Abstract
Fragile X Syndrome (FXS) is a neurodevelopmental disorder caused by a CGG expansion in the FMR1 gene located at Xq27.3. Patients with the premutation in FMR1 present specific clinical problems associated with the number of CGG repeats (55–200 CGG repeats). Premutation carriers have elevated FMR1 mRNA expression levels, which have been associated with neurotoxicity potentially causing neurodevelopmental problems or neurological problems associated with aging. However, cognitive impairments or neurological problems may also be related to increased vulnerability of premutation carriers to neurotoxicants, including phenobarbital. Here we present a study of three sisters with the premutation who were exposed differentially to phenobarbital therapy throughout their lives, allowing us to compare the neurological effects of this drug in these patients.
Keywords: Premutation, Fragile X, FMR1, Neurotoxicity, Phenobarbital, Pesticides
1. Introduction
Fragile X Syndrome (FXS) is a neurodevelopmental disorder caused by a CGG expansion in the FMR1 gene at q27.3 on the X-chromosome. FXS affects 1 in 4000 males and 1 in 8000 females (Crawford et al., 2001), and is the most common inherited form of intellectual disability and autism spectrum disorder (ASD) (Hagerman, 2008). The full mutation (CGG repeats of >200) leads to a decrease or absence of the FMR1 protein (FMRP) (Loesch et al., 2004). Individual carriers of the fragile X premutation (CGG repeats between 55 and 200) are at high risk for developing a neurodegenerative disorder called Fragile X-associated tremor/ataxia syndrome (FXTAS) (Garcia-Arocena and Hagerman, 2010). Additionally, carriers can develop neuropathy, fertility problems, executive function deficits, attention deficits, memory problems, language dysfluencies, affective disorders, anxiety, attention deficit/hyperactivity disorder (ADHD) and sleep problems (Sullivan et al., 2011; Seltzer et al., 2012; Losh et al., 2012; Hagerman and Hagerman, 2013). Approximately 20% of the female carriers develop fragile-X-associated primary ovarian insufficiency (FXPOI) (Sullivan et al., 2011). Approximately 1 in 130–259 females and 1 in 468–813 males in the general population have a premutation in the FMR1 gene (Seltzer et al., 2012).
Clinical problems associated with the FMR1 premutation are thought to be caused by neurotoxic effects of elevated cellular FMR1 mRNA levels observed in carriers (Hagerman and Hagerman, 2013). While there is a direct correlation between the CGG repeat length and the level of mRNA, there is considerable clinical heterogeneity amongst FMR1 premutation carriers. The reasons why some carriers are affected while others are not remain unknown (Hagerman and Hagerman, 2013), but suggest the possibility that environmental factors may interact with genetic susceptibility factors to influence clinical outcome in FMR1 premutation carriers. In support of this hypothesis, several case reports have suggested that the onset, progression and severity of neurological deterioration in FXTAS may be exacerbated by exposure to environmental neurotoxicants released from chemical plants near the patient’s home (Paul et al., 2010), chemotherapy for breast cancer with the chemotherapeutic agent carboplatin (O’Dwyer et al., 2005) and a history of substance abuse (opiates, alcohol, cocaine and methamphetamine) (Muzar et al., 2014; Muzar et al., 2015; Martínez-Cerdeño et al., 2015)
Anti-epileptic drugs (AEDs) may constitute another environmental modifier of FXTAS outcome or neurodevelopmental problems associated with the premutation. Seizures occur in approximately 8–13% of FMR1 premutation carriers, and in boys with the premutation, seizures are associated with autism spectrum disorder (ASD) (Chonchaiya et al., 2012). Cognitive impairment without FXTAS is unusual in premutation carriers, but seizures can exacerbate cognitive deficits in young carriers (Chonchaiya et al., 2012). In general, seizures in early life are known to dysregulate FMRP even in those without an FMR1 mutation, because FMRP moves away from the dendrites and into the perinuclear region with early life seizures (Bernard et al., 2014). However, cognitive impairment has also been associated with the use of anti-epileptic drugs, particularly the first generation AEDs, which include phenobarbital, phenytoin, carbamazepine, valproate and the benzodiazepines (Mula and Trimble, 2009). Randomized double-blind crossover studies in healthy volunteers found that of these first generation AEDs, phenobarbital produced the greatest cognitive impairment (Mula and Trimble, 2009; Park and Kwon, 2008).
The goal of this paper is to present three sisters who are FMR1 premutation carriers with cognitive dysfunction, various neurological problems and disparate history of phenobarbital use.
2. Materials and methods
2.1. Clinical data
Clinical histories are reported for three female patients who are first degree relatives (sisters) in a family with the FMR1 premutation who are from a small town in Colombia. Clinical data include in-depth clinical history and physical examination, as well as brain anatomy as assessed by 3 T magnetic resonance imaging with intravenous gadolinium injection.
