Abstract
Limitations of preclinical models of human memory contribute to the pervasive view that rodent models do not adequately predict therapeutic efficacy in producing cognitive impairments or improvements in humans. We used a source-memory model (i.e. a representation of the origin of information) we developed for use in rats to evaluate possible drug-induced impairments of both spatial memory and higher order memory functions in the same task. Memory impairment represents a major barrier to use of NMDAR antagonists as pharmacotherapies. The scaffolding protein postsynaptic density 95kDa (PSD95) links NMDARs to the neuronal enzyme nitric oxide synthase (nNOS), which catalyzes production of the signaling molecule nitric oxide (NO). Therefore, interrupting PSD95-nNOS protein-protein interactions downstream of NMDARs represents a novel therapeutic strategy to interrupt NMDAR-dependent NO signaling while bypassing unwanted side effects of NMDAR antagonists. We hypothesized that the NMDAR antagonist MK-801 would impair source memory. We also hypothesized that PSD95-nNOS inhibitors (IC87201 and ZL006) would lack the profile of cognitive impairment associated with global NMDAR antagonists. IC87201 and ZL006 suppressed NMDA-stimulated formation of cGMP, a marker of NO production, in cultured hippocampal neurons. MK-801, at doses that did not impair motor function, impaired source memory under conditions in which spatial memory was spared. Thus, source memory was more vulnerable than spatial memory to impairment. By contrast, PSD95-nNOS inhibitors, IC87201 and ZL006, administered at doses that are behaviorally effective in rats, spared source memory, spatial memory, and motor function. Thus, PSD95-nNOS inhibitors are likely to exhibit favorable therapeutic ratios compared to NMDAR antagonists.
Keywords: source memory, spatial memory, episodic memory, NMDAR, MK-801, PSD95-nNOS small molecule inhibitors
1. Introduction
The NMDAR signaling complex represents a therapeutic target for intervention in a wide range of pathologies that are induced or worsened by excessive glutamate stimulation and resulting excitotoxicity. Clinical trials using NMDAR antagonists (e.g. ketamine and dextromethorphan), show efficacy in alleviating symptoms in a wide range of neuropathologies, including neuropathic pain [1, 2] and depression (for review see Sanacora and Schatzberg [3]). However, the efficacy of NMDAR blockade as a therapeutic intervention is undermined by the fact that high-affinity NMDAR antagonists produce debilitating cognitive side effects [2, 4]. The NMDAR signaling complex is implicated in learning and memory consistent with the presence of NMDARs in the hippocampus, a brain structure essential for memory. Inactivation of or lesions to the CA1 region of the hippocampus produces severe deficits in delay dependent spatial working memory [5]. NMDAR antagonists also produce dose dependent memory deficits [5-8]. These results underscore the adverse pharmacological effects associated with global NMDAR antagonists, consistent with the broad role of NMDAR signaling in cell physiology and function.
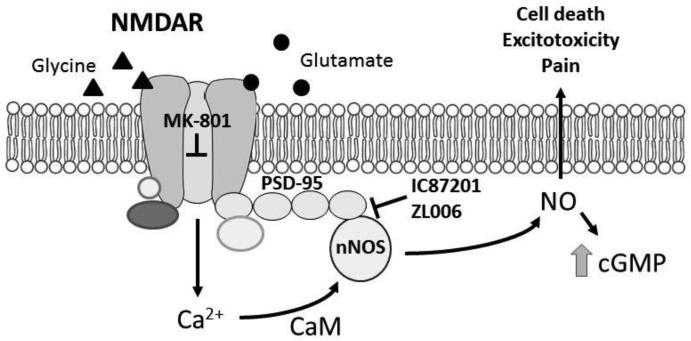
The scaffolding protein postsynaptic density 95 kDA (PSD-95) tethers the NMDAR to other proteins, forming an NMDAR signaling complex (Figure 1). The postsynaptic membrane is specialized to receive glutamate signals and, through a network of various PSD-95 protein-protein interactions, transduce these signals in the postsynaptic cell [9]. One such protein that interacts with PSD-95 is the enzyme neuronal nitric oxide synthase (nNOS), which catalyzes formation of the signaling molecule nitric oxide (NO). PSD-95 tethers nNOS to the NMDAR signaling complex. NMDAR-dependent formation of NO is implicated in the development and maintenance of numerous pathological states, including pain, depression, posttraumatic stress disorder and stroke. Thus, the PSD95-nNOS interface is a promising therapeutic target for ameliorating an array of neurological and neuropsychiatric disorders.
Figure 1.
NMDAR activation facilitates the development and maintenance of numerous pathological states by generating the signaling molecule nitric oxide (NO). MK-801, a non-competitive antagonist, prevents influx of ions through this ionotropic receptor. IC87201 and ZL006, small molecule inhibitors of PSD95-nNOS protein-protein interactions, suppress NMDAR-dependent NO and cGMP formation. We hypothesize that interrupting protein-protein interactions downstream of NMDARs represents a novel therapeutic strategy that bypasses unwanted side effects of global NMDAR activation. “T” indicates that compounds inhibit at this step.
