Abstract
Euthanasia by anesthetic agents is commonly performed prior to tissue collection in order to minimize pain and distress to the animal. However, depending on their mechanism of action as well as administration regimen, different methods of anesthesia may trigger an acute stress response through engaging the hypothalamic-pituitary-adrenal (HPA) axis, which can impact numerous other physiological processes that the researcher may wish to examine as endpoints. We investigated the effects of the commonly used anesthetic agent isoflurane on two different endpoints related to the stress response: plasma corticosterone levels and gene expression of the glucocorticoid receptor (GR) as well as several of its regulators including FK506-binding protein 51 (Fkbp5) in the hippocampus of male and female rats. Our results indicate that brief exposure to anesthesia by isoflurane prior to decapitation can alter plasma corticosterone levels differentially in male and female rats within minutes without impacting gene expression in the hippocampus. We conclude that collection methods can influence stress-related physiological endpoints in female rats and the potential influence of even brief anesthesia as well as sex differences in response to anesthesia should be evaluated during the experimental design process and data interpretation. This finding is particularly important in light of new NIH standards regarding sex and reproducibility, and care should be taken to be certain that sex differences in endpoints of interest are not an artifact of sex differences in response to collection paradigms.
Keywords: acute stress, isoflurane, anesthesia, corticosterone, glucocorticoid receptor, FKBP5
Introduction
The stress response can profoundly impact functional outcomes due to the regulatory role of stress-related systems in other physiological processes in the body. In studies examining terminal endpoints in particular, the effects of the stress response triggered by tissue collection methods must be distinguished from those of the experimental manipulations. In many areas of biomedical research utilizing animals, sedation and/or euthanasia by anesthetic agents is commonly performed prior to tissue collection in order to minimize pain and distress to the animal. However, depending on their particular mechanisms of action as well as administration regimen, different methods of anesthesia may induce undetected alterations in physiology through engaging the stress endocrine system [1, 2]. These subtle changes may subsequently impact numerous other physiological processes that may include the endpoints of focus, thus producing a confounding effect on the metrics of interest. Therefore, when designing a study, the effects of anesthetic agents and tissue collection methods should be considered regarding not only the desired experimental endpoints but also stress-related outcomes. While anesthetics like isoflurane are often used in the performance of euthanasia in laboratory settings, certain anesthetic agents have reported neurotoxic effects [3, 4]. Even if present in the system for a brief period before euthanasia, anesthetic agents may cause persistent effects in tissue and potentially affect cellular processes [5]. Many of the common anesthetic agents modulate ion channels such as the N-methyl D-aspartate (NMDA) and the γ-aminobutyric acid A (GABAA) receptors and likely downstream signaling molecules important in neuronal survival and cell death. Therefore, humane methods of euthanasia that do not require anesthesia, such as rapid decapitation, have been used whenever possible in neurochemical studies.
Despite the potential effects of anesthetic agents on the experimental outcome of interest, one must strive to minimize animal pain and distress. The American Veterinary Medical Association makes the following statement regarding physical means of euthanasia: “When properly used by skilled personnel with well-maintained equipment, physical methods of euthanasia may result in less fear and anxiety and be more rapid, painless, humane, and practical than other forms of euthanasia”[6]. The physical means of euthanasia most applicable to laboratory research are cervical dislocation, decapitation, and microwave irradiation [5]. However, cervical dislocation is not suitable for large rats due to the need for increased handling as well as reports that it induces more stress than decapitation in 6-month-old rats [7]. Considering the fact that microwave irradiation requires the use of specially designed equipment, rapid decapitation remains a commonly used physical method of euthanasia without the use of anesthesia.
Here we compared the effects of the anesthetic agent isoflurane followed by euthanasia to those of rapid decapitation on two different endpoints related to the stress response: plasma corticosterone levels and gene expression of the several genes involved in mediating the stress response. Expression of the glucocorticoid receptor (GR, gene name: Nr3c1) – the main effector of the HPA axis-mediated stress response – as well as its negative regulator FK506-binding protein 51 (gene name: Fkbp5) and positive regulators FK506-binding protein 52 (gene name: Fkbp4) and peptidyl-prolyl isomerase D (gene name: Ppid) were examined in the hippocampus, a brain region densely populated with GR and sensitive to the effects of stress [8]. Although isoflurane can alter gene expression patterns in the brain over the course of several hours or days [9, 10], it is not clear whether it can induce rapid changes in the expression of genes involved in the stress response in the span of minutes – a question particularly relevant for studies using isoflurane as anesthesia prior to euthanasia. Although the impact of isoflurane on serum hormone and brain cholinesterase levels have been examined in male and female rats [1], to our knowledge, the current study is the first to investigate isoflurane’s effects on stress-related gene expression in the male and female rat brain. Our results indicate that anesthesia by isoflurane prior to decapitation can alter stress-related hormone concentrations differentially in male and female rats without impacting gene expression in the brain.
