disease-free survival
- EMAST
elevated microsatellite alterations at selected tetranucleotide repeats
- E/L
EMAST /MSI-L
- FS
frame shift
- MSI-H
high levels of microsatellite instability
- LOH
loss of heterozygosity
- MSI-L
low levels of microsatellite instability
- MSI
microsatellite instability
- MS
microsatellite stable
- OS
overall survival
- ROH
retention of heterozygosity
- SFR
survival following recurrence
- SOR
smallest overlapping region
- 9p24.2- or 9p24.2-LOH
chromosome 9p24.2 loss of heterozygosity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: study concept and design (M.K.), acquisition of data (M.G., M.K., H.I.); analysis and interpretation of data (M.K., Y.O.); drafting of the manuscript (M.K., J.M.C. and C.R.B.); critical revision of the manuscript for important intellectual content (M.K., J.M.C. and C.R.B.); statistical analysis (Y.O., Y.T., M.K., Y-H.C., B.M.); obtained funding (C.R.B., M.K., and J.M.C.); material support (C.C., H-R.K., J.K., H.H., T.N., T.K., M.K.); study supervision (M.K.).
Disclosures: The authors disclose no conflicts of interest.
References
- 1.Siegel R, DeSantis C, Jemal A. Colorectal Cancer Statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.O’Dwyer PJ, Eckhardt SG, Haller DG, et al. Priorities in colorectal cancer research: recommendations from the gastrointestinal scientific leadership council of the coalition of cancer cooperative groups. J Clin Oncol. 2007;25:2313–2321. doi: 10.1200/JCO.2006.08.6900. [DOI] [PubMed] [Google Scholar]
- 3.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 4.Srivastava G, Renfro LA, Behrens RJ, et al. Prospective multicenter study of the impact of oncotype DX colon cancer assay results on treatment recommendation in stage II colon cancer patients. Oncologist. 2014;19:492–497. doi: 10.1634/theoncologist.2013-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Network TCGA. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vilar E, Tabernero J. Molecular dissection of microsatellite instable colorectal cancer. Cancer Discov. 2013;3:502–511. doi: 10.1158/2159-8290.CD-12-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budinska E, Popovici V, Tejpar S, et al. Gene expression patterns unveil a new level of molecular heterogeneity in colorectal cancer. J Pathol. 2013;231:63–76. doi: 10.1002/path.4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marisa L, de Reynies A, Duval A, et al. Gene expression classification of colon cancer into molecular subtypes: characterization, validation, and prognostic value. PLoS Med. 2013;10:e1001453. doi: 10.1371/journal.pmed.1001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phipps AI, Limburg PJ, Baron JA, et al. Association between molecular subtypes of colorectal cancer and patient survival. Gastroenterology. 2015;148:77–87. doi: 10.1053/j.gastro.2014.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinicrope FA, Shi Q, Smyrk TC, et al. Molecular markers identify prognostically distinct subtypes of stage III colon cancer patients. Gastroenterology. 2015;148:88–99. doi: 10.1053/j.gastro.2014.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fearon ER, Carethers JM. Molecular subtyping of colorectal cancer: Time to explore both intertumoral and intratumoral heterogeneity to evaluate patient outcome. Gastroenterology. 2015;148:10–13. doi: 10.1053/j.gastro.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carethers JM, Jung BH. Genetics and genetic biomarker in sporadic colorectal cancer. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umar A, Kunkel TA. DNA-replication fidelity, mismatch repair and genome instability in cancer cells. Eur J Biochem. 1996;238:297–307. doi: 10.1111/j.1432-1033.1996.0297z.x. [DOI] [PubMed] [Google Scholar]
- 14.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 15.Halford S, Sasieni P, Rowan A, et al. Low-level microsatellite instability occurs in most colorectal cancers and is a nonrandomly distributed quantitative trait. Cancer Res. 2002;62:53–57. [PubMed] [Google Scholar]
- 16.Bacolla A, Larson JE, Collins JR, Li J, Milosavljevic A, Stenson PD, et al. Abundance and length of simple repeats in vertebrate genomes are determined by their structural properties. Genome Res. 2008;18:1545–1553. doi: 10.1101/gr.078303.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haugen A, Goel A, Yamada K, et al. Genetic instability caused by loss of MutS homologue 3 in human colorectal cancer. Cancer Res. 2008;68:8465–8472. doi: 10.1158/0008-5472.CAN-08-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada K, Kanazawa S, Koike J, et al. Microsatellite instability at tetranucleotide repeats in sporadic colorectal cancer in Japan. Oncol Rep. 2010;23:551–561. [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SY, Chung H, Devaraj B, et al. Microsatellite alterations at selected tetranucleotide repeats are associated with morphologies of colorectal neoplasias. Gastroenterology. 2010;139:1519–1525. doi: 10.1053/j.gastro.