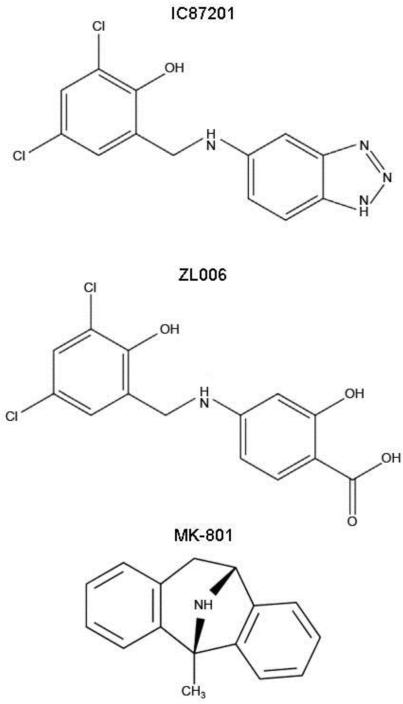
IC87201 (Figure 2) is a first in class small molecule inhibitor of PSD95-nNOS protein-protein interactions [10]. IC87201 was identified in a high-throughput screen using a binding assay consisting of the protein binding domains of PSD95 and nNOS [10]. IC87201 and a structurally related analog ZL006 (Figure 2)[12] inhibit the interaction between PSD95 and nNOS proteins in vitro [11] and attenuate glutamate-induced increases in NO production without affecting either the catalytic activity of nNOS [10, 12] or NMDAR-mediated excitatory postsynaptic currents [12]. We recently reported that IC87201 and ZL006 show efficacy in rodent models of chronic pain with similar ED50's but lack the profile of motor impairment exhibited by the NMDAR antagonist MK-801 (Figure 2)[11]. These PSD95-nNOS disruptors are brain penetrant and show efficacy in mouse models of depression [13] and rat models of conditioned fear [14]. ZL006 also decreases ischemic damage in a preclinical model of stroke and inhibits association of PSD95 with nNOS in wildtype but not nNOS−/− mice [12].
Figure 2.
Chemical structures of the small molecule PSD95-nNOS inhibitors IC87201 and ZL006 and non-competitive NMDAR antagonist MK-801.
NMDAR and nNOS play critical roles in learning and memory [12, 15, 16]. ZL006 did not produce impairment in the Morris water maze, a test of spatial memory [12]. Thus, these agents may exhibit fewer untoward side effects compared to NMDAR antagonists. However, spatial memory tests do not assess types of memory that are impaired in human diseases [17]. Whether PSD95-nNOS inhibitors spare memory functions in preclinical tasks that closely model human cognitive impairment, remains poorly understood. In the present work, we used a source memory rat model to compare the memory profile of an NMDAR antagonist (MK-801) with two novel protein-protein interaction disruptors (IC87201 and ZL006) that act downstream of NMDARs to disrupt NMDAR-dependent NO signaling.
Source memory, a representation of the origin (i.e. source) of information, is a key feature of episodic memory in humans and is hippocampal CA3 dependent [18-21]. We used our source memory model to demonstrate that episodic memories in rats are structured as bound representations, a structure similar to that of people [21]. We also showed that source memory is dissociable from spatial working memory [18-21]. Therefore, this source-memory preparation provides a unique opportunity to examine potential cognitive impairments of NMDAR antagonists and PSD95-nNOS inhibitors in a preclinical model that recapitulates critical features of human cognition. Impairments in source memory is characteristic of Alzheimer's disease [22], mild cognitive impairment [23], amnesia [24], and normal aging [25]. We also used hippocampal neuronal cultures to verify efficacy of PSD95-nNOS inhibitors in suppressing NMDA-stimulated cGMP formation, a marker of NO production in comparison with the NMDAR antagonist MK-801.
2. Methods and materials
2.1. Drugs
PSD95-nNOS inhibitors IC87201 and ZL006 were synthesized (by P.K.) in the laboratory of Ganesh Thakur. MK-801 was purchased from Sigma-Aldrich (St. Louis, MO).
2.2. In vitro methods
2.2.1. Materials for cell culture
The following reagents were obtained from commercial sources: interleukin-1 (IL-1) (Biogen Inc.); Dulbecco's Modified Eagle's Medium (DMEM) and fetal calf serum (GIBCO), transforming growth factor-@1 (TGF+1) (all from British Biotechnology), basic fibroblast growth factor (Boehringer Mannheim); H7, W7, calmidazolium, nifedipine, and Bay-K8644 (all from Research Biochemicals Inc.); (S,)- and (RJ-adenosine 3’:5’-cyclic phosphorothioate [S,)- and (&,)-CAMPS] (all from Biolog Life Science Institute, Bremen, Germany). All other reagents were from Sigma-Aldrich.
2.2.2. Cell culture
Primary hippocampal cultures were prepared from hippocampi of 17 day old Sprague-Dawley rat embryos as previously described [26] with minor modification. Briefly, hippocampi were dissected and incubated for 15 min at 37°C in Tryple Expres (Life technology). The tissue was triturated with fire-polished pipets in warm media (Neurobasal media containing B27). The cells were dissociated and collected by centrifugation, and resuspended in Neurobasal media containing B27. The cells were plated onto plastic culture dishes (0.8 × 105 cells per 96-well poly-L-lysine-coated plates) and were maintained at 37 °C, 5% CO2. Three days after plating, 50% of the medium was changed and the medium was subsequently changed every 3 to 4 days.
