Abstract
Viruses represent an important class of pathogens that have had an enormous impact on the health of the human race. They are extraordinarily diverse; viral particles can range in size from ~80 nanometers to ~10 microns in length, and contain genomes with RNA or DNA strands. Regardless of their genome type, RNA species are frequently generated as a part of their replication process, and for viruses with RNA genomes, their loading into virion represent a critical step in the creation of infectious particles. RNA imaging tools represent a powerful approach to gain insight into fundamental viral processes, including virus entry, replication, and virion assembly. Imaging viral processes in live cells is critical due to both the heterogeneity of these processes on a per cell basis, and the inherent dynamics of these processes. There are a number of methods for labeling RNA in live cells; we’ll introduce the myriad of methods and then focus on one approach for labeling viral RNA, using multiply-labeled tetravalent RNA imaging probes (MTRIPs), which do not require engineering of the target RNAs. We feel this approach is advantageous given many viral genomes may not tolerate large nucleotide insertions into their sequences.
Motivation for imaging RNA
There are several reasons for imaging RNA. For cellular messenger RNAs, the expression level alone, of a particular mRNA, is not the sole regulator of translation into proteins.[1] The translation and stability of an mRNA are controlled through a variety of mechanisms. The spatial localization of a particular RNA within a cell helps to control its translation and can reflect the developmental fate of a particular cell or organism, as has been observed in yeast, Drosophila melanogaster, Xenopus oocytes, and fibroblasts[2]. In addition, a RNA can be regulated by the type and number of RNA binding proteins (RBPs) or miRNAs with which it interacts. For example, ribosomal assembly and translation initiation is highly dependent on the recognition of an mRNA 5′ cap by the appropriate eukaryotic initiation factors (eIFs). These eIFs also interact with polyA binding protein to alter the structure of the mRNA to facilitate translation[3]. Another example is the RNA binding protein HuR, which binds to one or more AU rich sequences in the 3′ untranslated regions (UTRs) of mRNAs in order to stabilize them [4].
In the case of viral RNA, the regulation and specific localization of viral messenger RNAs is critical to the production of viral proteins, and the localization of genomic RNA is essential to both replication and assembly of viral particles. One important example of this is influenza virus. The proteins that help form influenza genomic ribonucleoproteins (RNPs), such as its nucleoprotein and matrix protein, assist in the regulation of the trafficking, transcription, replication, and packaging of its segmented genome, ensuring that a virion contains every segment with high fidelity[5]. Influenza genomic RNA must traverse the cytosol upon entry, enter the nucleus in order to replicate, and then, newly formed genomic RNPs must be trafficked from the nucleus to the plasma membrane, the site of virion assembly. Clearly, imaging can help preserve spatial information for a specific RNA and given sufficient resolution, can assist in elucidating the dynamics, localization, and even structures that the RNA and RBPs form.
RNA imaging techniques
Fixed and live cell RNA imaging has been pursued as a way to determine the spatial localization, dynamics, and interactions of RNA molecules. Fluorescent, fixed cell RNA imaging was popularized by fluorescence in situ hybridization (FISH)[6]. This technique involves “fixing” cells or tissue by exposing them to aldehydes or alcohols, and introducing short (~20–50 nt), antisense oligonucleotides that are labeled with fluorophores or dioxigenin. It was used by Sundell et al.[6] to show that β-actin mRNAs localize to the periphery of chicken embryonic fibroblasts when the actin cytoskeleton is intact. It has also been used by Long et al. to show the localization of lacZ induced transcripts in yeast cells, with and without deletions to introns that might inhibit nuclear export[7]. By swapping the fluorophores for gold reporters, this method was also used to determine RNA localization on actin filaments at the ultrastructural level [8]. However, many of these early fluorescence studies lacked single molecule sensitivity, which is important considering that there are far fewer mRNAs than proteins in a cell. They also were limited to observing gross temporal effects; subtler effects like motor driven transport occur on timescales that are far too short to observe using fixed cell techniques.
The issue of single molecule sensitivity was solved by Femino et al. [9] where many, multiply-labeled fluorescent 50 nt antisense DNA strands were used against an mRNA target, and later by Raj et al., where multiple single-fluorescently labeled ~20 nt antisense DNA strands were employed [10]. In both cases, the results were similar; sufficient DNA strands bind to the mRNA molecule to generate a fluorescent “puncta”, brighter than the non-specific, hazy background, and easy to detect in most microscopes. Typically, if approximately 20–30 organic fluorophores are contained within the puncta, then single RNAs can be detected.