2.2. Molecular analyses
To determine CCG repeat size in the FMR1 gene of each patient, genomic DNA was isolated from whole blood using standard methods, and analyzed by PCR and Southern blotting as previously described (Tassone et al., 2008; Filipovic-Sadic et al., 2010). The activation ratio (AR) was measured using ratios of the computer-quantified signal intensity of chosen Southern blot bands as previously described (Tassone et al., 1999). To assess copy number variants, a genome-wide assessment of 2,391,739 SNPs was conducted using the Illumina Omni 2.5 M BeadChip in concordance with manufacturer specifications (Illumina, San Diego, CA). Copy Number Variants (CNVs) were identified using PennCNV (Wang et al., PMID: 17921354), which uses a hidden Markov model (HMM) based algorithm to identify CNVs, taking into account distance between adjacent SNPs, signal intensity, allelic ratio, and allele frequency of each SNP. To search for CNVs that may have been missed and to validate calls made by PennCNV, we manually assessed plots of log R ratio (LRR) and B allele frequency (BAF) within each chromosomal band for each sample using custom UCSC genome browser. To ensure quality, only samples with a standard deviation of LRR <0.30 and correlation of LRR to the wave model was >−0.2 and <0.4. The frequency of each CNV call with a reciprocal overlap >0.5 within a control dataset (Cooper et al., PMID: 21841781) and the database of genomic variants (DGV) was calculated. CNV calls obtained from PennCNV using a series of filters to remove copy-number polymorphisms and variants observed commonly in typical developing controls were analyzed. We assessed the frequency of each of the CNVs in the Database of Genomic Variants and from a published set of controls (n = 8,329) (Cooper et al., Nature Genetics, 2011, PMID: 21841781).
3. Patients’ clinical history
3.1. Case 1—JT
This patient is a 53 year old, Hispanic woman carrier of the FMR1 premutation. She had a normal vaginal birth, was breast fed for 2 years and exhibited normal developmental milestones with sitting at 6 months of age, crawling at 8 months and walking at 12 months. She was hyperactive in childhood with occasional physical aggression and social disinhibition but she was sociable without abnormal behavior. She is a G1P1A0 patient with late onset menarche at 16 years of age and early onset of menopause at 42 years old. Her father was a carrier of the premutation, her other 2 sisters have the premutation and her only child has FXS (Fig. 1).
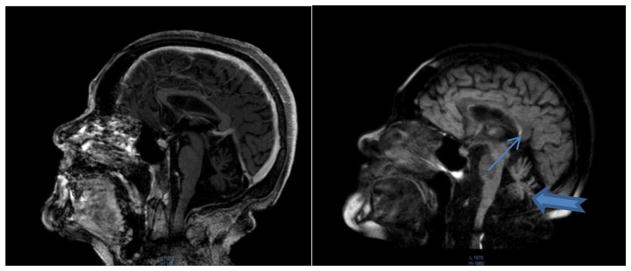
Fig. 1.
MRI of Case 1 at 53 years of age.
Note cerebellar atrophy (thick arrow) and hyperintensity of the splenium or posterior aspect of the corpus callosum (thin arrow).
She developed generalized seizures at the age of 6, which were managed with phenytoin (2 capsules of 100 mg daily) until 43 years of age when it was suspended due to therapeutic failure (1 seizure per month). Her seizures lead to an accident in which she fractured her hip. Because of socioeconomic difficulties, the fracture was not corrected surgically as needed, and she is unable to walk independently and uses a wheel chair for mobility. Since the age 43 years, she has been treated with phenobarbital (2 capsules of 100 mg daily), which improved seizure control as evidenced by reduction in the number of seizures to 1 per year. However, since starting phenobarbital, the patient experienced progressive deterioration of motor coordination and speech, lost the ability to write and speak fluently or to perform mental processes, such as counting to 10, which she could do before the pharmacological shift. The patient lost her ability to manage money and to socialize. She now presents with a cerebellar syndrome, severe dysarthria, swallowing difficulty, loss of writing and fine motor skills required for simple tasks such as taking a spoon to her mouth for eating.
On examination at age 53, her height was 1.67 m; weight, 52 kg; BMI, 18.7; head circumference, 53.5 cm. She has a long face, bilateral temporal muscle atrophy, long non-prominent ears (7 × 4 cm) and mandibular prognathism with grade III dental malocclusion. Her left wrist and fingers 3, 4 and 5 of the same hand have fixed flexion deformities with fixed adduction of finger 5. The interphalangeal articulations of the right hand are also deformed: fingers 4 and 5 are deviated towards the middle axis with hyperextension of finger 5. Scoliosis is evident. Her hips have a flexion deformity on the right secondary to her unrepaired fracture. Her cardiovascular examination was normal.
A recent 3 T cerebral MRI with Gadolinium contrast was done at age 53, which showed a significant reduction of the hippocampal volume on the right side associated with alterations in the signal intensity, a finding suggestive of mesial sclerosis. The cerebellar volume loss was significant but there was no loss of temporoparietal volume.
3.2. Case 2—MET
This patient, who is a sister of case 1, is a 58 year old carrier of the premutation who had a normal vaginal birth and was breast fed for 2 years. She exhibited normal developmental milestones with sitting at 6 months, crawling at 8 months and walking at 12 months. As a child she was shy, anxious, solitary and had poor eye contact. She never connected socially with others and did not marry. She had menarche at age 15 and menopause early at age 40. She suffered from recurrent panic attacks and was very irritable but not aggressive nor hyperactive.
She developed generalized seizures at the age of 9, which were managed with phenytoin (100 mg capsules 3 times a day) until she was 48 years old, but did not provide adequate control because she had 1 to 3 seizures/week. Phenytoin was changed to phenobarbital (100 mg capsules 3 times a day) at the age of 48 years, which reduced her seizures to one per month. Nevertheless, despite better control of the seizures, the patient became more socially isolated and lost her ability to speak and move; becoming permanently asthenic and adynamic. Because of concern for phenobarbital toxicity, she was switched to valproic acid as an anticonvulsant at age 57. The patient continued with seizures of approximately 15 to 20 min at a frequency of 2 per month. The patient lost sphincter control, became aphagic and adynamic, lost significant weight and finally passed away in the hospital approximately 8 months after beginning the valproic acid treatment.