2.2.3. cGMP determination
In Experiment 1, typical 8 day hippocampal cortical neuron cultures were used for cGMP detection [10, 12]. Cells were washed once with magnesium free phosphate buffered saline (PBS) and pre-incubated at 37 °C for 10 minutes in the presence of PBS, MK-801 (1 μM), ZL006 (20 μM) or IC87201 (20 μM). Following pre-incubation, NMDA (100 μM) was added to stimulate the cells. The stimulation was stopped after 2 min by aspiration and the cells lysed by addition of 30 μL of 1x AlphaLisa lysis buffer with 200 uM IBMX. cGMP levels were measured by AlphaLisa assay, according to the manufacturer suggested protocol. Briefly, 10 μL of the cell lysate was incubated with 10 μL of acceptor beads for 1 h at room temperature. Donor beads with tracer were added to the above mixture and incubated for 1 h at room temperature. The AlphaLisa signal present in each well was determined using a Perkin-Elmer Enspire Alpha plate reader.
2.3. In vivo methods
2.3.1. Subjects for in vivo studies
Seven male Long-Evans rats (Rattus norvengicus; Harlan, Indianapolis, IN; ~1.5 years; 451 g., on average, at the start of the experiment) were individually housed with light onset and offset in the colony at 7 a.m. and 7 p.m. EST, respectively. One rat failed to revisit the replenishment location at initial stages of training and was excluded from Experiments 2 and 3. The rats received 45-mg chow and chocolate pellets (F0165 and F0299, respectively; Bio-Serv, French town, NJ) during experimental sessions and 15 g/day of 5012-Rat-Diet (PMI Nutrition International, St. Louis, MO) after completing each session. Water was available ad lib, except when the rat was in the maze. Prior to the onset of the present study, the rats received training in the source memory task described in Crystal and Smith [21].
2.3.2. Apparatus
An 8-arm radial maze (described in Babb and Crystal [27]) was used. The maze consisted of a central hub, eight runways, guillotine doors, and a food trough and pellet dispenser at the distal end of each arm. Experimental events, movement of guillotine doors and activation of food dispensers, were controlled by computers. During testing, the experimenter is not present in the testing room, with the exception of placement of the subject on the apparatus (described in Behavioral Procedure). Data collection resumes after the experimenter exited the testing room. Data were recorded (10-ms resolution) with MED-PC software (version 4.1); thus no subjective ratings of behavior by an experimenter are involved in assessments of either spatial memory or source memory in our task. Chocolate- and chow-flavored pellets were placed underneath each food trough in order to keep food odors constant throughout all parts of the experiment.
2.3.3. Behavioral procedure
Prior to the current study, the rats received source memory training [21]. In our source memory paradigm, rats are taught that whether or not a distinctive food item (i.e. chocolate-flavored food pellets) will replenish is based upon the method by which the rat encountered the item (i.e. the source information). Rats discovered the chocolate location either by running to the location (i.e. self-generated food seeking) or by being placed at the chocolate location (i.e. experimenter-generated food seeking). The self-generated chocolate location replenished after a retention interval, whereas the experimenter-generated chocolate location did not replenish. Other locations were baited with standard chow-flavored food, which never replenish.
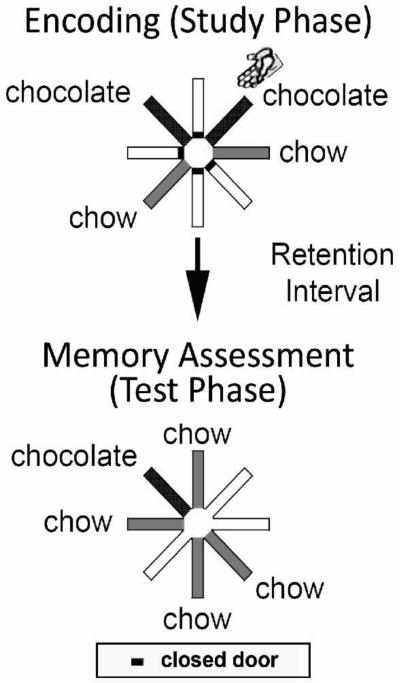
In the current study, daily unique sessions contained the following (Figure 3): a study phase (first helpings of food), a retention interval, and a test phase (second helpings of food). In the study phase (first helpings), the rats were given access to four out of eight locations. To initiate the study phase, rats were placed in the hub; orientation of the rats was pseudo random. Two of the four arms provided 1 chow pellet; the remaining two arms provided 3 chocolate pellets. Locations during the study phase did not replenish food on subsequent revisits. Controlled access to individual arms allowed the experimenter to place the rat in one of the designated chocolate arms (randomly selected); the rat was placed at the distal end of the arm near the food trough and facing the trough and dispenser. Therefore, in the study phase, each rat visited one chocolate location by running down the runway (self-generated food seeking) and the second chocolate location by being placed by the experimenter in the arm (experimenter-generated food seeking). The study phase ended when food was dispensed at all four accessible locations. The rat was removed from the maze and placed in the home cage for a retention interval of 5 minutes. The rat was returned to the central hub and all 8 arms opened to begin the test phase (second helpings). In the test phase, 1 chow pellet was provided at each of the four previously unvisited arms, and 3 chocolate pellets were provided at the self-generated chocolate location (replenishment). The other chocolate location did not replenish (nonreplenishment).