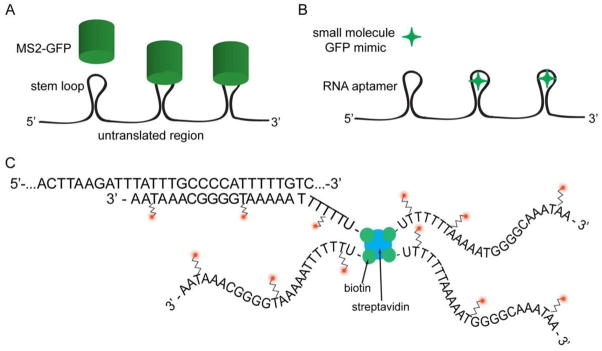
Live cell techniques solved the temporal problem of fixed cell techniques (Fig. 1). They include the MS2-GFP[11] aptamer binding system, along with λN-GFP[12], Pumilio homology domains (Pum-HD)[13], and RNA aptamers such as Spinach[14,15]. The most popular, MS2- GFP, involves transfecting cells such that they express an RNA binding protein fused to GFP (specifically MS2, a bacteriophage coat protein) along with a target mRNA that contains multiple hairpin repeats[11]. MS2-GFP utilizing six stem-loop sequences was first used by Bertrand et al. to show the localization of ASH1 mRNA to the budding tip of yeast cells [11]. In order to achieve single molecule sensitivity needed to observe both localized and non-localized mRNAs, more stem-loop repeats were needed. In the work by Fusco et al., the authors introduced 24 stem-loop repeats into inducible mRNA reporters that contained the 3′ UTR of β-actin, and imaged the mRNA at a rate of 9 Hz in order to show that mRNA can undergo directed motion at a velocity of up to 1 μm/s, contingent on an intact cytoskeletal network [16]. Similarly, the work of Shav-Tal et al. used a 24 stem-loop inducible reporter mRNA to examine nuclear transport; the motion described was much more complex than simple diffusion[17]. More recently, the MS2-GFP system was taken a step further by engineering a transgenic mouse to stably express the target mRNA engineered with 24 aptamer stem loops in its 3′-UTR [18].
Figure 1. Schematic of RNA imaging approaches.
A) MS2-GFP fusion binds to aptamer sequences encoded in the RNA. Typically 24 repeats are utilized to achieve single molecule sensitivity. B) Here a reduced form of GFP binds to a new aptamer, Spinach, and fluoresces upon binding. C) MTRIP probes for RNA imaging which use native sequences. The multiply labeled tetravalent imaging RNA probe consists of a 4 oligonucleotide arms (actual RSV probe sequence shown, with one arm hybridized to its target sequence). The oligonucleotides are internally modified to contain amines, which are then labeled with fluorophores. They are also end labeled with biotin to allow for tetramerization using streptavidin.
An alternative system to MS2-GFP is the λN-GFP system[12]. This system utilizes a 22 amino acid peptide, λN, fused to 3 copies of a GFP and a nuclear localization sequence. The peptide binds to a 15 nucleotide stem-loop called boxB that is expressed as part of a reporter mRNA, with only four repeats of boxB needed to label the mRNA. Originally described by Daigle et al., the system was used to examine the localization of reporters containing β-actin zip code protein binding sequences[12]. It was also used to examine the localization of an mRFP fused reporter containing signal recognition particle receptor subunit B, thereby allowing the authors to observe the localization of the reporter in one wavelength and the translated protein in another wavelength simultaneously. The λN-GFP system was additionally combined with MS2- GFP system by Lange et al., demonstrating the colocalization of two different mRNA species within the budding tip of yeast[19]. It should be noted though, the original work by Daigle did not demonstrate single molecule sensitivity.
Recently a new set of RNA aptamers were discovered to supplement the RBP-FP strategy discussed above. These new RNA sequences bind organic molecules mimicking the fluorophore in GFP and EGFP and were found by systematic enrichment and directed evolution[20]. These aptamers, named Spinach, Spinach2, and Broccoli, can be encoded into a reporter RNA and when the appropriate organic mimic ligand is delivered to cells and binds to the aptamer, will fluoresce[14,15,21]. These aptamers have been used in mammalian cells to track the noncoding RNA 5S. However, there are several unique caveats to using this method. First, the folding of the aptamer might be perturbed by changes in temperature, cellular ion concentration, or adjacent sequences within the reporter RNA. The sensitivity to adjacent sequences might necessitate the insertion of further nucleotides or “spacer” sequences, increasing the length of the aptamer. Also, multiple Spinach repeats are needed to achieve sequence specificity. Finally, depending on the particular reporter, post-transcriptional regulation and interactions with native RBPs might be perturbed by the presence of the aptamer, potentially changing localization. In addition this approach has not been shown to be single molecule sensitive as of yet; it is yet unknown whether sufficient repeats of the aptamer can be inserted in a RNA, and properly fold, to achieve this level of sensitivity.
There are also caveats to the MS2-GFP approach. This approach hinges on the assumption that cells can be transfected or modified to express RNAs containing aptamers. This assumption might not hold true for all mRNAs or viral RNAs in which the addition of so many repeat sequences might interfere with RNA secondary structure and RBP sites necessary for proper post-transcriptional processing. Additionally, it might change RNA: RBP stoichiometry, leading to improper regulation. As for imaging the RNAs, given that the fluorescent protein fusion is constitutively expressed, high backgrounds from unbound proteins can reduce the signal to noise ratio and make it hard to distinguish single puncta (although MS2 is typically engineering with a nuclear localization sequence to diminish this problem). For viruses, an important metric for success of any inserted sequence or co-transfection of a viral RNA, is the effect of this addition on viral titers, a measure of viral replication and assembly. These approaches, given the size of the modifications for single molecule sensitivity, may affect viral titer and this clearly has to be tested for any given virus application. In addition to the above caveats, more recently Garcia and Parker[22], described in yeast that mRNA that contain the MS2 binding aptamers are not degraded appropriately and that the degradation intermediates can bind to MS2 making image interpretation difficult. This clearly needs to be examined in mammalian cells, and with viral RNA, as it would greatly diminish the power of this approach.