Materials and Methods
Animals
Adult Wistar male and female rats (n = 12–13, 300–400 g) were purchased from Charles River Laboratories (Wilmington, MA), housed in an AAALAC-approved facility under standard laboratory conditions, and maintained on a 14:10 reverse light:dark cycle with free access to food and water. The Emory University Institutional Animal Care and Use Committee approved all animal use procedures. Animal experimentation was carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experimental Design: Rapid Decapitation vs. Isoflurane Anesthesia Prior to Decapitation
Prior to the start of the experiments, rats were randomly assigned into one of three treatment groups (n = 4–5 per group). All animals were allowed to sit undisturbed in a quiet environment for 3 hours following removal from the animal facility and prior to euthanasia. Collection took place at least 3 hours prior to the end of the animals’ light cycle. A separate room was designated for isoflurane induction and decapitation each, and both of these rooms were separated from the rat holding room. One animal was euthanized at a time, and the induction chamber was cleaned with 70% ethanol and water between each animal. Rats were either euthanized by rapid decapitation using a guillotine (RD, no anesthesia) or exposed to one of two different dosing regimens of the anesthetic isoflurane (Slow or Fast induction) prior to decapitation by guillotine. The slow-induction regimen consisted of exposing each individual rat to an induction chamber with 2% isoflurane (in oxygen) for 2 min followed by 5% isoflurane for 3 min. Rats in the fast-induction group were exposed individually to 5% isoflurane (in oxygen) in an induction chamber for 5 minutes. Animals in the isoflurane groups were euthanized by decapitation after making sure the animal was completely unresponsive to tail pinch. The personnel were kept constant throughout the entirety of the experiment.
Blood and Tissue Collection
Following euthanasia by rapid decapitation, trunk blood was immediately collected from animals in all groups and centrifuged at 1800 rcf. Plasma was then transferred into fresh tubes and stored at -80°C until used for hormone analysis. At the same time as blood collection, brains were rapidly removed, flash frozen on dry ice, and stored at −80°C until they were used for quantitative RT-PCR analyses.
Endocrine Analyses
Total plasma corticosterone levels were assayed using the ELISA kit purchased from Enzo Life Sciences (sensitivity: 27 pg/ml, Farmingdale, NY, USA) according to the manufacturer’s instructions. Samples were run in duplicates, and the coefficient of variance among the duplicates was less than 15%.
Quantitative RT-PCR
The hippocampus was dissected under RNAse-free conditions, homogenized, and RNA was extracted using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. RNA integrity was assessed by a Nano-Drop 2000 spectrophotometer (ThermoScientific, Wilmington, DE, USA) and RNA samples were reverse transcribed using the High Capacity RNA to cDNA Kit (Applied Biosystems, Foster City, CA, USA). To ensure uniform amounts of total cDNA across groups, cDNA was quantified via the PicoGreen Assay (Invitrogen, Carlsbad, CA), then standardized so that all samples started quantitative RT-PCR with 1 μg cDNA. The rat gene Gapdh was determined to be the optimal endogenous control based on an inter-group variance of less than 10% across RD, Slow induction, and Fast induction groups. Primers for Fkbp5 (forward: TGGAGGTGAACCCTCAGAAC, reverse: TCTTGCTCAATGCTTTGCTG), Nr3c1 (forward: GGAAGGTCTGAAGAGCCAAG, reverse: GATGATTTCAGCTAACATCTCTGG), Fkbp4 (forward: GGGAAGGAAAGGTTCCAGAT, reverse: AGTACACGGTGCCCCTTTCT), Ppid (forward: AGAACCCGCGAGTCTTCTTT, reverse: GCAGAGCTTTCCCAGTTGTC), and Gapdh (forward: GAGGTGACCGCATCTTCTTG, reverse: CCGACCTTCACCATCTTGTC) were designed and purchased from Applied Biosystems (Foster City, CA). The universal two-step RT-PCR cycling conditions used on the 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA) were: 50°C (2 min), 95°C (10 min), 40 cycles of 95°C (15 s) and 60°C (1 min). Samples were run in triplicate, and the coefficient of variation within the triplicates was no more than 4%. Individual samples within each group were also analyzed through Grubb’s test for statistical outliers. Fold changes in gene expression were calculated by the comparative 2−ΔΔCT quantification method relative to the RD group.