Figure 3.
A schematic of the maze used in Experiments 2 and 3. In the study phase, two randomly selected locations provide chocolate (shown in dark grey) – one encountered via self-exploration (self-generated chocolate location) and the other encountered through placement by the experimenter (experimenter-generated chocolate location; indicated by the hand icon). The experimenter-generated chocolate location replenished after a retention interval, whereas the self-generated chocolate location did not replenish. Other locations were baited with standard chow-flavored food, which never replenished.
Source memory includes information about the context in which an episodic memory was formed. In our task, the rats need to remember what occurred (chow vs. chocolate flavored food), where it occurred (location on the maze), and the source of what-where information (self-generated exploration of the maze vs. experimenter placement). Accurate source memory performance is documented by a high revisit rate to the replenishing chocolate location and a low revisit rate to the nonreplenishing chocolate location. Our index of source memory is the replenishment rate minus the nonreplenishment rate; therefore, intact source memory is documented by a positive value, and the elimination of source memory is documented by zero.
2.3.4. Drug preparation and administration for in vivo studies
MK-801 was dissolved in saline and administered intraperitoneally (i.p.) in a within subjects dosing paradigm in order of increasing dose (0.1, 0.2, and 0.3 mg/kg). IC87201 (1, 4 and 10 mg/kg) and ZL006 (10 mg/kg) were dissolved in a vehicle containing 3% DMSO with the remaining 97% comprised of 1:1:18 of emulphor (Alkamuls EL 620L; Solvay):ethanol (Sigma-Aldrich):0.9% NaCl (Aquilite System; Hospira, Inc, Lake Forest, IL). Active compounds were compared with equivalent volumes of the appropriate vehicle in each case. MK-801, IC87201, and ZL006 were administered 30 minutes prior to behavioral testing. All drugs were administered intraperitoneally (i.p.) in a volume of 1 ml/kg.
2.3.5. Experimental procedures
In Experiment 2, our objective was to determine whether the NMDAR antagonist MK-801 produced cognitive impairment in a source memory procedure, and compare the impact of this agent on both spatial and source memory performance. Both encoding and retrieval of memories occurred after drug administration. Behavioral testing was performed with an inter-trial interval of at least 48 hours within each dose condition. An inter-trial interval of one week separated testing of escalating doses. One additional rat was not included in the 0.2 mg/kg i.p. MK-801 condition due to motor impairment that prevented navigation on the maze. All rats showed motor impairment at 0.3 mg/kg i.p. MK-801, which precluded behavioral testing.
Experiment 3 was conducted to determine the possible memory-impairment profile of the PSD95-nNOS inhibitors, IC87201 and ZL006, at doses showing therapeutic efficacy in attenuating depression [15], conditioned fear [14] and pathological pain in rodents [11]. Behavioral testing was identical to Experiment 2. Behavioral testing was performed with an inter-trial interval of one week for three consecutive weeks, within dose condition. A two week interval separated conditions, during which washout trials determined that all subjects were at baseline performance. Drug-induced motor impairment was not observed following either IC87201 or ZL006 treatment, consistent with our previously published work [11].
2.3.6. Data analysis
Source memory was measured as the difference between the probabilities of a rat revisiting the replenishing (self-generated) and non-replenishing (experimenter-generated) locations. The probability of revisiting chocolate was calculated as reported in our previous work [21], as follows: The probability of at least one revisit to the chocolate location during the first five choices in the test phase was calculated (1 if at least one revisit occurred; 0 otherwise); the probability expected by chance (i.e., random arm entries) is 0.487 (calculated with a geometric distribution). For estimates of accuracy in spatial working memory (i.e. avoiding chow-flavored locations), a correct visit was defined as visiting an arm that was baited with chow in the test phase, and the analysis of the first four choices was restricted to the six non-chocolate arms; accuracy expected by chance (i.e. random arm entries) is 0.518. Memory impairment was analyzed by a repeated measure ANOVA followed by a LSD post-hoc test. All statistical analysis was performed using IBM SPSS Statistics version 22 (SPSS Inc., Chicago, IL, USA). Statistical tests were considered significant at alpha level of p = 0.05.
3. Results
3.1. IC87201 and ZL006 inhibit cGMP formation following NMDA stimulation
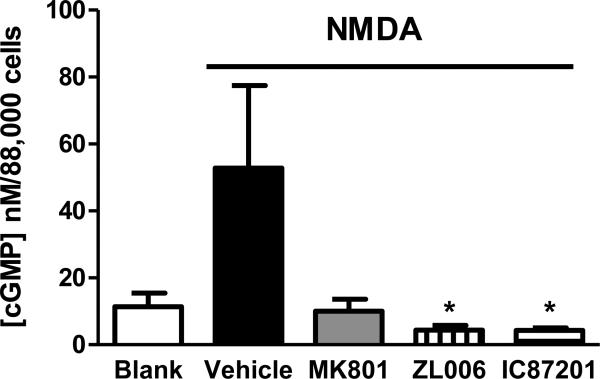
In cultured hippocampal neurons, PSD95-nNOS inhibitors IC87201 (20 μM) and ZL006 (20 μM) suppressed NMDA-stimulated cGMP formation relative to vehicle (p = 0.029; p < 0.05 for each comparison; Figure 4). MK-801 (1 μM) showed a trend towards reducing NMDA-stimulated cGMP levels in the same assay conditions (p = 0.067). NMDA-stimulated cGMP levels in cultured neurons treated with PSD95-nNOS inhibitors did not differ from levels observed following incubation with MK-801 or from basal levels determined in the absence of NMDA. Thus, PSD95-nNOS inhibitors suppress NO formation in vitro, consistent with interruption of NMDAR-dependent NO signaling by the small molecules.