In order to image unmodified RNAs, other groups have turned to using antisense oligonucleotide based probes that bind to target mRNAs using Watson-Crick pairing. One such probe is the molecular beacon (MB)[23], which consists of a hairpin-structured oligonucleotide with a fluorophore bound to one end and a quencher to the other. The 5′ and 3′ end sequences are complimentary to each other, so when there is no target present the molecular beacon exists as a stem-loop. The presence of a target RNA allows the stem to open and the beacon to bind to the target sequence, separating the fluorophore from the quencher spatially and allowing fluorescence. This system was first described by Tyagi et al., and has since been used within cells[24]. In live cells, the beacons have been microinjected to track native mRNAs in Drosophila oocytes and native β-actin mRNAs, albeit using a ratio with a control molecule beacon for the latter case[24,25]. While the idea of an activatable mRNA probe is compelling, they have two caveats for their use. First, they need to bind efficiently and with few non-specific interactions, and second, the approach still needs to be single molecule sensitive, and bright enough for trafficking studies. DNA-based MBs, because of the thermodynamic barrier to opening, do not tend to bind quickly unless the number of binding sites is very high or if microinjected into the cell at high concentration; this though limits assay throughput. Also, given MBs contain only one organic fluorophore, they typically require at least 20–30 to bind for them to be single molecule sensitive. With some specific microscopy equipment, fewer probes can bind, but the signal is noisy and will likely photobleach quickly. One approach to augment binding kinetics was to use a linear oligonucleotide, but to increase its affinity using modified bases. One example of this is the work of Molenaar et al., in which the authors used linear 2′-O- methyl RNA oligonucleotides with fluorophores to image small nuclear RNAs, rRNA, polyA mRNA, and human cytomegalovirus gene IE1 RNA within the nucleus of a live cell using microinjection[26]. It is likely that the authors targeted nuclear RNA because microinjection is able to flood the nucleus with high numbers of probes. A more delicate approach is the delivery of nucleic acids using the bacterial pore-forming toxin streptolysin-O (SLO), which was compared to traditional transfection methods using a modified oligonucleotide by Spiller et al.[27]. SLO gives probes direct access to the cytosol but limits the amount that can be delivered. Therefore, this approach favors linear probes that bind at lower concentrations within the cytosol.
Using these approaches for imaging viral RNA
To date, the most successful use of live-cell viral RNA imaging has been for human immunodeficiency virus (HIV) using the MS2 and related BglG system. Both the Hu lab at the National Cancer Institute and the Simon and Bieniasz labs at Rockefeller University have used these systems to image HIV genomic RNA dynamics and loading into viral particles using total internal reflection fluorescence (TIRF) microscopy[28–30]. It should be noted though, that in the case of Simon and Bieniasz, the infectivity of the modified virus was 10 fold less than wild type, indicating some effect on the virus. In addition to this work, MS2 has been recently applied to flavivirus localization[31]. Also, molecular beacons were used to detect bovine and human respiratory syncytial virus (RSV) genomes[32,33] as well as influenza mRNA[34], but single molecule sensitivity was not achieved using these approaches.
Given the possibility that many viral genomes may not tolerate the aptamer insertions, and single molecule sensitivity is a necessity, there was clearly a need for a new approach. This approach would have to be sensitive enough to detect single genomic RNAs, enable observation for long periods of time, and it should not significantly interfere with viral replication (as defined by changes in viral morphology and/or logarithmic decreases in titer). The multiply labeled, tetravalent RNA imaging probes (MTRIPs), first described in Santangelo et al. 2009[35] satisfy most of these criteria, as the following text should demonstrate. Furthermore, the use of this probe enables variations in chemistry and it is compatible with multiple imaging techniques.
MTRIPs applied to viral RNA imaging
MTRIP design and delivery and first uses
The Santangelo lab previously reported the use of MTRIPs for fixed and live cell imaging of both native mRNA and viral RNA, targeting the RSV genome[35,36]. The probes themselves consist of four fluorescently labeled (typically 2–3 fluorophores per oligo) oligonucleotides, end- labeled with biotin, that are complexed together with streptavidin. Each probe contains ~10 fluorophores per probe, and often 2–3 probes are utilized to target an RNA molecule. This yields approximately ~30 fluorophores per RNA, sufficient for single RNA detection, using most microscopes. The oligonucleotides are a combination of 2′-O-Methyl RNA and DNA to prevent pattern recognition receptor activation or direct RNase interactions with probes bound to target RNA. For messenger RNA, the 3′UTR is frequently targeted, avoiding miRNA and RBP sites, in order to prevent any interference with translation. In addition, no effects on RNA degradation rates have been observed[37]. Combining the modified probes with the streptolysin O (SLO) delivery enables sufficient probes to enter the cell cytoplasm and bind to cytoplasmic RNAs[38]. This design does not require the use of streptavidin, as multi-arm PEG molecules have also been used for the probe core[39], though streptavidin has certain advantages, as peptides can easily be attached to facilitate RNA-protein interaction assays[37].