Statistical Analysis
A two-way ANOVA with sex and treatment as main factors were used for assessment of corticosterone concentrations. Planned comparisons were conducted within females given established knowledge regarding greater stress-responsivity in females than males. Gene expression was normalized to the RD group within each sex, and separate one-way ANOVAs were performed for males and females. The alpha value was set to 0.05, and GraphPad Prism 6.0 was used for all statistical analyses.
Results
Isoflurane elevates plasma corticosterone in female rats
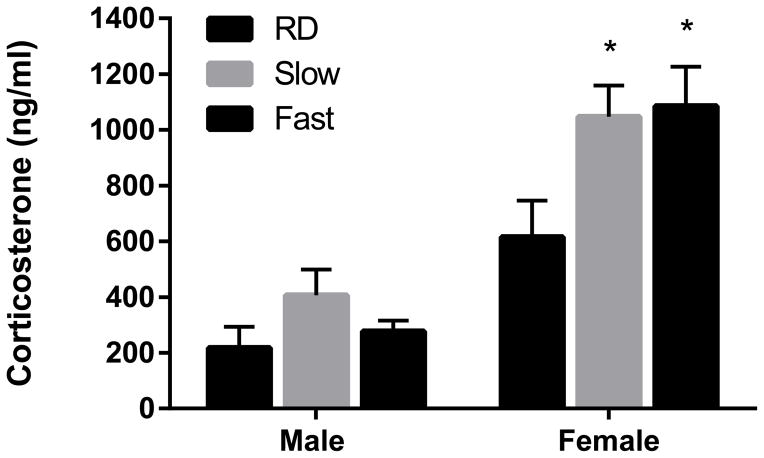
A two-by-three analysis of variance (male vs female X RD vs. Slow vs. Fast induction) demonstrated a main effect of sex [F(1,19) = 47.45, p < 0.0001]. Females displayed significantly higher plasma corticosterone levels [M =915.81, SD=260.79] compared to males [M =300.49, SD=96.4]. Results also indicated a main effect of treatment [F(2,19) = 4.571, p < 0.05] but no interaction [F(1,19) = 1.799, p > 0.05)] (Fig. 1). Subsequently, a Tukey’s multiple comparison test showed that plasma corticosterone levels of females in both the Slow and Fast induction groups were significantly elevated compared to that of the RD condition (p < 0.05). No significant effect of either isoflurane regimen was detected in males (p > 0.05).
Figure 1. Anesthesia by brief exposure to isoflurane prior to decapitation elevates total plasma corticosterone in female, but not male, rats.
Females displayed significantly higher total plasma corticosterone compared to males (p < 0.0001). In addition, a main effect of treatment was discovered (p < 0.05) but no interaction effect (p > 0.05). A Tukey’s post-hoc test showed that in females, both Slow and Fast induction treatments increased total plasma corticosterone compared to total plasma corticosterone in the RD condition (p < 0.05). No effect of isoflurane was detected in males (p > 0.05). RD, rapid decapitation (n = 4); Slow induction by isoflurane (n = 4), Fast induction by isoflurane (n = 5). Data are presented as mean ± SEM. * p < 0.05 (Tukey’s multiple comparison test) compared to the same-sex RD group.
Isoflurane does not impact hippocampal expression of genes related to the stress response
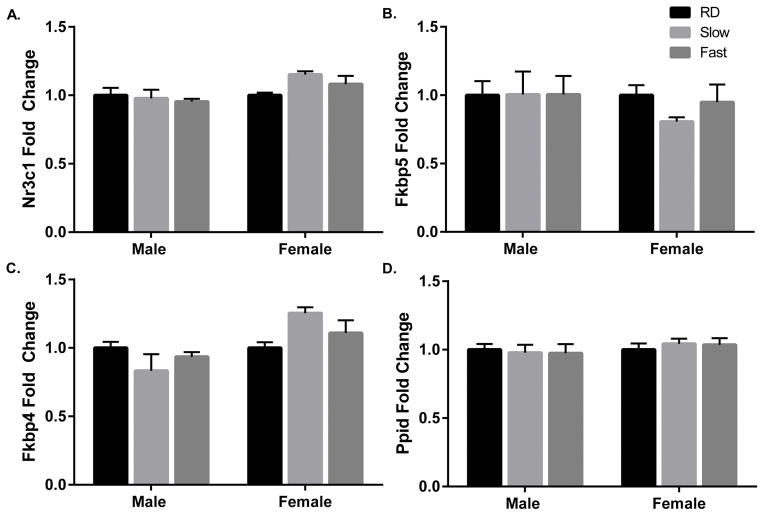
Results of one-way ANOVAs indicated no effect of isoflurane treatment within either sex on hippocampal expression of Nr3c1 (males: F(2,9) = 0.2125, p = 0.8125; females: F(2,10) = 2.721, p = 0.1139; Fig. 2A), Fkbp5 (males: F(2,9) = 0.00063, p = 0.9994; females: F(2,10) = 0.9985, p = 0.4024; Fig. 2B), Fkbp4 (males: F(2,9) = 1.181, p = 0.3505; females: F(2,10) = 3.031, p = 0.0936; Fig. 2C), and Ppid (males: F(2,9) = 0.05994, p = 0.7992; females: F(2,10) = 0.2292, p = 0.9422; Fig. 2D).