The patient did not consume alcohol, drugs nor psychotoxic substances. No other pathologies were reported. There is no history of surgeries or allergies, only one hospitalization in the last year of her life after three episodes of generalized seizures of more than 15 mineach.
On examination at age 57, she was 1.58 m tall and weighed 54 kg, with a BMI of 21 and head circumference of 54.5 cm. She had a long face, bilateral temporal muscle atrophy, cachectic appearance, long prominent ears (7.5 × 4 cm) and mandibular prognathism with grade III dental malocclusion. Her cardiovascular and respiratory examination was normal. There were no abdominal abnormalities. The patient was usually nonresponsive to verbal stimuli, but did follow a few orders. She was not oriented in time or space, showing clear neurological compromise.
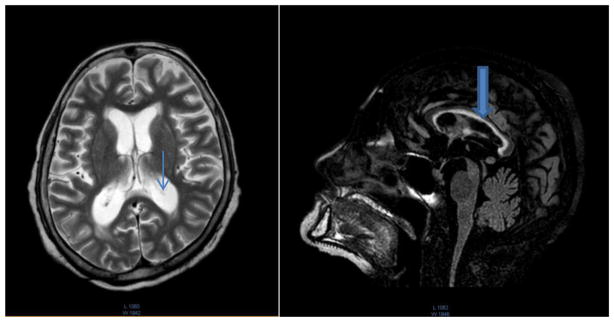
A brain MRI with contrast was done at age of 58 years; the results show a severe bilateral loss of hippocampal volume, with alterations in the signal intensity of the hippocampus and its internal architecture. These findings are suggestive of a bilateral mesial temporal sclerosis. There is a global loss of brain volume and ventricular dilation, in addition to loss of volume of the corpus callosum. Changes were also seen in the subependymal white matter signal (Fig. 2).
Fig. 2.
MRI of case 2 at 58 years of age.
Note global brain atrophy and dilated ventricles (thin arrow) with the thin corpus callosum (thick arrow).
3.3. Case 3—RT
This sister of cases 2 and 3 is a 57 year old Hispanic woman with FMR1 premutation who had a normal vaginal birth, was breast fed for 2 years, but had developmental delays with walking at 3 years old and mutism since birth.
She never used alcohol or psychotropic medication, nor has she had chronic pathology, surgery, hospitalizations or allergy. She had a late onset of menarche at age 16 with menopause at age 48 and was never sexually active. She has no history of seizures or medication with antiepileptic drugs
On examination at age 57, her height is 1.53 m; weight, 44.5 kg, BMI, 19; head circumference, 51.5 cm. She has a long narrow face, normal length ears (6 cm) and mandibular prognathism with grade III dental malocclusion. She is nonverbal. No previous MRI or CT-Scans are available for comparison. A recent 3 T cerebral MRI with Gadolinium contrast reported normal results.
3.4. Molecular measures
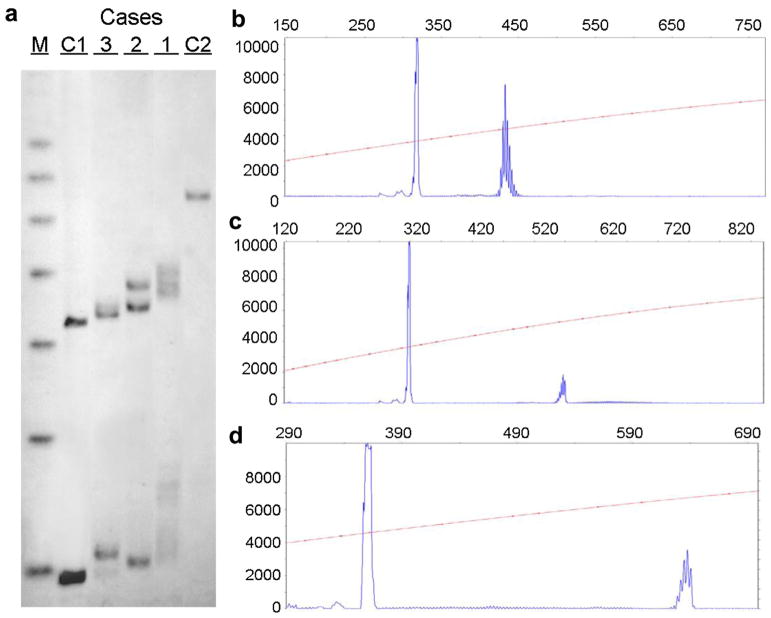
DNA analysis showed the presence of a premutation allele in all three cases. The CGG repeat number was 45, 135 in case 1; 30, 110 CGG repeats in case 2; and 30, 69 in case 3 (Fig. 3). The activation ratio, which expresses the fraction of cells carrying the normal allele on the active X-chromosome was 0.5 in case 1, 0.4 in case 2 and 0.2 in case 3.
Fig. 3. Genetic analyses of Cases 1–3.
The CGG allele size and the methylation status as determined by Southern blotting (SB) and capillary electrophoresis (CE) are illustrated in the three sisters. The SB analysis on the left demonstrates the presence of a premutation allele in all three cases, which were sized by PCR as shown in b–d. (CGG = 30,69; 30,110 and 45,135 in Case 3, 2 and 1, respectively). M = 1 KB DNA size ladder marker; C1 = normal female, negative control and C2 = full mutation male, positive control. The X-axis indicates the size of the alleles in base pairs while the Y-axis indicates the fluorescence intensity of each allele.