A detailed protocol for the design and fluorescent labeling of the oligonucleotide probes can be found below:
Probe sequences are chosen by scanning a reference sequence for the viral genome (GenBank M74568.1 in NCBI Nucleotide database for the case of RSV). The ideal candidate sequence (or sequences) satisfy the following criteria: they are 17–20 nt in length; they have ~50% GC content; they are not complimentary to any endogeneous cellular mRNAs (according to NCBI Blast results for the host cell species); they do not spontaneously fold into secondary structures easily (according Mfold/UNAfold results at 37 °C and 150 mM sodium chloride).
Oligonucleotides are synthesized by a third party (Biosearch Technologies) with internal amines (dT-C6 amines), 2′-O-methyl modified bases, and a 5′ biotin.
Oligonucleotides are labeled with fluorophores by reacting with at least a 4X excess (per amine) of Cy3B NHS ester dye in 0.1 M sodium bicarbonate buffer pH 8 for 6 hr at room temperature.
Excess dye is removed by filtering with nuclease free water in a 3 kDa molecular weight cutoff centrifugal filter at least 5 times. The concentration and degree of labeling is assessed by absorption measurement at 260 nm (for the oligonucleotide, corrected for any dye absorption at this wavelength) and 565 nm (the absorption maximum for Cy3B). Ideally the degree of labeling should be 2–3 fluorophores per oligonucleotide.
The labeled oligonucleotides can be stored at −20 °C until further use.
Immediately before delivering the probes to infected cells (for labeling and extracting virus), the MTRIP probes are assembled according to the following protocol:
The oligonucleotides are added to streptavidin (or neutravidin in our case) in a 5:1 ratio in 1X PBS. The concentration of streptavidin is determined by the final concentration of probes to be delivered to the cells, after being diluted in reduced media (OptiMEM) and SLO. For 1 mL of 30 nM probe delivery solution, 5 μL of 30 μM oligonucleotide is added to 5 μL of 6 μM streptavidin. This mixture is incubated at room temperature for 1 hr.
The SLO must be activated by reduction. In our case, 100 μL of a 2 U/mL solution of SLO is activated by adding 1.5 μL of a 0.5M TCEP solution (Bond Breaker TCEP neutral pH, Thermo Scientific). This mixture is incubated at 37 °C for 1 hr.
After incubation, the assembled probes are filtered to removed excess oligonucleotides in a 30 kDa molecular weight cutoff centrifugal filter using 1X PBS for one spin. The remaining probe solution (~20 μL) is placed in a new tube
The reduced media (900 μL) is added to the assembled probes. The SLO solution is added to the assembled probes. This mixture is referred to as the probe delivery solution.
The cells are washed with calcium free Dulbecco’s PBS to remove any dead cells.
The delivery solution is added to the cells and allowed to incubate for 10 min at 37 °C. The delivery solution is removed and completed growth media is added to the cells. The cells are incubated for 15 min at 37 °C.
The cells can be chemically fixed and immunofluorescently stained for proteins and fixed cell imaging (Fig. 2), placed in imaging buffer for live cell imaging, or the virus can be isolated.
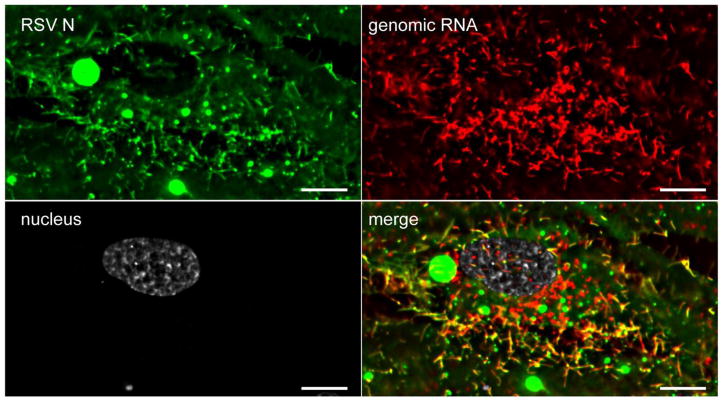
Figure 2. Image of Respiratory syncytial virus infected cell where MTRIPs were used to detect viral RNA, and immunostaining post-fixation to detect the viral nucleoprotein 24 hours post-infection with a multiplicity of infection of 1.0.
An RSV infected BJ fibroblast immnuofluorescently stained for RSV N (green) that has had MTRIP probes targeting the genomic RNA (red) delivered to it via Streptolysin O permeabilization. Nucleus also shown (white). Single plane widefield deconvolved image shown. Scale bar 10 microns.
Users should keep some concerns in mind when attempting to label any RNA with MTRIPs, or similar methods:
Underlabeling of the oligonucleotide probe or designing an insufficient number of probe sequences could result in the accumulation of less than 30 fluorophores per RNA molecule, preventing the ability to image single RNA molecules. The fluorescence intensity of single probes can be checked by adsorbing the probes to coverglass and examining the intensity distribution of the puncta.
The accuracy of the binding of delivered probes to the correct RNA must be verified against an untargeted or double stranded probe control case. It can also be checked by using FISH with probes that are labeled with a different fluorophore and checking for colocalization.