Figure 4.
PSD95-nNOS inhibitors IC87201 and ZL006 suppress NMDA-stimulated cGMP formation in cultured hippocampal neurons (P < 0.03). Effects of PSD95-nNOS inhibitors did not differ from NMDAR antagonist MK-801. NMDA-stimulated cGMP levels in NMDAR antagonist and PSD95-nNOS inhibitor treated groups did not differ from basal levels, measured in absence of NMDA. *p < 0.05. Data are shown as means + SEM.
3.2. NMDAR antagonist MK-801 selectively impaired source memory under conditions in which spatial working memory was spared
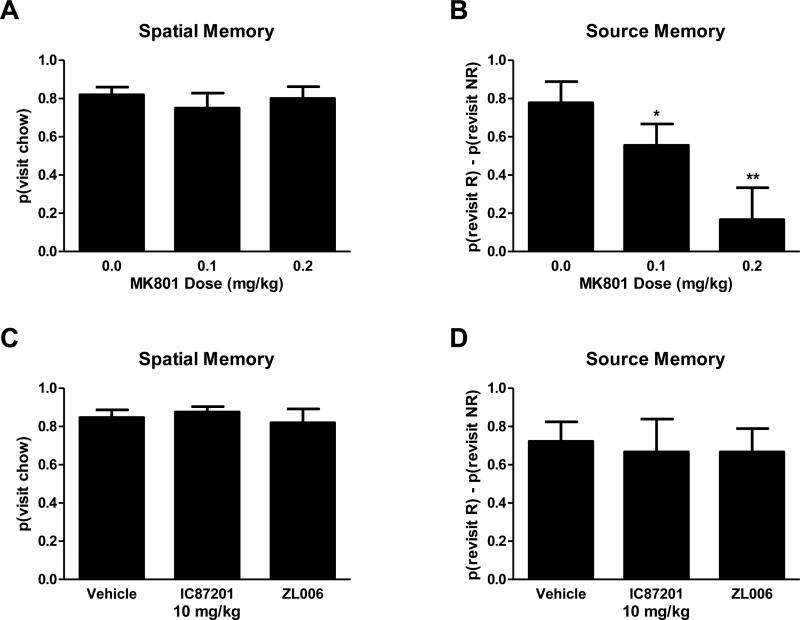
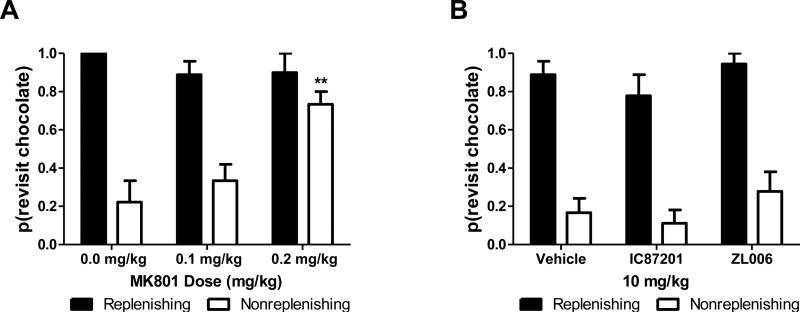
Rats used in the present study traversed the maze, indicating that motor side effects of pharmacological manipulations did not prevent completion of the radial arm maze tasks. Rats showed no impairment on measures of spatial working memory at doses of MK-801 (0.1-0.2 mg/kg; i.p.) that did not impair motor function (Figure 5A; p = 0.394). However, MK-801 dose-dependently impaired source memory relative to vehicle (Figure 5B; F(2,6) = 23.4, p = 0.001); both the low (p = 0.015) and the high dose of MK-801 (p = 0.006) impaired source memory relative to vehicle. Source memory impairment could be attributable to either forgetting the spatial or source information of the chocolate locations. Therefore, we compared the revisit probabilities between the replenishing and nonreplenishing chocolate locations. We observed a significant interaction between MK-801 dose and probability that the rat would return to the nonreplenishing chocolate location (Figure 6A; F(2,6) = 23.4, p = 0.001). At 0.2 mg/kg i.p. MK-801, the probability of revisiting the nonreplenishing chocolate location increased compared to vehicle (p = 0.006), consistent with a maximal impairment of source memory at the highest dose of the NMDAR antagonist. In all dose conditions, rats correctly revisited the chocolate locations that were about to replenish (0.97, 0.03; mean, SEM).
Figure 5.