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson MMC, Berg M, Soreide K. Prevalence and implication of elevated microsatellite alterations at selected tetranucleotides in cancer. Br J Cancer. 2014;111:823–827. doi: 10.1038/bjc.2014.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia M, Choi C, Kim H-R, et al. Moderate Levels of Microsatellite Instability are associated with Recurrent Distant Metastasis in Stage II and III Primary Colorectal Cancers. Gastroenterology. 2012;143:48–50. doi: 10.1053/j.gastro.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carethers JM, Koi M, Tseng-Rogenski SS. EMAST is a form of microsatellite instability that is initiated by inflammation and modulates colorectal cancer progression. Genes. 2015;31:185–205. doi: 10.3390/genes6020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campregher C, Schmid G, Ferk F, et al. MSH3-deficiency initiates EMAST without oncogenic transformation of human colon epitherial cells. PLoS One. 2012;7:e50541. doi: 10.1371/journal.pone.0050541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laghi L, Bianchi P, Delconte G, et al. MSH3 protein expression and nodal status in MLH1-dificient colorectal cancers. Clin Cancer Res. 2012;18:3142–3153. doi: 10.1158/1078-0432.CCR-12-0175. [DOI] [PubMed] [Google Scholar]
- 25.Van Oers JM, Edwards Y, Chahwan R, et al. The MutS β complex is a modulator of p53-driven tumorigenesis through its functions in both DNA double-strand break repair and mismatch repair. Oncogene. 2013;33:3939–3946. doi: 10.1038/onc.2013.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toiyama Y, Tanaka K, Kitajima T, et al. Elevated serum angiopoietin-like protein 2 correlates with the metastatic properties of colorectal cancer: A serum biomarker for early diagnosis and recurrence. Clin Cancer Res. 2014;20:6175–6186. doi: 10.1158/1078-0432.CCR-14-0007. [DOI] [PubMed] [Google Scholar]
- 27.Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. Oncologist. 2008;13:51–64. doi: 10.1634/theoncologist.2007-0142. [DOI] [PubMed] [Google Scholar]
- 28.Tseng-Rogenski SS, Chung H, Wilk MB, et al. Oxidative stress induces nuclear-to-cytosol shift of hMSH3, a potential mechanism for EMAST in colorectal cancer cells. PLoS One. 2012;7:e50616. doi: 10.1371/journal.pone.0050616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Arita M, Koike J, et al. Downregulation of MutS homolog 3 under hypoxic condition in human colorectal cancer cells. BBA-Mol Cell Res. 2012;1823:889–899. doi: 10.1016/j.bbamcr.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tseng-Rogenski SS, Hamaya Y, Choi DY, et al. Interleukin 6 Alters localization of MSH3, leading to DNA mismatch repair defects in colorectal cancer cells. Gastroenterology. 2015;148:579–589. doi: 10.1053/j.gastro.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SY, Miyai K, Han HS, et al. Microsatellite instability, EMAST, and morphology associations with T cell infiltration in colorectal neoplasia. Dig Dis Sci. 2012;57:72–78. doi: 10.1007/s10620-011-1825-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nosho K, Baba Y, Tanaka N, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010;222:350–366. doi: 10.1002/path.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoon HH, Orrock JM, Foster NR, et al. Prognostic impact of FoxP3+ regulatory T cells in relation to CD8+ T lymphocyte density in human colon carcinomas. PLoS One. 2012;7:e42274. doi: 10.1371/journal.pone.0042274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mei Z, Liu Y, Liu C, et al. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer. 2014;110:1595–1605. doi: 10.1038/bjc.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enquist IB, Good Z, Jubb AM, et al. Lymph node-independent liver metastasis in a model of metastatic colorectal cancer. Nat Commun. 2014;5:3530. doi: 10.1038/ncomms4530. [DOI] [PubMed] [Google Scholar]
- 36.Hamaya Y, Guarinos C, Tseng-Rogenski SS, et al. Efficacy of adjuvant 5-fluorouracil therapy for patients with EMAST-positive stage II/III colorectal cancer. PloS One. 2015;10:e0127591. doi: 10.1371/journal.pone.0127591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roth AD, Tejpar S, Delorenzl M, et al. Prognostic role of KRAS and BRAF in Stage II and III resected colon cancer: Results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466–474. doi: 10.1200/JCO.2009.23.3452. [DOI] [PubMed] [Google Scholar]
- 38.Richman SD, Seymour MT, Chambers P, et al. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but not preclude benefit from oxaliplatin or irinotecan: Results from MRC FOCUS Trial. J Clin Oncol. 2009;27:5931–5937. doi: 10.1200/JCO.2009.22.4295. [DOI] [PubMed] [Google Scholar]
- 39.Carethers JM, Murali B, Yang B, et al. Influence of race on microsatellite instability and CD8+ T cell infiltration in colon cancer. PloS One. 2014;9:e100461. doi: 10.1371/journal.pone.0100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