Imaging of virion structure using MTRIPs in live cells
Just as Hu, Bieniasz and Simon have been interested in HIV assembly[28–30], the Santangelo lab has similarly been interested in human respiratory syncytial virus (RSV) assembly[32,33,40,41]. RSV is a non-segmented, negative-sense RNA virus that infects humans, cows, and sheep. It causes upper respiratory tract infections in infants, immunocompromised individuals, and the elderly. The global disease burden in children under 5 years of age was 33.8 million cases, with at least 3.4 million cases requiring hospitalization. The virus causes acute lower respiratory infections (coughing, wheezing, etc.), bronchiolitis (infection and inflammation of distal bronchiolar airways), and pneumonia[42,43]. Current treatment is limited to the prophylactic administration of a monoclonal antibody against the RSV fusion protein (palivizumab) in high risk individuals and the antiviral ribavirin, which has been hampered by safety, efficacy, and cost concerns[44]. Vaccine attempts have been unsuccessful to date, possibly due to poor maturation of high affinity antibodies. The failure of a formalin inactivated vaccine in the 1960s was extremely problematic, as it actually led to enhanced disease in children when challenged[42]. Given this, we have turned our attention to studying viral assembly and replication, as it may lead to new targets for interventions. One issue with RSV has been the lack of information regarding the structure of its virion, which is pleomorphic, generating both spherical-like and filamentous virions. Liljeroos et al. reported the first structures of RSV via cryo-electron tomography (cryo-ET), but filamentous structures longer than 1 μm could not be imaged[45]. In order to assist Elizabeth Wright’s group to further investigate RSV virion structure via cryo-ET, the Santangelo lab used MTRIPs in live cells to image the RSV genome, and then utilized dSTORM imaging to more clearly interrogate the filamentous virion at ~20–30 nm resolution in the xy plane, and approximately ~50 nm along the z-axis[41,46].
The protocol used in Alonas et al. [41] for labeling and isolating the virions from cells is detailed below:
Inoculate a T75 flask of 100% confluent HEp-2 cells with RSV A2 virus at a multiplicity of infection (MOI) of 0.01 in 3 mL of serum free media. Incubate for 1 hr at 37 °C
Add 12 mL of complete media after inoculation.
Add 5mL of complete media for each day until the cells have achieved >90% cytopathological effect (CPE) by phase microscopic inspection (approximately 4 days post infection).
Deliver MTRIP probes at 100 nM concentration in a 3 mL final volume according to the aforementioned delivery protocol.
After adding the complete media, wash the cells once in complete media to help remove unbound probes.
To extract virus, scrape entire growth surface with cell scraper. Wash the growth surface multiple times using a serological pipette and the media in the flask
Aliquot and freeze the media at −80 °C.
It is important to note that this protocol is specific to RSV, which remains mostly cell associated in lab culture conditions. Other viruses that bud effectively from cells might not require further purification.
The most important concern after labeling any virus is to verify the infectivity remains similar to the unlabeled, wild-type (within an order of magnitude). This can be done multiple ways:
Titering the virus by plaque assay.
Determining the viral mRNA transcripts (qRT-PCR) made in cells infected with labeled or unlabeled virus at a few timepoints post infection.
Determining the amount of protein (Western blot and/or ELISA) made in cells infected with labeled or unlabeled virus at a few timepoints post infection.
Determining the morphology of the virion produced in cells infected with labeled or unlabeled virus using transmission electron microscopy (TEM).
All of these verification assays were done for RSV, demonstrating that labeling with MTRIPs did not affect the infectivity[41].
Once the virus is labeled and the infectivity is verified, it is possible to examine individual virions on glass (Fig. 3), using both conventional widefield fluorescence microscopy and super resolution localization microscopy. For RSV, we were particularly interested in the distribution of the genomic RNA along the filamentous virions. However, the filamentous virions had to be isolated from cell debris as much as possible before attempting to immunofluorescently stain them. The protocol used for filtering and isolating viral filaments on coverglass can be found below:
Figure 3. Viral filaments imaged on glass coverslips.
MTRIP probes were introduced into RSV infected cells and the virus was isolated, filtered, adsorbed to glass, and immnunofluorescently stained for the N and F proteins according to the protocol in this review. Each individual channel and a merged image are shown. Widefiled deconvolved fluorescence images, scale bar 5 μm.
Clean coverslips with 70% ethanol and place in 24 well plate. Add 0.5 mL poly-L-lysine per well and incubate for 1 hr at 37 °C.
Thaw frozen virus aliquot in water bath at 37 °C.
Place virus 5 μm membrane centrifugal filter and spin at 5,000 x g for 4 min.
Place filtered virus in 0.45 μm membrane centrifugal filter and spin at 5,000 x g for 1 min.
Add 200 μL media to the top of the filter and pipette up and down to retrieve the virus from the top of the filter.
Wash poly-L-lysine off of the coverslips with PBS and add virus.
Centrifuge virus onto coverslips using plate rotor spinning at 1,000 x g for 30 min at 4 °C.
Remove media from coverslips, fix virus with 4% paraformaldehyde at room temperature for 10 min, permeabilize, block, and begin immunostaining.
A few concerns that the reader should keep in mind when attempting this protocol are:
The membrane filtering pertains to isolating only cell-associated filamentous virions, like RSV. It is important to check that most of the infectivity is preserved after the filtering process by plaque assay.
Either voxel based or blob-based colocalization with multiple markers should be performed in order to increase the certainty that a viral particle is in fact an infectious virion.