Figure 2. Anesthesia by brief exposure to isoflurane prior to decapitation does not impact hippocampal expression of stress-related genes immediately following induction.
Expressions of Nr3c1 (A), Fkbp5 (B), Fkbp4 (C), and Ppid (D) were normalized to that of the endogenous control Gapdh, and fold changes in gene expression are expressed relative to the mean expression of the same-sex, RD group. RD, rapid decapitation (n = 4); Slow induction by isoflurane (n = 4); Fast induction by isoflurane (n = 5). Data are presented as mean fold change 2−ΔΔCt ± SEM.
Discussion
Here we investigated the effects of a brief general anesthesia by isoflurane prior to euthanasia by decapitation on stress-related hormone and gene expression in the blood and brain of adult male and female rats. The data demonstrate that isoflurane elevated plasma corticosterone levels of female, but not male, rats. Our findings are consistent with those of several other studies using male rats which have reported no changes in corticosterone levels following similar exposures to isoflurane [2, 11, 12]. A substantial body of literature shows that female rats display greater HPA axis activity including higher corticosterone levels compared to males at both baseline and in reaction to an acute stressor [13–15]. Therefore, it is possible that the isoflurane-induced increase in plasma corticosterone in female rats demonstrated here reflects this underlying sex difference of HPA axis activity. We also found that a brief exposure to isoflurane does not impact the hippocampal expression of several stress response-related genes including the GR, a ligand-activated transcription factor, and its regulators FKBP5, FKBP4, and PPID. Upon binding of corticosterone, GR translocates into the nucleus and regulates transcriptional pathways involved in a variety of physiological processes [8]. The proteins examined here regulate GR’s activity at ligand binding (FKBP5) as well as nuclear translocation (FKBP5, FKBP4, and PPID), and would therefore be expected to be involved in corticosterone signaling [16].
Although changes in gene expression in the brain have been reported following isoflurane exposure in the span of several hours or even days [9, 10], many studies report no impact on gene expression within minutes of isoflurane exposure. For example, the expression of the immediate early genes c-fos and c-jun were unchanged in the brain following 5 minutes of 2% isoflurane in male rats compared to unanesthetized rats [17]. Similarly, a 1-minute exposure to 4% isoflurane did not alter the expression of eight representative genes in the rat hippocampus [18]. The same isoflurane treatment also did not alter the expression of corticotrophin-releasing hormone, arginine vasopressin and oxytocin in the rat hypothalamus [2]. The lack of change in hippocampal expression of stress-related genes despite an increase in plasma corticosterone levels in female rats is likely observed due to the relatively short period of time from the onset of isoflurane exposure to the time of euthanasia. Specifically, the time delay in peripheral levels of corticosterone to reach deeper brain structures such as the hippocampus and for gene transcription to be initiated upon binding of corticosterone can account for the divergent corticosterone and gene expression findings in female rats. In addition to time, insufficient power may account for the lack of a statistical difference in expression of Fkbp4 and Nr3c1 in females after the slow induction paradigm and type II error cannot be ruled out in the case of these two genes. The divergent patterns of expression among the genes, although not significant in any case, illustrate that future studies should assess the potential for sex differences in anesthesia exposure on a case by case basis when establishing the experimental design and control groups.
The extent of isoflurane-induced changes to corticosterone concentrations can be substantially less than that induced by other forms of anesthesia including CO2 [1]. However, our data demonstrate that even isoflurane can induce a robust increase in plasma corticosterone in female rats. We conclude that collection methods can influence stress-related physiological endpoints differentially in male and female rats and the potential influence of even brief anesthesia should be evaluated during the experimental design process and data interpretation. This finding is particularly important in light of new NIH standards regarding sex and reproducibility and care should be taken to be certain that sex differences in endpoints of interest are not an artifact of sex differences in response to collection paradigms.