To determine whether additional genomic changes were present in any of the three sisters that might explain their severe phenotype, a genome-wide assessment of 2,391,739 SNPs was conducted. We found no large (>50 kbp) known disease-associated CNVs or potentially pathogenic rare CNVs in these three probands.
4. Discussion
In this study we report on three sisters all of whom have the FMR1 premutation and present with developmental problems. FMR1 premutation is known to cause neurodegeneration with aging in a subgroup of carriers, most commonly males beginning in their 60s (Hagerman and Hagerman, 2013; Berry-Kravis et al., 2007). However, developmental problems can also occur in those with the premutation, the most common including ADHD, ASD and social anxiety (Chonchaiya et al., 2012; Farzin et al., 2006; Cordeiro et al., 2015).
Case 1 had FMR1 allele in the intermediate range (45 CGG repeats) and one in the high premutation range (135 CGG repeats) with an activation ratio (AR) of 0.5. Case 2 also had a relatively high CGG repeat number (110 CGG repeats) and an unfavorable AR of 0.4, which likely led to toxicity due to the increased expression levels of FMR1 mRNA observed in premutation females (Tassone et al., 2000). Although case 3 had a premutation allele of 69 CGG repeats, her AR was 0.2, thus, most of her premutation allele is on the active X-chromosome. Case 3 did not have exposure to phenobarbital but she had developmental problems including mutism associated with developmental delay. Her mutism may be related to the anxiety problems seen in premutation carriers (Cordeiro et al., 2015), and it is usually responsive to a selective serotonin reuptake inhibitor such as sertraline as well as speech and language therapy when treatment is started early.
The reason why some premutation carriers may have more developmental problems than others remains unknown. Approximately 20% of carriers with neurodevelopmental problems, ASD or neurological problems early in life have a second genetic hit such as a copy number variant (Lozano et al., 2014). However, we did not find this to be the case in the patients reported herein. Another possibility is suggested by the observation that seizures occur in 8–16% of carriers (Chonchaiya et al., 2012; Bailey et al., 2008), and in boys with FMR1 premutation, seizures are associated with a worsening of ASD symptoms (Chonchaiya et al., 2012). That this may be a causal relationship is suggested by reports that seizures cause FMRP dysregulation (Bernard et al., 2014) and, conversely, expression of the premutation increases electrical burst activity in mouse neurons (Cao et al., 2012).
In addition, or alternatively, the medication used to treat seizures may worsen neurological symptoms in FMR1 premutation carriers. Phenobarbital is a first generation antiepileptic drug (AED) that acts primarily on the gamma aminobutyric acid (GABA) receptors to enhance inhibitory neurotransmission (Mula and Trimble, 2009). Prolonged use of phenobarbital has been associated with cognitive decline and depression in older children and adults (Mula and Trimble, 2009; Park and Kwon, 2008), and experimental animal models confirm that treatment with phenobarbital is sufficient to impair cognitive and motor behavior (Verrotti et al., 2014; Forcelli et al., 2010, 2011a,b; Bhardwaj et al., 2012). Phenobarbital is widely used in Ricaurte, Colombia, a small, relatively isolated township whose residents are mainly peasants with limited economic income, education level and access to resources and medical care (Saldarriaga et al., 2014). This is consistent with the World Health Organization (WHO) recommendations that phenobarbital be used as first-line monotherapy in lower income settings because of its relatively low cost, convenient dosing and broad spectrum of activity (World Health Organization, 2012). However, a recent clinical study of low-income patients in Zambia reported memory problems and depression associated with use of phenobarbital (Elafros et al., 2014).
The mechanism(s) by which chronic exposure to phenobarbital causes cognitive dysfunction in children and adults are thought to be related to suppression of neuronal excitability and/or enhancement of inhibitory neurotransmission (Park and Kwon, 2008). More is known about the developmental neurotoxicity of phenobarbital (Verrotti et al., 2014; Bittigau et al., 2002). In rodent models, postnatal exposure to phenobarbital causes excessive neuronal apoptosis, presumably due to its GABA mimetic actions, impairs neurogenesis, causes focal mitochondrial degeneration in diverse brain regions and dysregulates the cortical proteome (Saldarriaga et al., 2014; Elafros et al., 2014; Merola and Eubig, 2012). Exposure to phenobarbital at levels that fall within the moderate to high range of clinically relevant doses impairs the morphological and physiological maturation of both excitatory and inhibitory synapses in the rodent striatum, a brain region that displays reduced volume in human imaging studies of adults exposed to AEDs early in life (National Pesticide Information Center Agency USEP, 2015). These impairments coincide with cognitive dysfunction in weanling rodents (Forcelli et al., 2011b). Interestingly, neurons derived from the hippocampus of mice expressing Fmr1 premutations also exhibit impaired neuronal morphogenesis (Wolansky and Harrill, 2008), enhanced cell death or apoptosis (Garcia-Arocena and Hagerman, 2010), and irregularities in the number, mobility and metabolic function of mitochondria during early stages of neuronal differentiation (Kaplan et al., 2012). Such observations raise the intriguing question of whether phenobarbital converges on pathways of neurotoxicity inherent in neurons of FMR1 premutation carriers, resulting in an exacerbation of neurological symptoms.