Multiple fields of view from each sample can then be imaged using conventional widefield fluorescence microscopy or super resolution localization microscopy (e.g. dSTORM). For the imaging of RSV using dSTORM, we showed that the distribution of N and the genomic RNA vary, suggesting that not all N proteins might be bound to the genomic RNA. In contrast, the fusion protein (F) was evenly distributed along the filament. Also, we found that the matrix (M) and M2-1 proteins were also evenly distributed along the length of the filament, but the M2-1 protein was in a layer closer to the center of the virion, and might serve as an adaptor between the genomic RNA and the M layer[41,46].
Imaging of virus entry and early replication events
The imaging of single virions can reveal entry pathways and early replication events. The ability to label infectious virions in an innocuous manner is a pre-requisite to imaging. For RSV, we used MTRIP labeled virions to infect cells and watch for filament fusion, possible compartmentalization, and early replication of the genomic RNA. A protocol for the inoculation and imaging of single filaments as they enter into cells is shown below:
Grow HEp-2 or Vero cells into a semi-confluent layer in a 35 mm coverglass-bottomed dish.
Chill cells on ice at 4 °C for 20 min.
Filter labeled virus according to the aforementioned filtering protocol. Reconstitute to at least 1 mL volume in chilled serum free media.
Add filtered, labeled virus to cells and spin in centrifuge at 500 x g for 30 min at 4 °C to inoculate virus.
Remove viral media and wash cells with chilled media before adding warm growth media and transferring to a live cell imaging stage that is preheated to 37 °C.
Locate cells and virions of interest and begin recording images.
Once the images are recorded, the processing of the single filament images took place as follows:
Track single filaments using manual tracking (Volocity, Perkin Elmer).
-
Export position and velocity data for all filaments into MATLAB and run a custom script to do the following:
Segment the particle tracks over a rolling window of 4 minutes
Calculate the velocities and the radius of gyration (an extent parameter) over each segment.
Compile the segments into histograms and compared them to a threshold for active and passive transport
It is important for the reader to note that:
During the initial moments of recording images, it is possible, depending on the live cell chamber utilized on the microscope, that a temperature dependent transient will be present due to the warming of the dish in the live cell chamber. In our experimental setup, we found this to occur during the first 5 minutes of recording. Data from this period was discarded from the analysis. This should be determined for each individual experimental setup prior to acquiring any data.
The threshold between active and passive transport can be determined by carefully simulating the two transport modes via Monte Carlo for a particle that is of a similar size and shape to the virions.
For the case of RSV, we found the temporal duration of active states had median time 6.3 × 10−2 s, indicating that the motion of filaments was largely diffusive [41].
The above live cell imaging protocol can be adapted for assessing the compartmentalization of viral RNA, either by fixing and immunofluorescently staining the cells at various timepoints, or by also transducing the cells to express a fluorescent protein fusion. For RSV, we checked colocalization of viral RNA and proteins via staining with EEA1 (early endosomes), CD63 (late endosomes), and LAMP1 (lysosomes) and also performed live cell imaging in cells transduced with Rab5-GFP. Labeled genomic RNA from filamentous virion did not appear to interact with any of these markers, suggesting the RSV filament fusion occurs before any compartmentalization[41]. This type of assay can be used to compare the virus of interest to other viruses whose entry pathway is better understood (e.g. the endocytosis of influenza).
Additionally, the live cell imaging of cells in the process of being infected with MTRIP labeled virus can also be used to observe cells slightly later in the infection. However, it is recommended to attempt this with subconfluent cells that have been grown on a patterned surface to restrict their migration. For imaging RSV at 2, 6, and 10 hr post infection, we plated HEp-2 cells on 35 mm coverslip bottomed dishes with 50 μm fibronectin islands patterned on to them. By delivering MTRIPs labeled with a different fluorophore, we were able to measure the replication of the genomic RNA and show that replication was confined to certain regions in the cytoplasm[41].
Future work using MTRIPs to interrogate RSV will include determining the factors that influence genomic RNA loading into viral filaments and also elucidate the role of M2-1 during replication, specifically how it interacts directly with the genome during viral mRNA production.
Conclusions
Imaging RNA using either unmodified targets and MTRIPs or MS2-GFP, etc., for modified targets depends very much on whether the genome of your virus or mRNA generated by the virus can tolerate insertions. Some genomes have a significant amount of secondary structure, which could be perturbed by large genomic insertions. In general, the infectivity and titer of the virus should be checked no matter what approach is being used. In general, RNA imaging is a powerful tool for the study of viral processes, which open the door to new interventions against a number of pathogens which we currently have few defenses.
Highlights.
Summarizes techniques for imaging single RNA molecules in cells using FISH, MS2-GFP, Spinach aptamers, and multiply labeled tetravalent imaging probes (MTRIPs).
Protocols are provided for using MTRIPs to label the genomic RNA of respiratory syncytial virus (RSV).
The labeled virus can be used for single virion and single cell imaging via multiple imaging approaches.