Highlights.
Rapid anesthesia by isoflurane may impact stress-related markers.
Plasma corticosterone and hippocampal gene expression were examined in rats.
Brief exposure to isoflurane elevated corticosterone in female, but not male, rats.
Expression of the glucocorticoid receptor and its regulators were unchanged.
Possible interactions of sex and anesthesia should be evaluated in study designs.
Acknowledgments
This research was funded by R01NR014886 from the National Institute of Nursing Research (GNN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Deckardt K, et al. The effects of inhalation anaesthetics on common clinical pathology parameters in laboratory rats. Food Chem Toxicol. 2007;45(9):1709–18. doi: 10.1016/j.fct.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Wu XY, et al. Effect of pentobarbital and isoflurane on acute stress response in rat. Physiol Behav. 2015;145:118–21. doi: 10.1016/j.physbeh.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Clarkson AN. Anesthetic-mediated protection/preconditioning during cerebral ischemia. Life Sci. 2007;80(13):1157–75. doi: 10.1016/j.lfs.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 4.Soriano SG, Anand KJ. Anesthetics and brain toxicity. Curr Opin Anaesthesiol. 2005;18(3):293–7. doi: 10.1097/01.aco.0000169238.36927.c2. [DOI] [PubMed] [Google Scholar]
- 5.Karmarkar SW, Bottum KM, Tischkau SA. Considerations for the use of anesthetics in neurotoxicity studies. Comp Med. 2010;60(4):256–62. [PMC free article] [PubMed] [Google Scholar]
- 6.Gianaros PJ, et al. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. Neuroimage. 2007;35(2):795–803. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venkataraman BV, Shetty PS, Joseph T. Variations in brain and heart acetylcholine content in rat: cervical dislocation vs guillotine technique. Indian J Physiol Pharmacol. 1981;25(3):289–91. [PubMed] [Google Scholar]
- 8.De Kloet ER, et al. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19(3):269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 9.Pekny T, et al. Short general anaesthesia induces prolonged changes in gene expression in the mouse hippocampus. Acta Anaesthesiol Scand. 2014;58(9):1127–33. doi: 10.1111/aas.12369. [DOI] [PubMed] [Google Scholar]
- 10.Rampil IJ, Moller DH, Bell AH. Isoflurane modulates genomic expression in rat amygdala. Anesth Analg. 2006;102(5):1431–8. doi: 10.1213/01.ane.0000202384.96269.51. [DOI] [PubMed] [Google Scholar]
- 11.Desaulniers D, et al. Effects of anesthetics and terminal procedures on biochemical and hormonal measurements in polychlorinated biphenyl treated rats. Int J Toxicol. 2011;30(3):334–47. doi: 10.1177/1091581810397774. [DOI] [PubMed] [Google Scholar]
- 12.Zardooz H, et al. Plasma corticosterone, insulin and glucose changes induced by brief exposure to isoflurane, diethyl ether and CO2 in male rats. Physiol Res. 2010;59(6):973–8. doi: 10.33549/physiolres.931896. [DOI] [PubMed] [Google Scholar]
- 13.Kant GJ, et al. Comparison of stress response in male and female rats: pituitary cyclic AMP and plasma prolactin, growth hormone and corticosterone. Psychoneuroendocrinology. 1983;8(4):421–8. doi: 10.1016/0306-4530(83)90021-5. [DOI] [PubMed] [Google Scholar]
- 14.Lesniewska. Sex Differences in Adrenocortical Structure and Function. 1990 [Google Scholar]
- 15.Weinstock M, et al. Gender differences in sympathoadrenal activity in rats at rest and in response to footshock stress. Int J Dev Neurosci. 1998;16(3–4):289–95. doi: 10.1016/s0736-5748(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 16.Galigniana NM, et al. Regulation of the glucocorticoid response to stress-related disorders by the Hsp90-binding immunophilin FKBP51. J Neurochem. 2012;122(1):4–18. doi: 10.1111/j.1471-4159.2012.07775.x. [DOI] [PubMed] [Google Scholar]
- 17.Hamaya. The Effects of Pentobarbital, Isoflurane, and Propofol on Immediate-Early Gene Expression in the Vital Organs of the rat. doi: 10.1097/00000539-200005000-00034. [DOI] [PubMed] [Google Scholar]
- 18.Staib-Lasarzik I, et al. Anesthesia for euthanasia influences mRNA expression in healthy mice and after traumatic brain injury. J Neurotrauma. 2014;31(19):1664–71. doi: 10.1089/neu.2013.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]