It is noteworthy that Case 3 had no history of phenobarbital exposure but clearly presented with neurologic symptoms at an early age. Exposure to neurotoxicants have previously been suggested to exacerbate neurologic symptoms in FMR1 premutation carriers, including chemotherapy for breast cancer with carboplatin (O’Dwyer et al., 2005), occupational exposure to Agent Orange (Paul et al., 2010) or residential proximity to chemical plants emitting hexachloropentadiene, a synthetic precursor to chlorinated cyclodiene pesticides or 2,4- or 2,6-toulene diisocyanate and dichloromethane used in the manufacture of polyurethane foam (Paul et al., 2010). Many residents in Ricaurte, Colombia are chronically exposed to pesticides with neurotoxic potential. The most commonly used pesticides include avermectin (abamectin), neonicotinoids (imadacloprid, thiacopril), pyrethroids (lambdacialotrin, gamaciolotrin and deltramethrin) and organophosphorus (OP) compounds (chlorpyrifos, monocrotofos and metamidofos). Cases 1 and 2 were exposed to these pesticides daily while working in their home gardens. Case 3 did not work actively in the garden due to her hearing disability, but was in direct contact with these pesticides since they were stored inside the house and sometimes in their rooms due to the lack of space (Anon., 2015).
While avermectin (Merola and Eubig, 2012), neonicotinoids (National Pesticide Information Center Agency USEP, 2015), pyrethroids (Wolansky and Harrill, 2008) and OP pesticides (Pope, 1999) all interfere with neurotransmission in both target (insects) and non-target (human) species, the pyrethroids and OPs pose the most risk to the human brain. Pyrethroids are synthetic pyrethrins analogs with high selectivity for insects. While the canonical mechanism of action for pyrethroids is neuronal hyperexcitation as a consequence of binding to and depolarization of voltage-gated sodium channels (Bal-Price et al., 2015), bifenthrin, a type I pyrethroid, was recently demonstrated to alter synchronous Ca2+ oscillations in primary mouse cortical neurons independent of changes in voltage-gated sodium channels (Cao et al., 2014). The changes in Ca2+ oscillations were coincident with increased neurite outgrowth and both effects were blocked by a selective antagonist of mGluR5. Acute pyrethroid poisoning of humans can cause either “tremor syndrome” (type I pyrethroids) or choreoathetosis/salivation syndrome (type II pyrethroids). In a recent case study, acute ingestion of type I pyrethroids was reported to cause tonic-clonic seizures in a 19-month old female patient (Giampreti et al., 2013). However, because high doses are required to cause effects in humans, a causative role for pyrethroids in the cases described herein is questionable.
OPs are the most widely used pesticides worldwide, including Colombia (Catalina Rodríguez et al., 2013), and there is documented widespread human exposure to OPs (National Pesticide Information Center Agency USEP, 2015). The mechanism of their insecticidal activity is inhibition of acetylcholinesterase, resulting in a buildup of acetylcholine at cholinergic synapses with subsequent overstimulation of postsynaptic cholinergic receptors. While a similar mechanism of action contributes to the acute neurotoxicity of OPs in humans, emerging evidence suggests that subacute and chronic effects of OPs, including developmental neurotoxicity, may be mediated by mechanisms other than or in addition to cholinesterase inhibition (Rohlman et al., 2011; Jett and Lein, 2006). Much of the research on OP neurotoxicity has focused on chlorpyrifos, which has been linked, along with other OPs, to cognitive and affective disorders in humans after acute intoxication and following chronic exposures that do not cause cholinergic crisis (Rohlman et al., 2011; Crane et al., 2013; Freire and Koifman, 2013; Zhang et al., 2015). The mechanism(s) by which chlorpyrifos disrupts cognitive and affective behavior are not known, but experimental models demonstrate that chlorpyrifos interferes with neuronal morphogenesis in primary culture (Howard et al., 2005; Yang et al., 2008) and in vivo (Yang et al., 2011), impairs axonal transport and mitochondrial dynamics and movement in neurons (Hernandez et al., 2015; Salama et al., 2014; Middlemore-Risher et al., 2011), induces neuronal apoptosis in vitro (Caughlan et al., 2004) and activates the ryanodine receptor (Morisseau et al., 2009), a calcium ion channel embedded in the membrane of the endoplasmic reticulum that plays a critical role in modulating intracellular Ca2+ fluxes in neurons (Pessah et al., 2010). Collectively, these observations suggest the feasibility of the hypothesis that FMR1 premutation carriers have increased susceptibility to adverse neurological outcomes associated with pesticides that are neurotoxic.
All of the cases presented here had some clinical features associated with the FMR1 premutation including early menopause, seizures, learning problems, anxiety or facial features typical of carriers. It is likely that case 1 and perhaps case 2 had possible FXTAS because of deteriorating neurological function and brain atrophy combined with limited white matter disease (Hagerman and Hagerman, 2013). However, for Case 2, autopsy could not be performed to look for intranuclear inclusions, the neuropathological hallmark of FXTAS (Berry-Kravis et al., 2007; Greco et al., 2006). Because of the deteriorating function in two of these premutation carriers upon treatment with phenobarbital, it is recommended that further studies be performed at a cellular level regarding the toxicity of phenobarbital in carriers.
Patients with the FMR1 premutation appear to be more vulnerable to environmental toxicity (Paul et al., 2010; Muzar et al., 2014; Muzar et al., 2015). It has also been reported that exposure to illicit drugs can lead to earlier onset of FXTAS symptoms (Muzar et al., 2014; Muzar et al., 2015; Martínez-Cerdeño et al., 2015). Therefore counseling for premutation carriers should include the avoidance of environmental toxins discussed here (Polussa et al., 2014).