Acknowledgments
Funding for this work was provided by NIH R01GM094198 (P.J.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schwanhäusser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, et al. Global quantification of mammalian gene expression control. Nature. 2011;473:337–342. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- 2.Martin KC, Ephrussi A. mRNA localization: gene expression in the spatial dimension. Cell. 2009;136:719–730. doi: 10.1016/j.cell.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simone LE, Keene JD. Mechanisms coordinating ELAV/Hu mRNA regulons. Curr Opin Genet Dev. 2013;23:35–43. doi: 10.1016/j.gde.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisfeld AJ, Neumann G, Kawaoka Y. At the centre: influenza A virus ribonucleoproteins. Nat Rev Microbiol. 2015;13:28–41. doi: 10.1038/nrmicro3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sundell CL, Singer RH. Requirement of microfilaments in sorting of actin messenger RNA. Science. 1991;253:1275–1277. doi: 10.1126/science.1891715. [DOI] [PubMed] [Google Scholar]
- 7.Long RM, Elliott DJ, Stutz F, Rosbash M, Singer RH. Spatial consequences of defective processing of specific yeast mRNAs revealed by fluorescent in situ hybridization. Rna. 1995;1:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 8.Bassell GJ, Powers CM, Taneja KL, Singer RH. Single mRNAs visualized by ultrastructural in situ hybridization are principally localized at actin filament intersections in fibroblasts. The Journal of Cell Biology. 1994;126:863–876. doi: 10.1083/jcb.126.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Femino AM, Fay FS, Fogarty K, Singer RH. Visualization of single RNA transcripts in situ. Science. 1998;280:585–590. doi: 10.1126/science.280.5363.585. [DOI] [PubMed] [Google Scholar]
- 10.Raj A, van den Bogaard P, Rifkin SA, van Oudenaarden A, Tyagi S. Imaging individual mRNA molecules using multiple singly labeled probes. Nat Meth. 2008;5:877–879. doi: 10.1038/nmeth.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertrand E, Chartrand P, Schaefer M, Shenoy SM, Singer RH, Long RM. Localization of ASH1 mRNA particles in living yeast. Mol Cell. 1998;2:437–445. doi: 10.1016/s1097-2765(00)80143-4. [DOI] [PubMed] [Google Scholar]
- 12.Daigle N, Ellenberg J. LambdaN-GFP: an RNA reporter system for live-cell imaging. Nat Meth. 2007;4:633–636. doi: 10.1038/nmeth1065. [DOI] [PubMed] [Google Scholar]
- 13.Ozawa T, Natori Y, Sato M, Umezawa Y. Imaging dynamics of endogenous mitochondrial RNA in single living cells. Nat Meth. 2007;4:413–419. doi: 10.1038/nmeth1030. [DOI] [PubMed] [Google Scholar]
- 14.Paige JS, Nguyen-Duc T, Song W, Jaffrey SR. Fluorescence imaging of cellular metabolites with RNA. Science. 2012;335:1194–1194. doi: 10.1126/science.1218298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strack RL, Disney MD, Jaffrey SR. A superfolding Spinach2 reveals the dynamic nature of trinucleotide repeat-containing RNA. Nat Meth. 2013;10:1219–1224. doi: 10.1038/nmeth.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fusco D, Accornero N, Lavoie B, Shenoy SM, Blanchard JM, Singer RH, et al. Single mRNA molecules demonstrate probabilistic movement in living mammalian cells. Curr Biol. 2003;13:161–167. doi: 10.1016/s0960-9822(02)01436-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shav-Tal Y, Darzacq X, Shenoy SM, Fusco D, Janicki SM, Spector DL, et al. Dynamics of single mRNPs in nuclei of living cells. Science. 2004;304:1797–1800. doi: 10.1126/science.1099754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grünwald D, Singer RH. In vivo imaging of labelled endogenous β-actin mRNA during nucleocytoplasmic transport. Nature. 2010;467:604–607. doi: 10.1038/nature09438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lange S, Katayama Y, Schmid M, Burkacky O, Bräuchle C, Lamb DC, et al. Simultaneous transport of different localized mRNA species revealed by live-cell imaging. Traffic. 2008;9:1256–1267. doi: 10.1111/j.1600-0854.2008.00763.x. [DOI] [PubMed] [Google Scholar]
- 20.You M, Jaffrey SR. Structure and Mechanism of RNA Mimics of Green Fluorescent Protein. Annu Rev Biophys. 2015;44:187–206. doi: 10.1146/annurev-biophys-060414-033954. [DOI] [PubMed] [Google Scholar]
- 21.Filonov GS, Moon JD, Svensen N, Jaffrey SR. Broccoli: rapid selection of an RNA mimic of green fluorescent protein by fluorescence-based selection and directed evolution. J Am Chem Soc. 2014;136:16299–16308. doi: 10.1021/ja508478x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia JF, Parker R. MS2 coat proteins bound to yeast mRNAs block 5″ to 3″ degradation and trap mRNA decay products: implications for the localization of mRNAs by MS2-MCP system. Rna. 2015;21:1393–1395. doi: 10.1261/rna.051797.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyagi S, Kramer FR. Molecular beacons: probes that fluoresce upon hybridization. Nat Biotechnol. 1996;14:303–308. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]