MK-801 selectively impairs source memory, while small molecule PSD95-nNOS inhibitors IC87201 and ZL006 spared both spatial working memory and source memory. A,C. High spatial working memory performance is represented by a high revisit rate to previously unvisited chow locations. Treatment with the NMDAR antagonist MK-801, and PSD95-nNOS small molecule inhibitors IC87201 and ZL006 did not impair spatial working memory. B,D. Positive source memory scores document that rats preferentially revisit the chocolate location that is about to replenish while avoiding the non-replenishing chocolate location. *p < 0.05, **p < 0.01. Data are shown as means + SEM.
Figure 6.
Source memory impairment observed in Experiment 2 was due to MK-801-induced selective impairment of source rather than spatial information. High source memory performance is documented by a high revisit rate to the location that is about to replenish (replenishing), and a low revisit rate to the location that is not about to replenish (nonreplenishing). A. Following treatment with MK-801 (0.1-0.2 mg/kg i.p.), rats returned to the non-replenishing chocolate location. Source memory errors are shown by an increase in revisits to the non-replenishing chocolate location following MK-801 treatment. B. Following treatment with IC87201 and ZL006, rats did not return to the non-replenishing location, documenting that PSD95-nNOS inhibitors did not impair source memory. **p < 0.01. Data are shown as means + SEM.
3.3. PSD95-nNOS inhibitors spared spatial working memory and source memory
IC87201 and ZL006 did not produce impairment in either spatial working memory or source memory (Figure 5C-D; spatial working memory: p = 0.745; source memory: p = 0.942). Lack of impairment in either spatial working memory or source memory was additionally observed at doses of 1 and 4 mg/kg i.p. (data not shown), and results were identical to those observed with the high dose of IC87201 (10 mg/kg). The probability of revisiting a chocolate location did not change as a result of drug condition (Figure 6B; p = 0.095) suggesting that the source information was successfully remembered; rats revisited the chocolate location that was about to replenish and avoided the non-replenishing chocolate location. Additionally, spatial and source memory accuracy did not differ between saline and vehicle groups (p = 0.587).
4. Discussion
The NMDAR antagonist MK-801 preferentially impaired source memory under conditions in which spatial memory was spared. By contrast, PSD95-nNOS inhibitors IC87201 and ZL006 spared source memory, spatial memory, and motor function. This is the first evaluation of the impact of pharmacological manipulations – the NMDAR antagonist MK-801 and PSD95-nNOS interaction inhibitors IC8721 and ZL006 – using a paradigm (i.e. source memory) that taps into episodic memory function. In our study, MK-801 selectively impaired source memory in a dose-dependent manner. By examining the probability of revisiting the individual chocolate locations, it is apparent that the spatial information for the chocolate locations is retained as rats consistently returned to both locations to receive a second helping of chocolate. This high return rate to both chocolate locations indicates that, while the spatial information was retained, the rats were unable to store and/or retrieve the source information. We have hypothesized that our source memory task taps into the binding of information into an episodic memory of an event or series of events, which is evolutionarily old and shared between rats and humans [21]. Selective impairment of source information supports our model of source memory as a component of memory independent of spatial working memory. Additionally, as source memory was observed to be more susceptible to impairment than spatial memory, we propose that our source memory procedure is a sensitive preclinical measure for detecting undesirable side effects. Moreover, our results with MK-801 also indicate that failure of pharmacological interventions to disrupt spatial memory in the radial arm maze does not mean that memory impairment is necessarily absent.
The PSD95-nNOS inhibitors, IC87201 and ZL006, administered at doses used by our lab and others to suppress pathological pain ([11]; 2 - 10 mg/kg i.p. (data not shown)), depression [13] and conditioned fear[14], did not produce memory impairments typical of the NMDAR antagonist MK-801. In fact, IC87201 and ZL006 spared both spatial working memory and source memory. By contrast, MK-801, at doses that show comparable therapeutic efficacy as analgesics [11], produced both motor [11] and memory (present study) impairments. Our studies are consistent with work of Zhou and colleagues which suggests that ZL006 does not produce general impairments in spatial memory in the Morris water maze [12].
The lack of impairment produced by MK-801 in spatial working memory scores in the present study was unexpected as memory impairment has been repeatedly reported (0.15-0.2 mg/kg i.p.) at systemic doses similar to those used here [6, 28]. We propose that this difference is due to evaluation of doses that (1) show therapeutic efficacy and (2) do not impair motor performance (i.e. impact navigation on the maze). It should be noted that there is disagreement in the literature as to the threshold at which MK-801 produces reliable impairments in spatial working memory with doses as high as 0.33 mg/kg failing to produce impairments [8]. MK-801 reliably attenuates hyperalgesia and alleviates pain behavior at a dose of 0.1 mg/kg [2, 11, 29, 30]. However, the therapeutic range of 0.1-0.3 mg/kg is also associated with cognitive impairments, followed closely by motor impairments at doses of 0.3 mg/kg and greater [6-8, 28]. We, thus, propose that the absence of MK-801-induced impairment in spatial working memory observed here is due to our use of a therapeutically-effective low dose of MK-801 that did not impair motor behavior in our task.