Acknowledgments
This research was supported by the United States National Institute of Child Health and Human Development (grants R01 HD036071 and U54 HD079125), the MIND Institute, United States National Institute of Environmental Health (grant P01 ES011269), the United States Environmental Protection Agency (grant R83543201) and Universidad del Valle (grant 1771). The contents of this work are solely the responsibility of the grantee and do not necessarily represent the official views of the USEPA or NIEHS. Further, the USEPA and NIEHS do not endorse the purchase of any commercial products or services mentioned in the publication.
References
- Oral communication: pesticides used for agriculture in Ricaurte. 2015. [Google Scholar]
- Bailey DB, Raspa M, Olmsted M, Holiday DB. Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. Am J Med Genet A. 2008;146A(16):2060–2069. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- Bal-Price A, Crofton KM, Sachana M, Shafer TJ, Behl M, Forsby A, et al. Putative adverse outcome pathways relevant to neurotoxicity. Crit Rev Toxicol. 2015;45(1):83–91. doi: 10.3109/10408444.2014.981331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard PB, Castano AM, Bayer KU, Benke TA. Necessary, but not sufficient: insights into the mechanisms of mGluR mediated long-term depression from a rat model of early life seizures. Neuropharmacology. 2014;84:1–12. doi: 10.1016/j.neuropharm.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis E, Abrams L, Coffey SM, Hall DA, Greco C, Gane LW, et al. Fragile X-associated tremor/ataxia syndrome: clinical features, genetics, and testing guidelines. Mov Disord. 2007;22(14):2018–2030. doi: 10.1002/mds.21493. quiz 2140. [DOI] [PubMed] [Google Scholar]
- Bhardwaj SK, Forcelli PA, Palchik G, Gale K, Srivastava LK, Kondratyev A. Neonatal exposure to phenobarbital potentiates schizophrenia-like behavioral outcomes in the rat. Neuropharmacology. 2012;62(7):2337–2345. doi: 10.1016/j.neuropharm.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittigau P, Sifringer M, Genz K, Reith E, Pospischil D, Govindarajalu S, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci. 2002;99(23):15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Hulsizer S, Tassone F, Tang H, Hagerman RJ, Rogawski MA, et al. Clustered burst firing in FMR1 premutation hippocampal neurons: amelioration with allopregnanolone. Hum Mol Genet. 2012;21(13):2923–2935. doi: 10.1093/hmg/dds118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Cui Y, Nguyen HM, Jenkins DP, Wulff H, Pessah IN. Nanomolar bifenthrin alters synchronous Ca2+ oscillations and cortical neuron development independent of sodium channel activity. Mol Pharmacol. 2014;85(4):630–639. doi: 10.1124/mol.113.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalina Rodríguez D, Carvajal S, Peñuela G. Effect of chlorpyrifos on the inhibition of the enzyme acetylcholinesterase by cross-linking in water-supply samples and milk from dairy cattle. Talanta. 2013;111:1–7. doi: 10.1016/j.talanta.2013.03.036. [DOI] [PubMed] [Google Scholar]
- Caughlan A, Newhouse K, Namgung U, Xia Z. Chlorpyrifos induces apoptosis in rat cortical neurons that is regulated by a balance between p38 and ERK/JNK MAP kinases. Toxicol Sci. 2004;78(1):125–134. doi: 10.1093/toxsci/kfh038. [DOI] [PubMed] [Google Scholar]
- Chonchaiya W, Au J, Schneider A, Hessl D, Harris SW, Laird M, et al. Increased prevalence of seizures in boys who were probands with the FMR1 premutation and co-morbid autism spectrum disorder. Hum Genet. 2012;131(4):581–589. doi: 10.1007/s00439-011-1106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro L, Abucayan F, Hagerman R, Tassone F, Hessl D. Anxiety disorders in fragile X premutation carriers: Preliminary characterization of probands and non-probands. Intractable Rare Dis Res. 2015;4(3):123–130. doi: 10.5582/irdr.2015.01029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane AL, Abdel Rasoul G, Ismail AA, Hendy O, Bonner MR, Lasarev MR, et al. Longitudinal assessment of chlorpyrifos exposure and effect biomarkers in adolescent Egyptian agricultural workers. J Expo Sci Environ Epidemiol Nat Am. 2013;23(4):356–362. doi: 10.1038/jes.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DC, Acuña JM, Sherman SL. FMR1 and the fragile X syndrome: human genome epidemiology review. Genet Med. 2001;3(5):359–371. doi: 10.1097/00125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elafros MA, Bui E, Birbeck GL. Medication side effects among people with epilepsy taking phenobarbital in Zambia. Epilepsy Res. 2014;108(9):1680–1684. doi: 10.1016/j.eplepsyres.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin F, Perry H, Hessl D, Loesch D, Cohen J, Bacalman S, et al. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr. 2006;27(Suppl 2):S137–44. doi: 10.1097/00004703-200604002-00012. [DOI] [PubMed] [Google Scholar]