- 24.Bratu DP, Cha BJ, Mhlanga MM, Kramer FR, Tyagi S. Visualizing the distribution and transport of mRNAs in living cells. Proc Natl Acad Sci USa. 2003;100:13308–13313. doi: 10.1073/pnas.2233244100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tyagi S, Alsmadi O. Imaging native beta-actin mRNA in motile fibroblasts. Biophysical Journal. 2004;87:4153–4162. doi: 10.1529/biophysj.104.045153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molenaar C, Marras SA, Slats JC, Truffert JC, Lemaître M, Raap AK, et al. Linear 2′ O-Methyl RNA probes for the visualization of RNA in living cells. Nucleic Acids Research. 2001;29:E89–9. doi: 10.1093/nar/29.17.e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spiller DG, Giles RV, Grzybowski J, Tidd DM, Clark RE. Improving the intracellular delivery and molecular efficacy of antisense oligonucleotides in chronic myeloid leukemia cells: a comparison of streptolysin-O permeabilization, electroporation, and lipophilic conjugation. Blood. 1998;91:4738–4746. [PubMed] [Google Scholar]
- 28.Sardo L, Hatch SC, Chen J, Nikolaitchik O, Burdick RC, Chen D, et al. Dynamics of HIV-1 RNA Near the Plasma Membrane during Virus Assembly. Journal of Virology. 2015;89:10832–10840. doi: 10.1128/JVI.01146-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Nikolaitchik O, Singh J, Wright A, Bencsics CE, Coffin JM, et al. High efficiency of HIV-1 genomic RNA packaging and heterozygote formation revealed by single virion analysis. Proceedings of the National Academy of Sciences. 2009;106:13535–13540. doi: 10.1073/pnas.0906822106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jouvenet N, Simon SM, Bieniasz PD. Imaging the interaction of HIV-1 genomes and Gag during assembly of individual viral particles. Proceedings of the National Academy of Sciences. 2009;106:19114–19119. doi: 10.1073/pnas.0907364106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miorin L, Maiuri P, Marcello A. Visual detection of Flavivirus RNA in living cells. Methods. 2015 doi: 10.1016/j.ymeth.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santangelo PJ, Bao G. Dynamics of filamentous viral RNPs prior to egress. Nucleic Acids Research. 2007;35:3602–3611. doi: 10.1093/nar/gkm246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santangelo P, Nitin N, LaConte L, Woolums A, Bao G. Live-cell characterization and analysis of a clinical isolate of bovine respiratory syncytial virus, using molecular beacons. Journal of Virology. 2006;80:682–688. doi: 10.1128/JVI.80.2.682-688.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang W, Cui ZQ, Han H, Zhang ZP, Wei HP, Zhou YF, et al. Imaging and characterizing influenza A virus mRNA transport in living cells. Nucleic Acids Research. 2008;36:4913–4928. doi: 10.1093/nar/gkn475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santangelo PJ, Lifland AW, Curt P, Sasaki Y, Bassell GJ, Lindquist ME, et al. Single molecule sensitive probes for imaging RNA in live cells. Nat Meth. 2009;6:347–349. doi: 10.1038/nmeth.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lifland AW, Zurla C, Yu J, Santangelo PJ. Dynamics of native β-actin mRNA transport in the cytoplasm. Traffic. 2011;12:1000–1011. doi: 10.1111/j.1600-0854.2011.01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jung J, Lifland AW, Zurla C, Alonas EJ, Santangelo PJ. Quantifying RNA-protein interactions in situ using modified-MTRIPs and proximity ligation. Nucleic Acids Research. 2013;41:e12. doi: 10.1093/nar/gks837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walev I, Palmer M, Valeva A, Weller U, Bhakdi S. Binding, oligomerization, and pore formation by streptolysin O in erythrocytes and fibroblast membranes: detection of nonlytic polymers. Infect Immun. 1995;63:1188–1194. doi: 10.1128/iai.63.4.1188-1194.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lifland AW, Zurla C, Santangelo PJ. Single Molecule Sensitive Multivalent Polyethylene Glycol Probes for RNA Imaging. Bioconjugate Chem. 2010;21:483–488. doi: 10.1021/bc9003876. [DOI] [PubMed] [Google Scholar]
- 40.Shaikh FY, Cox RG, Lifland AW, Hotard AL, Williams JV, Moore ML, et al. A critical phenylalanine residue in the respiratory syncytial virus fusion protein cytoplasmic tail mediates assembly of internal viral proteins into viral filaments and particles. mBio. 2012;3 doi: 10.1128/mBio.00270-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alonas E, Lifland AW, Gudheti M, Vanover D, Jung J, Zurla C, et al. Combining single RNA sensitive probes with subdiffraction-limited and live-cell imaging enables the characterization of virus dynamics in cells. ACS Nano. 2014;8:302–315. doi: 10.1021/nn405998v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res. 2011;162:80–99. doi: 10.1016/j.virusres.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collins PL, Graham BS. Viral and Host Factors in Human Respiratory Syncytial Virus Pathogenesis. Journal of Virology. 2008;82:2040–2055. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meng J, Stobart CC, Hotard AL, Moore ML. An overview of respiratory syncytial virus. PLoS Pathog. 2014;10:e1004016. doi: 10.1371/journal.ppat.1004016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liljeroos L, Krzyzaniak MA, Helenius A, Butcher SJ. Architecture of respiratory syncytial virus revealed by electron cryotomography. Proceedings of the National Academy of Sciences. 2013;110:11133–11138. doi: 10.1073/pnas.1309070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kiss G, Holl JM, Williams GM, Alonas E, Vanover D, Lifland AW, et al. Structural Analysis of Respiratory Syncytial Virus Reveals the Position of M2-1 Between the Matrix Protein and the Ribonucleoprotein Complex. Journal of Virology. 2014;88:7602–7617. doi: 10.1128/JVI.00256-14. [DOI] [PMC free article] [PubMed] [Google Scholar]