Three lines of evidence support an improved therapeutic ratio for PSD95-nNOS inhibitors compared to NMDAR antagonists. First, we verified that IC87201 and ZL006 disrupt NMDA-stimulated formation of the signaling molecule nitric oxide; in cultured hippocampal neurons, the PSD95-nNOS inhibitors were highly efficacious in suppressing NMDA-stimulated cGMP formation, a marker of NO production. Second, at therapeutically effective doses, ZL006 and IC87201 failed to produce impairments in spatial working memory; this finding is consistent with previous suggestions that ZL006 does not produce spatial memory impairment in the Morris water maze [12]. Moreover, the PSD95-nNOS inhibitors spared source memory, which was eliminated by the NMDAR antagonist MK-801. Clinical trials have shown that NMDAR antagonists are efficacious in alleviating symptoms in a wide range of neuropathologies such as depression [15], traumatic brain injury, stroke, dementia, and neuropathic pain [1, 2]. NMDAR antagonists, such as ketamine and dextromethorphan, are already used widely in clinical settings to treat patients with neuropathic pain and have been proposed as antidepressants with rapid onset efficacy [3]; however, patients report adverse side effects on both cognitive and motor function [2]. By successfully avoiding impairments in memory and motor function, it is likely that PSD95-nNOS inhibitors exhibit favorable therapeutic ratios in humans compared to global NMDAR antagonists. The present work validates the use of a source memory task to study side effect profiles (i.e., memory impairment of novel therapeutic interventions) of pharmacotherapies. Collectively, our findings suggest that targeting PSD95-nNOS protein-protein interactions downstream of NMDARs will show a more limited profile of unwanted side effects compared to NMDAR antagonists.
Research Highlights.
- Source memory was more vulnerable than spatial memory to impairment in rats
- NMDAR antagonist MK-801 preferentially impaired source memory over spatial memory
- PSD95-nNOS inhibitors failed to produce spatial memory or source memory impairments
- IC87201 and ZL006 disrupt NMDA-stimulated formation of nitric oxide in vitro
- PSD95-nNOS disruptors may lack unwanted side effects of NMDAR antagonists
Acknowledgements
This work was supported by AG044530 (to JDC), MH098985 (to JDC and AGH), DA037673 (to AGH and YL), an Indiana University Collaborative Research Grant (to AGH and YL) and a Lilly Presidential Life Science Professorship (to AGH). All procedures were approved by the Bloomington Institutional Animal Care and Use Committee at Indiana University and followed national guidelines.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Funding and Disclosure
Yvonne Lai is co-founder of Anagin, LLC, otherwise, the other authors declare no conflict of interest.
References
- 1.Muir KW. Glutamate-based therapeutic approaches: clinical trials with NMDA antagonists. Curr Opin Pharmacol. 2006;6:53–60. doi: 10.1016/j.coph.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Zhou HY, Chen SR, Pan HL. Targeting N-methyl-D-aspartate receptors for treatment of neuropathic pain. Expert Rev Clin Pharmacol. 2011;4:379–88. doi: 10.1586/ecp.11.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanacora G, Schatzberg AF. Ketamine: promising path or false prophecy in the development of novel therapeutics for mood disorders? Neuropsychopharmacol. 2015;40:259–67. doi: 10.1038/npp.2014.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao YH, Tang JS, Corlett PR, Wang XY, Yang M, Chen HX, et al. Reduced Dorsal Prefrontal Gray Matter After Chronic Ketamine Use. Biol Psychiat. 2011;69:42–8. doi: 10.1016/j.biopsych.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 5.Zhang XH, Liu SS, Yi F, Zhuo M, Li BM. Delay-dependent impairment of spatial working memory with inhibition of NR2B-containing NMDA receptors in hippocampal CA1 region of rats. Mol Brain. 2013;6:13. doi: 10.1186/1756-6606-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butelman ER. A Novel Nmda Antagonist, Mk-801, Impairs Performance in a Hippocampal-Dependent Spatial-Learning Task. Pharmacol Biochem Be. 1989;34:13–6. doi: 10.1016/0091-3057(89)90345-6. [DOI] [PubMed] [Google Scholar]
- 7.Li HB, Matsumoto K, Yamamoto M, Watanabe H. NMDA but not AMPA receptor antagonists impair the delay-interposed radial maze performance of rats. Pharmacol Biochem Be. 1997;58:249–53. doi: 10.1016/s0091-3057(97)00015-4. [DOI] [PubMed] [Google Scholar]
- 8.Ward L, Mason SE, Abraham WC. Effects of the NMDA antagonists CPP and MK-801 on radial arm maze performance in rats. Pharmacol Biochem Behav. 1990;35:785–90. doi: 10.1016/0091-3057(90)90359-p. [DOI] [PubMed] [Google Scholar]
- 9.Sheng M. The postsynaptic NMDA-receptor--PSD-95 signaling complex in excitatory synapses of the brain. J Cell Sci. 2001;114:1251. doi: 10.1242/jcs.114.7.1251. [DOI] [PubMed] [Google Scholar]