- Filipovic-Sadic S, Sah S, Chen L, Krosting J, Sekinger E, Zhang W, et al. A novel FMR1 PCR method for the routine detection of low abundance expanded alleles and full mutations in fragile X syndrome. Clin Chem. 2010;56(3):399–408. doi: 10.1373/clinchem.2009.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcelli PA, Janssen MJ, Stamps LA, Sweeney C, Vicini S, Gale K. Therapeutic strategies to avoid long-term adverse outcomes of neonatal antiepileptic drug exposure. Epilepsia. 2010;3(3):18–23. doi: 10.1111/j.1528-1167.2010.02603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcelli PA, Kozlowski R, Snyder C, Kondratyev A, Gale K. Effects of neonatal antiepileptic drug exposure on cognitive, emotional, and motor function in adult rats. J Pharmacol Exp Ther. 2011a;340(3):558–566. doi: 10.1124/jpet.111.188862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcelli PA, Gale K, Kondratyev A. Early postnatal exposure of rats to lamotrigine, but not phenytoin, reduces seizure threshold in adulthood. Epilepsia. 2011b;52(4):e20–2. doi: 10.1111/j.1528-1167.2010.02971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire C, Koifman S. Pesticides, depression and suicide: a systematic review of the epidemiological evidence. Int J Hyg Environ Health. 2013;216(4):445–460. doi: 10.1016/j.ijheh.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Garcia-Arocena D, Hagerman PJ. Advances in understanding the molecular basis of FXTAS. Hum Mol Genet. 2010;19(R1):R83–9. doi: 10.1093/hmg/ddq166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giampreti A, Lampati L, Chidini G, Rocchi L, Rolandi L, Lonati D, et al. Recurrent tonic-clonic seizures and coma due to ingestion of Type I pyrethroids in a 19-month-old patient. Clin Toxicol (Phila) 2013;51(6):497–500. doi: 10.3109/15563650.2013.808747. [DOI] [PubMed] [Google Scholar]
- Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129(Pt. 1):243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- Hagerman R, Hagerman P. Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. 2013;12(8):786–798. doi: 10.1016/S1474-4422(13)70125-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman PJ. The fragile X prevalence paradox. J Med Genet. 2008;45(8):498–499. doi: 10.1136/jmg.2008.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez CM, Beck WD, Naughton SX, Poddar I, Adam BL, Yanasak N, et al. Repeated exposure to chlorpyrifos leads to prolonged impairments of axonal transport in the living rodent brain. Neurotoxicology. 2015;47:17–26. doi: 10.1016/j.neuro.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Howard A, Bucelli R, Jett D, Bruun D, Yang D, Lein P. Chlorpyrifos exerts opposing effects on axonal and dendritic growth in primary neuronal cultures. Toxicol Appl Pharmacol. 2005;207(2):112–124. doi: 10.1016/j.taap.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Jett DA, Lein PJ. Toxicology of Organophosphate and Carbamate Compounds. Elsevier; 2006. Toxicology of organophosphate and carbamate compounds; pp. 233–245. [Google Scholar]
- Kaplan ES, Cao Z, Hulsizer S, Tassone F, Berman RF, Hagerman PJ, et al. Early mitochondrial abnormalities in hippocampal neurons cultured from Fmr1 pre-mutation mouse model. J Neurochem. 2012;123(4):613–621. doi: 10.1111/j.1471-4159.2012.07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesch DZ, Huggins RM, Hagerman RJ. Phenotypic variation and FMRP levels in fragile X. Ment. Retard Dev Disabil Res Rev. 2004;10(1):31–41. doi: 10.1002/mrdd.20006. [DOI] [PubMed] [Google Scholar]
- Losh M, Klusek J, Martin GE, Sideris J, Parlier M, Piven J. Defining genetically meaningful language and personality traits in relatives of individuals with fragile X syndrome and relatives of individuals with autism. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(6):660–668. doi: 10.1002/ajmg.b.32070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano R, Hagerman RJ, Duyzend M, Budimirovic DB, Eichler EE, Tassone F. Genomic studies in fragile X premutation carriers. J Neurodev Disord. 2014;6(1):27. doi: 10.1186/1866-1955-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Cerdeño V, Lechpammer M, Lott A, Schneider A, Hagerman R. Fragile X-associated tremor/ataxia syndrome in a man in His 30s. JAMA Neurol. 2015;72(9):1070–1073. doi: 10.1001/jamaneurol.2015.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merola VM, Eubig PA. Toxicology of avermectins and milbemycins (macrocylic lactones) and the role of P-glycoprotein in dogs and cats. Vet Clin North Am Small Anim Pract. 2012;42(2):313–333. vii. doi: 10.1016/j.cvsm.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlemore-Risher ML, Adam BL, Lambert NA, Terry AV. Effects of chlorpyrifos and chlorpyrifos-oxon on the dynamics and movement of mitochondria in rat cortical neurons. J Pharmacol Exp Ther. 2011;339(2):341–349. doi: 10.1124/jpet.111.184762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisseau C, Merzlikin O, Lin A, He G, Feng W, Padilla I, et al. Toxicology in the fast lane: application of high-throughput bioassays to detect modulation of key enzymes and receptors. Environ Health Perspect. 2009;117(12):1867–1872. doi: 10.1289/ehp.0900834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mula M, Trimble MR. Antiepileptic drug-induced cognitive adverse effects. CNS Drugs. 2009;23(2):121–137. doi: 10.2165/00023210-200923020-00003. [DOI] [PubMed] [Google Scholar]
- Muzar Z, Adams PE, Schneider A, Hagerman RJ, Lozano R. Addictive substances may induce a rapid neurological deterioration in fragile X-associated tremor ataxia syndrome: a report of two cases. Intractable Rare Dis Res. 2014;3(4):162–165. doi: 10.5582/irdr.2014.01023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzar Z, Lozano R, Schneider A, Adams PE, Faradz SMH, Tassone F, et al. Methadone use in a male with the FMRI premutation and FXTAS. Am J Med Genet A. 2015;167(6):1354–1359. doi: 10.1002/ajmg.a.37030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Pesticide Information Center Agency USEP. Active Ingredient Fact Sheets [Internet] 2015 Recuperado a partir de: http://npic.orst.edu/ingred/aifact.html.