- 10.Florio SK, Loh C, Huang SM, Iwamaye AE, Kitto KF, Fowler KW, et al. Disruption of nNOSPSD95 protein-protein interaction inhibits acute thermal hyperalgesia and chronic mechanical allodynia in rodents. Br J Pharmacol. 2009;158:494–506. doi: 10.1111/j.1476-5381.2009.00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee WH, Xu Z, Ashpole NM, Hudmon A, Kulkarni PM, Thakur GA, et al. Small molecule inhibitors of PSD95-nNOS protein-protein interactions as novel analgesics. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou L, Li F, Xu HB, Luo CX, Wu HY, Zhu MM, et al. Treatment of cerebral ischemia by disrupting ischemia-induced interaction of nNOS with PSD-95. Nat Med. 2010;16:1439–43. doi: 10.1038/nm.2245. [DOI] [PubMed] [Google Scholar]
- 13.Doucet MV, Levine H, Dev KK, Harkin A. Small-Molecule Inhibitors at the PSD-95/nNOS Interface have Antidepressant-Like Properties in Mice. Neuropsychopharmacol. 2013;38:1575–84. doi: 10.1038/npp.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shekar A, Fitz SD, Johnson PL, Hohmann AG, Widlanski T, Lai YY. Post-trauma disruption of nNOS-PSD95 protein-protein interaction is an effective means to ameliorate conditioned fear.. 42nd Annual Meeting of the Society-for-Neuroscience; New Orleans, LA, USA. 2012; Society for Neuroscience Abstract Viewer and Itinerary Planner; [Google Scholar]
- 15.Doucet MV, Harkin A, Dev KK. The PSD-95/nNOS complex: new drugs for depression? Pharmacol Ther. 2012;133:218–29. doi: 10.1016/j.pharmthera.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Zhou L, Zhu DY. Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide. 2009;20:223–30. doi: 10.1016/j.niox.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Crystal JD, Glanzman DL. A biological perspective on memory. Current Biology. 2013;23:R728–R31. doi: 10.1016/j.cub.2013.07.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crystal JD, Alford WT, Zhou WY, Hohmann AG. Source Memory in the Rat. Current Biology. 2013;23:387–91. doi: 10.1016/j.cub.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crystal JD. Animal models of source memory. J Exp Anal Behav. 2016;105:56–67. doi: 10.1002/jeab.173. [DOI] [PubMed] [Google Scholar]
- 20.Crystal JD, Alford WT. Validation of a rodent model of source memory. Biol Lett. 2014;10:20140064. doi: 10.1098/rsbl.2014.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crystal JD, Smith AE. Binding of episodic memories in the rat. Curr Biol. 2014;24:2957–61. doi: 10.1016/j.cub.2014.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallo DA, Chen JM, Wiseman AL, Schacter DL, Budson AE. Retrieval monitoring and anosognosia in Alzheimer's disease. Neuropsychology. 2007;21:559–68. doi: 10.1037/0894-4105.21.5.559. [DOI] [PubMed] [Google Scholar]
- 23.Schmitter-Edgecombe M, Woo E, Greeley DR. Characterizing Multiple Memory Deficits and Their Relation to Everyday Functioning in Individuals With Mild Cognitive Impairment. Neuropsychology. 2009;23:168–77. doi: 10.1037/a0014186. [DOI] [PubMed] [Google Scholar]
- 24.Gold JJ, Smith CN, Bayley PJ, Shrager Y, Brewert JB, Stark CEL, et al. Item memory, source memory, and the medial temporal lobe: Concordant findings from fMRI and memory-impaired patients. P Natl Acad Sci USA. 2006;103:9351–6. doi: 10.1073/pnas.0602716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schacter DL, Kaszniak AW, Kihlstrom JF, Valdiserri M. The Relation between Source Memory and Aging. Psychol Aging. 1991;6:559–68. doi: 10.1037//0882-7974.6.4.559. [DOI] [PubMed] [Google Scholar]
- 26.Carroll FY, Beart PM, Cheung NS. NMDA-mediated activation of the NO/cGMP pathway: Characteristics and regulation in cultured neocortical neurones. J Neurosci Res. 1996;43:623–31. doi: 10.1002/(SICI)1097-4547(19960301)43:5<623::AID-JNR12>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 27.Babb SJ, Crystal JD. Discrimination of what, when, and where: Implications for episodic-like memory in rats. Learn Motiv. 2005;36:177–89. [Google Scholar]
- 28.Haller J, Nagy R, Toth M, Pelczer KG, Mikics E. NR2B subunit-specific NMDA antagonist Ro25-6981 inhibits the expression of conditioned fear: a comparison with the NMDA antagonist MK-801 and fluoxetine. Behav Pharmacol. 2011;22:113–21. doi: 10.1097/FBP.0b013e328343d7b2. [DOI] [PubMed] [Google Scholar]
- 29.Davar G, Hama A, Deykin A, Vos B, Maciewicz R. MK-801 blocks the development of thermal hyperalgesia in a rat model of experimental painful neuropathy. Brain Res. 1991;553:327–30. doi: 10.1016/0006-8993(91)90844-l. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki R, Matthews EA, Dickenson AH. Comparison of the effects of MK-801, ketamine and memantine on responses of spinal dorsal horn neurones in a rat model of mononeuropathy. Pain. 2001;91:101–9. doi: 10.1016/s0304-3959(00)00423-1. [DOI] [PubMed] [Google Scholar]