- O’Dwyer JP, Clabby C, Crown J, Barton DE, Hutchinson M. Fragile X-associated tremor/ataxia syndrome presenting in a woman after chemotherapy. Neurology. 2005;65(2):331–332. doi: 10.1212/01.wnl.0000168865.36352.53. [DOI] [PubMed] [Google Scholar]
- Park SP, Kwon SH. Cognitive effects of antiepileptic drugs. J Clin Neurol. 2008;4(3):99–106. doi: 10.3988/jcn.2008.4.3.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Pessah IN, Gane L, Ono M, Hagerman PJ, Brunberg JA, et al. Early onset of neurological symptoms in fragile X premutation carriers exposed to neurotoxins. Neurotoxicology. 2010;31(4):399–402. doi: 10.1016/j.neuro.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah IN, Cherednichenko G, Lein PJ. Minding the calcium store: ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol Ther. 2010;125(2):260–285. doi: 10.1016/j.pharmthera.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polussa J, Schneider A, Hagerman R. Molecular advances leading to treatment implications for fragile x premutation carriers. Brain Disord Ther. 2014;3 doi: 10.4172/2168-975X.1000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CN. Organophosphorus pesticides: do they all have the same mechanism of toxicity. J Toxicol Environ Health B Crit Rev. 1999;2(2):161–181. doi: 10.1080/109374099281205. [DOI] [PubMed] [Google Scholar]
- Rohlman DS, Anger WK, Lein PJ. Correlating neurobehavioral performance with biomarkers of organophosphorous pesticide exposure. Neurotoxicology. 2011;32(2):268–276. doi: 10.1016/j.neuro.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama M, El-Morsy D, El-Gamal M, Shabka O, Mohamed WM. Mitochondrial complex I inhibition as a possible mechanism of chlorpyrifos induced neurotoxicity. Ann Neurosci. 2014;21(3):85–89. doi: 10.5214/ans.0972.7531.210303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldarriaga W, Tassone F, González-Teshima LY, Forero-Forero JV, Ayala-Zapata S, Hagerman R. Fragile X syndrome. Colomb Med. 2014;45(4):190–198. [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Baker MW, Hong J, Maenner M, Greenberg J, Mandel D. Prevalence of CGG expansions of the FMR1 gene in a US population-based sample. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(5):589–597. doi: 10.1002/ajmg.b.32065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S, Welt C, Sherman S. FMR1 and the continuum of primary ovarian insufficiency. Semin Reprod Med. 2011;29(4):299–307. doi: 10.1055/s-0031-1280915. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Iklé DN, Dyer PN, Lampe M, Willemsen R, et al. FMRP expression as a potential prognostic indicator in fragile X syndrome. Am J Med Genet. 1999;84(3):250–261. [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Chamberlain WD, Hagerman PJ. Transcription of the FMR1 gene in individuals with fragile X syndrome. Am J Med Genet. 2000;97(3):195–203. doi: 10.1002/1096-8628(200023)97:3<195::AID-AJMG1037>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Tassone F, Pan R, Amiri K, Taylor AK, Hagerman PJ. A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile X (FMR1) gene in newborn and high-risk populations. J Mol Diagn. 2008;10(1):43–49. doi: 10.2353/jmoldx.2008.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrotti A, Scaparrotta A, Cofini M, Chiarelli F, Tiboni GM. Developmental neurotoxicity and anticonvulsant drugs: a possible link. Reprod Toxicol. 2014;48:72–80. doi: 10.1016/j.reprotox.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Wolansky MJ, Harrill JA. Neurobehavioral toxicology of pyrethroid insecticides in adult animals: a critical review. Neurotoxicol Teratol. 2008;30(2):55–78. doi: 10.1016/j.ntt.2007.10.005. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Mental Health Gap Action Programme (mhGAP): mhGAP EvidenceResource Center E and S. WHO|Standard antiepileptic drugs (phenobarbital, phenytoin, carbamazepine, valproic acid) for management of convulsive epilepsy in adults and children. 2012 http://www.who.int/mental_health/mhgap/evidence/epilepsy/q7/en/
- Yang D, Howard A, Bruun D, Ajua-Alemanj M, Pickart C, Lein PJ. Chlorpyrifos and chlorpyrifos-oxon inhibit axonal growth by interfering with the morphogenic activity of acetylcholinesterase. Toxicol Appl Pharmacol. 2008;228(1):32–41. doi: 10.1016/j.taap.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Lauridsen H, Buels K, Chi LH, La Du J, Bruun DA, et al. Chlorpyrifos-oxon disrupts zebrafish axonal growth and motor behavior. Toxicol Sci. 2011;121(1):146–159. doi: 10.1093/toxsci/kfr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wu M, Yao H, Yang Y, Cui M, Tu Z, et al. Pesticide poisoning and neurobehavioral function among farm workers in Jiangsu, People’s Republic of China. Cortex. 2015 doi: 10.1016/j.cortex.2015.09.006. [DOI] [PubMed] [Google Scholar]